Molecular investigation of the multi-phase photochemistry of Fe(iii)–citrate in aqueous solution†
Abstract
Iron (Fe) is ubiquitous in nature and found as FeII or FeIII in minerals or as dissolved ions Fe2+ or Fe3+ in aqueous systems. The interactions of soluble Fe have important implications for fresh water and marine biogeochemical cycles, which have impacts on global terrestrial and atmospheric environments. Upon dissolution of FeIII into natural aquatic systems, organic carboxylic acids efficiently chelate FeIII to form [FeIII–carboxylate]2+ complexes that undergo a wide range of photochemistry-induced radical reactions. The chemical composition and photochemical transformations of these mixtures are largely unknown, making it challenging to estimate their environmental impact. To investigate the photochemical process of FeIII–carboxylates at the molecular level, we conduct a comprehensive experimental study employing UV-visible spectroscopy, liquid chromatography coupled to photodiode array and high-resolution mass spectrometry detection, and oil immersion flow microscopy. In this study, aqueous solutions of FeIII–citrate were photolyzed under 365 nm light in an experimental setup with an apparent quantum yield of (φ) ∼0.02, followed by chemical analyses of reacted mixtures withdrawn at increment time intervals of the experiment. The apparent photochemical reaction kinetics of Fe3+–citrates (aq) were expressed as two generalized consecutive reactions of 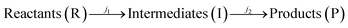 with the experimental rate constants of j1 ∼ 0.12 min−1 and j2 ∼ 0.05 min−1, respectively. Molecular characterization results indicate that R and I consist of both water-soluble organic and Fe–organic species, while P compounds are a mixture of water-soluble and colloidal materials. The latter were identified as Fe–carbonaceous colloids formed at long photolysis times. The carbonaceous content of these colloids was identified as unsaturated organic species with low oxygen content and carbon with a reduced oxidation state, indicative of their plausible radical recombination mechanism under oxygen-deprived conditions typical for the extensively photolyzed mixtures. Based on the molecular characterization results, we discuss the comprehensive reaction mechanism of FeIII–citrate photochemistry and report on the formation of previously unexplored colloidal reaction products, which may contribute to atmospheric and terrestrial light-absorbing materials in aquatic environments.
with the experimental rate constants of j1 ∼ 0.12 min−1 and j2 ∼ 0.05 min−1, respectively. Molecular characterization results indicate that R and I consist of both water-soluble organic and Fe–organic species, while P compounds are a mixture of water-soluble and colloidal materials. The latter were identified as Fe–carbonaceous colloids formed at long photolysis times. The carbonaceous content of these colloids was identified as unsaturated organic species with low oxygen content and carbon with a reduced oxidation state, indicative of their plausible radical recombination mechanism under oxygen-deprived conditions typical for the extensively photolyzed mixtures. Based on the molecular characterization results, we discuss the comprehensive reaction mechanism of FeIII–citrate photochemistry and report on the formation of previously unexplored colloidal reaction products, which may contribute to atmospheric and terrestrial light-absorbing materials in aquatic environments.

- This article is part of the themed collections: Atmospheric chemistry and Chemistry of Atmospheric Pollutants


 Please wait while we load your content...
Please wait while we load your content...
