Sodium-coupled electron transfer reactivity of metal–organic frameworks containing titanium clusters: the importance of cations in redox chemistry†
Abstract
Stoichiometric reduction reactions of two metal–organic frameworks (MOFs) by the solution reagents 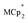 (M = Cr, Co) are described. The two MOFs contain clusters with Ti8O8 rings: Ti8O8(OH)4(bdc)6; bdc = terephthalate (MIL-125) and Ti8O8(OH)4(bdc-NH2)6; bdc-NH2 = 2-aminoterephthalate (NH2-MIL-125). The stoichiometry of the redox reactions was probed using solution NMR methods. The extent of reduction is greatly enhanced by the presence of Na+, which is incorporated into the bulk of the material. The roughly 1 : 1 stoichiometry of electrons and cations indicates that the storage of e− in the MOF is tightly coupled to a cation within the architecture, for charge balance.
(M = Cr, Co) are described. The two MOFs contain clusters with Ti8O8 rings: Ti8O8(OH)4(bdc)6; bdc = terephthalate (MIL-125) and Ti8O8(OH)4(bdc-NH2)6; bdc-NH2 = 2-aminoterephthalate (NH2-MIL-125). The stoichiometry of the redox reactions was probed using solution NMR methods. The extent of reduction is greatly enhanced by the presence of Na+, which is incorporated into the bulk of the material. The roughly 1 : 1 stoichiometry of electrons and cations indicates that the storage of e− in the MOF is tightly coupled to a cation within the architecture, for charge balance.

- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...