A responsive nanoprobe for ratiometric florescence detection of hydroxyl radicals in macrophage polarization†
Mazen
Alanazi
,
Miaomiao
Wu
,
Jiaxi
Yong
,
Zexi
Zhang
,
Huayue
Zhang
,
Dihua
Tian
and
Run
Zhang
 *
*
Australian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, St Lucia, QLD 4072, Australia. E-mail: r.zhang@uq.edu.au
First published on 8th October 2024
Abstract
Quantification of hydroxyl radicals (˙OH), one form of reactive oxygen species (ROS), plays critical roles in early diagnosis and treatment monitoring of various diseases. In this work, we report the development of a responsive nanoprobe for ratiometric fluorescence detection and imaging of ˙OH in macrophage polarization. The nanoprobe, BSA-CCA@LDH-SRB, was designed and prepared using coumarin 3-carboxylic acid (CCA) as the sensing unit for ˙OH, and sulforhodamine B (SRB) loaded on layered double hydroxide (LDH) served as the fluorescent reference component. The coupling of CCA to bovine serum albumin (BSA) and the loading of BSA–CCA on the surface of LDH enabled the nanoprobe for fluorescence detection of ˙OH with high sensitivity and minimal interference from other biomolecules, ions, and ROS. The emission of the prepared BSA-CCA@LDH-SRB at 444 nm emerged and the intensity was increased according to the concentration of ˙OH, while the emission at 580 nm was maintained, allowing the nanoprobe for ratiometric fluorescence (F444/580) detection of ˙OH. Loading of the BSA protein on the LDH surface and the biocompatibility and colloidal stability of the LDH-based fluorescent nanoprobe were further improved, facilitating the detection of ˙OH generation in macrophage polarization stimulated by both biomolecules and physical ultrasound irradiation. This study thus offers a new nanoprobe as the tool for investigating ˙OH evolutions, advancing the biomedical investigations of macrophage polarization associated inflammation.
1. Introduction
The macrophage polarization mechanism is commonly known for biological and pathological functions. As they circulate throughout the human body, macrophages (M0) are differentiated into two different phenotypes in terms of their reactivity with inflammation.1 Firstly, pro-inflammatory macrophages (M1) are responsible for the production of pro-inflammatory cytokines to attack the invading molecules. The second phenotype is anti-inflammatory macrophages (M2) which initiate tissue remodeling functions to maintain healthy cells after the phagocytosis process.2 The inflammation induction in M1 would increase the reactive oxygen species (ROS) generation, while M2 stimulation would induce antioxidant activity to reduce the ROS level.3 Thus, the equilibrium condition of macrophage polarization is highly associated with redox reactions. Based on this widely known hypothesis, monitoring redox species, including ROS and antioxidants, is essential for understanding the macrophage polarization process.4–6ROS participate extensively in redox reactions leading to a profound impact on the cellular environment.7 In living cells, oxygen is involved in reduction or oxidation reactions to regulate ROS generation.8 The redox imbalance is one of the significant contributors for oxidative stress, which is known as a risk factor for a lot of diseases, such as chronic inflammation,9 cancer,10 cardiovascular,11 retinal,12 diabetes mellitus,13 and neurodegenerative diseases.14,15 During inflammation, macrophages as convertible cells polarize to different phenotypes (M0, M1, and M2) to respond to ROS in different manners.16 The detection of ROS can be utilized as an efficient approach to monitor cell modifications, apoptosis resistance, and the progress of macrophage polarization.
As one of most aggressive ROS, the hydroxyl radical (˙OH) has received significant research attention recently owing to its high reactivity, short lifetime, and low concentration.17–19 The generation of ˙OH can occur through the Fenton reaction when H2O2 reacts with metal ions, like Fe2+ and Cu+. Moreover, ˙OH can be produced from other ROS like hypochlorous (HOCI), peroxynitrite (ONOO−), and singlet oxygen (1O2) via different reactions in living organisms. For this reason, ˙OH is considered as a highly aggressive molecule towards living cells.14 To reduce the excessive generation of ˙OH and other free radicals and maintain the redox balance, antioxidants (e.g. glutathione, GSH) promote a defensive method by scavenging ˙OH and other ROS in the cell. In the first stage of inflammation and cancer formation, antioxidants control redox balance, enhancing cell survival by inhibiting apoptosis. In contrast, the high-level ROS generation causes oxidative stress-inducing apoptosis, inflammation, and tumors.20 In this regard, two opposite therapeutic approaches could be proposed to treat chronic inflammation and cancerous diseases.21,22
To detect ˙OH in the living cells, many analytical methods have been developed recently, such as electron paramagnetic resonance (EPR),23 ultraviolet visible (UV-vis) spectroscopy,24 electrochemical sensors,25 chromatography techniques,26 chemiluminescence,27,28 and fluorescence spectroscopy.29–32 Among various analytical techniques, fluorescent analysis using responsive nanoprobes offer specific features regarding selectivity, specificity, real-time detection in biological systems, and well qualified for ˙OH detection in vivo and in vitro.33–36 The most well-investigated example for ˙OH-fluorescence quantitative methods is coumarin-based probes that show “OFF/ON” fluorescence response to ˙OH.37–39 Compared with “OFF/ON” switch-based nanoprobes, ratiometric nanoprobes with two fluorescent units can assist to minimize measurement errors,40,41 which are more likely to occur with single fluorescence-based nanoprobes.33,42 Probes with ratiometric fluorescence response could contribute to eliminating background noise that affects the quality of measurements, offering high precision and accuracy without fluctuating results.43,44 In addition, the design of nanoprobes requires low cytotoxicity, biocompatibility, and high dispersity, which are essential for the development of responsive probes for biomedical applications in living systems.33,45 In our previous research, we have developed a series of functional bioclay (layered double hydroxide (LDH)) nanoparticles as the carrier for biomedical applications, such as theranostic nanomedicine for drug and gene delivery.46,47 The LDH nanoparticles could be stabilized in electrolyte solutions through a bovine serum albumin (BSA) pre-coating strategy.48 The prepared nanoparticles exhibited excellent biocompatibility both in vitro and in vivo, enhanced uptake in diseased and normal cells, facilitating LDH to be further investigated as the nanoplatform for the development of ratiometric fluorescence nanoprobes for the detection and visualization of ˙OH during macrophage polarization.
Herein, we report the development of a LDH nanoparticle based responsive nanoprobe for ratiometric fluorescence detection of ˙OH in situ during macrophage polarization. The probe was designed by conjugating a coumarin-based ˙OH-responsive unit, CCA, into BSA coated LDH nanoparticles (Scheme 1). Ratiometric fluorescence analysis was achieved by incorporating an additional fluorescent dye (SRB) into the LDH nanoparticles, as SRB is not sensitive for ˙OH generation. LDH nanoparticles with high biocompatibility served as a skeleton to combine BSA–CCA and SRB for ratiometric fluorescence analysis of ˙OH. In this nanoprobe, CCA in BSA–CCA conjugation on LDH nanoparticles is weakly fluorescent in the absence of ˙OH while in the presence of ˙OH, CCA can react with ˙OH to yield the highly fluorescent 7-HCCA, switching on the blue channel emission. SRB in LDH served as a reference dye for the ratiometric fluorescent detection of ˙OH. The fluorescence ratio (F444/580) was determined using various analytical techniques, including fluorescence spectroscopy, flow cytometry, and confocal microscopy, to determine the concentration of ˙OH in solution and in macrophage cells. This nanoprobe was then applied to the detection and imaging of ˙OH in macrophage polarization at different activations, including biomolecules and external physical ultrasound irradiation.
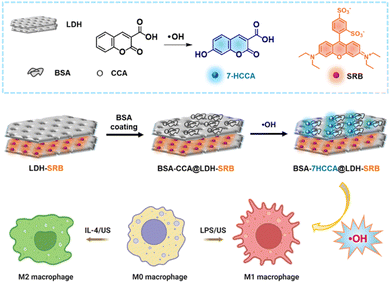 | ||
| Scheme 1 A schematic design of the nanoprobe (BSA-CCA@LDH-SRB) for ˙OH detection in macrophage polarization activated by lipopolysaccharide (LPS), interlukin-4 (IL-4) and ultrasound treatment. | ||
2. Experimental
2.1. Materials
Unless otherwise noted, all experiments were conducted at the Australian Institute for Bioengineering and Nanotechnology (AIBN), and all chemicals were used as received. Sodium chloride, potassium chloride, magnesium chloride hexahydrate, copper chloride dihydrate, and sodium hydroxide were purchased from Chem-Supply Australia. Coumarin 3-carboxylic acid (CCA), sulforhodamine B (SRB), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysulfosuccinimide (sulfo-NHS), 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT), zinc chloride, cobalt chloride hexahydrate, iron chloride tetrahydrate, sodium phosphate dibasic heptahydrate, manganese chloride tetrahydrate, and bovine serum albumin (BSA) were purchased from Sigma Aldrich. Aluminum chloride hexahydrate was purchased from Scharlau. Dimethyl sulfoxide (DMSO) was purchased from Thermo Scientific.2.2. Synthesis of LDH nanoparticles
Coprecipitation of two distinct types of metal ions, typically divalent and trivalent metals, is the most used method for the synthesis of LDH nanoparticles. Following the protocol established in our previous research,46 0.6 M magnesium chloride and 0.2 M aluminum chloride were added into 5 mL of distilled water and the solution was stirred continuously for 30 min at 4 °C. Concurrently, a solution of 320 mg of NaOH dissolved in 20 mL of distilled water was also stirred for 30 min at 4 °C. These two solutions were then combined and stirred at room temperature for another 20 min. The resultant suspension was separated via centrifugation and washed twice with distilled water. Finally, hydrothermal treatment at 100 °C for 2 h was applied to further crystallize the LDH nanoparticles.2.3. Synthesis of BSA-CCA@LDH-SRB
To form LDH–SRB, 0.5 mM of the fluorescent dye (SRB) (0.25% of Al3+) was added into NaOH solution. Then, this solution was mixed with metal salts using the same protocol of the LDH nanoparticle preparation. Prior to the preparation of the BSA-CCA@LDH-SRB nanoprobe, BSA–CCA conjugation was synthesized through the EDC-sulfo-NHS protocol to facilitate the formation of covalent amide bonds between CCA and the amine groups of BSA. The BSA–CCA conjugate was purified using the dialysis membrane to remove the free CCA molecules, which are not conjugated to BSA. Conjugated CCA on BSA was determined by a standard curve method obtained from the measurement of absorbance of CCA at 320 nm. BSA also functioned as a stabilizer and a coating agent for the functionalisation of LDH nanoparticles. The mass ratio of LDH to BSA was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Typically, the LDH suspension was added into BSA solution drop by drop, and the mixture was vigorously stirred for 30 min in the dark to ensure saturated stabilisation.
5. Typically, the LDH suspension was added into BSA solution drop by drop, and the mixture was vigorously stirred for 30 min in the dark to ensure saturated stabilisation.
2.4. Characterization of the nanoparticles
The size distribution and zeta potential of all LDH nanoparticle variants were assessed by dynamic light scattering (DLS, Zetasizer Nano, Malvern). The morphology of LDH nanoparticles and corresponding nanoprobes were characterized using a transmission electron microscope (TEM, Hitachi HT7700). All nanoparticles were collected in powder form for the Fourier transform infrared (Nicolet 5700 ATR-FTIR spectrometer) spectrum and X-ray diffraction (XRD, Bruker D8 Advanced powder XRD) pattern analysis. All BSA@LDH nanoparticles underwent additional centrifugation for characterization.The quantification of fluorescent dyes on the nanoprobes was carried out by utilizing standard curves obtained via UV-vis spectroscopy (Shimadzu UV-2450 spectrophotometer). The linearity equations obtained from UV-vis analyses (y = 0.1248x − 0.0035) for SRB and (y = 0.0058x + 0.0026) for CCA were used to estimate the concentration of fluorescent dyes.
The emission spectra were measured using a fluorescence spectrometer (Shimadzu RF-5301PC), with an excitation wavelength of 390 nm for CCA. This excitation wavelength and excitation/emission slit (10/10 nm) are constant in all experiments to obtain a robust ratiometric fluorescence response or a nanoprobe to ˙OH. Additionally, the fluorescence intensity of the nanoprobe was monitored over various levels of the analyte (˙OH).
2.5. Cellular uptake
RAW 264.7 macrophages (ATCC® TIB-71™) were obtained from the American Type Cell Collection. The cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin. And the incubator was set at 37 °C with 5% CO2. The cell culture media were replaced every two days. The experiments were conducted when the level of cell confluency reached 80%.Cells (5 × 104 cells per mL) were seeded in 24-well plates for 24 h incubation. Then, the old media were replaced with new media containing different concentrations of the BSA-CCA@LDH-SRB nanoprobe (0 to 100 μg mL−1). After 6 h incubation with the nanoprobe, the cells were washed twice with PBS before testing. For the time-dependent experiment, the nanoprobe incubation period varied from 0.5 to 24 h, and the concentration (50 μg mL−1) was constant.
2.6. Cell viability
RAW 264.7 cells (1 × 104 cells per mL) were seeded in 96-well plates for 24 h incubation. Then, the culture media were replaced with fresh media containing the BSA-CCA@LDH-SRB nanoprobe at concentrations of 0 to 100 μg mL−1. After incubation for 24 and 48 h, the old media was replaced with new media, and 20 μL of 5 mg mL−1 MTT solution was added into each well, and then, the cells were further incubated for 4 h at 37 °C. Next, the culture media were discarded and washed twice with PBS of pH 7.4. Then, 100 μL of DMSO solution was added into each well. After this, the absorbance was tested at 565 nm using a plate reader, Tecan M200 Pro Infinite.2.7. Cell analyses using flow cytometry
Following the protocol of cellular uptake, cells (5 × 104 cells per mL) were seeded in 24-well plates for 24 h. Next, the old media were replaced with new media, and pro-inflammatory induced macrophages (M1) were stimulated by 100 ng mL−1 of LPS, while anti-inflammatory induced macrophages (M2) were activated by 20 ng mL−1 of IL-4. After another 24 h for macrophage stimulation, cells were incubated with the BSA-CCA@LDH-SRB probe for 6 h, and the cells were washed twice by PBS before running the test by flow cytometry (CytoFLEX-Flow Cytometer, Beckman Coulter).For ultrasound irradiation-dominated macrophage polarisation, cells (5 × 104 cells per mL) were seeded in 24-well plates and incubated for 24 h at 37 °C. The cells were then treated with: group (i) control groups including cells only; cells incubated with 100 ng mL−1 of LPS for 24 h; cells incubated with 20 ng mL−1 of IL-4 for 24 h; cells with ultrasound at 0.5 W cm−2 for 8 min; cells incubated with 100 ng mL−1 of LPS for 24 h and ultrasound at 0.5 W cm−2 for 8 min; and cells incubated with 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe for 6 h; group (ii) 100 ng mL−1 of LPS for 24 h and then incubated with 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe for 6 h; group (iii) 100 ng mL−1 of LPS for 24 h and ultrasound (0.5 W cm−2) for 8 min at 24 h of LPS stimulation, and then incubated with 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe for 6 h; group (iv) 100 ng mL−1 of LPS for 48 h and ultrasound (0.5 W cm−2) for 8 min at 24 and 48 h, and then incubated with 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe for 6 h; group (v) 20 ng mL−1 of IL-4 for 24 h and then incubated with 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe for 6 h; group (vi) 20 ng mL−1 of IL-4 for 24 h and ultrasound (0.5 W cm−2) for 8 min at 24 h of IL-4 stimulation, and then incubated with 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe for 6 h.
2.8. Cell analyses using fluorescence microscopy
To obtain high quality imaging, coverslips were placed on the surface of 6-well plates. Then, RAW264.7 cells (3 × 105 cells per mL) were seeded on the plate and incubated for 24 h. To obtain M1 and M2 macrophage cells, LPS and IL-4 were used to stimulate M1 and M2, respectively, using the protocol described above. Next, the cell culture media were replaced by new media containing 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe. After incubation for another 6 h, all cells were washed three times with PBS for confocal fluorescence images (Leica Sp8 Microscopy). The ultrasound irradiation mediated macrophage polarization was analysed with similar treatment of flow cytometry analysis in Section 2.7. The generation of intracellular ˙OH in different macrophage phenotypes was determined by the F444/580 ratio.3. Results and discussion
The MgAl/LDH nanoparticle was utilized as a nanoplatform for the development of the nanoprobe due to its excellent biocompatibility, cellular internalisation, low cost and facile preparation. In this work, considering the layered structure of LDH with a positive surface charge, the ratiometric fluorescence nanoprobe, BSA-CCA@LDH-SRB, was developed, where the SRB as a fluorescent reference dye with a negative charge was intercalated between positively charged LDH layers, while cross-linked BSA–CCA works as the ˙OH recognition unit was coated on the surface of LDH (Scheme 1). Using the standard curve obtained from UV-vis absorbance of free SRB, the loading amount of SRB was determined by a standard curve method to be 1.43 μM for the prepared LDH–SRB nanoparticles (Fig. S1 and S2, ESI†). As shown in Fig. S3 (ESI†), BSA showed absorption about 280 nm with negligible absorption at 320 nm, attributing to absorptions of BSA and CCA, respectively. By measuring absorbance at 320 nm at different concentrations of CCA, the standard curve for determining the amount of CCA in BSA–CCA conjugation was obtained (Fig. S4, ESI†). The amount of CCA in BSA–CCA conjugation was then calculated to be 104.44 μM using the standard curve.As shown in Fig. 1A and B, the average size of plain LDH was 75 nm, and the charge (zeta potential) was recorded as 47.2 mV. After loading SRB, the nanoparticle's size was increased up to 97.8 nm, while the zeta potential was reduced by ∼50% to 24.7 mV. To observe BSA coating of LDH nanoparticles, free BSA molecules were loaded into the surface of plain LDH. BSA@LDH nanoparticles (108.2 nm) fairly showed a larger size compared with the plain LDH (75 nm). The zeta potential showed a dramatic shift from positive (47.2 mV) to negative (−5.7 mV) after BSA loading. Similarly, after loading BSA–CCA on LDH–SRB, the size and zeta potential of BSA-CCA@LDH-SRB were recorded as 125.6 nm and −12.10 mV, respectively, indicating the successful loading of BSA–CCA on LDH nanoparticles. These findings demonstrate that the BSA-CCA@LDH-SRB nanoprobe has a preferable size distribution and zeta potential measurements, concluding that this nanoprobe has great ability to resist aggregation with the contributions from BSA coating and stabilization.
As shown in Fig. 1C and Fig. S5 (ESI†), with LDH and LDH–SRB FTIR spectra, a highly noticeable peak was observed in the range of 3250–3550 cm−1 attributed to O–H stretch and H2O molecules inserted between LDH layers. Similarly, water molecules are shown via the faint peak at 1650 cm−1. The presence of carbonate ions is elucidated by the intensive peak at 1370 cm−1, concluding that LDH and LDH–SRB showed the three characteristic peaks of LDH nanoparticles. The FT-IR spectra of BSA@LDH showed the two characteristic peaks of BSA at 1540 cm−1 that could be attributed to the amide II band from the N–H bending vibration of the amine group, and 1650 cm−1 referred to the amide I band which is caused by the carbonyl stretch (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). When the whole system was combined to form BSA-CCA@LDH-SRB, the main peaks of BSA at 1650 and 1540 cm−1 clearly emerged and the peak of LDH at 1370 cm−1 appeared, concluding that LDH nanoparticles were stabilized by the BSA-coating method. As shown in Fig. 1D, polydispersity index (PDI) measurements were recorded between 0.12 and 0.22 for all forms of LDH nanoparticles, indicating identical monodispersed nanoparticles.
O). When the whole system was combined to form BSA-CCA@LDH-SRB, the main peaks of BSA at 1650 and 1540 cm−1 clearly emerged and the peak of LDH at 1370 cm−1 appeared, concluding that LDH nanoparticles were stabilized by the BSA-coating method. As shown in Fig. 1D, polydispersity index (PDI) measurements were recorded between 0.12 and 0.22 for all forms of LDH nanoparticles, indicating identical monodispersed nanoparticles.
Furthermore, TEM images were obtained to validate the characterization of nanoparticles. As demonstrated in Fig. 1E, plain LDH as well as all modified forms of LDH showed monodispersing nanoparticles with the identical hexagonal shape of LDH, suggesting that BSA efficiently stabilized the LDH nanoparticles. Therefore, BSA@LDH would preserve exceptional colloidal stability which plays a crucial role in minimizing the occurrence of detrimental nanoparticle aggregation. Powder XRD patterns of the BSA-CCA@LDH-SRB nanoprobe, BSA@LDH and LDH–SRB are identical to that of the pure LDH nanoparticle (Fig. S6, ESI†), suggesting the excellent crystallinity of prepared nanoparticles and the nanoprobe.
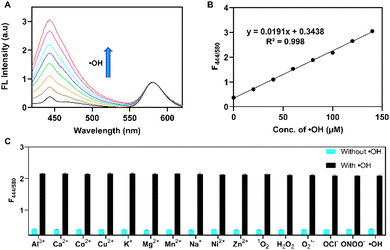
Prior to the detection of the ˙OH using the BSA-CCA@LDH-SRB nanoprobe, validation of the capability of BSA-CCA and BSA-CCA@LDH for fluorescence responses to ˙OH was performed in PBS of pH 7.4. As shown in Fig. S7 (ESI†), BSA–CCA showed a significant enhancement in fluorescence at 444 nm upon responding to ˙OH, which could be attributed to the transformation of CCA to 7-HCCA in the presence of ˙OH (Scheme 1) that is generated by the Fenton reaction (H2O2 + Fe2+ → ˙OH + Fe3+ + OH−). Loading of BSA–CCA on LDH nanoparticles, the formed BSA-CCA@LDH showed weak fluorescence at 444 nm, and the emission was gradually enhanced with the addition of ˙OH (Fig. S8, ESI†). Then, titration measurements of the BSA-CCA@LDH-SRB nanoprobe to ˙OH were conducted in PBS buffer to validate the ratiometric fluorescence response to ˙OH. The fluorescence (λex./em. = 390/444 nm) would be switched “ON” accompanied with transformation of CCA to 7-HCCA, whereas the emission of SRB (λem. = 580 nm) would be consistent as a reference dye, allowing for the fluorescence ratiometric (F444/580) detection of ˙OH. As shown in Fig. 2A, BSA-CCA@LDH-SRB emitted two remarkable peaks, which are attributed to the emission of CCA and SRB. As expected, the peak at 444 nm demonstrated the gradual increase of the fluorescence intensity in parallel with the increase of the ˙OH concentration, while the second peak at 580 nm referring to SRB did not show any significant change, as SRB is stable over a wide range of ˙OH. Energy transfer from CCA to SRB would be limited as of the (i) large distance between CCA (conjugation with 66 kDa BSA with a size of about 7 nm) and SRB mostly in the interlayer of LDH and (ii) small overlap of CCA's emission with SRB's absorption (Fig. S9, ESI†). In addition, utilizing the ratio of two fluorophores can enhance the sensitivity toward the targeting molecule (˙OH) to reduce the error measurements. Fig. 2B shows the linearity of BSA-CCA@LDH-SRB over a wide concentration of ˙OH. This linearity for ˙OH detection with the equation (y = 0.0191x + 0.3438, R2 = 0.998) indicates the capability of this nanoprobe for quantification of ˙OH. The limit of detection (LOD) and the limit of quantification (LOQ) were determined to be 12.6 and 38.4 nM, respectively, according to the method defined by the International Union of Pure and Applied Chemistry (IUPAC).49 The selectivity experiment was designed to monitor the fluorescence ratio F444/580 over various metals and ROS with and without ˙OH. Fig. 2C shows high selectivity of the BSA-CCA@LDH-SRB nanoprobe for ˙OH due to the hydroxylation on the CCA structure making it highly fluorescent compared with its native state.
The fluorescence stability over time of the nanoprobe and the product after responding ˙OH was investigated. The fluorescence ratio F444/580 was measured every 2.5 min for 30 min. As shown in Fig. S10A (ESI†), time dependent measurements of BSA-CCA@LDH-SRB without ˙OH showed low fluorescence intensity of CCA at 444 nm over 30 min, while the addition of ˙OH to BSA-CCA@LDH-SRB resulted in high fluorescence intensity at 444 nm. In contrast, the emission intensity of SRB at 580 nm was stable over 30 min and not changed in the presence of ˙OH (Fig. S10B, ESI†). The F444/580 ratio was determined to be ∼0.5 in the absence of ˙OH and increased to ∼2.0 after responding to ˙OH (Fig. S10C, ESI†). This remarkable change in the F444/580 ratio is attributed to the conversion of CCA into 7-HCCA, and 7-HCCA maintained its fluorescence intensity stable over 30 min. The F444/580 ratio was not changed in 30 min, suggesting the high fluorescence stability of the nanoprobe and the corresponding products. Moreover, emission of BSA-CCA@LDH-SRB at 444 nm was significantly increased within 2 min of ˙OH addition, suggesting the fast response of the BSA-CCA@LDH-SRB nanoprobe to ˙OH.
Further investigation involved the response of the BSA-CCA@LDH-SRB nanoprobe in different pHs of the PBS buffer. The emission intensity of the BSA-CCA@LDH-SRB nanoprobe at 444 and 580 nm and the F444/580 ratio were monitored over a wide range of pH (3 to 12) in the absence and presence of ˙OH. As shown in Fig. S11A (ESI†), the highest fluorescence intensity ratio of F444/580 was recorded with pH 7.4. The emission intensity at 580 nm was retained in the pH range of 3–12 in the absence of and presence of ˙OH (Fig. S11B, ESI†), while, in contrast, the F444/580 ratio was reduced when pH <7 (Fig. S11C, ESI†). Similarly, the F444/580 ratio was gradually decreased when the reaction takes place under high basic pH conditions. This could be attributed to the precipitation of iron ions (in the Fenton reaction to produce ˙OH) under basic conditions (Fe2+ + OH− → Fe(OH)2), which inhibit the generation of ˙OH in the solution.50
Prior to the investigation of ˙OH in polarized macrophage cells, the MTT assay was conducted to determine the biocompatibility of the BSA-CCA@LDH-SRB nanoprobe to RAW 264.7 macrophages. BSA-CCA@LDH-SRB at different concentrations (0, 5, 10, 25, 50, and 100 μg mL−1) was incubated with cells and the cell viability was measured after 24 and 48 h. As shown in Fig. S12 (ESI†), BSA-CCA@LDH-SRB showed a low level of cytotoxicity at 24 and 48 h as the cell viability of RAW 264.7 cells does not change significantly. By way of illustration, when the nanoprobe's concentration was 100 μg mL−1, the cell viability was recorded as 87% and 79.6% at 24 and 48 h, respectively, suggesting that BSA-CCA@LDH-SRB has minimal interruption for cell growth, allowing for further experiments on investigating ˙OH in macrophage cells.
As shown in Fig. S13A (ESI†), the cellular uptake was monitored with different concentrations (0 to 100 μg mL−1) of BSA-CCA@LDH-SRB in M0. The SRB channel showed a gradual increase in parallel with the probe concentration, concluding that more BSA-CCA@LDH-SRB nanoprobes are being internalized into the cells. Clearly, the CCA channel showed a small increase, which could be attributed to the increased internalization of the nanoprobe as well as the partly conversion of CCA to 7-HCCA due to the response of the nanoprobe to endogenous ˙OH. Next, 50 μg mL−1 of the nanoprobe was selected to conduct time-dependent measurements for the cellular uptake over 24 h. As shown in Fig. S13B (ESI†), the nanoprobe could be internalized within 30 min incubation and the MFI showed a direct correlation with time, i.e. greater cellular uptake with a longer incubation time.
Considering the fact that macrophages at varied polarizations produced different levels of ˙OH, the BSA-CCA@LDH-SRB nanoprobe was then applied to determine the ˙OH levels in polarized macrophages, including M0, M1, and M2, through measuring the F444/580 ratio via confocal microscopy imaging. BSA-CCA@LDH-SRB was internalized to track the generation of ˙OH in 420–460 (CCA) and 560–590 nm (SRB) channels. As shown in Fig. 3A, the control group of macrophages showed non-fluorescence in both (CCA) and (SRB) channels. After incubation of M0 cells with 50 μg mL−1 of the BSA-CCA@LDH-SRB nanoprobe for 6 h, the fluorescence image showed intense fluorescence in the SRB channel and weak fluorescence in the CCA channel. Treatment of the macrophages with LPS and IL-4, pro-inflammatory M1 and anti-inflammatory M2 were formed, respectively.1 When M1-stimulated macrophages were incubated with the nanoprobe, the CCA channel noticeably showed fluorescence signals, while M2-stimulated macrophages via the same channel showed a low level of fluorescence signals compared with M1 macrophages. These images suggest the response of conjugated CCA to largely expressed ˙OH in M1. In the SRB channel, the fluorescence signal was significantly produced with M0, M1, and M2 macrophages. In this context, SRB as a fluorescence dye is not reacting with ˙OH (Fig. S10B, ESI†). Through analyzing fluorescence ratiometric images (FCCA/SRB ratio) (Fig. 3B), the highest ratio was obtained in M1, about 6 and 3 times higher than that of M0 and M2 phenotypes, respectively, which implies that high levels of ˙OH were generated in the pro-inflammatory macrophage (M1). The confocal fluorescence imaging and image analyses confirmed the capability of the BSA-CCA@LDH-SRB nanoprobe for the ratiometric fluorescence imaging of ˙OH in polarized macrophage cells. To further validate the data obtained from fluorescence imaging, flow cytometry analysis was conducted to investigate ˙OH generation in polarized macrophage cells. As shown in Fig. 3C, the MFI ratio obtained from flow cytometric analysis showed a significant increase in M1 compared to those in M0 and M2. Results from these studies indicate that the BSA-CCA@LDH-SRB nanoprobe is able to detect ˙OH levels in polarized macrophage cells.
Upon exposure to ultrasound (US) in aqueous solution, oscillatory behavior of minute gas bubbles produces transient cavitation that is followed by the formation of ˙OH through the sonolysis of water (H2O → ˙OH + ˙H; ˙H + H2O → H2 + ˙OH).51–54 This sonolysis has been well documented for the ˙OH generation locally for therapeutic applications.55,56 To determine the ultrasound-induced ˙OH generation, BSA-CCA@LDH-SRB was added into PBS buffer of pH 7.4 with ultrasound exposure (2.0 W cm−2) for 0–12 min. As shown in Fig. S14 (ESI†), emission of the CCA channel was gradually increased over the exposure period, indicating the generation of ˙OH during sonication. The produced ˙OH could be monitored with ratiometric fluorescence response using the BSA-CCA@LDH-SRB nanoprobe. Encouraged by this result, we then examined the capability of the BSA-CCA@LDH-SRB nanoprobe for visualizing ˙OH formation in macrophage cells with sonication. As shown in Fig. 4, control groups of macrophages are non-fluorescent in both CCA and SRB channels. After internalization of the BSA-CCA@LDH-SRB nanoprobe, intense red emission of the SRB channel and weakly blue emission of the CCA channel were observed before sonication. With the sonication of cells for 2–8 min (2.0 W cm−2), the emission from the CCA channel was observed and increased with the sonication period, while changes of SRB channel emission were not observed. The results of this fluorescence imaging experiment suggest that the ultrasound-induced ˙OH generation in cells could be monitored using the BSA-CCA@LDH-SRB nanoprobe. In addition, changes of fluorescence signals at both CCA and SRB channels were recorded by ratiometric fluorescence analysis, in which an increase of the ratio was observed over the 0–8 min sonication (Fig. S15, ESI†).
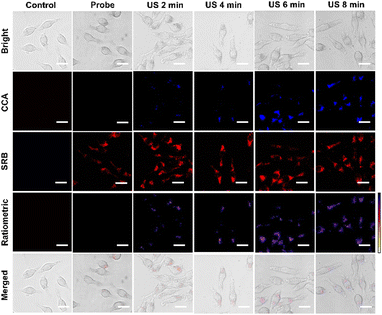 | ||
| Fig. 4 Confocal images of RAW264.7 cells incubated with BSA-CCA@LDH-SRB for US treatment over different time points (0 to 8 min). The scale bar is 20 μm. | ||
In addition to the macrophage activation by LPS and IL-4, recent studies have shown that the macrophage polarization could be modulated by low-intensity pulsed ultrasound activation.57–59 Ultrasound irradiation at low intensity could attenuate pro-inflammatory responses of macrophages to form M1 and contribute to shifting the M1 to the M2 phenotype.60,61 Inspired by these biological studies, we then used the BSA-CCA@LDH-SRB nanoprobe to determine the ˙OH generation during ultrasound-mediated macrophage polarization. As shown in Fig. 5A, control groups of macrophage cells before and after treatments, including LPS/IL-4 stimulation, ultrasound irradiation showed negligible fluorescence at both CCA and SRB channels prior to the incubation of the BSA-CCA@LDH-SRB nanoprobe. Similar to the above experiments (Fig. 3), the BSA-CCA@LDH-SRB nanoprobe stained macrophage cells showed high fluorescence at the SRB channel, and the LPS stimulated macrophage (M1) showed intense emission at both CCA and SRB channels. In sharp contrast, intracellular fluorescence was remarkably decreased for the LPS stimulated macrophage cells with low intensity ultrasound treatments. This could be attributed to (i) the attenuation of inflammation by inhibiting the polarization of the macrophage to M1 and (ii) the M1 to M2 phenotypic shift upon the physical stimulation, including ultrasound.59 As a result, the ˙OH generation in the low intensity ultrasound treatment groups was inhibited, presenting as the low fluorescence signals at the CCA channel. Further experiments involved the ultrasound treatment of IL-4 stimulated macrophage cells. As shown in Fig. 5A, negligible intracellular fluorescence at both IL-4 and IL-4 + US groups was observed, indicating the low concentrations of ˙OH in these macrophage cells. This is also in agreement with the published research about ultrasound-mediated macrophage polarization,62 showing that the low intensity ultrasound could promote M2 polarization while not inducing a shift polarization of M2 to M1.
Corresponding ˙OH generation in ultrasound mediated macrophage polarization was then evaluated by flow cytometric analyses. As shown in Fig. 5B, weak fluorescence (MFI) at both CCA and SRB channels was observed for control groups. After incubation with the BSA-CCA@LDH-SRB nanoprobe, SRB channel fluorescence was remarkably increased and a small increase of fluorescence at the CCA channel was observed as a result of staining with CCA. Upon treatment with LPS, fluorescence at the CCA channel was remarkably increased, indicating the generation of ˙OH in the M1 cells. Similar to the results obtained from fluorescence imaging, significant increases of the fluorescence intensity at the CCA channel for the US treated group were not observed. The MFI ratio change at both channels confirmed that the inhibition of ˙OH generation in LPS stimulated macrophage cells with ultrasound treatment (Fig. 5C).
4. Conclusions
In conclusion, we have developed a new ratiometric nanoprobe that quantifies intracellular ˙OH during macrophage polarization. To achieve this, the blue-emissive CCA was employed as the ˙OH recognition unit and SRB as a reference dye. The nanoprobe was prepared by coating LDH nanoparticles with BSA–CCA conjugation, and intercalated SRB between the LDH layers. In the presence of ˙OH, conversion of CCA to 7-HCCA increased the emission of the CCA channel at 444 nm, while the SRB emission was not changed, allowing for the ratiometric fluorescence (F444/580) detection of ˙OH with LOD and LOQ values to be 12.6 and 38.4 nM, respectively. The BSA-CCA@LDH-SRB nanoprobe demonstrated high sensitivity and selectivity for ˙OH, while exhibiting minimal cytotoxicity towards RAW 264.7 cells. This nanoprobe offers a new and effective method for differentiating between M0, M1, and M2 macrophage phenotypes through detecting the levels of ˙OH generation. This capability holds promise for early diagnosis and treatment monitoring of several diseases, offering significant potential for enhancing clinical outcomes.Author contributions
Conceptualization: M. Alanazi and R. Zhang; data curation: M. Alanazi and R. Zhang; formal analysis: M. Alanazi, M. Wu, J. Yong, Z. Zhang, H. Zhang, D. Tian, and R. Zhang; validation: M. Alanazi, M. Wu, J. Yong, and R. Zhang; visualization: M. Alanazi, M. Wu, J. Yong, Z. Zhang, and H. Zhang; investigation: M. Alanazi and R. Zhang; methodology: M. Alanazi, M. Wu, J. Yong, Z. Zhang, H. Zhang, D. Tian, and R. Zhang; writing – original draft: M. Alanazi; writing – review and editing: M. Alanazi and R. Zhang; resources: R. Zhang; supervision: R. Zhang; funding acquisition: R. Zhang; project administration: R. Zhang. All authors have read and agreed to the published version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.† Data will be made available upon request.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors gratefully acknowledge the financial support from the National Health and Medical Research Council (APP1175808). M. A. acknowledges the sponsorship from the Saudi Arabian Cultural Mission, Australia. We also sincerely thank Professor Zhi Ping Xu at the Shenzhen Bay Laboratory for the project discussion. Assistance of Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis (CMM) and the Queensland Node of the Australian National Fabrication Facility (ANFF-Q), the University of Queensland, are also acknowledged.References
- S. Chen, A. F. U. H. Saeed, Q. Liu, Q. Jiang, H. Xu, G. G. Xiao, L. Rao and Y. Duo, Signal Transduction Targeted Ther., 2023, 8, 207 CrossRef PubMed.
- J. Liu, X. Geng, J. Hou and G. Wu, Cancer Cell Int., 2021, 21, 1–7 CrossRef.
- H.-Y. Tan, N. Wang, S. Li, M. Hong, X. Wang and Y. Feng, Oxid. Med. Cell. Longevity, 2016, 2795090 CrossRef.
- H. Deng, L. Xu, J. Ju, X. Mo, G. Ge and X. Zhu, Biomaterials, 2022, 290, 121824 CrossRef CAS PubMed.
- E. J. Park, J. W. Song, H. J. Kim, C.-S. Kim, Y. J. Song, D. H. Yang, H. Yoo, J. W. Kim and K. Park, J. Ind. Eng. Chem., 2020, 92, 158–166 CrossRef CAS.
- Y. Huang, J. Lu, L. Zhao, X. Fu, S. Peng, W. Zhang, R. Wang, W. Yuan, R. Luo and X. Wang, Free Radicals Biol. Med., 2023, 206, 162–179 CrossRef CAS PubMed.
- L. C. D'Souza, J. G. Paithankar, H. Stopper, A. Pandey and A. Sharma, Antioxid. Redox Signaling, 2024, 40, 691–714 CrossRef.
- L. Diebold and N. S. Chandel, Free Radicals Biol. Med., 2016, 100, 86–93 CrossRef CAS.
- S. Rizwan, P. ReddySekhar and B. MalikAsrar, Antioxid. Redox Signaling, 2014, 1126–1167 Search PubMed.
- S. Saikolappan, B. Kumar, G. Shishodia, S. Koul and H. K. Koul, Cancer Lett., 2019, 452, 132–143 CrossRef CAS PubMed.
- Z. Zhang, R. Dalan, Z. Hu, J. W. Wang, N. W. Chew, K. K. Poh, R. S. Tan, T. W. Soong, Y. Dai and L. Ye, Adv. Mater., 2022, 34, 2202169 CrossRef CAS PubMed.
- M.-L. Ko, P.-H. Peng, M.-C. Ma, R. Ritch and C.-F. Chen, Free Radicals Biol. Med., 2005, 39, 365–373 CrossRef CAS PubMed.
- C. M. O. Volpe, P. H. Villar-Delfino, P. M. F. Dos Anjos and J. A. Nogueira-Machado, Cell Death Dis., 2018, 9, 119 CrossRef.
- H. Sies, V. V. Belousov, N. S. Chandel, M. J. Davies, D. P. Jones, G. E. Mann, M. P. Murphy, M. Yamamoto and C. Winterbourn, Nat. Rev. Mol. Cell Biol., 2022, 23, 499–515 CrossRef CAS PubMed.
- C. H.-L. Hung, S. S.-Y. Cheng, Y.-T. Cheung, S. Wuwongse, N. Q. Zhang, Y.-S. Ho, S. M.-Y. Lee and R. C.-C. Chang, Redox Biol., 2018, 14, 7–19 CrossRef CAS.
- Y.-C. Liu, X.-B. Zou, Y.-F. Chai and Y.-M. Yao, Int. J. Biol. Sci., 2014, 10, 520 CrossRef PubMed.
- M. Alanazi, J. Yong, M. Wu, Z. Zhang, D. Tian and R. Zhang, Chem. – Asian J., 2024, 19, e202400105 CrossRef CAS PubMed.
- J.-T. Hou, M. Zhang, Y. Liu, X. Ma, R. Duan, X. Cao, F. Yuan, Y.-X. Liao, S. Wang and W. X. Ren, Coord. Chem. Rev., 2020, 421, 213457 CrossRef CAS.
- J. Wu, Y. Zhao, K. Li, S. Muhammad, M. Ju, L. Liu, Y. Huang, B. Wang, W. Ding and B. Shen, TrAC, Trends Anal. Chem., 2022, 116734 CrossRef CAS.
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, Exp. Mol. Med., 2020, 52, 192–203 CrossRef CAS PubMed.
- V. I. Sayin, M. X. Ibrahim, E. Larsson, J. A. Nilsson, P. Lindahl and M. O. Bergo, Sci. Transl. Med., 2014, 6, 221ra215 Search PubMed.
- K. Le Gal, M. X. Ibrahim, C. Wiel, V. I. Sayin, M. K. Akula, C. Karlsson, M. G. Dalin, L. M. Akyürek, P. Lindahl and J. Nilsson, Sci. Transl. Med., 2015, 7, 308re308 Search PubMed.
- T. Oka, S. Yamashita, M. Midorikawa, S. Saiki, Y. Muroya, M. Kamibayashi, M. Yamashita, K. Anzai and Y. Katsumura, Anal. Chem., 2011, 83, 9600–9604 CrossRef CAS PubMed.
- Y. Xue, Q. Luan, D. Yang, X. Yao and K. Zhou, J. Phys. Chem. C, 2011, 115, 4433–4438 CrossRef CAS.
- L. Li, A. Zhu and Y. Tian, Chem. Commun., 2013, 49, 1279–1281 RSC.
- L. Linxiang, Y. Abe, Y. Nagasawa, R. Kudo, N. Usui, K. Imai, T. Mashino, M. Mochizuki and N. Miyata, Biomed. Chromatogr., 2004, 18, 470–474 CrossRef PubMed.
- P. Geng, J. Lv, L. Zhao and Y. Wang, Anal. Methods, 2023, 15, 5233–5238 RSC.
- Y. Hu, Z. Zhang and C. Yang, Ultrason. Sonochem., 2008, 15, 665–672 CrossRef CAS PubMed.
- B. Tang, N. Zhang, Z. Chen, K. Xu, L. Zhuo, L. An and G. Yang, Chem. – Eur. J., 2008, 14, 522–528 CrossRef CAS.
- Z. Li, T. Liang, S. Lv, Q. Zhuang and Z. Liu, J. Am. Chem. Soc., 2015, 137, 11179–11185 CrossRef CAS.
- G. M. Ganea, P. E. Kolic, B. El-Zahab and I. M. Warner, Anal. Chem., 2011, 83, 2576–2581 CrossRef CAS PubMed.
- H. Gao, L. Sun, J. Li, Q. Zhou, H. Xu, X.-N. Ma, R. Li, B.-Y. Yu and J. Tian, Adv. Sci., 2023, 10, 2303926 CrossRef CAS PubMed.
- J. Liu, M. Wu, R. Zhang and Z. P. Xu, View, 2021, 2, 20200139 CrossRef CAS.
- X. Hai, Z. Guo, X. Lin, X. Chen and J. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 5853–5861 CrossRef CAS PubMed.
- Q. Guo, Y. Liu, Q. Jia, G. Zhang, H. Fan, L. Liu and J. Zhou, Anal. Chem., 2017, 89, 4986–4993 CrossRef CAS PubMed.
- R. Liu, L. Zhang, Y. Chen, Z. Huang, Y. Huang and S. Zhao, Anal. Chem., 2018, 90, 4452–4460 CrossRef CAS PubMed.
- B. Zhang, L. Xu, Y. Zhou, W. Zhang, Y. Wang and Y. Zhu, Luminescence, 2020, 35, 305–311 CrossRef CAS PubMed.
- X. Zhang, Y. Fu, G. Qian, R. Zhang and Z. P. Xu, J. Mater. Chem. B, 2020, 8, 5420–5424 RSC.
- D. Zhou, H. Huang, Y. Wang, Y. Wang, Z. Hu and X. Li, J. Mater. Chem. B, 2019, 7, 3737–3744 RSC.
- N. Kaur, S. Chopra, G. Singh, P. Raj, A. Bhasin, S. K. Sahoo, A. Kuwar and N. Singh, J. Mater. Chem. B, 2018, 6, 4872–4902 RSC.
- L. Zhou, X. Zhang, Q. Wang, Y. Lv, G. Mao, A. Luo, Y. Wu, Y. Wu, J. Zhang and W. Tan, J. Am. Chem. Soc., 2014, 136, 9838–9841 CrossRef CAS PubMed.
- X. Sun and Y. Lei, TrAC, Trends Anal. Chem., 2017, 89, 163–180 CrossRef CAS.
- Y. Nosaka and A. Y. Nosaka, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed.
- M. Zhang, S. Zhang, X. Guo, Z. Xun, L. Wang, Y. Liu, W. Mou, T. Qin, Z. Xu, L. Wang, X. Chen, B. Liu and X. Peng, J. Hazard. Mater., 2024, 465, 133104 CrossRef CAS PubMed.
- W. Freinbichler, L. Bianchi, M. A. Colivicchi, C. Ballini, K. F. Tipton, W. Linert and L. Della Corte, J. Inorg. Biochem., 2008, 102, 1329–1333 CrossRef CAS PubMed.
- J. Liu, Y. Wu, C. Fu, B. Li, L. Li, R. Zhang, T. Xu and Z. P. Xu, Small, 2020, 16, 2002115 CrossRef CAS PubMed.
- J. Yong, M. Wu, B. J. Carroll, Z. P. Xu and R. Zhang, Trends Genet., 2024, 40, 352–363 CrossRef CAS PubMed.
- Z. Gu, H. Zuo, L. Li, A. Wu and Z. P. Xu, J. Mater. Chem. B, 2015, 3, 3331–3339 RSC.
- D. Eaton, J. Photochem. Photobiol., B, 1988, 2, 523–531 CrossRef CAS PubMed.
- S. Sathe, I. Chakraborty, V. S. Cheela, S. Chowdhury, B. Dubey and M. Ghangrekar, Bioresour. Technol., 2021, 341, 125850 CrossRef CAS PubMed.
- G. Mark, A. Tauber, R. Laupert, H.-P. Schuchmann, D. Schulz, A. Mues and C. von Sonntag, Ultrason. Sonochem., 1998, 5, 41–52 CrossRef CAS PubMed.
- C. C. Y. Wong, L.-L. Sun, M.-J. Liu, E. Stride, J. L. Raymond, H.-H. Han, J. Kwan and A. C. Sedgwick, Chem. Commun., 2023, 59, 4328–4331 RSC.
- P. Riesz, D. Berdahl and C. L. Christman, Environ. Health Perspect., 1985, 64, 233–252 CrossRef CAS PubMed.
- B. Miljevic, F. Hedayat, S. Stevanovic, K. E. Fairfull-Smith, S. E. Bottle and Z. D. Ristovski, Aerosol Sci. Technol., 2014, 48, 1276–1284 CrossRef CAS.
- M. Wu, J. Yong, H. Zhang, Z. Wang, Z. P. Xu and R. Zhang, Adv. Healthcare Mater., 2023, 12, 2301497 CrossRef CAS PubMed.
- I. Rosenthal, J. Z. Sostaric and P. Riesz, Ultrason. Sonochem., 2004, 11, 349–363 CrossRef CAS PubMed.
- Z. Xu, S. Li, L. Wan, J. Hu, H. Lu and T. Zhang, J. Orthop. Res., 2023, 41, 919–929 CrossRef CAS PubMed.
- S. A. A. Gouda, B. E. Aboulhoda, O. M. Abdelwahed, H. Abdallah, L. Rashed, R. E. Hussein and N. Sharawy, Life Sci., 2023, 314, 121338 CrossRef CAS PubMed.
- Z.-C. Zhang, Y.-L. Yang, B. Li, X.-C. Hu, S. Xu, F. Wang, M. Li, X.-Y. Zhou and X.-Z. Wei, Biomed. Pharmacother., 2019, 120, 109499 CrossRef CAS PubMed.
- H. C. Qin, Z. W. Luo and Y. L. Zhu, World J. Stem Cells, 2022, 14, 214–218 CrossRef PubMed.
- H. Kawai, A. Ito, A. Kawaguchi, M. Nagai-Tanima, R. Nakahara, S. Xu and H. Kuroki, Sci. Rep., 2023, 13, 11494 CrossRef CAS PubMed.
- C.-H. Hsu, Y.-J. Pan, Y.-T. Zheng, R. Y. Lo and F.-Y. Yang, CNS Neurosci. Ther., 2023, 29, 4113–4123 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis and fluorescence spectroscopy, XRD, FT-IR characterisation, fluorescence analysis of ˙OH, MTT assay, and flow cytometry analyses. See DOI: https://doi.org/10.1039/d4tb01934b |
| This journal is © The Royal Society of Chemistry 2024 |