Stereoselective semi-synthesis of the neuroprotective natural product, serofendic acid†
Abstract
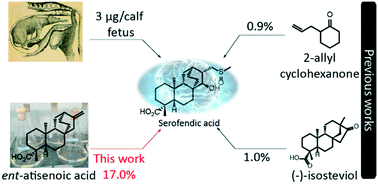
We have recently demonstrated a synthetic biology-enabled semi-synthesis of the potent neuroprotective compound, serofendic acid. An engineered bacterium produces ent-atis-16-en-19-oic acid, which has six of eight chiral carbons configured with the appropriate stereochemistry. Setting the configuration of the C15 hydroxyl group and C16 methylene is a critical step that occurs late in each published total or formal synthesis. Here we explore the use of alternative reducing reagents, stereochemical directing agents, reaction order, and product recycling to improve the diastereoselectivity of this step. We find that installing and oxidizing the C17 methylsulfide prior to reducing the C15 ketone provides the greatest yield of the desired C15,C16 diastereomer. This represents an improved total synthesis of serofendic acid.
- This article is part of the themed collection: Natural Products


 Please wait while we load your content...
Please wait while we load your content...
