Synergistic stabilization of lead halide perovskites by univalent cations under electric field stress
Nikita A.
Emelianov
 *a,
Victoria V.
Ozerova
*a,
Victoria V.
Ozerova
 a,
Yuri S.
Fedotov
ab,
Mikhail V.
Zhidkov
a,
Yuri S.
Fedotov
ab,
Mikhail V.
Zhidkov
 a,
Lavrenty G.
Gutsev
a,
Lavrenty G.
Gutsev
 ac,
Eugeniy V.
Golosov
a,
Rasim R.
Saifutyarov
d,
Lyubov A.
Frolova
ac,
Eugeniy V.
Golosov
a,
Rasim R.
Saifutyarov
d,
Lyubov A.
Frolova
 a and
Pavel A.
Troshin
a and
Pavel A.
Troshin
 ea
ea
aFederal Research Center of Problems of Chemical Physics and Medicinal Chemistry, Russian Academy of Sciences, Academician Semenov Prospect 1, 141432 Chernogolovka, Moscow Region, Russia. E-mail: emelianov@icp.ac.ru
bInstitute of Solid State Physics, Russian Academy of Sciences, Academician Osipyan Str. 2, Chernogolovka, 142432 Moscow Region, Russia
cInstitute for Micromanufacturing, Louisiana Tech University, Ruston, LA 71272, The United States
dNational Research Centre “Kurchatov Institute”, Moscow 123182, Russia
eZhengzhou Research Institute of HIT, 26 Longyuan East 7th, Jinshui District, Zhengzhou, Henan Province 450000, China
First published on 9th December 2025
Abstract
The low operational stability of perovskite solar cells, primarily caused by ion migration under photogenerated electric fields, remains one of the key barriers to their practical deployment. In the present paper, we report the results of a comparative study of the field-induced aging dynamics in lead iodide perovskite films with different univalent cation compositions: MAPbI3, FAPbI3, Cs0.15FA0.85PbI3 and Cs0.1MA0.15FA0.75PbI3. By employing a complementary suite of techniques including IR s-SNOM, PL microscopy, SEM/EDX, and ToF-SIMS mapping, alongside AIMD simulations of hydrogen on the surface of FAPbI3, we visualized the dynamic behavior of cations and anions during aging and identified the corresponding reaction products. The simulations revealed that surface-based hydrogen can destabilize the lattice by abstracting surface iodine, in agreement with experimentally observed degradation of FAPbI3, where volatile species are produced. It is shown that the formamidinium cations have a significantly higher resistance to electric fields when compared to the methylammonium cations. Univalent cation induced phase segregation has been observed for multication perovskite films upon electric field exposure. The results obtained provide a deep insight into the mechanistic pathways of the electrodegradation of differently composed lead halide perovskites and pave the way for the rational design of a new generation of perovskite absorber materials that can resist electric field-induced damage.
1 Introduction
Recently, impressive progress has been made in the development of perovskite solar cells (PSCs), which has rapidly allowed them to achieve an efficiency of 27%, an efficiency which nearly matches the PCE of crystalline silicon solar cells.1 Moreover, when compared to silicon solar panels, PSCs have a number of important advantages. In particular, the power-to-weight ratio for PSCs, with an absorber layer thickness of <1 µm, is several orders of magnitude higher than for silicon solar cells. For comparison, a 1 MW photovoltaic (PV) power plant requires 18 kg of perovskite absorber or 7 tons of silicon of 99.999999999% purity to achieve nearly the same results.2 Furthermore, roll-to-roll solution-based film coating technology offers great opportunities for scaling up the production and reducing costs. Also, this method can produce PV panels on flexible substrates for various applications, such as powering of the Internet of Things (IoT) devices.3 Despite all these promising applications PSCs remain limited by their insufficient operational stability, which prevents their commercialization.4 Encapsulation of the solar cell could suppress extrinsic degradation mechanisms related to the effects of moisture and oxygen;5 however, it would not fully prevent intrinsic degradation mechanisms, and the devices under the operational conditions will continue to degrade over time. The electric field, generated by the built-in potential due to the difference of the electrode work functions, and the photogenerated potential formed during solar cell operation lead not only to the transport of electrons, but also the diffusion of ions, which subsequently causes the degradation of the perovskite absorber layer's crystal lattice.6 Consequently, the influence of the electric field on the aging of PSCs is one of the key factors affecting device operational stability.7 However, unlike the photochemical and thermal degradation pathways,8 the field-induced degradation remains relatively sparsely studied.Methylammonium lead iodide (MAPbI3) has demonstrated anodic oxidation of iodide anions I− to molecular iodine I2, which further reacts with I− to yield triiodide I3− and polyiodides that stay in the films and can be converted back to the iodide anions upon reduction.9 On the contrary, the methylammonium cations (MA or CH3NH3+) undergo electrochemical reduction with the formation of volatile products, which leave the film and thus make the degradation irreversible.10
Formamidinium (CH(NH2)2+ or FA) cations and also their combination with Cs+ cations within the lead halide perovskite lattice, demonstrate significantly higher thermal and photochemical stability.11 As such, lead halide perovskites with the formamidinium and cesium cations are expected to possess enhanced electrochemical stability. However, data regarding the electric field-induced aging of the formamidinium-based perovskites remain fragmentary and incomplete. Previously, the degradation of the formamidinium lead iodide/gold electrode interface was visualized by time-of-flight secondary ion mass spectrometry (ToF-SIMS).12 Depth-resolved XPS/UPS/REELS profiling of PSCs based on the Cs0.05FA0.85MA0.10Pb(I0.85Br0.15)3 absorber material has revealed the presence of electrically-passive trace amounts of PbI2 and I2 throughout the device, while scanning from the top Cu electrode to the bulk of the perovskite film.13 Also electron microscopy combined with X-ray microanalysis and ToF-SIMS were utilized to directly investigate the halide redistribution in the mixed-halide FA0.87Cs0.13Pb(I0.87Br0.13)3 perovskite solar cells.14 Overall, however, the cation dynamics remains unexplored mostly due to the difficulty in its direct visualization.
Herein, we address this challenge using infrared scanning near-field optical microscopy (IR s-SNOM), which provides a unique opportunity to investigate the cation dynamics upon electric field-induced aging with the nanometer spatial resolution. These studies were complemented by using photoluminescence (PL) microscopy, which is a sensitive tool to observe the anion dynamics, whereas ToF-SIMS and SEM/EDX provided essential information on the evolution of the film composition and morphology. The range of the studied absorber materials included the series of model compositions with different univalent cations such as MAPbI3, FAPbI3, Cs0.15FA0.85PbI3, and Cs0.1MA0.15FA0.75PbI3. Thus, in this work, we report for the first time the results of a comparative study of the electric field-induced aging dynamics of complex lead halide perovskites with varied A-site cation compositions under bias stress relevant to the operational conditions of PSCs.
2 Results and discussion
Two-terminal lateral resistor-type devices (Fig. 1a) represent a commonly used model device architecture for studying dynamic processes in perovskite films with high spatial resolution.15,16 Thermally evaporated gold electrodes were used to minimize contact resistance, as elevated values are known to promote irreversible degradation at the electrode–perovskite interface.10To identify perovskite absorber compositions with enhanced long-term operational stability, we investigated the field-driven dynamics of mobile cations under electric fields, comparable to those experienced at the maximum power point.17 Therefore, we used the bias voltage to generate a field of 1 V µm−1 in average.
IR s-SNOM represents an advanced microscopy technique that combines atomic force microscopy (AFM) study of the film topography with nanoscale infrared spectroscopy mapping. The nanoscale spatial resolution of the IR spectroscopy mapping (25 nm) is achieved due to the near-field effect, so the conductive cantilever serves as an antenna and thus enables signal collection predominantly from the area comparable to the tip radius. It is well established that the IR s-SNOM signal intensity is proportional to the surface concentrations of the probed species.18
In our case, we could directly visualize the dynamics of the organic cations under exposure of the perovskite films to electric bias. The results presented in Fig. 1 demonstrate that methylammonium cations are completely depleted from the MAPbI3 film surface after one week of aging under an electric field.19
Interestingly, the MA cations appear on the surface of the gold electrodes in the aged samples. We attribute this effect to the electric field-induced degradation of MAPbI3 leading to the formation of organic species (methylamine, I2, and probably also HI) and their subsequent partial adsorption on the gold electrodes promoting reverse reactions regenerating MAI or similar species. The electric field-induced degradation of MA-containing species is accompanied by the formation of deep cavities on the perovskite/cathode interface.15
The formamidinium cations appear to be much more resistant with respect to field-induced aging. For FAPbI3, even after a week of polarization, some of the FA cations remained on the channel's surface, concentrating in the near-cathode region. The topography of the samples after bias exposure showed the formation of small caverns at the anode, but the near-cathode region showed growth of the dendrite-like particles, which feature one of the aging products. A complete disappearance of cations from the FAPbI3 film surface occurred only after 40 days of polarization. (Fig. S1, SI).
A partial substitution of formamidinium by cesium cations in Cs0.15FA0.85PbI3 had a positive effect on stability: only a small gradient of the formamidinium ion concentration was observed after 7 days of the field-induced aging in the device channel (Fig. 1). Even after 40 days, FA cations were retained in the film, accumulating in the near-cathode region (Fig. S1, SI). The Cs0.15FA0.85PbI3 films also showed the accumulation of a new phase at the near-cathode area after 7 days of electric field exposure, similar to FAPbI3 (Fig. 1).
For the triple-cation Cs0.1MA0.15FA0.75PbI3 perovskite films, we observed a different distribution pattern of FA cations. The cation-depleted region was located in the center of the channel, whereas their concentration was relatively high near both electrodes after a week of polarization. In contrast to MAPbI3, the MA cations were retained on the film surface in a narrow strip near the cathode even after 7 days of electric field exposure. Thus, we could directly observe the separation of MA- and FA-rich phases in space upon electric field-induced aging of the multication perovskite. Long-term exposure of Cs0.1MA0.15FA0.75PbI3 to the electric field leads to a complete depletion of methylammonium cations in the perovskite film surface and their partial redeposition on the surface of the electrodes. As in the case of other compositions, FA cations exhibited significantly higher resistance to the electric field, thus persisting in the near-cathode region even after 40 days of electric field exposure (Fig. S1, SI).
Scanning electron microscopy coupled with energy dispersive X-ray analysis (SEM/EDX) demonstrated the partial erosion of the material leading to the formation of the deep caverns near the cathode for the MAPbI3 film. The presence of gold extending from the anode to the cathode supports the findings of ref. 8 indicating that electric field-driven degradation leads to the oxidation of gold at the anode and the subsequent formation of AuI2− complexes, which migrate toward the cathode under the influence of the applied electric field (Fig. 2).
For the FAPbI3 film, the electric field exposure resulted in the formation of multiple voids along the entire length of the channel with the highest density nearby the cathode. These voids could have the same origin as caverns in the case of the MAPbI3 film and are likely related to the partial material decomposition with the loss of the film material via volatile products. Similar behavior was observed for the FA-rich perovskite films aged upon exposure to light or elevated temperature for a considerable time.20 Occurrence of voids also could be attributed to a phase transition occurring within individual grains of the film, in which the photoactive α-phase converts to the δ-phase under applied bias voltage.12
In the case of the Cs0.15FA0.85PbI3 films, the formation of voids was observed only at the cathode side, which is associated with partial suppression of the spontaneous phase transition process by introducing cesium cations.21 A new “bright” phase was observed in SEM micrographs, manifesting as needle-like structures near the anode and flake-like aggregates closer to the cathode. EDX data show spatial overlap between Cs and I signals and a lack of Pb in these areas, consistent with the formation of a CsI-rich phase. Thus, electrochemical reduction of FA+ at the cathode likely triggers perovskite lattice destabilization, leading to the segregation and precipitation of CsI as a secondary phase.
For Cs0.1MA0.15FA0.75PbI3, the CsI phase was also observed but in much smaller amounts. Clearly, the presence of MA cations in the perovskite film composition suppressed the formation and/or segregation of CsI upon the electric field-induced aging. Thus, the triple-cation perovskite formulation appears to be more resistant to segregation into Cs-rich and FA-rich domains as compared to the double-cation system. However, the PL microscopy of Cs0.1MA0.15FA0.75PbI3 films demonstrated field-induced phase segregation to MA-rich and FA-rich domains, as can be concluded from the shape of the spectra (Fig. S3, SI).
The PL quenching was the most intense near the anode for all perovskite formulations (Fig. 3). This observation suggests that I2 and associated polyiodide species (e.g., I3−) formed due to the anodic oxidation of I− behave as traps and promote nonradiative recombination of charge carriers.22 The double-cationic perovskite film retained the most intense PL closer to the center of the channel, which suggests that the polyiodide formation on the anode side and the material degradation with the CsI formation at the cathode side are both responsible for the charge trapping and their non-radiative recombination.
 | ||
| Fig. 3 The PL microscopy data for MAPbI3, FAPbI3, Cs0.15FA0.85PbI3 and Cs0.1MA0.15FA0.75PbI3 pristine channels (left column) and after 40 days of electric field-induced aging (right column). | ||
For the aged FAPbI3 and Cs0.1MA0.15FA0.75PbI3 films, the highest luminescence intensity was retained closer to the cathode, thus suggesting that the anodic oxidation of I− provides stronger contribution to the material aging than the cathodic reduction of organic cations and Pb2+. The lowest stability was observed for the MAPbI3 films that showed the complete luminescence quenching over the entire channel (Fig. 3).
The ToF-SIMS mapping results confirm almost complete depletion of organic cations from the aged films of MAPbI3. However, the MA cations were much better retained in the film of the triple cation perovskite Cs0.1MA0.15FA0.75PbI3. Here, the MA+ concentration decreases only slightly—remaining within one order of magnitude—whereas in MAPbI3, the surface MA+ content diminishes by two orders of magnitude, indicating nearly complete decomposition. Importantly, the surface concentration of FA+ cations shows low variation—remaining within one order of magnitude—across all perovskite formulations investigated. However, for FAPbI3, strong depletion in the near-cathode region was observed. Much better retention of the FA cations was observed for the Cs0.1MA0.15FA0.75PbI3 films, which might have been explained by higher activity of MA cations undergoing the cathodic reduction; however, the films of Cs0.15FA0.85PbI3 demonstrated an even higher stability in terms of the formamidinium cation retention. In the last case, the concentration of FA did not change significantly after the field-induced aging.23 The Cs cations demonstrate notable aggregation (Fig. 4), which corroborated with the SEM/EDX results and also confirmed the formation of Cs-rich domains due to the field-induced phase segregation (or liberation of the CsI phase).
The broadening of the Pb+ distribution profile in the cathodic region after the electric field exposure also proves the reduction of Pb2+ to metallic lead for all of the studied perovskites.24 The expansion of the area delivering an intense I2− signal towards both the anode and cathode sides could be caused by redeposition of the volatile substances formed during the degradation process.25 Noticeably, very similar changes after the field-induced aging were observed for all of the studied perovskites (Fig. S4–S7, SI).
It should be noted that the chemical processes occurring during the electric field-induced degradation were described previously for MAPbI3 (ref. 9, 10 and 15) and Cs0.1MA0.15FA0.75PbI3.26 Using this background knowledge, along with the new results obtained in this work, we summarize the major pathways of the field-induced degradation of the lead halide perovskite films as follows:
(1) Phase segregation with the formation of Cs-rich, MA-rich and FA-rich domains in the case of the multication systems;
(2) Formation of caverns (MAPbI3) and voids (FA-based formulations) due to the material degradation and the formation of volatile species and the α-to δ-phase transition;
(3) Cathodic reduction of organic methylammonium and formamidinium cations:
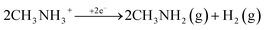 | (1) |
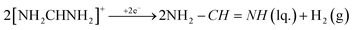 | (2) |
It was previously shown that the reduction of the methylammonium cation produces methylamine, which was expected. The reduction of the formamidinium cation, due to the low stability of neutral formamidine, leads to its further decomposition into NH3 and HCN. Furthermore, HCN can be converted to the s-triazine and aminomalonic dinitrile, which is also not stable and can undergo further transformations.27
(4) Cathodic reduction of Pb2+ to metallic lead:
| Pb2+ + 2e− → Pb0 | (3) |
(5) Liberation of the free phase of CsI in the case of Cs0.15FA0.85PbI3 due to its partial field-induced decomposition;
(6) Anodic oxidation of iodide anions I− with the formation of molecular iodine as well as polyiodides:
| 2I− → I2 | (4a) |
| I2 + I− → I3− | (4b) |
(7) Anodic oxidation of gold and its diffusion into the perovskite phase:
| Au0 − e− → Au+ | (5) |
To complement the experimental findings, we focused our theoretical analysis on FAPbI3, which displayed the most unusual degradation behavior. Notably, ToF-SIMS and SEM revealed significant structural changes near the cathode even before the application of an external electric field. Upon field exposure, we observed a co-localized depletion of formamidinium (FA+) and iodide (I−) ions, accompanied by void formation. These findings strongly suggest the evolution of a volatile degradation product, such as hydrogen iodide (HI), under cathodic conditions.
To elucidate the mechanism underlying these observations, ab initio molecular dynamics (AIMD) simulations were performed on a 3 × 3 × 3 supercell of the FAPbI3 (100) surface with hydrogen added. Our AIMD trajectory, run for a total of 50 ps, revealed a distinct progression in hydrogen behavior. During the initial 25 ps, the surface-based hydrogen preferentially adopts two bonding motifs: Pb–I–H and Pb–H–I. In the latter half of the simulation (25–50 ps), the Pb–H–I motif exhibited increased mobility, ultimately enabling the detachment of iodine from lead and the spontaneous formation of hydroiodic acid (HI) on the surface (Fig. 5). This product has been previously identified28 in experimental degradation studies of FAPbI3 by mass spectrometry, lending further support to our theoretical interpretation.
Together, these results offer a clear atomistic mechanism for field-free cathodic degradation: surface-based hydrogen abstracts surface iodine atoms to form HI, leaving behind iodine vacancies. This pathway aligns closely with the experimental evidence of iodine loss, void formation, and FA+ depletion in the cathodic region.
Fig. 6 summarizes the dominant degradation mechanisms observed in perovskite absorbers of varying composition under operational bias.
 | ||
| Fig. 6 Schematic illustration of degradation mechanisms for lead halide perovskites under electric field stress. | ||
Cathode- and anode-driven redox processes underpin void formation and phase segregation, thereby governing the stability of the perovskite absorber. As previously shown,21 the anodic oxidation of iodide (I−) to I2 is reversible under operational conditions. In contrast, cathodic reduction of organic ammonium cations (e.g., MA+ and FA+) is irreversible, as it produces volatile species such as methylamine, ammonia, and hydrogen iodide that escape the perovskite lattice. Therefore, the stability of organic ammonium cations—particularly their resistance to cathodic reduction—is a key factor influencing the operational durability of perovskite absorbers. Our data demonstrate that in multicationic perovskites, a synergistic stabilization arises from the complementary roles of organic cations, collectively suppressing key degradation pathways.
3 Conclusion
Herein, we performed a comparative study of four model lead halide perovskites, MAPbI3, FAPbI3, Cs0.15FA0.85PbI3 and Cs0.1MA0.15FA0.75PbI3, in the context of their intrinsic stability with respect to the electric field. A set of complementary techniques such as PL microscopy, AFM, IR s-SNOM, SEM/EDX, and ToF-SIMS provided a deep insight into the aging behavior of the studied materials and allowed us to unravel the predominant aging pathways. MAPbI3 was the least stable and degraded very fast due to both the cathodic reduction of methylammonium cations and anodic oxidation of the I− anions. Interestingly, for the series of FA-based perovskite absorber materials the lowest stability was observed for the FAPbI3 films, which revealed the formation of voids and massive loss of formamidinium cations upon aging. On the contrary, the double-cation perovskite formulation Cs0.15FA0.85PbI3 appeared to be the most stable since it retained the vast majority of organic cations in the bulk and preserved the highest PL intensity after the aging. Mixing three univalent cations in Cs0.1MA0.15FA0.75PbI3 films resulted in the remarkable stabilization of the material: both MA and FA cations survived the field-induced degradation much better than in the case of the corresponding single-cation perovskite formulations MAPbI3 and FAPbI3. Replacing MA with FA clearly improves the stability of the perovskite films in the cathodic region, so the I− oxidation with the polyiodide formation becomes the major aging pathway. Furthermore, the most promising multication perovskite formulations also suffer from the field-induced phase segregation. However, the polyiodide formation and phase segregation processes could be reversible under certain conditions.10 In support of these findings, AIMD simulations revealed that hydrogen formed near the FAPbI3 surface can destabilize the lattice by abstracting iodine atoms and generating volatile HI, offering an atomistic mechanism for the experimentally observed cathodic degradation.The obtained results feature the importance of controlling ion migration in perovskite films as the most promising approach to more robust materials tolerating the exposure to the electric field. The ion dynamics could be suppressed by using appropriate grain surface passivation or via the introduction of the compact buffer interlayers blocking the parasitic redox processes at both cathode and anode sides.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5se01048a.Acknowledgements
This work was supported by the Russian Science Foundation (project No. 19-73-30020P). The theoretical team acknowledges Louisiana Optical Network Infrastructure (LONI) for the computational infrastructure used to complete this project.References
- Best Research-Cell Efficiency Chart, https://www.nrel.gov/pv/cell-efficiency.html, accessed on 18 May 2025.
- M. Graetzel, Perovskite Photovoltaics for Space Applications. PVSPACE-2024, Istanbul, 2024, pp. 15–18 Search PubMed.
- L. Zhang, Y. Wang, X. Meng, J. Zhang, P. Wu, M. Wang, F. Cao, C. Chen, Z. Wang, F. Yang, X. Li, Y. Zou, X. Jin, Y. Jiang, H. Li, Y. Liu, T. Bu, B. Yan, Y. Li, J. Fang, L. Xiao, J. Yang, F. Huang, Sh. Liu, J. Yao, L. Liao, L. Li, F. Zhang, Y. Zhan, Y. Chen, Y. Mai and L. Ding, The issues on the commercialization of perovskite solar cells, Mater, Futures, 2024, 3, 022101, DOI:10.1088/2752-5724/ad37cf.
- P. Zhu, Ch. Chen, J. Dai, Y. Zhang, R. Mao, S. Chen, J. Huang and J. Zhu, Toward the Commercialization of Perovskite Solar Modules, Adv. Mater., 2024, 36, 15–2307357, DOI:10.1002/adma.202307357.
- N. A. Belich, A. A. Petrov, P. A. Ivlev, N. N. Udalova, A. A. Pustovalova, E. A. Goodilin and A. B. Tarasov, How to stabilize standard perovskite solar cells to withstand operating conditions under an ambient environment for more than 1000 hours using simple and universal encapsulation, J. Energy Chem., 2023, 78, 246–252, DOI:10.1016/j.jechem.2022.12.010.
- X. Yan, W. Fan, F. Cheng, H. Sun, C. Xu, L. Wang, Zh. Kang and Y. Zhang, Ion migration in hybrid perovskites: Classification, identification, and manipulation, Nano Today, 2022, 44, 101503, DOI:10.1016/j.nantod.2022.101503.
- J. Thiesbrummel, S. Shah, E. Gutierrez-Partida, F. Zu, F. Peña-Camargo, S. Zeiske, J. Diekmann, F. Ye, K. P. Peters, K. O. Brinkmann, P. Caprioglio, A. Dasgupta, S. Seo, F. A. Adeleye, J. Warby, Q. Jeangros, F. Lang, S. Zhang, S. Albrecht, T. Riedl, A. Armin, D. Neher, N. Koch, Y. Wu, V. M. Le Corre, H. Snaith and M. Stolterfoht, Ion-induced field screening as a dominant factor in perovskite solar cell operational stability, Nat. Energy, 2024, 9(6), 1–13, DOI:10.1038/s41560-024-01487-w.
- N. Li, Y. Luo, Z. Chen, X. Niu, X. Zhang, J. Lu, R. Kumar, J. Jiang, H. Liu, X. Guo, B. Lai, G. Brocks, Q. Chen, S. Tao, D. P. Fenning and H. Zhou, Microscopic Degradation in Formamidinium-Cesium Lead Iodide Perovskite Solar Cells under Operational Stressors, Joule, 2020, 4, 1743–1758, DOI:10.1016/j.joule.2020.06.005.
- O. R. Yamilova, A. V. Danilov, M. Mangrulkar, Y. S. Fedotov, S. Y. Luchkin, S. D. Babenko, S. I. Bredikhin, S. M. Aldoshin, K. J. Stevenson and P. A. Troshin, Reduction of methylammonium cations as a major electrochemical degradation pathway in MAPbI3 perovskite solar cells, J. Phys. Chem. Lett., 2020, 11, 221–228, DOI:10.1021/acs.jpclett.9b03161.
- S. Yu. Luchkin, A. F. Akbulatov, L. A. Frolova, M. P. Griffin, A. Dolocan, R. Gearba, D. A. Vanden Bout, P. A. Troshin and K. J. Stevenson, Reversible and Irreversible Electric Field Induced Morphological and Interfacial Transformations of Hybrid Lead Iodide Perovskites, ACS Appl. Mater. Interfaces, 2017, 9(39), 33478–33483, DOI:10.1021/acsami.7b01960.
- A. F. Akbulatov, S. Yu. Luchkin, L. A. Frolova, N. N. Dremova, K. L. Gerasimov, I. S. Zhidkov, D. V. Anokhin, E. Z. Kurmaev, K. Stevenson and P. A. Troshin, Probing the intrinsic thermal and photochemical stability of hybrid and inorganic lead halide perovskites, J. Phys. Chem. Lett., 2017, 8, 1211–1218, DOI:10.1021/acs.jpclett.6b03026.
- M. N. Drozdov, P. A. Yunin, V. V. Travkin, A. I. Koptyaev and G. L. Pakhomov, Direct imaging of current-induced transformation of a perovskite/electrode interface, Adv. Mater. Interfaces, 2019, 6, 1900364, DOI:10.1002/admi.201900364.
- J. Hu, P. Chen, D. Luo, D. Wang, N. Chen, S. Yang, Z. Fu, M. Yu, L. Li, R. Zhu and Z.-H. Lu, Tracking the evolution of materials and interfaces in perovskite solar cells under an electric field, Commun. Mater., 2022, 3, 39, DOI:10.1038/s43246-022-00262-2.
- Z. Xu, R. A. Kerner, S. P. Harvey, K. Zhu, J. J. Berry and B. P. Rand, Halogen Redox Shuttle Explains Voltage-Induced Halide Redistribution in Mixed-Halide Perovskite Devices, ACS Energy Lett., 2023, 8(1), 513–520, DOI:10.1021/acsenergylett.2c02385.
- Y. Yuan, J. Chae, Y. Shao, Q. Wang, Z. Xiao, A. Centrone and J. Huang, Photovoltaic Switching Mechanism in Lateral Structure Hybrid Perovskite Solar Cells, Adv. Energy Mater., 2015, 5, 1500615, DOI:10.1002/aenm.201500615.
- L. A. Frolova, N. N. Dremova and P. A. Troshin, The chemical origin of the p-type and n-type doping effects in the hybrid methylammonium–lead iodide (MAPbI3) perovskite solar cells, Chem. Commun., 2015, 51, 14917–14920, 10.1039/C5CC05205J.
- U. Erdil, M. Khenkin, M. Remec, Q. Emery, V. Sudhakar, R. Schlatmann, A. Abate, E. A. Katz and C. Ulbrich, Mimicking Outdoor Ion Migration in Perovskite Solar Cells: A Forward Bias, No-Light Accelerated Aging Approach, ACS Energy Lett., 2025, 10(3), 1529–1537, DOI:10.1021/acsenergylett.5c00376.
- I. Niehues, L. Mester, E. Vicentini, D. Wigger, M. Schnell and R. Hillenbrand, Identification of weak molecular absorption in single-wavelength s-SNOM images, Opt. Express, 2023, 31, 7012–7022 CrossRef CAS PubMed.
- N. A. Emelianov, V. V. Ozerova, Yu. S. Fedotov, M. V. Zhidkov, R. R. Saifutyarov, M. S. Malozovskaya, M. S. Leshchev, E. V. Golosov, L. A. Frolova and P. A. Troshin, Direct Nanoscale Visualization of the Electric-Field-Induced Aging Dynamics of MAPbI3 Thin Films, Materials, 2023, 16, 4277, DOI:10.3390/ma16124277.
- M. I. Ustinova, M. N. Sarychev, N. A. Emelianov, Y. Li, Y. Zhuo, T. Zheng, S. D. Babenko, E. D. Tarasov, P. P. Kushch, N. N. Dremova, G. A. Kichigina, A. V. Rasmetyeva, A. I. Kukharenko, D. P. Kiryukhin, E. Z. Kurmaev, X. Xu, P. A. Troshin, L. A. Frolova and I. S. Zhidkov, Towards Better Perovskite Absorber Materials: Cu+ Doping Improves Photostability and Radiation Hardness of Complex Lead Halides, EcoMat, 2025, 7, e12512, DOI:10.1002/eom2.12512.
- Y. Liang, F. Li, X. Cui, T. Lu, C. Stampfl, S. P. Ringer, X. Yang, J. Huang and R. Zheng, Toward stabilization of formamidinium lead iodide perovskites by defect control and composition engineering, Nat. Commun., 2024, 15, 1707, DOI:10.1038/s41467-024-46044-x.
- N.-G. Park, Perovskite solar cells: An emerging photovoltaic technology, Mater. Today, 2015, 18, 65–72, DOI:10.1016/j.mattod.2014.07.007.
- A. A. Govyadinov, I. Amenabar, F. Huth, P. Scott Carney and R. Hillenbrand, Quantitative Measurement of Local Infrared Absorption and Dielectric Function with Tip-Enhanced Near-Field Microscopy, J. Phys. Chem. Lett., 2013, 4, 1526–1531, DOI:10.1021/jz400453r.
- T. Zhang, X. Meng, Y. Bai, S. Xiao, C. Hu, Y. Yang, H. Chen and S. Yang, Profiling the organic cation-dependent degradation of organolead halide perovskite solar cells, J. Mater. Chem. A, 2017, 5, 1103, 10.1039/C6TA09687E.
- N. A. Emelianov, V. V. Ozerova, Yu. S. Fedotov, E. V. Shchurik, N. A. Slesarenko, M. V. Zhidkov, E. V. Golosov, R. R. Saifutyarov, L. A. Frolova and P. A. Troshin, Electric-Field-Induced Aging Dynamics of Triple-Cation Lead Iodide Perovskite at Nanoscale, Sol. Energy Mater. Sol. Cells, 2024, 282, 113305, DOI:10.1016/j.solmat.2024.113305.
- R. A. Kerner, L. Zhao, S. P. Harvey, J. J. Berry, J. Schwartz and B. P. Rand, Low Threshold Voltages Electrochemically Drive Gold Migration in Halide Perovskite Devices, ACS Energy Lett., 2020, 5, 3352–3356, DOI:10.1021/acsenergylett.0c01805.
- A. F. Akbulatov, V. M. Martynenko, L. A. Frolova, N. N. Dremova, I. Zhidkov, S. A. Tsarev, S. Y. Luchkin, E. Z. Kurmaev, S. M. Aldoshin, K. J. Stevenson and P. A. Troshin, Intrinsic thermal decomposition pathways of lead halide perovskites APbX3, Sol. Energy Mater. Sol. Cells, 2020, 213, 110559, DOI:10.1016/j.solmat.2020.110559.
- L. A. Frolova, S. Y. Luchkin, Yu. Lekina, L. G. Gutsev, S. A. Tsarev, I. S. Zhidkov, E. Z. Kurmaev, Z. X. Shen, K. J. Stevenson, S. M. Aldoshin and P. A. Troshin, Reversible Pb2+/Pb0 and I−/I3− Redox Chemistry Drives the Light-Induced Phase Segregation in All-Inorganic Mixed Halide Perovskites, Adv. Energy Mater., 2021, 11, 2002934, DOI:10.1002/aenm.202002934.
| This journal is © The Royal Society of Chemistry 2026 |




