A dual-functional photosensitizer for mitochondria-targeting photodynamic therapy and synchronous polarity monitoring†
Liu
Yang
a,
Shenglong
Gan
cd,
Jie
Zhang
cd,
Yin
Jiang
*b,
Qingxin
Chen
*c and
Hongyan
Sun
 *cd
*cd
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan 410083, P. R. China
bSchool of Biomedical and Pharmaceutical Sciences, Guangdong University of Technology, Guangzhou, Guangdong, 510006, China. E-mail: yjiang@gdut.edu.cn
cDepartment of Chemistry and COSDAF (Centre of Super-Diamond and Advanced Films), City University of Hong Kong, 83 Tat Chee Avenue, Kowloon, Hong Kong, China. E-mail: qchen88-c@my.cityu.edu.hk
dKey Laboratory of Biochip Technology, Biotech and Health Centre, Shenzhen Research Institute of City University of Hong Kong, Shenzhen, 518057, P. R. China. E-mail: hongysun@cityu.edu.hk
First published on 26th September 2024
Abstract
Mitochondria-targeting photodynamic therapy (PDT) has been validated as an effective strategy for inducing cell death through the disruption of mitochondrial function. The mitochondrial microenvironment, such as viscosity, polarity, pH and proteins, undergoes dynamic changes during PDT treatment, and investigating these parameters is crucial for comprehending the intrinsic mechanisms at the cellular level. In this context, disclosure of mitochondrial microenvironment alterations holds significant importance. Nevertheless, a probe capable of visualizing mitochondrial polarity fluctuations during PDT treatment has not been reported. Importantly, a dual-functional photosensitizer (PS) with polarity detection capability is highly advantageous as it can mitigate potential metabolic and localization disparities between the PS and the polarity probe, thus improving the accuracy of detection. In this contribution, a series of potential PSs were prepared by integrating the 2,1,3-benzoxadiazole (BD) scaffold with various heteroatom-incorporated electron-withdrawing groups. Among them, BDI exhibited potent phototoxicity against cancer cells and remarkable sensitivity to polarity changes, establishing it as a dual-functional PS for both photodynamic therapy and polarity detection. Leveraging its polarity detection capability, BDI successfully discriminated mitochondrial polarity discrepancy between cancer cells and normal cells, and indicated mitochondrial polarity fluctuations during drug-induced mitophagy. Crucially, BDI was employed to unveil mitochondrial polarity variations during PDT treatment, underscoring its dual function. Altogether, the meticulous design of the dual-functional PS BDI offers valuable insights into intracellular microenvironment variations during the PDT process, thereby enhancing our understanding and guiding the optimization of PDT treatment.
Introduction
Polarity is a significant intracellular parameter influencing various cellular processes and reflecting specific protein functions or the cell status.1,2 Particularly, aberrant changes in polarity are closely associated with diverse diseases, including cancers.3,4 Thus, establishing reliable methods for intracellular polarity detection is of utmost importance. Fluorescence imaging technology, with its high sensitivity, selectivity, noninvasiveness, and excellent spatial–temporal resolution,5,6 has become a widely used tool for intracellular polarity visualization. Despite the plethora of reported fluorescent probes for polarity detection,7–14 the inadequate selectivity of most of these probes remains a main concern, as their fluorescence might be affected by other factors such as viscosity, pH and temperature.8 This limitation has spurred researchers to design innovative probes with improved selectivity toward polarity.11On the other hand, photodynamic therapy (PDT) stands out as a promising therapeutic modality, boasting advantages such as noninvasiveness, spatial–temporal controllability, and negligible drug resistance.15,16 Typically, PDT functions with three fundamental components including a photosensitizer (PS), oxygen and an external light source. While a PS alone barely exhibits toxicity, it becomes a potent inducer of toxic reactive oxygen species (ROS) in the presence of specific light irradiation, causing damage to bio-macromolecules and triggering cell death.15 In view of the pivotal role of the PS in PDT, considerable efforts have been directed towards designing PSs with superior attributes. These include low dark cytotoxicity,17,18 high singlet oxygen production efficiency,19–22 absorption in the red to near-infrared (NIR) region,23 and the ability to target specific organelles.24–27 Mitochondria, as cellular powerhouses, play a crucial role in various physiological activities, and their dysfunction is closely linked to cell apoptosis.28 Accumulative studies have manifested that PS targeting mitochondria can significantly enhance PDT efficiency,29 making the design of mitochondria-targeting PSs desirable for potential clinical applications.
According to previous studies, PDT treatment induces cell death through diverse pathways,24,27,30 and a series of intracellular parameters,31–35 such as viscosity, pH, proteins and DNA, undergo distinct changes during PDT-related cell death. Hitherto, dozens of fluorescent probes have been introduced to monitor these changes during PDT treatment.31–33,36 However, there is a lack of probes capable of revealing cellular polarity variations during PDT treatment. Furthermore, a dual-functional PS with polarity detection capability is highly advantageous as it can mitigate potential metabolic and localization disparities between the PS and the polarity probe, thus improving the accuracy of detection. To this end, designing a dual-functional PS that combines mitochondria-targeting PDT with simultaneous polarity detection poses an intriguing yet challenging endeavor.
Considering these factors, our objective is to design a dual-functional PS for mitochondria-targeting PDT as well as intracellular polarity detection during PDT treatment (Scheme 1). To fulfill the corresponding requirement, a series of 2,1,3-benzoxadiazole (BD) derivatives were designed by conjugating the BD scaffold with various electron-withdrawing groups. The BD scaffold was chosen for its ultra-sensitivity toward the microenvironment, and especially its potential for polarity detection.7,37 Additionally, heteroatoms were introduced into the electron-withdrawing groups to enhance their singlet oxygen production efficiency.15,31,38–40 Moreover, a cation moiety was incorporated to improve their targeting ability toward mitochondria. On the basis of the above design, we reckon that these BD derivatives might have dual functions as efficient mitochondria-targeting PSs and potential probes for polarity detection, thus being capable of self-reporting mitochondrial polarity variations during PDT treatment.
Experimental
Preparation and characterization of BD derivatives
All the BD compounds were obtained via Knoevenagel condensation reactions between compounds 1–4 and BD-CHO as illustrated in Scheme S1 (ESI†). Details of the preparation procedure, including materials and apparatus, synthetic routes and characterization data, are fully presented in the ESI.†Spectroscopic measurement
5 mM of BD derivatives were dissolved in DMSO as a stock solution and diluted to a final concentration of 5 μM. 530 nm was set as the excitation wavelength for the spectroscopic measurement.In vitro photodynamic therapy
1,3-Diphenylisobenzofuran (DPBF) and singlet-oxygen sensor green (SOSG) were employed to detect 1O2 production efficiency in DMSO and pure water, respectively, as reported.31 The phototoxicity of BD derivatives was assessed with the CCK-8 assay and a 590 nm LED (50 mW cm−2) was chosen as the light source. Live/dead cell staining was conducted with Calcein AM and propidium iodide (PI), respectively. Additionally, DCFH-DA was utilized for ROS detection in live cells, while mitochondrial membrane potential was detected by the JC-1 assay during PDT treatment. The detailed protocol is described in the ESI.†Polarity imaging in live cells
Cells were seeded in a confocal dish and cultured for 24 h with a density of about 104 cells, and then co-incubated with BDI for polarity imaging. 543 nm was set as the excitation wavelength, while 550–600 nm and 620–670 nm were selected for the green channel and red channel, respectively. Mito-Tracker™ Green was used for mitochondrial labeling in the colocalization imaging and rapamycin (100 nM) to induce mitophagy for the drug-induced polarity fluctuation imaging.Self-reporting mitochondrial polarity fluctuations during PDT treatment
HeLa/HepG2 cells were incubated with 5 μM of BDI for 15 min and then irradiated for 30 s. Then, time-lapse CLSM images were obtained at different time intervals. 543 nm was set as the excitation wavelength, while 550–600 nm and 620–670 nm were selected for the green channel and red channel, respectively.Results and discussion
Spectral response of BD derivatives toward polarity
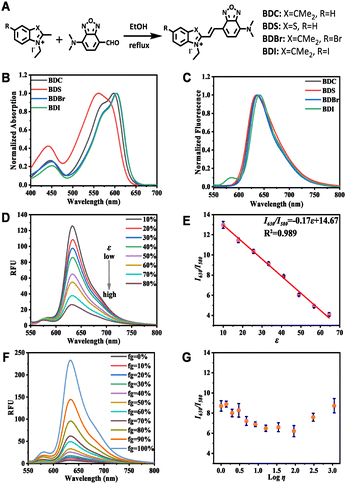
To validate our design strategy, four BD derivatives as depicted in Fig. 1A were synthesized and thoroughly characterized. Upon successful preparation of BD derivatives, their photophysical properties were investigated initially (Tables S1–S4, ESI†). As depicted in Fig. 1B, these compounds displayed similar absorption spectra with a maximum absorption at ca. 600 nm and a shoulder peak at ca. 450 nm. Meanwhile, all the BD derivatives exhibited a maximum emission at ca. 650 nm (Fig. 1C). Notably, BDI showed a distinctive emission between 550 and 600 nm compared with the other three counterparts. The spectroscopic behavior of the BD derivatives in various solvents with a wide range of polarities was then examined. Interestingly, the I630/I580 ratio of BDI demonstrated regular negative correlation with the polarity of the solvents, whereas the fluorescence of the other three BD derivatives did not show any regular correlation with the solvent polarity (Fig. S1, ESI†). This result implies that the fluorescence of BDI is likely to be sensitive to solvent polarity. Thereafter, the optical response of BD derivatives toward polarity was systematically investigated in the mixture of 1,4-dioxane and water with various polarities. By increasing the water fraction of the mixture, polarity could be finely tuned from low to high. Results (Fig. S2, ESI†) disclosed that the emission at ca. 650 nm of BD derivatives, including BDI, all decreased with the increase of polarity. In stark contrast, the emission at 580 nm of BDI did not show a significant change with the increase of polarity. Importantly, it was observed that the I630/I580 ratio of BDI displayed a linear correlation with solvent polarity (Fig. 1D and E).As underscored in previous literature,8 suboptimal selectivity of fluorescent probes toward polarity is a key issue that hampers their further application in a complex bio-system. To investigate the selectivity of BD derivatives toward polarity, optical response toward viscosity, pH as well as common bio-analytes were studied (Fig. S3–S5, ESI†). To our delight, the fluorescence of all the BD derivatives was not obviously affected by pH or the common bio-analytes (Fig. S4 and S5, ESI†). However, solvent viscosity exerted a pronounced influence on their fluorescence at approximately 650 nm (Fig. S3, ESI†), elucidating that the fluorescence of BD derivatives at ca. 650 nm is not specific for polarity detection. Encouragingly, although the fluorescence of BDI at 580 nm and 630 nm was both affected by viscosity, the I630/I580 ratio did not show drastic variations along with the change of viscosity (Fig. 1F and G). This observation suggests that the I630/I580 ratio of BDI is insensitive to viscosity changes, making BDI a highly selective probe for polarity detection with ratiometric fluorescence at 580 and 630 nm.
PDT efficiency of BD derivatives
Concurrently, the potential of BD derivatives as efficient photosensitizers was assessed. As depicted in Fig. S6 (ESI†), BD derivatives all possessed superior photostability, indicating their potential for PDT application. Meanwhile, singlet oxygen production efficiency was significantly enhanced after introducing heteroatoms into the indole ring (Fig. S7, S8, ESI,† and Fig. 2A and B), confirming the significant role of heteroatoms in improving PDT efficiency. Furthermore, all BD derivatives displayed negligible dark cytotoxicity (Fig. 2C), while BDI demonstrated higher phototoxicity toward HeLa cells compared with the other counterparts (Fig. 2D). This suggests BDI is a potential photosensitizer for efficient photodynamic therapy. Taken together, BDI was selected for further evaluation as a dual-functional photosensitizer for polarity detection and efficient photoablation of cancer cells. | ||
| Fig. 2 Singlet oxygen production efficiency of BD derivatives in (A) DMSO and (B) deionized water. (C) Dark cytotoxicity and (D) phototoxicity of BD derivatives toward HeLa cells. | ||
Intrinsic phototoxicity of BDI in live cells
Moving on to the first function of BDI as an efficient photosensitizer and its intrinsic phototoxicity, live/dead cell staining images disclosed that, under light irradiation, a significant portion of HeLa cells underwent cell death, while cells remained viable without light irradiation, demonstrating its phototoxic nature (Fig. S9, ESI†). Furthermore, BDI also exhibited phototoxicity toward other cancer cell lines beyond HeLa cells (Fig. S10, ESI†), suggesting its universal phototoxicity. Intriguingly, cells pretreated with BDI showed elevated intracellular ROS levels under light irradiation (Fig. S11, ESI†), suggesting that the phototoxicity of BDI is probably attributed to ROS generated under light irradiation.41,42 Additionally, the decrease in mitochondrial membrane potential under light irradiation further implied that the phototoxicity of BDI is closely related to the mitochondrial dysfunction (Fig. S12, ESI†).Polarity visualization with BDI in live cells
Thereafter, the second function of BDI in detecting intracellular polarity was studied in live cells. Prior to the bioimaging of polarity in live cells, the intracellular distribution of BDI was first explored. BDI was speculated to target mitochondria with a cation in its structure, as uncovered in previous research.9,11,31,38 Thus, colocalization imaging of BDI with a commercially available mitochondrial tracker was conducted in different cell lines. As depicted in Fig. 3, the fluorescence of BDI displayed excellent overlap with that of MitoTracker Green in different cancer cell lines, affirming its superior mitochondria-targeting capability. Consequently, BDI was established as a mitochondria-targeting photosensitizer, augmenting its potential for improving PDT efficiency.Following the colocalization imaging, BDI was utilized to visualize the mitochondrial polarities of cancer cells and normal cells. The result (Fig. 4) illustrated that a higher fluorescence intensity ratio (red/green) was observed in cancer cells compared with that in normal cells, implying lower mitochondrial polarity in cancer cells. In line with previous reports,9,11 these results validated the capability of BDI to assess mitochondrial polarity in live cells through ratiometric fluorescence imaging.
Referring to reported literatures,11,43,44 anticancer drugs have been reported to induce mitophagy, thereby inducing mitochondrial polarity fluctuations. Hence, BDI was further employed to visualize mitochondrial polarity variations in HeLa cells pretreated with rapamycin, a known inducer of mitophagy in a variety of cell types.45 Upon treatment of rapamycin, the fluorescence of MDC (dansylcadaverine, a commercial autophagy tracker) was enhanced over time (Fig. S13, ESI†), indicating the occurrence of mitophagy. Subsequently, time-lapse CLSM images of rapamycin-treated HeLa cells incubated with BDI were recorded. The results (Fig. 5) revealed that treatment with rapamycin resulted in a dramatic increase in the fluorescence intensity ratio (red/green) within 30 min, suggesting a decrease of mitochondrial polarity during mitophagy. Similar results were also observed in HepG2 cells (Fig. S14, ESI†), further demonstrating a mitochondrial polarity decrease in drug-induced mitophagy. Conversely, the fluorescence intensity ratio in cells without rapamycin treatment displayed negligible changes (Fig. S15, ESI†), thereby excluding the interference of other potential stimuli. Collectively, BDI could serve as a ratiometric probe for visualizing mitochondrial polarity variations during mitophagy.
Self-reporting mitochondrial polarity fluctuations during PDT treatment
After confirming the dual function of BDI as an efficient photosensitizer and a fluorescent probe for polarity detection, we endeavored to take advantage of this dual-functional photosensitizer to self-report mitochondrial polarity variations during the PDT process. Firstly, HeLa cells pretreated with BDI were irradiated to initiate the PDT process. Afterwards, fluorescence in the red and green channels was recorded at different time intervals. As displayed in Fig. 6, the fluorescence intensity ratio of red/green was significantly increased after light irradiation with the extension of incubation time, implying mitochondrial polarity decreased during PDT treatment. A similar increase in the fluorescence ratio (red/green) was observed in HepG2 cells after light irradiation (Fig. S16, ESI†), strongly suggesting ROS-induced mitochondrial polarity decrease was ubiquitous during the PDT treatment. Altogether, BDI could serve as a dual-functional photosensitizer, revealing mitochondrial polarity decrease during the PDT process.Conclusions
To sum up, a series of potential photosensitizers have been designed and prepared by incorporating the BD scaffold with different electron withdrawing groups. Among them, BDI displayed excellent selective response to polarity and high phototoxicity toward cancer cells, rendering it a dual-functional photosensitizer. BDI could specifically target mitochondria and differentiate cancer cells from normal cells with polarity discrepancy. In addition, decreased mitochondrial polarity was disclosed during anticancer drug-induced mitophagy with the ratiometric fluorescence of BDI. More importantly, BDI was successfully utilized to reveal mitochondrial polarity fluctuations during the PDT process. As a dual-functional photosensitizer, we believe BDI can not only fill in the toolbox of fluorescent probes for selective detection of intracellular polarity, but also help to shed light on intracellular polarity variations during the PDT process, thereby providing feedback of the PDT effect.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 22304188, 32122003, 22377017 and 21807014), the Natural Science Foundation of Hunan Province, China (Grant No. 2023JJ40712), the Research Grants Council of Hong Kong (Grant No. 11312422 and 11305221) and the City University of Hong Kong grant (Grant No. 9678275).References
- D. G. Drubin and W. J. Nelson, Cell, 1996, 84, 335 CrossRef PubMed.
- X. Michalet, S. Weiss and M. Jäger, Chem. Rev., 2006, 106, 1785 CrossRef PubMed.
- H. R. Pires and M. Boxem, J. Mol. Biol., 2018, 430, 3521 CrossRef CAS PubMed.
- A. Wodarz and I. Näthke, Nat. Cell Biol., 2007, 9, 1016 CrossRef CAS.
- J. Yin, L. Huang, L. Wu, J. Li, T. D. James and W. Lin, Chem. Soc. Rev., 2021, 50, 12098 RSC.
- M. Dai, Y. J. Yang, S. Sarkara and K. H. Ahn, Chem. Soc. Rev., 2023, 52, 6344 RSC.
- X. Qin, X. Yang, L. Du and M. Li, RSC Med. Chem., 2021, 12, 826 RSC.
- H. Xiao, P. Li and B. Tang, Coord. Chem. Rev., 2021, 427, 213582 CrossRef CAS.
- N. Jiang, J. Fan, F. Xu, X. Peng, H. Mu, J. Wang and X. Xiong, Angew. Chem., Int. Ed., 2015, 54, 2510 CrossRef CAS.
- K. Wang, L. Liu, D. Mao, S. Xu, C. Tan, Q. Cao, Z. Mao and B. Liu, Angew. Chem., Int. Ed., 2021, 60, 15095 CrossRef CAS.
- X. Li, X. Li and H. Ma, Chem. Sci., 2019, 11, 1617 RSC.
- Q. Li, J. Hong, S. Feng, S. Gong and G. Feng, Anal. Chem., 2022, 94, 11089 CrossRef CAS PubMed.
- W. Wang, L. Chai, X. Chen, Z. Li, L. Feng, W. Hu, H. Li and G. Yang, Biosens. Bioelectron., 2023, 231, 115289 CrossRef PubMed.
- B. Zheng, Y. Tian, S. Liu, J. Yang, F. Wu and H. Xiong, Anal. Chem., 2023, 95, 12054 CrossRef PubMed.
- T. C. Pham, V. N. Nguyen, Y. Choi, S. Lee and J. Yoon, Chem. Rev., 2021, 121, 13454 CrossRef PubMed.
- X. Zhao, J. Liu, J. Fan, H. Chao and X. Peng, Chem. Soc. Rev., 2021, 50, 4185 RSC.
- V. N. Nguyen, Y. Yan, J. Zhao and J. Yoon, Acc. Chem. Res., 2021, 54, 207 CrossRef PubMed.
- J. Miao, Y. Huo, G. Yao, Y. Feng, J. Weng, W. Zhao and W. Guo, Angew. Chem., Int. Ed., 2022, 61, e202201815 CrossRef.
- J. Tang, L. Wang, A. Loredo, C. Cole and H. Xiao, Chem. Sci., 2020, 11, 6701 RSC.
- Z. Wang, J. Zhao, A. Barbon, A. Toffoletti, Y. Liu, Y. An, L. Xu, A. Karatay, H. G. Yaglioglu, E. A. Yildiz and M. Hayvali, J. Am. Chem. Soc., 2017, 139, 7831 CrossRef CAS PubMed.
- H. Huang, S. Long, D. Huang, J. Du, J. Fan and X. Peng, Chem. Commun., 2021, 57, 9100 RSC.
- V. N. Nguyen, Y. Yim, S. Kim, B. Ryu, K. M. K. Swamy, G. Kim, N. Kwon, C. Y. Kim, S. Park and J. Yoon, Angew. Chem., Int. Ed., 2020, 59, 8957 CrossRef CAS PubMed.
- P. Chinna Ayya Swamy, G. Sivaraman, R. N. Priyanka, S. O. Raja, K. Ponnuvel, J. Shanmugpriya and A. Gulyani, Coord. Chem. Rev., 2020, 411, 213233 CrossRef CAS.
- M. Wu, X. Liu, H. Chen, Y. Duan, J. Liu, Y. Pan and B. Liu, Angew. Chem., Int. Ed., 2021, 60, 9093 CrossRef CAS.
- S. Kuang, L. Sun, X. Zhang, X. Liao, T. W. Rees, L. Zeng, Y. Chen, X. Zhang, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2020, 59, 20697 CrossRef CAS PubMed.
- X. Xue, C. Qian, H. Fang, H. K. Liu, H. Yuan, Z. Guo, Y. Bai and W. He, Angew. Chem., Int. Ed., 2019, 58, 12661 CrossRef CAS PubMed.
- H. Ma, Y. Lu, Z. Huang, S. Long, J. Cao, Z. Zhang, X. Zhou, C. Shi, W. Sun, J. Du, J. Fan and X. Peng, J. Am. Chem. Soc., 2022, 144, 3477 CrossRef CAS.
- B. C. Dickinson and C. J. Chang, Nat. Chem. Biol., 2011, 7, 504 CrossRef CAS PubMed.
- F. Lin, Y. Bao and F. Wu, Molecules, 2018, 23, 3016 CrossRef.
- N. Niu, Y. Yu, Z. Zhang, M. Kang, L. Wang, Z. Zhao, D. Wang and B. Tang, Chem. Sci., 2022, 13, 5929 RSC.
- L. Yang, Q. Chen, Y. Wan, S. Gan, S. Li, C. S. Lee, Y. Jiang, H. Zhang and H. Sun, Chem. Commun., 2022, 58, 9425 RSC.
- H. Chen, C. Ge, H. C. X. Zhang, L. Zhang, L. Jiang, P. Zhang and Q. Zhang, Dalton Trans., 2019, 48, 17200 RSC.
- R. Zhang, C. Zhang, C. Chen, M. Tian, J. H. C. Chau, Z. Li, Y. Yang, X. Li and B. Tang, Adv. Sci., 2023, 10, 2301295 CrossRef CAS PubMed.
- P. S. Maharjan and H. K. Bhattarai, J. Oncol., 2022, 2022, 7211485 Search PubMed.
- A. El-Hussein, M. Harith and H. Abrahamse, Int. J. Photoenergy, 2012, 2012, 281068 CrossRef.
- J. Wang, L. Zhang, M. Chen, S. Gao and L. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 23248 CrossRef.
- L. Yang, S. Chen, D. Yi, Q. Chen, J. Zhang, Y. Xie and H. Sun, J. Mater. Chem. B, 2021, 9, 8512 RSC.
- F. Xu, H. Li, Q. Yao, H. Ge, J. Fan, W. Sun, J. Wang and X. Peng, Chem. Sci., 2019, 10, 10586 RSC.
- D. Ma, H. Bian, S. Long, P. Zhou, R. Tian, Y. Wu, H. Ge, M. Li, J. Du, J. Fan, Y. Zhang and X. Peng, Sci. China: Chem., 2022, 65, 563 CrossRef.
- W. Zhai, Y. Zhang, M. Liu, H. Zhang, J. Zhang and C. Li, Angew. Chem., Int. Ed., 2019, 58, 16601 CrossRef PubMed.
- J. Morgan and A. R. Oseroff, Adv. Drug. Deliver. Rev., 2001, 49, 71 CrossRef CAS PubMed.
- M. D. Yaqoob, L. Xu, C. Li, M. M. L. Leong and D. Xu, Photodiagn. Photodyn. Ther., 2022, 38, 102830 CrossRef CAS.
- X. Wang, Q. Chen, J. Ou, Y. Huang, C. Wang, Y. Chen, Y. J. Qin, M. Chen, Y. Feng, G. Xu and X. Meng, Sens. Actuators, B, 2022, 351, 130953 CrossRef CAS.
- T. Zhang, F. Huo, W. Zhang, F. Cheng and C. Yin, Chem. Eng. J., 2022, 437, 135397 CrossRef CAS.
- S. Sarkar and D. C. Rubinsztein, Mol. BioSyst., 2008, 4, 895 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01872a |
| This journal is © The Royal Society of Chemistry 2024 |