Examining the UV-vis absorption of RAFT chain transfer agents and their use for polymer analysis†
Katja
Skrabania
a,
Anna
Miasnikova
b,
Achille Mayelle
Bivigou-Koumba
b,
Daniel
Zehm
b and
André
Laschewsky
*ab
aFraunhofer Institute for Applied Polymer Research, Geiselberg-Str.69, 14476, Potsdam-Golm, Germany. E-mail: laschewsky@iap.fraunhofer.de; Fax: +49 331 568 3000; Tel: +49 331 568 1327
bDepartment of Chemistry, Universität Potsdam, Karl-Liebknecht-Str.24-25, 14476, Potsdam-Golm, Germany. E-mail: laschews@rz.uni-potsdam.de; Fax: +49 331 977 5036; Tel: +49 331 977 5225
First published on 17th June 2011
Abstract
The absorption characteristics of a large set of thiocarbonyl based chain transfer agents (CTAs) were studied by UV-vis spectroscopy in order to identify appropriate conditions for exploiting their absorbance bands in end-group analysis of polymers prepared by reversible addition–fragmentation chain transfer (RAFT) polymerisation. Substitution pattern and solvent polarity were found to affect notably the wavelengths and intensities of the π–π*- and n–π*-transition of the thiocarbonyl bond of dithioester and trithiocarbonate RAFT agents. Therefore, it is advisable to refer in end group analysis to the spectral parameters of low molar mass analogues of the active polymer chain ends, rather than to rely on the specific RAFT agent engaged in the polymerisation. When using appropriate conditions, the quantification of the thiocarbonyl end-groupsvia the π–π* band of the thiocarbonyl moiety around 300–310 nm allows a facile, sensitive and surprisingly precise estimation of the number average molar mass of the polymers produced, without the need of particular end group labels. Moreover, when additional methods for absolute molar mass determination can be applied, the quantification of the thiocarbonyl end-groups by UV-spectroscopy provides a good estimate of the degree of active end group for a given polymer sample.
Introduction
Controlled free radical polymerisation (CRP) methods have evolved rapidly in the past decade. Among them, reversible addition–fragmentation chain transfer (RAFT) is one of the most prominent methods.1–3 The RAFT process relies on the reversible degenerative chain transfer in the presence of especially designed thiocarbonylthio compounds to control the polymerisation. The efficiency of a chain transfer agent to control the polymerisation is determined by the substituents of the thiocarbonylthio moiety: the Z group activates the thiocarbonyl bond towards radical addition while the R group reinitiates the polymerisation.2–8 In consequence of the polymerisation mechanism, both R and Z groups are incorporated as α- and ω-end groups, respectively, in the final polymer. Whereas the R-group is permanently attached to the α-terminus of the polymer chain by a stable C–C– bond, the Z group is attached to the ω-end terminus via the labile thiocarbonylthio moiety. The thiocarbonyl group, and thus the Z-group, may be gradually lost due to the inherent termination reactions, or due to side reactions of the thiocarbonyl moiety,9e.g. by UV-irradiation,10hydrolysis,11–15 or by oxidation.16,17 Nevertheless, a high degree of ω-end-group functionalisation is generally achieved under appropriate conditions.18–20The synthetic potential of thiocarbonylthio end-groups has been exploited,21,22 for example in photopolymerisations,23 in cycloadditions,24,25 or by transforming the thiocarbonylthio groups into thiols13,26–35 and a variety of other telechelic functionalities.17,22,32,36–40 CTA functionalisation can also be advantageously used in 1H NMR for polymer characterisation.14,19,41–44 In fact, the determination of the molar mass poses problems for many polymers with whatsoever method. The currently preferred method of size exclusion chromatography (SEC) generally lacks appropriate calibration standards: only a few polymers such as polystyrene or poly(methyl methacrylate) benefit from a correct calibration. The problem becomes even more severe for copolymers or polymers of complex architecture. The molar mass value of star polymers, for example, is underestimated by SEC analysis when using linear standards. Hence, appropriate labeling of the R and/or Z group of the RAFT agent can be advantageous for molar mass determination through end group analysis, in particular via1H NMR spectroscopy. Recently, we demonstrated that aromatic or aliphatic trimethylsilyl groups are excellent candidates for this purpose.19,20,45 Such 1H-NMR based molar mass analysis is precise up to molar masses of at least 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, but still, the relative intensity of the end group signal becomes too weak for very large polymers. Also, the extra effort for implementing such special labels may be cumbersome, and as trimethylsilyl groups are bulky and strongly hydrophobic, they can pose problems in strongly polar solvents.45,46
000, but still, the relative intensity of the end group signal becomes too weak for very large polymers. Also, the extra effort for implementing such special labels may be cumbersome, and as trimethylsilyl groups are bulky and strongly hydrophobic, they can pose problems in strongly polar solvents.45,46
Alternatively, the absorption of thiocarbonyl groups in the UV-vis range allows a straightforward method for end-group analysis. This group is inherently present in all RAFT agents, and thus, RAFT generated polymers and macroRAFT agents are usually coloured in yellow, orange or red shades. The colouration derives from the weak absorption of the thiocarbonyl group in the visible range due to a forbidden n–π*-transition. Additionally, the thiocarbonyl moiety absorbs light in the near UV range due to an allowed π–π* transition. This UV band is a priori applicable to analyse high molar mass polymers because of the strong absorptivity of the transition. Hence, the thiocarbonylthio functionality can be used as an intrinsic chromophore label attached to the polymer chain.12,14,47,48 As polymers prepared by the RAFT technique bear at least one thiocarbonyl moiety per chain, this provides a convenient analytical tool for determining the molar mass by end-group analysis.
In fact, the absorption of the thiocarbonylthio end groups was exploited to study the RAFT pre-equilibrium,49 to confirm the presence or absence of RAFT end-groups,42,50 or to prove the homogeneous concentration of active end-groups over the whole molar mass distribution in size exclusion chromatography (SEC).51 Still, though a priori very convenient, studies that exploit the absorption of thiocarbonylthio groups in order to derive the absolute number average molar mass Mn are surprisingly rare yet.12,14,42,48,52–57
Apart from the assumption of full end group functionalisation, the quantification of end groups on the basis of Lambert–Beer's law has been mostly based on the approximation that λmax and the molar absorptivity ε do not change when the thiocarbonyl moiety is transferred from the primary CTA to the polymer chain. However, it has not been verified whether this assumption is true. There are at least two basic limitations: first, the substitution pattern of the thiocarbonylthio moiety can notably change in the course of the polymerisation when the R-group is replaced by the growing polymer chain, and therefore, the spectral characteristics may be modified. Second, the UV-vis absorption of the thiocarbonyl moiety may be affected by the polarity of the microenvironment, to which mostly the solvent contributes. However, it is not always possible to use the same solvent for the RAFT agent and the polymer.
Hence we explored the effect of substitution pattern and solvent polarity on the absorption characteristics of a large set of dithioester and trithiocarbonate RAFT agents (Fig. 1), complementing the results with literature data.58–64 Emphasis was put on RAFT agents, whose R groups are structurally similar to the repeating units found in common polymers, such as polystyrenes, poly(meth)acrylates and polyacrylamides (see Fig. 2).
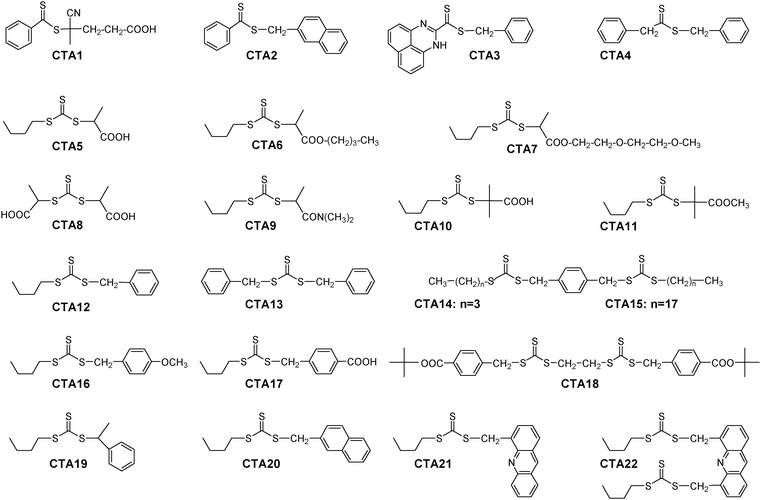 | ||
| Fig. 1 Structures of the studied thiocarbonylthio compounds. | ||
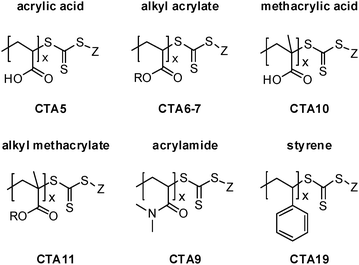 | ||
| Fig. 2 The structure of typical polymer chain ends, resulting from the use of trithiocarbonate RAFT agents. | ||
Experimental section
Materials
Experimental details for all materials used and the synthesis of the new RAFT agents and polymers are given in the ESI†. The syntheses of RAFT agents CTA1,1CTA4,65CTA5,66,67CTA6,48CTA8,68CTA10,69CTA12,51,55CTA13,27,42,70CTA14,42 and CTA1671 were reported before.Methods
1H and 13C NMR spectra were taken with an apparatus Bruker Avance 300. The chemical shifts are referenced to the respective solvent residual peak72 (CHCl3 7.26 ppm; DMSO 2.50 ppm). Elemental analysis was carried out using a Vario ELIII microanalyzer (Elementar Analysensysteme, Germany). IR-spectra were taken from KBr pellets using a FT-IR spectrometer Bruker IFS 66/s. Mass spectra were recorded by a GC/MS-system Trace DSQII (Thermo Scientific).SEC was run at 25 °C in THF or DMF (flow rate: 1.0 mL min−1) using a TSP apparatus (Thermo Separation Products from Thermo-Finnigan GmbH, Dreieich, Germany) equipped with a Shodex RI-71 refractive index detector, a TSP UV detector (260 nm), and a set of PSS SDV columns (styrene/divinyl benzene, 100 Å and 1000 Å porosity, 5 μm particle size). Polystyrene standards (PSS GmbH Mainz, Germany) were used for calibration.
The UV-vis absorption characteristics of the synthesised CTAs were studied by determining their maximum absorbance wavelengths λmax and the molar absorptivities ε of the π–π*- as well as the n–π*-transitions of the thiocarbonyl bond. All solvents were of spectrophotometric grade. The λmax values were reproducible within ±1 nm. The molar absorptivities ε at λmax were determined by a linear fit of absorbance vs. concentration (correlation coefficients (R2 ≥ 0.99)).
Knowing the molar absorptivity ε of the chromophore, and assuming the presence of exactly one trithiocarbonate moiety in every polymer chain, the number average molar mass of polymers made by the RAFT method can be derived. The concentration of the trithiocarbonate chromophore, and thus of the polymer, in a solution is calculated using the Beer–Lambert law:
| c = A/(l × ε) | (1) |
| c = m/(Mn × V) | (2) |
| Mn = (m × l × ε)/(A × V) | (3) |
Results and discussion
Structure of the chain transfer agents (CTAs) studied
The chemical structure of the RAFT agents studied (Fig. 1) comprises the two main classes employed so far, namely dithioesters (CTA1–CTA4) and trithiocarbonates (CTA5–CTA22). The new CTAs were synthesised by alkylation of the anions of the corresponding dithiocarboxylic and trithiocarbonic acids. The latter were prepared by the nucleophilic addition of thiolates to CS2. Alternatively, a modified ketoform reaction was applied in selected cases.73 For CTA2, the Grignard route to dithiobenzoic acid was used. The underlying dithiocarboxylic acid of CTA3, 1H-perimidine dithiocarbonic acid,74 was obtained by oxidising the corresponding benzylic halide with a mixture of sulfur and triethylamine in DMF (Fig. 3).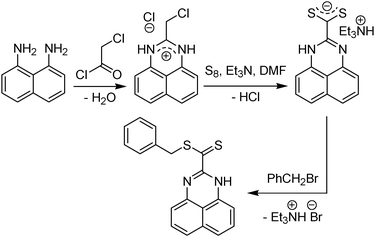 | ||
| Fig. 3 Synthetic route to RAFT agent CTA3. | ||
For the dithioesters studied, the Z groups were either phenyl (CTA1 and CTA2), perimidinyl in the case of CTA3, or benzyl for CTA4. CTA1 has been frequently used for RAFT polymerisations of (meth)acrylic and styrenic monomers.18 After deprotonation of the carboxylic acid functionality, this agent can be used for polymerisations in water.1,75 RAFT agents such as CTA4 are recommended to minimise retardation effects in the polymerisation of acrylic monomers.76–78 The intensely blue coloured perimidinyl derivative CTA3 may be seen alternatively as a derivative of 1,1-dithiooxalic acid, and thus represents a substitution pattern that so far has not been used in the RAFT polymerisation process.
For trithiocarbonates CTA5–CTA22, alkylsulfanyl, mostly n-butylsulfanyl moieties were chosen for the Z group. The analogues CTA14 and CTA15 are distinguished by the length of hydrophobic alkylsulfanyl moieties. While the R groups of CTA10–CTA11 are based on the 2-methyl propanoate-2-yl motif, in the case of CTA5–CTA9 they are based on the propanoate-2-yl motif, and in the case of CTA2–CTA4 as well as of CTA12–CTA22 they all derive from the benzyl motif. The latter two R-motifs are known to be particularly effective for the RAFT polymerisation of acrylic monomers.8,18,79 In analogy to dithioester CTA1, trithiocarbonates CTA5, CTA8 and CTA10 are useful for RAFT polymerisations in aqueous media, by virtue of their carboxylic acid moieties. The tertiary amide CTA9 may be also used for aqueous systems, as for similar RAFT agents,75 while the other RAFT agents are all strongly hydrophobic and thus aimed at the use in organic solvents. RAFT agents CTA2 and CTA20 contain both the naphthalene chromophore in their re-initiating R groups, while CTA21 and CTA22 contain the acridine chromophore. The absorbance bands of the R groups of the latter in the UV range are sufficiently resolved from the thiocarbonyl band to allow their separate analysis (Fig. 4). The new CTA3 is characterised by a strongly red-shifted and more intense n–π*-transition compared to the usual dithioesters, which allows a facile visual control of the presence of the Z group. Note that RAFT agents CTA8 and CTA13 are symmetrically substituted trithiocarbonates bearing two R groups, which allow for synthesising ABA triblock copolymers in two steps only. The same is true for the bis(trithiocarbonate)s CTA14, CTA15, CTA18, and CTA22, which bear two unsymmetrically substituted dithiocarbonyl moieties each.
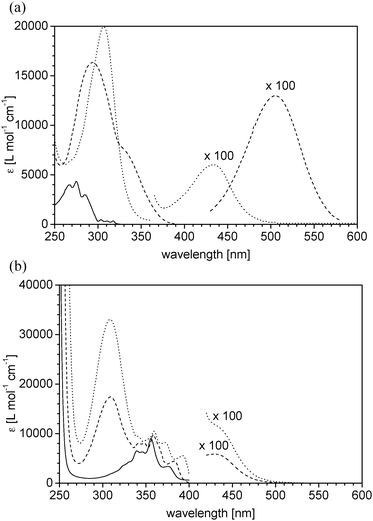 | ||
| Fig. 4 UV-vis spectra of UV chromophore substituted RAFT agents in n-hexane, in comparison to the spectra of the parent UV chromophores: (a) 2-ethylnaphthalene (solid line), dithiobenzoate CTA2 (dashed) and the trithiocarbonate CTA20 (dotted) bearing naphth-2-yl R groups (b) acridine (solid line), monofunctional CTA21 (dashed) and bifunctional CTA22 (dotted) bearing acridinyl R groups. | ||
The comparison of the formulae shown in Fig. 1 and 2 reveals that some of the trithiocarbonate RAFT agents studied here carry R groups with a substitution pattern that corresponds to common polymers. For instance CTA5 corresponds to poly(acrylic acid), CTA6 corresponds to poly(butyl acrylate), CTA9 corresponds to poly(N,N-dimethyl-acrylamide), CTA10 corresponds to poly(methacrylic acid), CTA11 corresponds to poly(methyl methacrylate), or CTA19 corresponds to poly(styrene).
UV-vis absorption characteristics of the chain transfer agents
A large variety of RAFT agents has been reported up to now.2,3,18 Their main categories according to the Z substituent are dithioesters,1 trithiocarbonates,27 xanthates80 and dithiocarbamates.81 Occasionally, more exotic RAFT agents, such as phosphoryl dithioesters,82 have been reported. The Z group of a CTA is the basic structural element that determines its chemical classification, activates or deactivates the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond towards radical addition and, additionally, determines its spectral characteristics. Some reports on the UV analysis of thiocarbonylthio compounds can be found in the early literature.60,62Thiocarbonyl compounds with different substituents (e.g. –Cl, –NH2, –F, –CF3, –CN, –CONH2) at the thiocarbonyl group have been extensively studied, as these compounds display pronounced and characteristic spectral shifts depending on electronic transition and substitution.58,63 Three types of bands were described: I (∼235 nm, strong), II (∼300 nm, strong) and III (420–500 nm, weak).58,60 Type I is ascribed to n–σ* transitions, type II to π–π* transitions and type III to forbidden n–π* transitions of the thiocarbonyl bond. Table 1 summarises some spectral data taken from the literature. While the allowed π–π* transitions have large molar absorptivities ε in the range of 8000–20
S double bond towards radical addition and, additionally, determines its spectral characteristics. Some reports on the UV analysis of thiocarbonylthio compounds can be found in the early literature.60,62Thiocarbonyl compounds with different substituents (e.g. –Cl, –NH2, –F, –CF3, –CN, –CONH2) at the thiocarbonyl group have been extensively studied, as these compounds display pronounced and characteristic spectral shifts depending on electronic transition and substitution.58,63 Three types of bands were described: I (∼235 nm, strong), II (∼300 nm, strong) and III (420–500 nm, weak).58,60 Type I is ascribed to n–σ* transitions, type II to π–π* transitions and type III to forbidden n–π* transitions of the thiocarbonyl bond. Table 1 summarises some spectral data taken from the literature. While the allowed π–π* transitions have large molar absorptivities ε in the range of 8000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1, and thus lead to intense bands in the range of 275–310 nm, the forbidden n–π* transitions are much weaker with molar absorptivities ε in the range of 15–120 L mol−1 cm−1 only, the absorbance maxima ranging from about 350 to 530 nm.
000 L mol−1 cm−1, and thus lead to intense bands in the range of 275–310 nm, the forbidden n–π* transitions are much weaker with molar absorptivities ε in the range of 15–120 L mol−1 cm−1 only, the absorbance maxima ranging from about 350 to 530 nm.
| Entry | Example | π → π* | n → π* | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| C6H12a | C2H5OH | CH3CN | C6H12a | C2H5OH | CH3CN | ||||
| a In cyclohexane. b In petroleum ether. c In isooctane. | |||||||||
| 1 | MeCS-SMe | 302 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
302 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
456 (15.5) | 446 (19.1) | 58 | |||
| 2 | MeCS-SEt | 306 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
59 | ||||||
| 3 | Ph-CSSH | 298 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
300 | 302 (9800) | 538 (70.8) | 518 | 522 (70.8) | 60 | |
| 4 | Ph-CS-SEt | 299 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
299 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
299 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) 800) |
508 (117.5) | 501 (117.5) | 497 (125.9) | 60 | |
| 5 | Ph-CS-S-iPr | 296 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) 400) |
299 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) 800) |
298 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
509 (117.5) | 503 (114.8) | 500 (128.8) | 60 | |
| 6 | Ph-CS-S-t-Bu | 296 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
298 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
296 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
526 (102.3) | 520 (93.3) | 515 (75.9) | 60 | |
| 7 | Ph-CS-S-Bn | 299 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
504 (107.0) | 64 | |||||
| 8 | HS-CS-SH | 288b (2400) | 59 | ||||||
| 9 | MeS-CS-SMe | 303 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
429 (28.2) | 58 | |||||
| 10 | PhS-CS-SPh | 310 (8900) | 460 (53.7) | 58 | |||||
| 11 | NH2-CS-SMe | 241 (6500) | 279 (8100) | 357 (39.8) | 59 | ||||
| 12 | (Et)2N-CS-S-Bn | 282 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
61 | ||||||
| 13 | EtO-CS-SEt | 221c (8700) | 278c (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
357c (52.5) | 58 and 59 | ||||
The comparison of the parent compounds dithiomethylacetate (entry 1) and dimethyltrithiocarbonate (entry 9) indicates that the π–π* absorption band has nearly the same energy, while the n–π* transition exhibits a marked hypsochromic shift for the trithiocarbonate. Compared to the dithioalkylacetates (entries 1 and 2), the conjugation of the phenyl moiety with the dithioester changes the transition energy for both the n–π* and π–π* bands, provoking a notable red-shift of the n–π* and a blue-shift of the π–π* transition, respectively, for this substitution pattern (entries 4–7). Moreover, the values for ε are higher. We see also that the parent dithioacids show lower ε values than their corresponding esters (compare entries 3 and 4, or 8 and 9). Additionally, the replacement of a primary or secondary alkyl substituent of the thiol sulfur atom by a tertiary alkyl group results in a red-shift of the n–π* transition, and seems to reduce the molar absorptivities of both the n–π* and the π–π* bands somewhat (compare entries 4 and 5 with 6). Characteristically, the substitution of one sulfur substituent of the thiocarbonyl chromophore in trithiocarbonates by nitrogen or oxygen substituents leads to a blue-shift of the π–π* and n–π* absorption (entries 11–13). In fact, thiocarbonyl compounds are cross-conjugated,59 and if the two substituents of the thiocarbonyl group differ markedly in their electron donor ability, as in xanthates and dithiocarbamates, two separate absorption maxima are observed (entries 11 and 13).
The spectral data of the synthesized RAFT agents are listed in Tables 2 and 3, which summarise the absorbance maxima λmax of the strong π–π*-transition as well as of the weak n–π*-transitions, and the ε values, respectively. The λmax values of the π–π*-transition of trithiocarbonates studied by us with secondary, tertiary and benzylic R groups are found between 305 nm and 309 nm in n-hexane, comparing well to the reported value of dimethyltrithiocarbonate (cf.Tables 1 and 2). For the dithiobenzoates CTA1 and CTA2, λmax of this transition is shifted to lower wavelengths (294–297 nm), while for the dithioacetate CTA4, λmax is at 309 nm. These findings also compare well with the literature data of structural analogues (cf.Table 1, entries 2 and 7). In the case of CTA3, the π–π*-transition of the thiocarbonyl moiety is superposed by the absorbance of the perimidine residue, and thus could not be resolved. Solvatochromic effects seem to be marginal only, studying a broad range of solvents of different polarity and of protic or aprotic character, namely ranging from n-hexaneviaCH2Cl2 and butyl acetate to butanol, acetonitrile and methanol.
| CTA | π → π* | n → π* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C6H14 | BuAc a | CH2Cl2 | CH3CN | C4H9OH | CH3OH | C6H14 | BuAc a | CH2Cl2 | CH3CN | C4H9OH | CH3OH | |
a
n-Butyl acetate.
b Not determined.
c Insufficient solubility.
d >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S band cannot be resolved.
e Data taken from ref. 65. S band cannot be resolved.
e Data taken from ref. 65.
|
||||||||||||
| CTA1 | 297 | 300 | 303 | 302 | 301 | 301 | —c | —b | 514 | —b | —b | 516 |
| CTA2 | 294 | 295 | 296 | 295 | 296 | 294 | 505 | 501 | 496 | 496 | 496 | —c |
| CTA3 | —b | —b | —d | —b | —b | —b | —c | —b | 618 | —c | —c | —c |
| CTA4 | 309e | —b | —b | —b | —b | —b | 463 | —b | —b | —b | —b | —b |
| CTA5 | 305 | 307 | 308 | —b | —b | 309 | 431 | 432 | 428 | —b | —b | 432 |
| CTA6 | 305 | 306 | 307 | 306 | 306 | 305 | 433 | 433 | 433 | 433 | 432 | 432 |
| CTA7 | —b | —b | 308 | 305 | —b | —b | —b | —b | —b | —b | —b | —b |
| CTA8 | —c | 304 | —c | —b | —b | 308 | —c | 431 | —c | —b | —b | 432 |
| CTA9 | 308 | 309 | 309 | 309 | 308 | 307 | 429 | 430 | 430 | 431 | 429 | 430 |
| CTA10 | 306 | 308 | 308 | 309 | 309 | 311 | 441 | 442 | 440 | 442 | 441 | 442 |
| CTA11 | 307 | 307 | 308 | 307 | 307 | 307 | 442 | 441 | 440 | 440 | 440 | 439 |
| CTA12 | 306 | 308 | 310 | —b | —b | 307 | 433 | 433 | 433 | —b | —b | 433 |
| CTA13 | 306 | 308 | 309 | —b | —b | 308 | 433 | 433 | 433 | —b | —b | 433 |
| CTA14 | 307 | 309 | 310 | 309 | 309 | 309 | 432 | 433 | 432 | 434 | 432 | —c |
| CTA15 | 308 | —c | 310 | —c | —c | —c | —c | —c | 433 | —c | —c | —c |
| CTA16 | 307 | 309 | 310 | —b | —b | 309 | 434 | 434 | 433 | —b | —b | 433 |
| CTA17 | —b | —b | 308 | 307 | —b | —b | —b | —b | —b | —b | —b | —b |
| CTA18 | —b | —b | 309 | —c | —b | —b | —b | —b | —b | —b | —b | —b |
| CTA19 | 308 | 312 | 312 | 308 | —b | —b | —b | —b | —b | —b | —b | —b |
| CTA20 | 307 | 309 | 310 | 308 | 308 | 308 | 433 | 432 | 433 | 433 | 432 | —c |
| CTA21 | 309 | 311 | 313 | —b | —b | 310 | 430 | 432 | 433 | —c | —c | —c |
| CTA22 | 309 | 310 | 311 | —b | —b | —c | 430 | 430 | 430 | —b | —b | —c |
| CTA | π → π* | n → π* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C6H14 | BuAc a | CH2Cl2 | CH3CN | C4H9OH | CH3OH | C6H14 | BuAc a | CH2Cl2 | CH3CN | C4H9OH | CH3OH | |
| a n-Butyl acetate. b Not determined. c Insufficient solubility. d Data taken from ref. 65. | ||||||||||||
| CTA1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
—c | —b | 114 | —b | —b | 114 |
| CTA2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
130 | 134 | 138 | 141 | 135 | —c |
| CTA3 | —b | —b | —b | —b | —b | —b | —b | —b | 1560 | —b | —b | —b |
| CTA4 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300d 300d |
—b | —b | —b | —b | —b | 45d | —b | —b | —b | —b | —b |
| CTA5 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
—b | —b | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
37 | 38 | 32 | —b | —b | 38 |
| CTA6 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
35 | 36 | 41 | 36 | 38 | 36 |
| CTA7 | —b | —b | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
—b | —b | —b | —b | —b | —b | —b | —b |
| CTA8 | —c | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
—c | —b | —b | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
—c | 35 | —c | —b | —b | 33 |
| CTA9 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
45 | 44 | 48 | 43 | 44 | 42 |
| CTA10 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
35 | 33 | 38 | 33 | 33 | 32 |
| CTA11 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
31 | 32 | 36 | 33 | 32 | 32 |
| CTA12 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
—b | —b | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
47 | 48 | 53 | —b | —b | 48 |
| CTA13 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
—b | —b | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
62 | 62 | 66 | —b | —b | 63 |
| CTA14 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
110 | 113 | 120 | 105 | 113 | —c |
| CTA15 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
—c | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
—c | —c | —c | —c | —c | 125 | —c | —c | —c |
| CTA16 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
—b | —b | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
56 | 58 | 63 | —b | —b | 59 |
| CTA17 | —b | —b | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
—b | —b | —b | —b | —b | —b | —b | —b |
| CTA18 | —b | —b | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
—c | —b | —b | —b | —b | —b | —b | —b | —b |
| CTA19 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
—b | —b | —b | —b | —b | —b | —b | —b |
| CTA20 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
61 | 62 | 65 | 61 | 65 | —c |
| CTA21 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
—b | —b | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
60 | 68 | 68 | —b | —b | —c |
| CTA22 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
—b | —b | —c | 118 | 124 | 140 | —b | —b | —c |
In the case of the n–π* absorption band, the findings seem more differentiated. As reported (cf.Table 1), the n–π* transition exhibits a marked bathochromic shift for the dithioesters in comparison to the trithiocarbonates. Dithioacetate CTA4 shows a band centered at 463 nm in hexane, while dithiobenzoate CTA2 exhibits the absorbance maximum at 505 nm, conferring a characteristic orange or, respectively, red colour to these compounds. The band of the CTA1 that bears a tertiary alkyl substituent is even shifted to higher λmax values. CTA2 shows moderate solvatochromism, with a blue-shift of about 10 nm in polar solvents such as acetonitrile or methanol. This result is again in good agreement with the literature data of structural analogues (cf.Table 1, entries 1, 4 and 7). The spectral behaviour of the perimidine derivative CTA3 is exceptional, as the absorbance maximum in the visible is shifted to about 620 nm, thus making this dithioester look blue. Apparently, the conjugation of the amidine functionality with the dithioester group is particularly effective.74
The trithiocarbonates studied exhibit a visible absorbance maximum in the range of 430–445 nm in hexane, thus making these CTAs look yellow. While trithiocarbonates with secondary alkyl (CTA5–CTA9) and benzylic (CTA12–CTA16) R groups show their maximum roughly at the same wavelength, namely in the range of 430–435 nm, λmax of trithiocarbonates bearing tertiary R groups (CTA10–CTA11) is red-shifted by nearly 10 nm (441–442 nm), in agreement with the data from the literature displayed in Table 1 (cf. entries 4, 5 and 7 with 6). In contrast to the dithiobenzoates, solvatochromic effects for the n–π* band are small at best in a broad range of solvents of different polarity and of protic or aprotic character.
Table 3 summarises the molar absorptivities of both the π–π* and the n–π* bands of the various thiocarbonyl compounds studied. For a meaningful comparison, the ε values for CTA14, CTA15, CTA18 and CTA22 must be divided by 2, as these compounds dispose of two trithiocarbonate moieties. Still, in contrast to the relatively uniform λmax values for compounds with similar R substituents, the molar absorptivities for both transitions in n-hexane differed strongly with the detailed substitution of the thiocarbonyl moiety. Within the trithiocarbonate class, the values of ε range from about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 21
000 to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 for the π–π* transition, and from 30 to 62 L mol−1 cm−1 for the n–π* transition. Even for very similar R substituents, such as the secondary R groups with ester moieties as in the group CTA5–CTA8, the molar absorptivities can differ by up to 20% in a given solvent. Nevertheless, some trends are evident. The intensities of the π–π* as well as the n–π* transition of compounds bearing benzylic R groups (CTA12–CTA19) are generally increased compared to secondary or tertiary alkyl groups for R. In dichloromethane, for instance, CTA6 and CTA7 have a molar absorptivity of about 14
000 L mol−1 cm−1 for the π–π* transition, and from 30 to 62 L mol−1 cm−1 for the n–π* transition. Even for very similar R substituents, such as the secondary R groups with ester moieties as in the group CTA5–CTA8, the molar absorptivities can differ by up to 20% in a given solvent. Nevertheless, some trends are evident. The intensities of the π–π* as well as the n–π* transition of compounds bearing benzylic R groups (CTA12–CTA19) are generally increased compared to secondary or tertiary alkyl groups for R. In dichloromethane, for instance, CTA6 and CTA7 have a molar absorptivity of about 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1, while the value of ε is above 16
000 L mol−1 cm−1, while the value of ε is above 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 for all compounds with a benzylic substituent. Regarding the measurements in the visible range, the results have to be interpreted cautiously as in these very concentrated solutions mutual interactions of solute molecules cannot be excluded. Still, the measured values of ε are throughout higher for trithiocarbonates with benzylic substituents. The origins of the increased intensity for the absorption bands were not object of this study, but the findings underline that the molar absorptivity ε at the maximum absorption wavelength λmax must be assumed to change sensitively with the detailed substitution pattern of a RAFT agent.
000 L mol−1 cm−1 for all compounds with a benzylic substituent. Regarding the measurements in the visible range, the results have to be interpreted cautiously as in these very concentrated solutions mutual interactions of solute molecules cannot be excluded. Still, the measured values of ε are throughout higher for trithiocarbonates with benzylic substituents. The origins of the increased intensity for the absorption bands were not object of this study, but the findings underline that the molar absorptivity ε at the maximum absorption wavelength λmax must be assumed to change sensitively with the detailed substitution pattern of a RAFT agent.
The comparison of the spectral data in solvents of varying polarity reveals how sensitive the absorptivities of the thiocarbonyl moiety are towards changes in the polarity and/or in the ability for H-bonding of the micro-environment (Tables 2 and 3). Depending on the solvent, the intensities of both transitions can vary strongly for a given compound. Mostly, the highest ε values are found in hexane. However, there are no obvious trends following for instance the solvent polarity according to the ET(30)83 or π* scale.84 In any case, the findings demonstrate that a meaningful quantitative analysis of the thiocarbonyl chromophores in polymers made by the RAFT technique must be done in the same solvent, in which the spectral data were established.
Suitable model compounds for macroCTAs
End group analysis of polymers by UV-vis spectroscopy is based on the approximation that λmax and ε of the chromophore remain unchanged in the course of the reaction. However, the analysis of the spectral features of the various dithioesters and trithiocarbonates revealed marked differences among their UV-vis characteristics. Depending on the substitution pattern in the R group, the values of λmax and, more importantly, of the molar absorptivity ε vary for both the π–π* as well as the n–π* transitions. For instance, when acrylic monomers are polymerised in the presence of trithiocarbonate RAFT agents bearing tertiary R groups, variations in λmax will be observed for the polymers formed. This situation is frequently encountered in practice, as tertiary R groups are better leaving groups than the secondary alkyl moiety of the propagating chain ends of acrylic polymers and thus, establish rapidly the main equilibrium of the RAFT process.2 In fact, the resulting shift in λmax in secondary compared to tertiary dithiobenzoates has been exploited in studies on the pre-equilibrium of RAFT polymerisation.49 The blue shift of the visible band can even be followed visually as, e.g., the initially magenta polymerisation mixtures containing an acrylic monomer and CTA1 turn red when the main equilibrium is established.These findings exemplify that the use of the molar absorptivity of the primary RAFT agents engaged in a polymerisation, in order to quantify the polymer end groups, and therefrom to calculate the molar mass of the polymer, is inappropriate in most cases. The substitution pattern of the thiocarbonyl chromophore is most likely going to change in the course of the polymerisation, and so are the spectral characteristics such as the values of λmax and ε. The best method to access a polymer's molar absorptivity is therefore the calibration with samples of the identical polymer, which are of a well known molar mass and completely functionalised with the chromophore end group. Obviously, this method is difficult to implement in practice, in particular if less common monomers are used, or if special polymer architectures (such as block copolymers) are to be studied. Therefore, we propose to use more conveniently the spectral data derived from a low mass molecular analog of the polymer's chain end. When having the same substitution pattern as the polymer to be analysed, the model compound should exhibit, within the experimental error, a nearly identical molar absorptivity to the one of the polymer.
We have tested the RAFT agents shown in Fig. 1 in various polymerisation experiments of acrylic and styrenic monomers. All proved to be useful for achieving polymerisation control, with three notable exceptions: RAFT polymerisation of the model monomers styrene as well as butyl acrylate failed for agents CTA3, CTA21, and CTA22, for unknown reasons. We may speculate whether the failure is due to steric constraints in the case of CTA21 and CTA22. In the case of the blue dye CTA3, electronic reasons may be responsible for the failure, taking the strongly red-shifted visible band into account. Still, these putative explanations remain speculative at present.
To verify the validity of our hypothesis, we compared molar masses derived from UV-vis end group analysis to values obtained from other methods such as SEC and end group analysis by 1H NMR, for a range of polymers. Polystyrene served as a first model system by virtue of its easy characterisation by SEC using polystyrene standards. Employing CTA18, the extent of end group functionalisation can be estimated by 1H NMR spectroscopy due to the incorporation of functional moieties into the R and the Z groups of the primary CTA that give rise to well-resolved signals in the NMR spectra (a doublet at 7.80 ppm for the ortho-aryl protons of the benzoate moiety in the R group, and a singlet at 3.61 ppm of the central ethylene moiety of the Z group between the two trithiocarbonate functions). 1H NMR analysis suggests, within experimental precision, a complete preservation of end groups (ratio Z/R = 1) (Table 4). The molar masses derived from UV-vis analysis, using the molar absorptivity of the model RAFT agent CTA19 (Fig. 5), match closely the values for the number average molar mass Mn obtained by 1H NMR and SEC. This demonstrates the general usefulness of the trithiocarbonate moiety as inherent polymer end group label. In this particular example, the results in Table 4 are equally good if the molar absorptivity ε of the model RAFT agent for the growing chain end, here CTA19, is used for the calculation, or the ε value of the RAFT agent originally engaged, here CTA18, as both values are very close (cf.Table 3).
| Polymer sample | λ max a/nm | Number average molar mass Mn according to analysis | PDI | |||
|---|---|---|---|---|---|---|
| UV band based on RAFT agent engageda | UV band based on model RAFT agenta | 1H NMR | SEC | |||
| a In dichloromethane. b By end group analysis, employing ε of model CTA19. c By end group analysis, employing ε of model CTA7. d By comparing the integrated aryl signals in o-position of the R groups and the aryl signals of the phenyl groups of the constitutional repeat unit. e By comparing the integrated aryl signals in o-position of the R groups and the methoxy signal of the constitutional repeat unit. f Eluent THF, calibrated with PS standards. g Apparent values, eluent DMF, calibrated with PS standards. | ||||||
| PS-1 | 315 | 2050 | 2100b | 2200d | 2300f | 1.09f |
| PS-2 | 314 | 3800 | 3900b | 3700d | 3500f | 1.17f |
| PMDEGA-1 | 308 | 6000 | 4900c | 5600e | 4500g | 1.20g |
| PMDEGA-2 | 306 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500c 500c |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700e 700e |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800g 800g |
1.29g |
| PMDEGA-3 | 308 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200c 200c |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400e 400e |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400g 400g |
1.40g |
| PMDEGA-4 | 308 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400c 400c |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800e 800e |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500g 500g |
1.64g |
| PMDEGA-5 | 306 | 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800c 800c |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000e 000e |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g 000g |
1.90g |
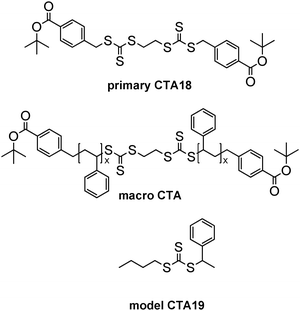 | ||
| Fig. 5 Structural comparison of the benzyl trithiocarbonate end groups in low molar mass RAFT agents CTA18, CTA19, and in a polystyrene macro-RAFT. | ||
In the following, we exemplify the importance of choosing the appropriate reference for the ε value to enable accurate end group quantification. Upon polymerisation of methoxy diethylene glycol acrylate (MDEGA) in the presence of RAFT agent CTA18, the substituent of the trithiocarbonate group changes from a primary to a secondary alkyl group, as the original benzyl R group is transformed into an acrylic chain end. CTA7 is a good model compound representing this transformation. According to Table 3, the molar absorptivities of the π–π*-transition at λmax deviate by nearly 20%, with CTA18 (ε/2 = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1) having the stronger absorbance band than CTA7 (ε = 13
000 L mol−1 cm−1) having the stronger absorbance band than CTA7 (ε = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 L mol−1 cm−1). Consequently, if the molar absorptivity of the engaged RAFT agent, i.e. of CTA18, is used for determining the molar masses of polyMDEGA, the concentration of end groups is underestimated, resulting in the overestimation of the true molar mass. The opposite scenario may also occur, if for example an acrylamide, represented by the model compound CTA9, is polymerised with the widely used RAFT agent CTA10. In this configuration, ε of CTA10 equals to 13
900 L mol−1 cm−1). Consequently, if the molar absorptivity of the engaged RAFT agent, i.e. of CTA18, is used for determining the molar masses of polyMDEGA, the concentration of end groups is underestimated, resulting in the overestimation of the true molar mass. The opposite scenario may also occur, if for example an acrylamide, represented by the model compound CTA9, is polymerised with the widely used RAFT agent CTA10. In this configuration, ε of CTA10 equals to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 L mol−1 cm−1, which is smaller than ε of CTA9, which equals 16
500 L mol−1 cm−1, which is smaller than ε of CTA9, which equals 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1. In this case, the concentration of end-groups may be overestimated if the calculation is based on the ε value of CTA10. To demonstrate this problem as well as the better reliability of UV-vis analysis using an appropriate absorptivity value, we synthesised a range of poly(methoxy diethylene glycol acrylate)s and compared the molar mass values resulting from 1H NMR, UV-vis and SEC characterisations (Table 4).
000 L mol−1 cm−1. In this case, the concentration of end-groups may be overestimated if the calculation is based on the ε value of CTA10. To demonstrate this problem as well as the better reliability of UV-vis analysis using an appropriate absorptivity value, we synthesised a range of poly(methoxy diethylene glycol acrylate)s and compared the molar mass values resulting from 1H NMR, UV-vis and SEC characterisations (Table 4).
The excellent agreement between the values derived from 1H-NMR data and UV-vis data using the molar absorptivity of the model CTA7 demonstrates once again the sturdiness of the latter characterisation technique. In contrast, the deviation of the Mn values calculated on the basis of the ε value of the used RAFT agent, namely of CTA18, illustrates the hazard of using an inappropriate ε. The deviations are not catastrophic, still, they are notable. Therefore, precise end-group analysis through UV-vis favourably requires the spectral data of a structurally closely related model compound. This may be possible by a pool of data, providing the correct absorptivities for identical monomer/Z group combinations. In this work we already report absorptivities of several model compounds suited for common polymers such as polystyrenes, polymethacrylates, polyacrylates and polyacrylamides.
Conclusions
The quantification of the thiocarbonyl end groups inherently present in polymers synthesised by the RAFT process is a convenient tool for determining easily the number average molar mass Mn. However, the spectral characteristics, namely the exact position of the absorbance maxima of the π–π* as well as the n–π* transitions in the near UV or in the visible range, respectively, and the molar absorptivities change notably with the detailed substitution pattern of the thiocarbonyl group. Therefore, it is most advisable to use the molar absorptivity values of low molar mass reference compounds with a substitution pattern identical to the active polymer chain end for calculating Mn values from the raw data, rather than the values of the RAFT agent employed in the polymerisation. In any case, the influence of the solvent on the spectral characteristics of a given thiocarbonyl compound is so strong that all measurements must be performed in the same solvent. If appropriate conditions are applied, and virtually full end group preservation is assured, the molar mass values obtained agree very well with the values determined by other methods. On the one hand, end group analysis provides thus a rapid and convenient access to a good estimate of the molar mass of polymers made by the RAFT process. This is particularly attractive for problematic cases, such as copolymers, associative systems, or strongly adsorbing samples, for which standard analytical methods such as e.g.SEC get very difficult or become impossible. On the other hand, if a second independent method for reliable molar mass determination can be applied, it is possible to quantify the share of polymer chains, which still bear an active thiocarbonyl moiety. This is most valuable e.g., in the synthesis of block copolymersvia macroRAFT agents.Acknowledgements
We thank Ch. Wieland and E. Wischerhoff (Fraunhofer Institute for Applied Polymer Research, Potsdam-Golm) for support with SEC measurements, and M. Heydenreich and A. Krtitschka (Universität Potsdam) for NMR measurements. Financial support was given by Deutsche Forschungsgemeinschaft DFG, grants LA611/7 and LA611/8.Notes and references
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS.
- Handbook of RAFT Polymerization, ed. C. Barner-Kowollik, Wiley-VCH, Weinheim, 2008 Search PubMed.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2009, 62, 1402–1472 CrossRef CAS.
- M. L. Coote and D. J. Henry, Macromolecules, 2005, 38, 1415–1433 CrossRef CAS.
- C. Barner-Kowollik, M. Buback, B. Charleux, M. L. Coote, M. Drache, T. Fukuda, A. Goto, B. Klumperman, A. B. Lowe, J. B. McLeary, G. Moad, M. J. Monteiro, R. D. Sanderson, M. P. Tonge and P. Vana, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5809–5831 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Acc. Chem. Res., 2008, 41, 1133–1142 CrossRef CAS.
- J. Chiefari, R. T. A. Mayadunne, C. L. Moad, G. Moad, E. Rizzardo, A. Postma, M. A. Skidmore and S. H. Thang, Macromolecules, 2003, 36, 2273–2283 CrossRef CAS.
- Y. K. Chong, J. Krstina, T. P. T. Le, G. Moad, A. Postma, E. Rizzardo and S. H. Thang, Macromolecules, 2003, 36, 2256–2272 CrossRef CAS.
- A. Favier, M.-T. Charreyre, P. Chaumont and C. Pichot, Macromolecules, 2002, 35, 8271–8280 Search PubMed.
- H. de Brouwer, M. A. J. Schellekens, B. Klumperman, M. J. Monteiro and A. L. German, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3596–3603 CrossRef CAS.
- M.-F. Llauro, J. Loiseau, F. Boisson, F. Delolme, C. Ladavière and J. Claverie, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5439–5462 CrossRef CAS.
- J.-F. Baussard, J.-L. Habib-Jiwan, A. Laschewsky, M. Mertoglu and J. Storsberg, Polymer, 2004, 45, 3615–3626 CrossRef CAS.
- D. B. Thomas, A. J. Convertine, R. D. Hester, A. B. Lowe and C. L. McCormick, Macromolecules, 2004, 37, 1735–1741 CrossRef CAS.
- M. Mertoglu, A. Laschewsky, K. Skrabania and C. Wieland, Macromolecules, 2005, 38, 3601–3614 CrossRef CAS.
- L. Albertin, M. H. Stenzel, C. Barner-Kowollik and T. P. Davis, Polymer, 2006, 47, 1011–1019 CrossRef CAS.
- T. Gruendling, R. Pickford, M. Guilhaus and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7447–7461 CrossRef CAS.
- M. Dietrich, M. Glassner, T. Gruendling, C. Schmid, J. Falkenhagen and C. Barner-Kowollik, Polym. Chem., 2010, 1, 634–644 RSC.
- A. Favier, C. Pichot and M.-T. Charreyre, Macromol. Rapid Commun., 2006, 27, 653–692 CrossRef CAS.
- M. Päch, D. Zehm, M. Lange, I. Dambowsky, J. Weiss and A. Laschewsky, J. Am. Chem. Soc., 2010, 132, 8757–8765 CrossRef CAS.
- J.-N. Marsat, M. Heydenreich, E. Kleinpeter, A. Laschewsky, H.v. Berlepsch and C. Böttcher, Macromolecules, 2011, 44, 2092 CrossRef CAS.
- G. Moad, Y. K. Chong, A. Postma, E. Rizzardo and S. H. Thang, Polymer, 2005, 46, 8458–8468 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Polym. Int., 2011, 60, 9–25 CrossRef CAS.
- R. Ran, Y. Yu and T. Wan, J. Appl. Polym. Sci., 2007, 105, 398–404 Search PubMed.
- A. J. Inglis, S. Sinnwell, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Macromolecules, 2008, 41, 4120–4126 CrossRef CAS.
- M. Glassner, J. P. Blinco and C. Barner-Kowollik, Polym. Chem., 2011, 2, 83–87 RSC.
- A. B. Lowe, B. S. Sumerlin, M. S. Donovan and C. L. McCormick, J. Am. Chem. Soc., 2002, 124, 11562–11563 CrossRef CAS.
- R. T. A. Mayadunne, E. Rizzardo, J. Chiefari, J. Krstina, G. Moad, A. Postma and S. H. Thang, Macromolecules, 2000, 33, 243–245 CrossRef CAS.
- Z. Wang, J. He, Y. Tao, L. Yang, H. Jiang and Y. Yang, Macromolecules, 2003, 36, 7446–7452 CrossRef CAS.
- R. T. A. Mayadunne, J. Jeffery, G. Moad and E. Rizzardo, Macromolecules, 2003, 36, 1505–1513 CrossRef CAS.
- A. Favier, C. Ladavière, M.-T. Charreyre and C. Pichot, Macromolecules, 2004, 37, 2026–2034 Search PubMed.
- C. Schilli, M. G. Lanzendörfer and A. H. E. Müller, Macromolecules, 2002, 35, 6819–6827 CrossRef CAS.
- M. Li, P. De, S. R. Gondi and B. S. Sumerlin, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5093–5100 CrossRef CAS.
- B. Yu, J. W. Chan, C. E. Hoyle and A. B. Lowe, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3544–3557 CrossRef CAS.
- B. Sasso, M. Dobinson, P. Hodge and T. Wear, Macromolecules, 2010, 43, 7453–7464 CrossRef CAS.
- V. Lima, X. Jiang, J. Brokken-Zijp, P. J. Schoenmakers, B. Klumperman and R.v. d. Linde, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 959–973 CrossRef CAS.
- X.-P. Qiu and F. M. Winnik, Macromol. Rapid Commun., 2006, 27, 1648–1653 CrossRef CAS.
- C. W. Scales, A. J. Convertine and C. L. McCormick, Biomacromolecules, 2006, 7, 1389–1392 CrossRef CAS.
- P. J. Roth, F. D. Jochum, R. Zentel and P. Theato, Biomacromolecules, 2010, 11, 238–244 CrossRef CAS.
- A. S. Goldmann, D. Quémener, P.-E. Millard, T. P. Davis, M. H. Stenzel, C. Barner-Kowollik and A. H. E. Müller, Polymer, 2008, 49, 2274–2281 CrossRef CAS.
- B. Le Droumaget and J. Nicolas, Polym. Chem., 2010, 1, 563–598 RSC.
- P. Lebreton, B. Ameduri, B. Boutevin and J.-M. Corpart, Macromol. Chem. Phys., 2002, 203, 522–537 Search PubMed.
- A. M. Bivigou-Koumba, J. Kristen, A. Laschewsky, P. Müller-Buschbaum and C. M. Papadakis, Macromol. Chem. Phys., 2009, 210, 565–578 CrossRef CAS.
- J. B. McLeary, F. M. Calitz, J. M. McKenzie, M. P. Tonge, R. D. Sanderson and B. Klumperman, Macromolecules, 2004, 37, 2383–2394 CrossRef CAS.
- N. Zhou, L. Lu, J. Zhu, X. Yang, X. Wang, X. Zhu and Z. Zhang, Polymer, 2007, 48, 1255–1260 Search PubMed.
- J. Weiss, C. Böttcher and A. Laschewsky, Soft Matter, 2011, 7, 483–492 RSC.
- J. Weiss and A. Laschewsky, Langmuir, 2011, 27, 4465–4473 Search PubMed.
- E. Rizzardo, J. Chiefari, B. Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Mejis, C. L. Moad, G. Moad and S. H. Thang, Macromol. Symp., 1999, 143, 291–307 CAS.
- K. Skrabania, H.v. Berlepsch, C. Böttcher and A. Laschewsky, Macromolecules, 2010, 43, 271–281 CrossRef CAS.
- P. Vana, T. P. Davis and C. Barner-Kowollik, Macromol. Rapid Commun., 2002, 23, 952–956 Search PubMed.
- J. Xu, J. He, D. Fan, W. Tang and Y. Yang, Macromolecules, 2006, 39, 3753–3759 CrossRef CAS.
- A. Bowes, J. B. McLeary and R. D. Sanderson, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 588–604 Search PubMed.
- M. S. Donovan, A. B. Lowe, B. S. Sumerlin and C. L. McCormick, Macromolecules, 2002, 35, 4123–4132 CrossRef CAS.
- P. Kujawa, F. Segui, S. Shaban, C. Diab, Y. Okada, F. Tanaka and F. M. Winnik, Macromolecules, 2006, 39, 341–348 CrossRef CAS.
- X.-P. Qiu and F. M. Winnik, Macromolecules, 2007, 40, 872–878 CrossRef CAS.
- D. Zehm, A. Laschewsky, P. Heunemann, M. Gradzielski, S. Prévost, H. Liang, J. P. Rabe and J.-F. Lutz, Polym. Chem., 2011, 2, 137–147 RSC.
- K. Skrabania, W. Li and A. Laschewsky, Macromol. Chem. Phys., 2008, 209, 1389–1403 CrossRef CAS.
- A. Laschewsky, M. Mertoglu, S. Kubowicz and A. F. Thünemann, Macromolecules, 2006, 39, 9337–9345 CrossRef CAS.
- J. Fabian, H. Viola and R. Mayer, Tetrahedron, 1967, 23, 4323–4329 Search PubMed.
- J. Fabian, Theor. Chim. Acta, 1968, 12, 200–205 CrossRef CAS.
- J. Fabian, S. Scheithauer and R. Mayer, J. Prakt. Chem., 1969, 311, 45–60 Search PubMed.
- T. Otsu and A. Kuriyama, Polym. Bull., 1984, 11, 135–142 Search PubMed.
- W. Rach and G. Gattow, Z. Anorg. Allg. Chem., 1988, 565, 47–53 Search PubMed.
- M. Petiau and J. Fabian, J. Mol. Struct., 2001, 538, 253–260 Search PubMed.
- J.-F. Baussard, PhD thesis, Université Catholique de Louvain, Louvain-la-Neuve, Belgium, 2004.
- M. Mertoglu, S. Garnier, A. Laschewsky, K. Skrabania and J. Storsberg, Polymer, 2005, 46, 7726–7740 CrossRef CAS.
- C. J. Ferguson, R. J. Hughes, B. T. T. Pham, B. S. Hawkett, R. G. Gilbert, A. K. Serelis and C. H. Such, Macromolecules, 2002, 35, 9243–9245 CrossRef CAS.
- A. Krieg, C. Pietsch, A. Baumgaertel, M. D. Hager, C. R. Becer and U. S. Schubert, Polym. Chem., 2010, 1, 1669–1676 RSC.
- S. Harrisson and K. L. Wooley, Chem. Commun., 2005, 41, 3259–3261 Search PubMed.
- K. Skrabania, A. Laschewsky, H.v. Berlepsch and C. Böttcher, Langmuir, 2009, 25, 7594–7601 CrossRef CAS.
- A. M. Bivigou-Koumba, E. Görnitz, A. Laschewsky, P. Müller-Buschbaum and C. M. Papadakis, Colloid Polym. Sci., 2010, 288, 499–517 Search PubMed.
- Q. Zhong, W. Wang, J. Adelsberger, A. Golosova, A. M. B. Koumba, A. Laschewsky, S. S. Funari, J. Perlich, S. V. Roth, C. M. Papadakis and P. Müller-Buschbaum, Colloid Polym. Sci., 2011, 289, 569–581 Search PubMed.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62, 7512–7515 CrossRef CAS.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754–6756 CrossRef CAS.
- W. Thiel, J. Fabian and B. Friebe, J. Prakt. Chem., 1986, 328, 812–823 Search PubMed.
- A. B. Lowe and C. L. McCormick, Prog. Polym. Sci., 2007, 32, 283–351 CrossRef CAS.
- A. Feldermann, A. Ah Toy, T. P. Davis, M. H. Stenzel and C. Barner-Kowollik, Polymer, 2005, 46, 8448–8457 CrossRef CAS.
- M. L. Coote, Macromolecules, 2004, 37, 5023–5031 CrossRef CAS.
- F. D'Agosto, R. Hughes, M.-T. Charreyre, C. Pichot and R. G. Gilbert, Macromolecules, 2003, 36, 621–629 CrossRef CAS.
- M. L. Coote, E. H. Krenske and E. I. Izgorodina, Macromol. Rapid Commun., 2006, 27, 473–497 CrossRef CAS.
- D. Charmot, P. Corpart, H. Adam, S. Z. Zard, T. Biadatti and G. Bouhadir, Macromol. Symp., 2000, 150, 23–32 CrossRef CAS.
- M. Destarac, D. Charmot, X. Franck and S. Z. Zard, Macromol. Rapid Commun., 2000, 21, 1035–1039 CrossRef.
- A. Alberti, M. Benaglia, M. Laus and K. Sparnacci, J. Org. Chem., 2002, 67, 7911–7914 CrossRef CAS.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- M. J. Kamlet, J.-L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details on the synthesis and molecular characterisation of the RAFT agents, monomer MDEGA, and the polymers made. See DOI: 10.1039/c1py00173f |
| This journal is © The Royal Society of Chemistry 2011 |
