Core–shell Fe3O4@CoFe2O4 nanoparticles as high-performance anode catalysts for enhanced oxygen evolution reaction†
Abstract
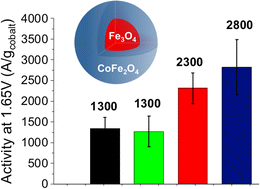
Water electrolysis is a promising and environmentally friendly means for renewable energy storage. Recent progress in the development of anion exchange membranes (AEMs) has provided new perspectives for high-performance anode catalysts based on transition metal oxides (TMOs) for the sluggish anodic oxygen evolution reaction (OER). Here, we report on core–shell nanoparticles (Fe3O4@CoFe2O4) which allow combining an electrocatalytic shell (CoFe2O4) with a conductive core (Fe3O4). Such an original approach significantly minimizes critical Co content in the catalyst and avoids addition of unstable conductive carbon black. The core–shell nanoparticles outperform Co(1−x)Fe(2+x)O4 nanoparticles and show an exceptional OER activity per Co unit mass (2800 A gcobalt−1 at 1.65 V vs. RHE) suggesting synergistic interaction between the core and the shell. Along with the core–shell structure, the size of the Fe3O4 core is a critical parameter, with a large conductive Fe3O4 core being beneficial for OER enhancement.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...