Open Access Article
Open Access ArticleAddressing preliminary challenges in upscaling the recovery of lithium from spent lithium ion batteries by the electrochemical method: a review†
Mohamad Arif Kasri ab,
Muhammad Zharfan Mohd Halizanb,
Irina Harunc,
Fadzli Irwan Bahrudind,
Nuraini Daude,
Muhammad Faiz Aizamddinf,
Siti Nur Amira Shaffeef,
Norazah Abd Rahmang,
Saiful Arifin Shafiee
ab,
Muhammad Zharfan Mohd Halizanb,
Irina Harunc,
Fadzli Irwan Bahrudind,
Nuraini Daude,
Muhammad Faiz Aizamddinf,
Siti Nur Amira Shaffeef,
Norazah Abd Rahmang,
Saiful Arifin Shafiee *a and
Mohd Muzamir Mahat
*a and
Mohd Muzamir Mahat *b
*b
aDepartment of Chemistry, Kulliyyah of Science, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, 25200 Kuantan, Pahang, Malaysia. E-mail: sabs@iium.edu.my
bFaculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia. E-mail: mmuzamir@uitm.edu.my
cDepartment of Environment, Faculty of Forestry and Environment, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia
dKulliyyah of Architecture & Environmental Design, International Islamic University Malaysia, Gombak, 53100 Kuala Lumpur, Selangor, Malaysia
eFaculty of Artificial Intelligence, Universiti Teknologi Malaysia, 54100 Kuala Lumpur, Malaysia
fGroup Research and Technology, PETRONAS Research Sdn. Bhd., Bandar Baru Bangi 43000, Selangor, Malaysia
gSchool of Chemical Engineering, College of Engineering, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
First published on 13th May 2024
Abstract
The paramount importance of lithium (Li) nowadays and the mounting volume of untreated spent LIB have imposed pressure on innovators to tackle the near-term issue of Li resource depletion through recycling. The trajectory of research dedicated to recycling has skyrocketed in this decade, reflecting the global commitment to addressing the issues surrounding Li resources. Although metallurgical methods, such as pyro- and hydrometallurgy, are presently prevalent in Li recycling, they exhibit unsustainable operational characteristics including elevated temperatures, the utilization of substantial quantities of expensive chemicals, and the generation of emissions containing toxic gases such as Cl2, SO2, and NOx. Therefore, the alternative electrochemical method has gained growing attention, as it involves a more straightforward operation leveraging ion-selective features and employing water as the main reagent, which is seen as more environmentally benign. Despite this, intensive efforts are still required to advance the electrochemical method toward commercialisation. This review highlights the key points in the electrochemical method that demand attention, including the feasibility of a large-scale setup, consideration of the substantial volume of electrolyte consumption, the design of membranes with the desired features, a suitable layout of the membrane, and the absence of techno-economic assessments for the electrochemical method. The perspectives presented herein provide a crucial understanding of the challenges of advancing the technological readiness level of the electrochemical method.
Introduction
Mitigating the global environmental issues encompassing climate change and greenhouse gases through decarbonisation of the transport sector has influenced the current shift in the global vehicle market from the domination of the internal-combustion engine (ICE) to electric vehicles (EVs) in support of the net-zero carbon emissions agenda.1,2 In the post-pandemic era, the EV sector has experienced a significant growth, with a projected revenue of USD 56.1 billion in the year 2023. Moreover, the market forecasts anticipate its expansion to a volume of USD 906.7 billion in 2028.3 As EVs are powered using Li-ion battery (LIB) technology, the boom in the EV industry has contributed significantly to the increasing lithium (Li) demand. In addition, the multiplying number of spent LIB from EVs in the next few years is a cause for concern. The International Energy Agency (IEA) forecasted a significant surge in LIB waste, with the EVs manufactured in 2019 are expected to generate 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of such waste, a figure that could escalate to 8 million tons by 2040.4,5 These trajectories highlight the critical dependence of the global community on Li and the opportunity for spent LIB to become the future Li market.6
000 tons of such waste, a figure that could escalate to 8 million tons by 2040.4,5 These trajectories highlight the critical dependence of the global community on Li and the opportunity for spent LIB to become the future Li market.6
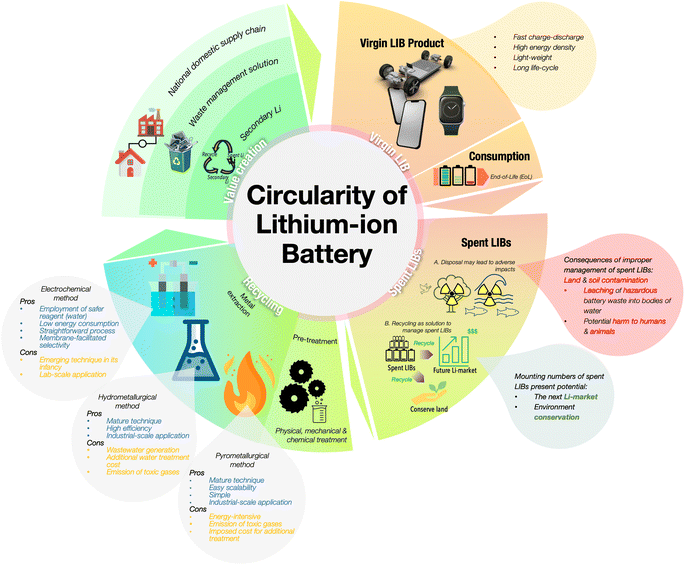
As the global dependence on Li is ever-growing, increasing the extraction and recovery efficiency of Li from spent LIB is pivotal to strike a balance between global Li reserves and consumption. The goal of recycling is threefold: (i) to decelerate resource depletion, (ii) to contribute to environmental conservation and (iii) to create a national domestic supply chain for the element.4,6,7 In addition to the issue of sustainable Li supply, spent LIB present alarming concerns in terms of their threat to the environment. Their occupation of landfills, leaching of the hazardous elements from the spent sources into the receiving water, and potential harm to the ecosystem from the exposure to contaminated water sources requires an imminent solution.7–10 Leachate from spent LIB may contain dissolved gases, heavy metals, additives, and electrolyte degradation products, leading to the potential release of heavy metals into the soil, nearby rivers, or lakes through rainwater runoff. The management of the spent LIB through recycling approaches not only benefits the environment, but also has potential in contributing to the establishment of a circular flow of the valuable elements in LIBs. The establishment of spent LIB recycling in the next few years would give local governments and the community a potential secondary supply, generating a secure supply chain as well as wealth, benefiting the local economy.
At present, the mature pathways for the recycling of Li and other precious metals from spent LIB are hydrometallurgy and pyrometallurgy. Pyrometallurgy employs the concept of heat treatment to induce chemical conversions in the mixture of metals in spent LIBs. Hydrometallurgy involves the separation or purification of the mixture of metals in spent LIB through leaching methods. Through hydrometallurgy, Li and other precious elements are leached into a leachate using either inorganic acid, organic acid, or bacteria. Despite the efficacy of metallurgical methods in extracting Li, the long-term setbacks are a matter requiring consideration. At the surface, the problems surrounding these techniques are excessive energy consumption, the use of toxic reagents, and the generation of wastewater, which highlights the need for an environmentally benign option for Li recovery.11–13 Fig. 1 illustrates the circularity of LIB from the virgin products to their end-of-life, followed by the need for their recycling to sustain the mineral supply while handling the substantial number of LIB that will be disposed of in the coming years.
In the recent years, the electrochemical approach to recycle Li mediated by membrane selectivity, current-induced reaction, and safer reagent consumption has garnered significant attention in academia and industry.14–17 This new approach has already been proposed as an alternative to pyro- and hydrometallurgy, and benefits from minimal-current operation and employment of water as the main reagent.11,14 However, to the best of our knowledge, there are not yet any comprehensive reports or patents for the up-scaled electrochemical technology in Li recycling. The method still requires solutions from both academia and industry before it can proceed to commercialisation. Despite the availability of metallurgical techniques like hydro- and pyrometallurgy at the industrial scale, their drawbacks in terms of adverse effects on the environment and high cost have ignited research into the emerging electrochemical recycling technique. This review aims to lay a foundation for addressing critical areas that remain to be tackled for the expansion of the electrochemical recycling technique and to provide insights into its path toward commercialisation.
Current trends in lithium consumption
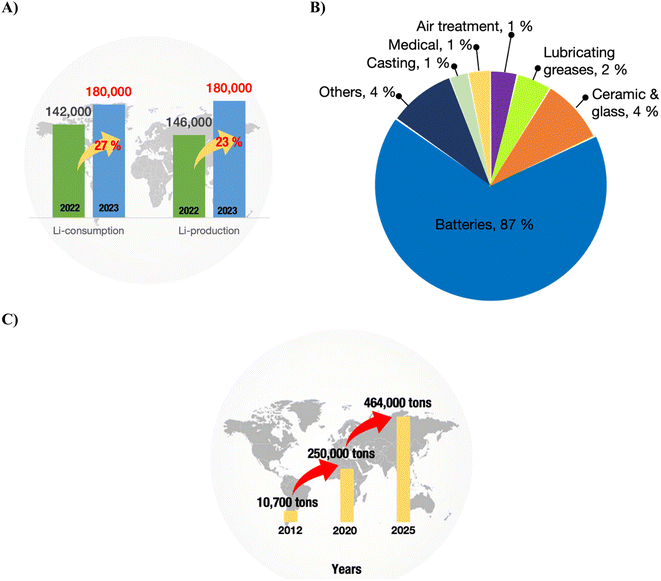
LIB are the forebearers of energy storage technology and stand at the core of the clean energy and net-zero emission agenda. The first-ever LIB were commercialised by SONY Corporation in 1991, based on Asahi Kasei Corporation's first assembly of Li1–xCoO2/C6.18 The path of rechargeable batteries based on Li has advanced robustly since then. Their rechargeability, extended life cycle, broad operational temperature range, and lightweight nature position LIB for versatile applications, spanning from personal appliances like smartphones, smartwatches, tablets, and laptops to vehicles and grid energy storage.19,20 As shown in Fig. 2A, from the year 2021 to 2022, Li production and consumption increased by 21% and 41%, respectively. Fig. 2B indicates that most of the Li consumed is directed toward battery manufacturing.21As of January 2024, global Li reserves stand at 28 million tons (Mt). The ceiling for this figure could potentially increase in coming years with the availability and readiness of advanced technologies that enhance the efficiency of Li production.22 However, despite this promising trend, the demand for Li is experiencing rapid growth. Electric vehicles (EVs) assume a pivotal role in achieving the pressing global agenda for net-zero emissions.1,2 A research conducted by Goldman Sachs has forecasted that EVs will constitute 50% of new car sales worldwide by 2035 (Goldman Sachs, 2023). In 2025, it is estimated that the amount of spent LIB could reach 464![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes, and the forecast from International Energy Agency states that by 2040, the amount of spent LIB accumulated from EVs manufactured in 2019 could reach as high as 8 million tons (see Fig. 2C).4,5,11 These unprecedented numbers present a detrimental threat to the environment and could hasten the shortage of global mineral reserves.4
000 tonnes, and the forecast from International Energy Agency states that by 2040, the amount of spent LIB accumulated from EVs manufactured in 2019 could reach as high as 8 million tons (see Fig. 2C).4,5,11 These unprecedented numbers present a detrimental threat to the environment and could hasten the shortage of global mineral reserves.4
Confronted with these circumstances, research endeavours have been directed towards a sustainable or circular-path of Li consumption through the recycling of LIB waste, which contains precious metals including Li, cobalt (Co), copper (Cu), manganese (Mn), and nickel (Ni). Spent LIB recycling is a developing industry that is gaining interest worldwide, driven by the economic returns derived from recovering the precious metals such as Li, Co, Mn, and Ni. This approach also serves as a barrier to protect the supply chains of critical materials for energy storage and production.12 Hydrometallurgy, pyrometallurgy, and electrochemical methods are being actively pursued for this purpose. However, the intricacies of these approaches have sparked a new concept of recycling spent LIBs. To relieve the raw material pressure for sustainable battery production, ongoing research on spent LIB recycling has focused on the concept of transforming or upcycling various types of degraded LIB cathodes, such as LiNi0.5Co0.2Mn0.3O2, LiMn2O4, and LiCoO2, into new regenerated forms for next-generation battery manufacturing.1,23,24 The advantages of this approach lie in the simplicity of the process, its low cost and environmental friendliness, and the ability to directly reuse the regenerated products.2 Recycling and upcycling uphold the greater goal of addressing the need for sustainable production of LIBs. It is notable that the diversity of approaches towards this common goal has ignited and contributed to the dynamic and rapid development of methods for the recycling of spent LIBs. Here, it is worth mentioning the work on spent LIB recycling has paid attention to the other integrated parts, such as the anode, which is composed of valuable materials such as copper, graphite and residual Li, and the liquid electrolyte, which is also of interest due to presence of the mineral Li.2 This demonstrates that the prospective recycling industry for spent LIB could prosper in the coming years with a united vision of protecting the mineral reserves while preserving battery production.
Given that recycling stands as a crucial pillar of energy security, it is imperative for academic and industry stakeholders to collaborate in refining both existing and upcoming recycling technologies to achieve a technology that is cost-effective, sustainable, and efficient in serving its purpose.
Commercialised lithium recycling methods
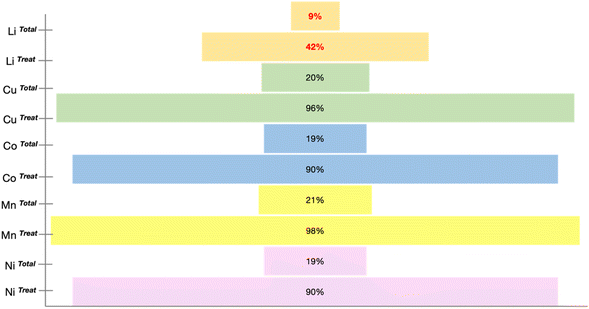
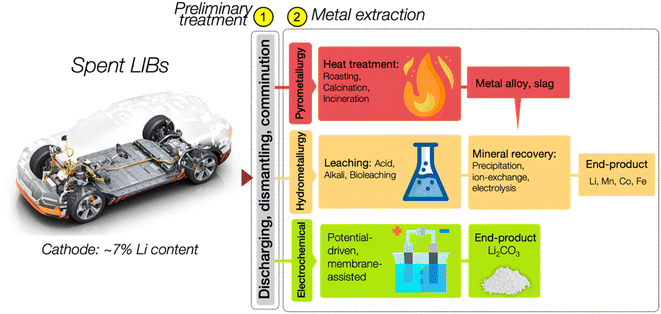
Precious metals such as Li, Mn, Cu, Co, and Ni are present in abundance in spent LIBs. The recycling rates of most of these elements exceed 70%; however, disconcertingly, the recycling rate for Li is below 50%, and the total percentage of recovered Li is merely ∼9% (see Fig. 3).25 The inconsistency in the recycling rates raises concerns about efficiency and stresses the urgency of Li recycling initiatives, highlighting the need for intensified efforts in the reality of escalating resource challenges. Fig. 3 details the recovery rates of minerals from spent LIBs, which encompasses the rates from the total generated spent LIB (XTotal) and the rates from the total spent LIB entering recycling plants (XTreat).25The prevalent approach to Li recycling involves metallurgical and electrochemical methods. As shown in Fig. 4, the processing route for the recovery of Li and other precious metals from spent LIB will include the following: (i) preliminary treatment/pretreatment and (ii) metal extraction. The pretreatment step involves discharging, dismantling, comminution, and separation of individual parts by means of physical methods like sieving, flotation, magnetic, or electromagnetic separation. This preliminary treatment plays a crucial role in achieving high purity for the recovered metals and contributes to the energy-saving aspect of the recycling process.12,26–29 Considering the differences in battery assembly and cathode chemistry, and the wide range of metal compositions, the pretreatment step is imperative to enable selective separation and recovery of metals from the waste.12,30 In addition to the high purity of the product and energy savings, the pretreatment is important is to guarantee the safety in the subsequent recycling processes. The reactivity of Li in the LIB system is a feature that is beneficial to its performance, but at the same time presents a major concern in the recycling process, and thus the established process of recycling the spent LIB would require pretreatment steps. Thus, to guarantee the safety in the recycling procedure, a pretreatment would ideally aim to make the battery reach the 0% state of charge (SoC) through deactivation by subjecting the battery to a nitrogen cooling environment. There are also discharging methods using a conducting solution or salt-solution immersion discharge and non-electrochemical discharge in a conducting powder.31–33
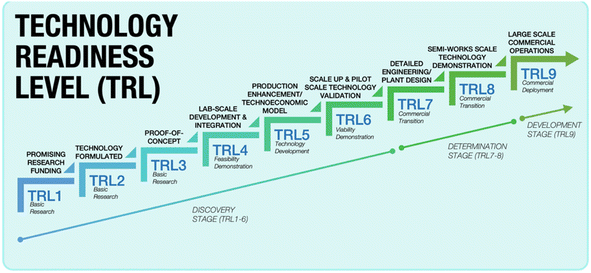
Once the pretreatment phase is concluded, the pre-treated spent LIBs, commonly referred to as black mass, are subsequently subjected to a metal extraction process. At this phase, a specific recycling method or a combination of processes is typically employed to extract Li, particularly using the pyrometallurgy, hydrometallurgy, and electrochemical methods. In the realm of Li recycling, pyro- and hydrometallurgical technologies are already mature and commercialised. They have been widely adopted by recycling companies worldwide, and are at Technology Readiness Level 9 (TRL9). The TRL offers a reliable measure of the maturity of a specific technology, which is assessed by the parameters assigned to each level.34,35 Fig. 5 briefly presents each TRL stage with its respective parameters. In comparison, the electrochemical method is currently at the lab-scale development and integration level which is TRL4, and hence, the critical phase now focuses on preparing it for an expanded scale of application.
These mature technologies have now been adopted in industry worldwide, but some regions would still require meticulous preparation before adopting these methods. Bahrudin et al., in their writing on the Malaysian perspective towards spent LIBs, highlighted crucial points that are relevant to any country heading toward the adoption of Li recycling technology locally.36 Emphasis is placed on the prerequisites for recycling interventions, infrastructure, and legislation to be researched and designed beforehand. This prior action should provide the government and local community with sufficient data, adequate infrastructure, and strong policy that sustains the local recycling industry.
Pyrometallurgy
The most studied method in Li and spent LIB recycling is metallurgy, a process that is utilised to extract or refine metals from their ore, or in this case, black mass.37 Pyrometallurgy is understood as a metallurgical process involving thermal treatment to trigger physical and chemical transformations in a multi-element source or black mass, thereby segregating the elements into individual entities.38The heat treatments used in the pyrometallurgy of black mass include roasting/calcination, incineration, pyrolysis, and smelting; these approaches are further classified into many processes based on their different extraction methods and operating atmospheres.39–41 The employment of high temperatures ranging from >500 °C to 2000 °C gives pyrometallurgy the advantage of greatly enhancing chemical reactions, surpassing the effects achievable at lower temperatures, at which only phase transitions and structural changes in metals occur.39,42,43 Companies worldwide including Umicore, Accurec, and Inmetco have employed pyrometallurgical treatment to give alloy metals and slag as the final products, which contain most of the Li remains.12 However, given its substantial energy consumption, association with high amounts of atmospheric emission, and limited range of recovered products, the pyrometallurgical method for Li recycling is inefficient, environmentally destructive, and unsustainable in the long run.12,13 It is important to mention the inability of the pyrometallurgical method to recycle Li directly. This is related to the greater oxygen affinity of Li, which contributes to Li slagging in the pyrometallurgical process.44 Thus, the pyrometallurgical method necessitates subjecting the slag to a post-treatment process. This will involve a leaching or hydrometallurgy process; these metallurgical methods will be elaborated upon in a later section. Such post-treatment leads to a higher energy consumption.45 Table 1 presents the diverse sets of parameters adopted by recycling companies worldwide for the recycling of spent LIB using the pyrometallurgy method. Here it is notable that in the approaches in which the products are not specifically subjected to post-treatment, Li is not recovered.
| Company | Country | Recycling capacity (t per year) | Types of batteries processed | Pyrometallurgic process | Post-treatments | Products | Secondary products | Waste | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Valdi (Eramet) | France | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
LIB and other battery types | (Not reported) | — | FeNi/FeMn alloy | — | — | 46 |
| Umicore | Belgium | 7000 | LIB, Ni–Cd, Ni–MH | Pyrolysis, 700 °C; smelting 1200–1450 °C | Leaching, solvent extraction | CoCl2, Co, Ni, Cu, Fe | Slag (Al, Si, Ca, Fe, Li, Mn) | Electrolyte, plastics, graphite | 47–49 |
| Xstrata (Glencore) | Switzerland | 7000 | All LIB chemistry | Conditioning (rotary kiln) and introduction into a Co–Ni winning process (EAF) | Hydrometallurgy | Alloy (Co–Ni–Cu) | Li slagged and ignorable metals | No recovery of Li, Al, electrolyte, graphite, plastics | 47 and 50 |
| Inmetco | USA | 6000 | LIB, Ni–Cd, Ni–MH | High-temperature melting recovery (HTMR), 1260 °C | Iron casting | Alloy (Co–Ni–Fe) | Li slag | The organic material used as a chemical reagent | 47 and 51 |
| Accurec | Germany | 6000 | All LIB chemistry | Pyrolysis, 250 °C; carbothermic reduction, 800 °C | Acid leaching | Li2CO3 Co-alloy | Metallic alloy | Electrolyte, polymers, graphite | 52–54 |
| JX Nippon mining and metals | Japan | 5000 | — | Smelting | Leaching, selective precipitation, electrowinning | Li2CO3, MnCO3, Co | — | Electrolyte | 46, 55 and 56 |
| SNAM | France | 300 | — | (Not reported) | — | Black mass (Cu, Ni, Co) | — | 47 | |
| Sumitomo | Japan | 150 | LIB (LiCoO2) | Calcination, 1000 °C | Acid leaching, hydrometallurgy | CoO | Co–Ni–Fe alloy Cu, Al, Fe | Electrolyte, plastics, Li, Ni, graphite | 41, 57, 58 |
| G&P batteries | UK | 145 | LIB | (Not reported) | (Not reported) | — | — | — | 59 |
| LithoRec | Germany | 2000 | — | Drying, calcination | Undisclosed leaching agent | Li2CO3, metal oxides | Al–Cu, plastic fractions | Electrolyte | 52 |
| GRS batteries | Germany | — | LiMnO2 | Vacuum distillation | — | Co, Ni, Cu, FeNi and FeMn | — | — | 59 |
| Battery resources | Germany | — | LIB, Ni–MH | Sintering | Leaching by NaOH, H2O2, H2SO4, Na2 CO3 | Li2CO3, metal oxides | Al–Cu, plastic fractions | Electrolyte | 52 |
Fig. 6 displays the recycling route carried out by Umicore, one of the recycling companies with a pyrometallurgical recycling approach.60 It is worth mentioning that the end-product of the Umicore process following the pyrometallurgy is in the form of alloy and slag. This product will usually necessitate further treatment to segregate the chemical entities of the desired minerals, such as Li.4,27,61–64 In addition to the additional cost required for the post-treatment of the alloy and slag, the pyrometallurgy process is a high-energy-demand process that contributes to greenhouse gas emissions (GHG).65,66 Moreover, environmental studies on pyrometallurgy have revealed possible risks including global warming, ozone layer depletion, photochemical ozone creation, carcinogenic and non-carcinogenic effects, and eutrophication due to the pyrometallurgical recycling process.66–69 These dilemmas of the pyrometallurgical method have directed the interest of academia and industry toward optimising the pyrometallurgical method in recent years. One strategy is minimising the energy consumption to mitigate the GHG emissions through strategies such as synergistic pyrolysis and the utilisation of green reactants such as bio-fuels, hydrogen, and ammonia.70,71
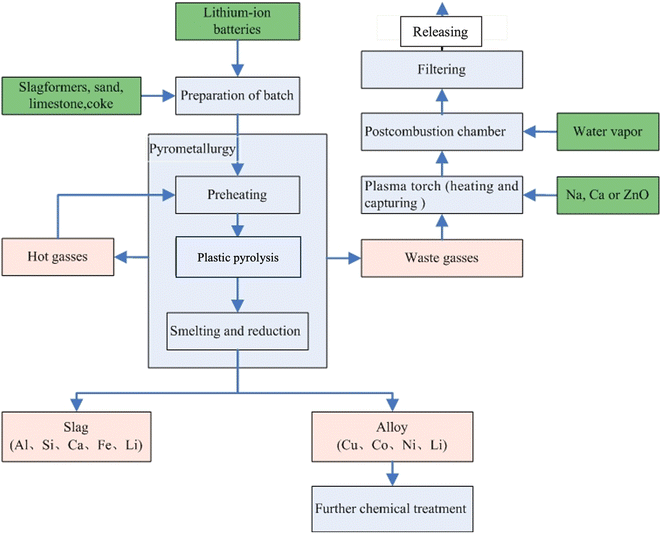 | ||
| Fig. 6 Umicore pyrometallurgical recycling route.60 The process involves initial preheating at a temperature <300 °C to evaporate the electrolyte, followed by pyrolysis of plastics at 700 °C, whereby the emitted hot gases are resupplied for the preheating. At the last stage, smelting/reduction takes place at 1200–1450 °C, resulting in slag (aluminium, silicon, calcium, iron) and alloy (copper, cobalt, nickel, Li, small quantities of iron). Beyond this stage, further leaching of the separated elements is required. | ||
Hydrometallurgy
The concept of hydrometallurgy is the transfer or leaching of valuable metals into a solution through a step referred to as ‘leaching’ employing a leaching agent from an alkali medium, acid medium or microorganisms like bacteria and fungi.39,72,73 Subsequently, metal recovery from the leachate solution takes place using various processes such as electrolysis, solvent extraction, chemical precipitation, and ion-exchange.72The primary advantage of utilising hydrometallurgy lies in its low energy consumption and higher recovery rates of metals in high purity compared to the pyrometallurgy technique. Despite that, the drawbacks associated with hydrometallurgy require careful consideration. In addition to the use of harsh chemicals as leaching agents in the metal leaching and recovery steps, research has progressed to the application of concentrated leachate to deliver a time-efficient and effective metal recovery. Table 2 lists the recoveries of Li and other precious minerals from spent LIB through hydrometallurgy techniques including alkali inorganic acid leaching, organic acid leaching, alkali leaching, and bioleaching. The hydrometallurgical method offers a solution to recover Li, which is not attainable using pyrometallurgy. As shown in the table, most studies successfully recovered Li. The flexibility of hydrometallurgy lies in the ability to choose among numerous reagents that can deliver the intended result. Compared with pyrometallurgy, hydrometallurgy operates at a lower temperature. Despite the ability to yield Li as the final product, the conventional use of inorganic acids as the reagent in hydrometallurgy generates toxic waste with adverse effects on the environment and human health, which has prompted a shift toward studies employing organic acids like citric acid and H2O2 (see Table 2). The efficiency of hydrometallurgical recycling is compromised by its substantial reagent consumption. When executed at the large industrial scale to process large quantities of spent LIBs, hydrometallurgy imposes significant chemical costs and additional expenses for wastewater treatment. Bioleaching hydrometallurgy for the recovery of Li has been recognized in recent years for its cost-effectiveness, environmental friendliness, low energy requirements, and high efficiency with minimal GHG emissions. However, challenges are encountered in the aspects of scalability and commercialisation, notably when there is an excessive input time compared to the traditional acid and alkali leaching methods in hydrometallurgy.74
| LIB type | Leaching agent | Leaching conditions | Recycling efficiency (%) | Ref. |
|---|---|---|---|---|
| Inorganic acid | ||||
| LiCoO2 | 2 M H2SO4 + 5 vol% H2O2 | 80 °C, 1 h, 50 g L−1 | Li = 99, Co = 99 | 75 |
| LiCoO2 | 2 M H2SO4 + 50 g per L glucose | 80 °C, 2 h, 35 g L−1 | Li = 92, Co = 88 | 76 |
| NCM | 1 M H2SO4 + 1 vol% H2O2 | 40 °C, 1 h, 40 g L−1 | Li/Co/Ni/Mn = 99.7 | 77 |
| NCA | 4 M HCl, 90 °C, 18 h with a 5% (w/v) solid to liquid ratio | 90 °C, 18 h, 5% (w/v) | Li/Co/Ni/Al = 100 | 78 |
| LiFePO4 | 2.5 M H2 SO4 | 60 °C, 4 h, 100 g L−1 | Li = 97, Fe = 98 | 79 |
| Mixture | 1.34 M H2SO4 + 0.45 g per g Na2S2O5 | 20 °C, 45 min, 10.9% | Mn = 94, Cd = 81, Zn = 99, Co = 96, Ni = 68 | 80 |
| Mixture | 1 M H2SO4 + 0.075 M NaHSO3 | 95 °C, 4 h, 20 g L−1 | Li = 97, Co = 92, Ni = 96, Mn = 88 | 81 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Organic acid | ||||
| LiCoO2 | 1.25 M citric acid + 1 vol% H2O2 | 90 °C, 0.5 h, 20 g L−1 | Li = 99, Co = 91 | 82 |
| LiCoO2 | 0.1 M citric acid + 0.02 M ascorbic acid | 80 °C, 6 h, 10 g L−1 | Li = 100, Co = 80 | 83 |
| NCM | 1 M citric acid + 12 vol% H2O2 | 60 °C, 40 min, 80 g L−1 | Total metals >98 | 84 |
| NCM | 3.5 M acetic acid + 4 vol% H2O2 | 60 °C, 1 h, 40 g L−1 | Li = 99.97, Co = 93.6, Ni = 92.7, Mn = 96.3 | 85 |
| Mixture | 1.5 M citric acid + 0.5 g per g glucose | 80 °C, 2 h, 20 g L−1 | Li = 99, Co = 92, Ni = 91, Mn = 94 | 86 |
| Mixture | 2 M tartaric acid + 4 vol% H2O2 | 70 °C, 0.5 h, 17 g L−1 | Li = 97.7, Ni = 98.2, Co = 98.9, Mn = 98.4 | 87 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Alkali leaching | ||||
| NCM | 367.5 g per L NH3·H2O + 140 g per L NH4HCO3 + 63.24 g per L H2 O2 | — | Li = 81.2, Co = 96.3, Ni = 96.4, Mn: hardly leached out | 88 |
| Mixture | 15 wt% NaOH | 50 °C, 1 h, 0.1 g mL−1 | Al = 58 | 89 |
| Mixture | 1.5 M NH3 + 1 M (NH4)2SO3 + 1 M NH4HCO3 | 60 °C, 3 h, 20 g L−1 | Li = 60.53, Co = 80.99, Ni = 96.32 | 90 |
| Mixture | 3 M (NH4)2SO4 + 0.75 M (NH4)2SO3 | 180 °C, 2 h, 83 g L−1 | Li = 98, Co = 81, Ni = 98, Mn = 92 | 91 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Bioleaching | ||||
| LiCoO2 | Bacteria: Acidithiobacillus ferrooxidans + 0.75 g per L copper ions | 35 °C,6 d | Co = 99.9 | 92 |
| LiCoO2 | Bacteria: Acidithiobacillus ferrooxidans + 0.02 g per L Ag+ | 35 °C, 7 d | Co = 98.4 | 93 |
| Mixture | Bacteria: Acidithiobacillus ferrooxidans + elemental sulphur 1% + 3 g per L Fe(II) | 30 °C, 20 d, 5 g L−1 | Li = 10, Co = 65 | 94 |
As described in the previous section, the implementation of pyrometallurgy mostly relies on post-treatment methods to leach or extract the metals from the alloy or slag formed, and thus includes hydrometallurgy as one of the necessary treatments, owing to its efficiency and selectivity. Worldwide, hydrometallurgy and combined pyro/hydrometallurgy techniques have been employed at the pilot and commercial scale. Provided in Table 3 are the companies that have committed to either the singular metallurgical method or combined metallurgical method. It is worth stating that the hydrometallurgical method is normally implemented to follow the recovery of metal through pyrometallurgy. The ability of hydrometallurgy to allow the recovery of most of the metals produced as alloy and slag in pyrometallurgy makes it a necessary post-treatment. On the other hand, the execution of the hydrometallurgy method alone can successfully recover Li and other precious metals from spent LIB without the need to perform pyrometallurgy. The use of thermal treatment to drive the reaction in pyrometallurgy leads to an excessive amount of energy being consumed compared to hydrometallurgy. It is unfair to say that hydrometallurgy is more cost-effective than the pyrometallurgy method by looking exclusively at the energy consumption, as the overall operation of hydrometallurgy imposes external costs including reagent costs and wastewater treatment expense. Nonetheless, when considering the energy consumption factor, it is simple to conclude the thermal treatment in pyrometallurgy is the most energy-consuming, followed by hydrometallurgy, as shown in Table 3 below.
| Company | Pyrometallurgy | Hydrometallurgy | Recovered mineral | Energy consumption | Status in the year 2020 |
|---|---|---|---|---|---|
| a High energy consumption reflects the employment of both the pyrometallurgy and hydrometallurgy methods together, while medium and low energy consumption reflect the employment of the hydrometallurgy method. | |||||
| America manganese | — | H2SO4, SO2 reductive leach | LiOH, Ni(OH)2, Co(OH)2, Mn(OH)2, Al | Low | Pilot plant |
| Brunpt recycling | — | H2SO4, H2O2 reductive leach, solvent extraction | Ni, Co, Mn, Al | Medium | Commercial plant |
| Duesenfeld | Calcination | Solvent leaching | Li2CO3 and metal oxide | High | Pilot plant |
| Li-cycle | — | H2SO4, H2O2, reductive leach, solvent extraction | NiSO4, CoSO4, MnCO3, Li2CO3 | Medium | Commercial plant |
| Lithion recycling | — | Leaching | Ni, Co, Mn, Al | Medium | Pilot plant |
| OnTo technology | Brief heating | Hydrothermal | Cathode material | Medium | Pilot plant |
| Sumitomo | Smelting | Electrowinning H2SO4 leaching | Ni, Co, Cu | High | Commercial plant |
| Umicore | Smelting | Leaching, solvent extraction, Co, Ni refining | Ni(OH)2, LiMeO2 | High | Commercial plant |
While the recycling output holds promise, and such pyro/hydrometallurgy recycling strategies have been implemented on the pilot and commercial scale, the pursuit of this strategy may prove more harmful and costly considering the metrics of energy and chemical consumption. The trade-offs made to maximize recycling benefits lead to increased recycling costs, raising concerns. In addition to the GHG emissions from the energy-demanding process of pyrometallurgy, the hydrometallurgy process produces gases such as chlorine (Cl2), sulfur dioxide (SO2), and nitrogen oxides (NOx), which are harmful to the environment and human health. A study also revealed that there is a risk of freshwater and terrestrial acidification from hydrometallurgy recycling, creating a requirement for wastewater treatment to mitigate the pollution threat in the receiving waters.12,69,72,96 This scenario introduces complexities in addressing health risks and environmental impacts from the recycling process, requiring a nuanced approach to balance the potential benefits and the drawbacks.
The risks associated with the current recycling methods have pushed the boundaries of Li recycling beyond metallurgy. In addition, based on the pursuit of sustainability, the quest for greener recycling methods has prevailed. In the past years, the electrochemical technique has appeared as the better solution. This method is anticipated to succeed as a commercialised method, but the following key areas require attention and effective solution from experts.
Electrochemical method
The innovative approach of Li recycling via the electrochemical method has garnered a significant attention from researchers and industry due to its more sustainable operational mechanism. The initial exploration was first undertaken by the Asl group in 2012, and mainly concentrated on developing a Li-liquid battery using spent LIB as the Li for the energy source.97 Building upon this pioneering study, the Bae group in 2016 leveraged the success achieved by Asl, directing their efforts towards a new emphasis on recycling Li from spent LIBs.11,14 Their venture marked a transformative development in the landscape of the recycling industry's future. The quest for electrochemical recycling of spent LIB has been cultivated since then, with studies moving in the direction of emulating the electrochemical cell to harvest Li from spent sources.Using the electrochemical concept, Li from spent LIB has been successfully harvested into one of the commercial precursor forms of Li2CO3 through reaction with water.11,14 The advantage of accomplishing recycling through the electrochemical technique, offers flexibility of choosing a safer reagent to recycle Li and other precious minerals compared with traditional hydrometallurgical approach. The avoidance of toxic or harmful reagents, along with the environmentally friendly operating conditions and the simplicity of the charge–discharge process, make this method an economical and sustainable substitute to existing metallurgical recycling approaches. Fig. 7 is a representation of the first electrochemical recycling attempt by Bae's group. In brief, the black mass from the pretreatment procedure is placed in the ‘waste compartment’. Water is used as the main reagent, and the presence of a small amount of electrolyte in the system is crucial to facilitate the movement of Li ions across the system. The system's selectivity for Li+ and its temporary intercalation are facilitated by the separator unit and graphite within the harvesting anode assembly, respectively. Following the completion of the charging process, the temporarily intercalated Li+ undergoes a subsequent discharging procedure to be transformed into the common precursor Li2CO3 through reaction with water. This profound exploration by Bae was the inspiration that has propelled contemporary research towards the electrochemical method of Li recycling.
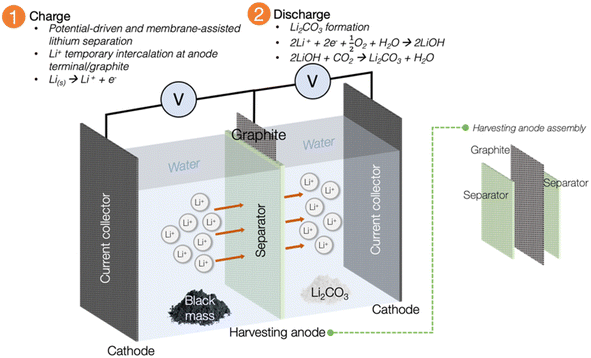 | ||
| Fig. 7 Illustration of the electrochemical setup and separator-integrated electrode assembly (adapted from ref. 11 and 14). Employing water as the main reagent could considerably cut the chemical consumption cost. The operational mechanism, which relies on a minimal current supply can presumably be less energy-consuming than pyrometallurgy, while the selectivity of the process towards the element of interest is facilitated by the high ionic conductivity of the membrane. | ||
Table 4 compiles the studies conducted on the electrochemical recycling of Li, and Fig. 8A–G are schematic diagrams of the concept from the respective studies. Over the years, there have been diverse selections of electrode, electrolyte, and separator materials, which have contributed to increased efficiency in the recycling process. Water has consistently played a crucial role as the primary reagent for the electrolyte across various attempts. It is more cost-effective than other hazardous and toxic reagents employed in the hydrometallurgy technique. Importantly, all attempts employing the electrochemical technique resulted in minimal-to-no wastewater generation. This not only reduces the environmental impact associated with disposal into receiving water, but also lowers the costs related to wastewater treatment. The compilation of studies highlights that the electrochemical recycling approach has proven to be an efficient and environmentally conscious method for Li recycling. Employing electrical energy to drive the reaction is assumed to be a superior option to the thermal treatment of pyrometallurgy, which consumes more energy and emits harmful gasses.
| Spent Li source | Operating temperature | Electrode | Membrane | Electrolyte | Recycling efficiency (%) | Product | Commercialisation status | Ref. |
|---|---|---|---|---|---|---|---|---|
| LiFePO4 | Room temperature | Spent LiFePO4 mixed with carbon black (cathode) stainless steel (anode) | Inorganic solid polymer electrolyte membrane | Water | — | Li metal | Laboratory scale research | 97 |
| LiFePO4, LiMn2O4, LiNi1/3 Co1/3 Mn1/3O2 | Room temperature | Copper (cathode) and graphite (anode) | Inorganic solid polymer electrolyte membrane | Water and LiPF6 in EC-DMC | Li = 75% | Li2CO3 | 14 | |
| LiFePO4 | Room temperature | Ruthenium-plated titanium | Anion-exchange membrane | Water and NaCl | Li = 98.0%, Fe = 96.0% | NaOH, FePO4, Li2CO3 | 16 | |
| LiFePO4 | Room temperature | Ruthenium-plated titanium | Cation-exchange membrane | Water, Li2SO4, and LiOH | Li = 96.0% | FePO4, LiOH·H2O | 15 | |
| LiMn2O4 | 90 °C | Graphite (cathode) and ruthenium-plated titanium (anode) | Filter cloth | Water, H2SO4 and MnSO4 | Li = 99.0%, Mn = 92.0% | Li2CO3, MnO2 | 99 | |
| LiCoO2 | Room temperature | Spent LixCoO2 and pristine LiCoO2 | Porous polymer membrane | 1.5 M Li2SO4 solution | — | Regenerated LiCoO2 | 100 | |
| LiCoO2 | 60 °C | Platinum-plated titanium base electrode plates | Acrylic fabric | Solution of mixed NH4HCO3 and (NH4)2SO3, and NaF | Li = 71.8%, Co = 55.4% | Regenerated LiCoO2 | 101 | |
| LiCoO2 | 100 °C | Pt cathode and Ni plate anode | — | LiOH solution | — | Regenerated LiCoO2 | 102 |
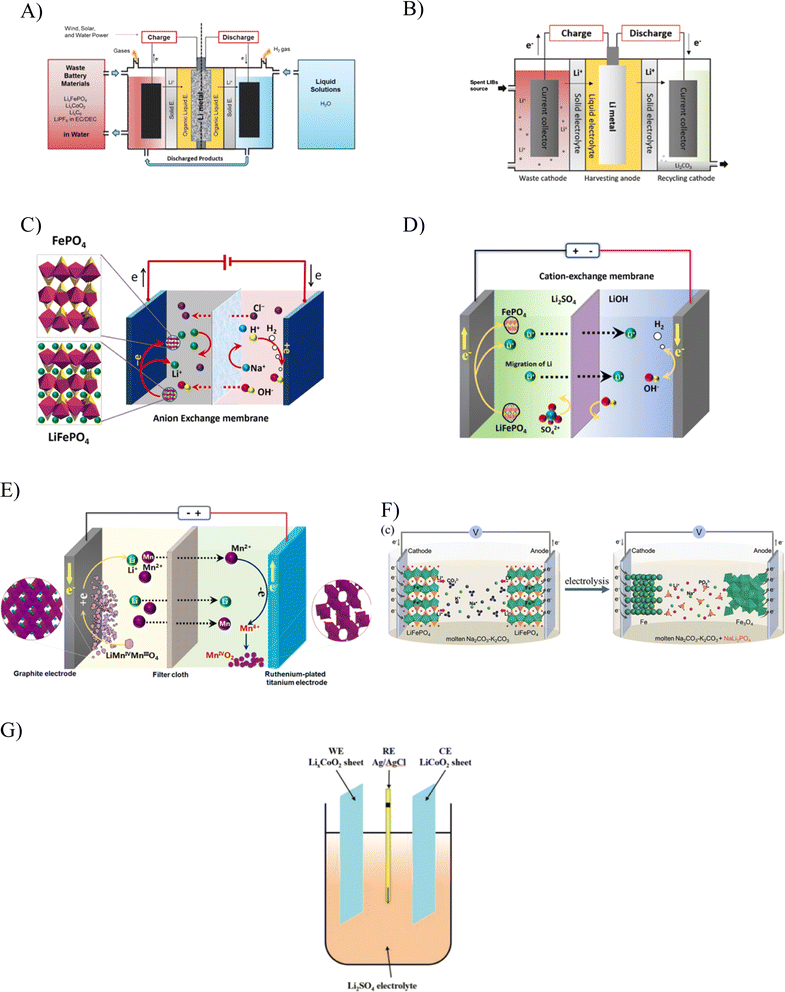 | ||
| Fig. 8 A–G are schematic illustrations of Li recycling studies using the electrochemical method. The displayed figures are representations from ref. 14–16, 97, 99, 103, and 104. The use of a current-driven process in the electrochemical method greatly reduces the consumption of reagent while avoiding Li loss in the process of separation from its spent sources. Studies using the electrochemical method have successfully recycled other precious minerals in addition to Li from spent LiFePO4, LiCoO2, LiMn2O4 and LiNi1/3Co1/3Mn1/3O2. A diverse range of setups have been explored in studies on the electrochemical recycling method, paving the way for its improvement, including improvement in the cost, which is dictated by the recovery efficiency. | ||
The exploration of the electrochemical method in the field of spent LIB recycling has seen its application in the direct recovery of precious elements. Additionally, the unique concept of the electrochemical method allows its application in the regeneration or rejuvenation of spent LIB cathodes. Compared with other regeneration approaches, the electrochemical regeneration process is less energy-consuming and uses less reagent compared to solid phase regeneration and hydrometallurgical regeneration.98 With lower cost and a facile process to produce a regenerated cathode, the electrochemical regeneration method has been deemed to be another attractive solution for sustainable battery production. Table 4 lists studies in which the electrochemical recycling method was attempted. The ability of the electrochemical method to make progress in achieving high efficiency of recovery is one of the excellent indicators of the performance of this method. With a facile operation and controlled environmental impact through usage of less detrimental reagents, this method is anticipated to be available in industrial scale in a few years. Apart from the recycling of minerals like Li, the electrochemical method is beneficial in the emerging technique of regenerating degraded cathodes from LIBs. In light of this electrochemical regeneration technique, which involves the relithiation of the Li-deficient spent cathode, it is important to understand that the reasons for the degradation of the electrochemical performance of LIB are not merely affected by the absence of Li. A wider perspective includes the influence of structural distortion and impurities on the spent cathodes. Therefore, an electrochemical regeneration process that includes the approach of the relithiation of degraded cathodes is undeniably needed to solve the identified issues. Hence, the electrochemical regeneration method, as well as other regeneration processes, must be able to handle the raw material impurities, the poor adaptability of the regenerated cathodes to the raw materials, and the inferior electrochemical performance of the regenerated products compared to virgin materials.98
Fig. 8 illustrates the electrochemical recycling concepts from several studies, which are referred to in Table 4. The initial discovery of Li recycling through the electrochemical technique was reported in the studies of Asl et al., who originally envisaged the potential to make Li-liquid batteries powered by spent LIBs.97 Their successful attempt to harvest Li powder from spent sources later inspired the studies of Li recycling using the electrochemical concept by Bae et al. in which more than 75% Li was recovered.14
Following these successful attempts, studies on the electrochemical recycling of Li prospered. Li et al. used a setup with a ruthenium-plated titanium electrode paired with the simple electrolyte of sodium chloride (NaCl), which delivered 98% Li recovery.16 Using a different electrolyte, Li et al. were able to achieve 96% recovery for Li.15 Using NaCl, the process was much more cost-saving, but it led to impurities, as reflected in the production of the by-product, sodium hydroxide (NaOH). Using a Li salt as the electrolyte avoids this scenario while also contributing to enhanced electrical conductivity.98 Despite the issue of impurities, choosing NaOH as the electrolyte seems to be more reliable, delivering the desired product with less energy consumption and cost-savings. The recovery of Li in the form of Li2CO3 and manganese as Mn2O4 from spent LiMn2O4 was also realised by the electrochemical method, as reported by Li et al.17 A 99% and 92% recovery efficiency for Li and Mn, respectively, were obtained in this trial, but the operating conditions rely on an elevated temperature, incurring an additional cost. The use of the electrochemical method to regenerate spent batteries, on the other hand, offers a very straightforward procedure, but heavily relies on the reagents used in the relithiation of the Li-deficient cathode. As discussed earlier, the issues with spent cathode of LIB is not exclusive only to Li-deficient. In a broader spectrum, the issues entail the structural distortion and impurities that require a much more holistic solution than the regeneration technique and this demands further extensive research.103,104
Despite the success of the electrochemical method to recover most metals with high efficiency, contemporary recycling research has underscored the deficient selectivity of the recycling methods in terms of the products. Although hydrometallurgical methods are an efficient process comparable to the electrochemical method, their selectivity remains problematic.105,106 Research navigating around the specific selectivity of recycling methods has involved the electrochemical recycling technique.105 A multifaceted strategy can be used to reach recycling output with high selectivity integrating the electrochemical method. Li et al. reported an approach to yield selective Li and Co recovery from LiCoO2 using electrochemical leaching and electrodeposition.105 The multiple electrochemical process strategy gave a recycling output with 97% of Li and 92% of Co recovered without contamination from impurities.
It is clear that the electrochemical recycling technique provides a solution. Its status as a safer and more economical method has positioned it as the keystone to sustaining the Li recycling industry. This contributes to the aforementioned threefold goal of recycling: to decelerate resource depletion, to contribute to environmental conservation, and to establish a national domestic supply chain for the elements. This vision, however, requires a collective effort from experts of multiple disciplines to ensure that this promising technique can be commercialised and mature as a technology to compete with and potentially replace the hydrometallurgy and pyrometallurgy techniques.
Challenges in upscaling the recovery of lithium from spent LIB using electrochemical methods
Despite the maturity of the pyro- and hydrometallurgical technology, there is a continuous pursuit of novel technologies that take into account the sustainability aspect. The intended refinements will encapsulate guarantees of environmental safety and cost-effectiveness. Although the electrochemical method appears to be the suitable pick, it is still a considerable time away from commercialisation. The spider plot in Fig. 9 delineates various metrics that serve as prominent aspects of comparison and portray the electrochemical method as a green alternative to metallurgy. However, the transformative potential of this method hinges on overcoming the challenge of upscaling, which is a crucial hurdle that demands immediate attention for the successful commercialisation of this ground-breaking approach.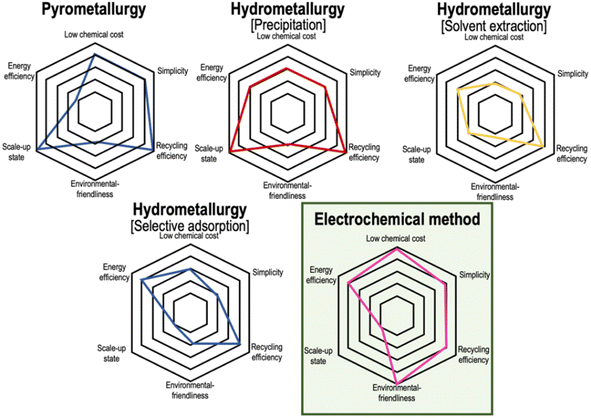 | ||
| Fig. 9 Spider plot highlighting a comparison of several metrics between recycling techniques. The electrochemical method is a much safer choice, but it is still some time away from commercialization (ref. 10). | ||
The subsequent section briefly explores the essential elements pivotal to the electrochemical technique, emphasizing a fresh perspective focused primarily on each integrated part or unit involved in its setup. This will serve as a basis for achieving the extensive and enduring application of the electrochemical method on a large scale.
Feasibility of a large-scale setup
For the recycling industry to embrace the electrochemical technology, the pivotal factor is feasibility assessment of this technology on a substantial scale. This stresses the critical need for larger dimensions and expansive setups enabling higher operational capacities.98 The anticipation is that these larger-scale operations will process substantial quantities of spent waste. This aspect of scalability gives rise to a vital concern regarding the feasibility of electrochemical setups capable of processing or recycling >1000 tonne per year of spent LIBs, which is equivalent to the industrial scale capacity. Table 5 below enumerates the global recycling companies that have effectively implemented recycling techniques on an industrial scale. The prevailing trend among these companies is the utilization of metallurgical methods, with none presently employing electrochemical techniques.| Scale | Company (country) | Recycling method |
|---|---|---|
| a Thermal: thermal pretreatment. Mech: mechanical treatment. Pyro: pyrometallurgy. Hydro: hydrometallurgy. Industrial scale: >1000 t/a. Pilot scale: <1000 t/a (e.g., a few hundred tons per year). Lab-scale: small-scale trials (a few grams or kilograms), or sometimes pilot-scale trials (a few hundred kilograms or more), but not yet commercialized. | ||
| Industrial scale (>1000 tonnes per year) | Umicore (Belgium) | Pyro + hydro |
| Accurec (Germany) | Thermal + mech + pyro + hydro | |
| Nickelhütte Aue (Germany) | Thermal + pyro + hydro | |
| SungEel (Korea) | Mech + (thermal +) hydro | |
| Kyoei Seiko (Japan) | Pyro | |
| Dowa (Japan) | Thermal + pyro + hydro | |
| Brunp (China) | Thermal + mech + hydro | |
| GEM (China) | Mech + hydro | |
| Huayou Cobalt (China) | Mech + hydro | |
| Ganzhou highpower (China) | Mech + pyro + hydro | |
| Pilot scale (<1000 tonnes per year) | SNAM (France) | |
| EDI (France) | Mech (aqueous shred + unknown) | |
| TES-AMM (formerly “Recupyl”) (France) | Mech (inert gas) | |
| AkkuSer (Finland) | Mech + unknown | |
| Duesenfeld (Germany) | Mech + hydro | |
| Promesa (Germany) | Mech (aqueous shred) + unknown | |
| Redux (Germany) | Thermal + Mech + unknown | |
| Retriev (US) | Mech (aqueous) + hydro | |
| Kobar (Korea) | Mech + hydro | |
| JX Nippon (Japan) | Thermal + mech + hydro | |
| Telerecycle (China) | Mech + hydro | |
| Guanghua (China) | Mech + hydro | |
| Lab scale | Erlos (Germany) | Mech + reconditioning (direct recycling) |
Most of these recycling companies employ pyro- and hydrometallurgy at the industrial scale, while the electrochemical method has yet to reach this scale. The full realisation of the potential and benefits offered by the electrochemical method as an alternative to metallurgical techniques hinges on intensive efforts to transition from laboratory-scale applications to industrial-scale operations. The inability to bridge this gap would mean that the true potential of this method would remain untapped, prolonging the dominance of traditional metallurgical approaches in the recycling industry. Early findings from Halizan et al. in 2023 revealed that a few companies had attempted to commercialise this method, including OnTo Technology, Farasis Energy, Green Li-ion and Evonik.108–110 This signals a future trend toward a potential transition of recycling technology to the electrochemical technique.
Despite uncertainties surrounding the feasibility of establishing an industry-suitable setup for electrochemical techniques, a potential solution lies in drawing inspiration from other successful electrochemical-related industries, including electrowinning and electroplating, which have successfully operated at industrial-scale capacities. Emulating their scale-up strategies could provide a pathway to leverage the advantages of electrochemical methods and facilitate the scalability of this infant technology. Fig. 10 depicts the setup for the electrowinning of copper in industry.111 This type of layout permits multiple electrowinning procedures to be performed simultaneously, directly impacting the efficiency and cost of the process. Apart from the compartment design, many nuances and intricacies were successfully addressed in transforming electrowinning technology from the laboratory-scale to the industrial-scale; most of the addressed concerns could shed light on addressing the upscaling dilemma in the electrochemical recycling technique.
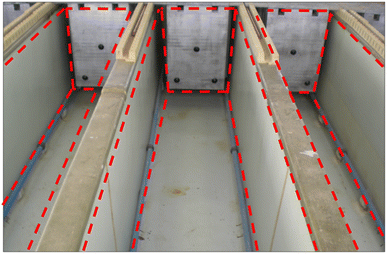 | ||
| Fig. 10 Industrial scale setup of copper electrowinning. The implementation of such a setup could be a ‘blueprint’ for feasible large-scale setup of the electrochemical recycling process. | ||
Substantial electrolyte consumption
In the broader perspective, it becomes evident that while laboratory-scale operation of the electrochemical recycling method only requires a manageable volume of electrolyte, the transition to an industrial scale, with its expansive setup, demands a comparable increase in electrolyte volume to accommodate a higher quantity of spent LIBs. The consumption of electrolyte emerges as a central concern in expanding the electrochemical method, not only impacting costs but also affecting the sustainability of this technique.Considering that a huge amount of electrolyte will be consumed and transformed into wastewater from the implementation of electrochemical technique, one approach to sustainable electrolyte consumption is to ‘reuse’ the used electrolyte with a prior treatment that is deemed necessary. In addition to this, while reusing the electrolyte is a sustainable step for minimising electrolyte consumption in the recycling process, the approach should ‘reduce’ any possible health effects and environmental threats from the disposal of the used electrolyte. This has been practiced in the electroplating industry to tackle the issue arising from the generation of considerable amounts of effluent with heavy metal ions such as iron (Fe), cobalt (Co), chromium (Cr), cadmium (Cd), nickel (Ni), lead (Pb), zinc (Zn), and mercury (Hg), which could bring about some detrimental health effects including kidney failure, thyroid dysfunction, sleeplessness, tiredness, rheumatoid arthritis, negative effects in the circulatory system and neural system, irritation of the gastrointestinal mucosa, and lung cancer.112 In the electrochemical recycling approach, similar concerns are probable for, the recycling of spent LIBs, as most of their composition is made up of heavy metal ions like Ni, Co, Fe, and manganese (Mn).
In the context of Malaysia, within the scheme of environmental regulations, the authority overseeing industrial wastewater is the Department of Environment (DOE). According to DOE, the wastewater generated from any processes in industrial premises is termed as “industrial effluent”. In line with the Environmental Quality Act of 1974, the DOE has enacted the “Environmental Quality (Industrial Effluent) Regulations 2009.” Within the scheme of these regulations, there exists a clear obligation for the owners or occupiers of any industrial premises to design and construct a system in strict compliance with the specifications issued by the DOE for treating industrial effluent on-site.113 Table 6 presents some of the regulation imposed by countries and regions including Malaysia (MY), China (CN), Singapore (SG), Korea (KR), and the European Union (EU).
| Imposed regulation | Region | Mechanism | Ref. |
|---|---|---|---|
| Regulation (EU) 2020/741 of the European Parliament and of the council of 25 May 2020 on minimum requirements for water reuse | EU | Wastewater reuse | 114 |
| Environmental quality (prescribed activities) (environmental impact assessment) order 2015 | MY | Wastewater treatment | 115 |
| Action plan for prevention and control of water pollution 4 | CN | Wastewater treatment | 116 |
| Environmental protection and management act 1999, part 5 water pollution Control | SG | Wastewater treatment | 117 |
| Wastes control act, article 18 | KR | Wastewater treatment | 118 |
Through the approach of reuse of the effluent and reduction of the adverse effects from the effluent, it is anticipated that these approaches will contribute to ‘Sustainable Blue Economies’. In detail, a blue economy is the sustainable use of ocean resources for economic growth, improved livelihoods and jobs, and ocean ecosystem health. One of the segments that is highlighted as contributing to the blue economy is waste disposal management, alongside with fisheries, tourism, maritime transport, climate change and renewable energy (see Fig. 11).119,120
Membrane separator features and layout
The membrane is a vital component in the mechanics of the electrochemical recycling setup, as underscored before. In attempts to upscale an electrochemical setup, the focal point is on the manufacturing or fabrication of a membrane with the features of interest and the design of the assembly of membranes in a large setup.The fabrication of the membrane in an electrochemical recycling technique, like the membrane separator in a battery cell, must take into account its ionic conductivity and mechanical stability. These are issues that require prominent focus. The ionic conductivity of the membrane guarantees Li+ ion mobility, which is conducive to high recycling efficiency. Fortunately, numerous approaches to fine-tune these properties have been explored in studies to ensure high Li+ mobility throughout the membrane, including the strategies of alternative electrolyte hosts, integration of fillers, blending materials, interpenetrating networks, and electrochemical and heat treatment of the membrane.64,121–126 Table 7 presents several approaches to enhance the ionic conductivity in the membrane separator of LIB systems. These approaches focus on enhancing the ionic conductivity as the main feature of the membrane. Assimilating these formulations and the strategies used in LIB systems in the development of membranes for electrochemical recycling technique is the most viable approach. This takes into account the similar purpose of the membrane in both LIB and the electrochemical technique, which is to facilitate in the mobility of Li ions across the system. The strategies of using either singular oxide-based or polymer-based materials or the use of composites is an interesting topic being explored in the development of membranes in LIBs. With successful outcomes being achieved using a wide range of materials, future membrane formulation and manufacturing must further probe the effectiveness of the membrane in its applications, the economic returns reflected from its manufacturing and outputs, and the feasibility, stability and cyclability of the membrane in large-scale setups of electrochemical recycling technique.
| Membrane materials | Li source | Approach | Conductivity achieved, S cm−1 | Ref. |
|---|---|---|---|---|
| Polyethylene oxide/Li6.4La3Zr1.4Ta0.6O12 | Li perchlorate (LiClO4) | Inorganic fillers | 1.70 × 10−4 at 30 °C | 127 |
| Polyethylene oxide/poly(propylene carbonate) | Li bis(trifluoromethanesulfonyl)imide (LiTFSI) | Materials compositing | 2.82 × 10−4 at 60 °C | 128 |
| Glycerol propoxylate triacrylate/poly(ethylene glycol) diacrylate/polymethylpyrrole | Li bis(trifluoromethanesulfonyl)imide (LiTFSI) | Electrochemical treatment | 7.0 × 10−4 at 70 °C | 126 |
| Aluminium-doped Li6.75La3Zr2O12 | Li metal | Thermal pulse treatment | 3.2 × 10−4 | 129 |
| Polyacrylonitrile | Li triflate (LiCF3SO3) | Alternative polymer host | 3.04 × 10−4 | 130 |
| Octakis(3-glycidyloxypropyldimethylsiloxy) octasilsesquioxane and amine-terminated polyethylene glycol | Li bis(trifluoromethanesulfonyl)imide (LiTFSI) | Interpenetrating network | 1.5 × 10−5 at 40 °C | 131 |
| Star-shaped siloxane cross-linker with the oligo(ethylene oxide) acrylate | Li perchlorate (LiClO4) | Interpenetrating network | 7.8 × 10−4 at 30 °C | 132 |
The absence of research investigating the aspects of feasibility, stability and cyclability of the membrane in electrochemical recycling techniques represents a notable void, obscuring insight into the sustainability of the technique. This gap stresses the need for intensive analyses to gain a full understanding and ensure the long-term viability of the process. The opportunity to tailor a membrane with significant cyclability, allowing one membrane to be subjected to multiple recycling cycles, is imperative to give the electrochemical recycling method superiority over the hydrometallurgical method in terms of cost-savings. This would potentially position the electrochemical method as a more efficient and cost-effective solution. Affecting the cyclability and stability is a factor called membrane aging, which is a common issue for electrochemical cells. The aging effect is unavoidable, but research towards its mitigation and strategies to develop a high-performance separator is intensely sought after, derived from the understanding of the aging fundamentals and mechanism.133,134 Establishing such important fundamentals and mechanism could advance membrane formulation and development for both LIB and electrochemical techniques to achieve much more stable and long-cycle-life membranes.
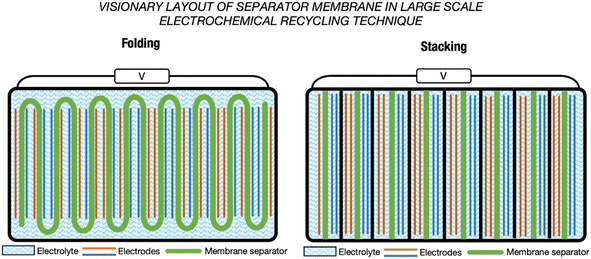
When further discussing the design of membranes for large electrochemical cells, the focus is often on a scheme that effectively addresses the concerns around the mechanical strength of the membrane while simultaneously mitigating pressure exertion throughout the entire cycle, especially when subjected to a substantial volume of electrolyte in longer-term operation. Failure to accomplish this delicate balance may lead to membrane separator leakage, in turn leading to unwanted side reactions and incidences that are detrimental to the overall operation. In the electrochemical cells of LIBs, every facet is precisely considered and embodied in the internal design to deliver a high performance battery, which has resulted in winding, stacking and folding designs for the electrode–separator composites (ESCs), as shown in Fig. 12.135–137 Emulating this design in a large-scale electrochemical recycling technique setup could mitigate the issues of stress or pressure on the separator to contribute to stable and safe electrochemical operation.137 Fig. 13 shows envisioned layouts of the ESC for electrochemical recycling methods. Inspired by the designs implemented in LIBs, real-world scale and larger-dimension electrochemical recycling setups integrating such a separator require further studies to determine the efficiency of these layouts. Practically, the processing of a large membrane would require consideration of the raw material cost and availability. Additionally, the properties of the membrane formulated in bulk size may require several compensations in order for it to serve its practical purpose, which will demand further fine-tuning and validation of the formulation to fit the intended application. In the folding design, the benefits are the facile assembly of the membrane and whole setup compared with the stacking design. However, the folding design would face the issues of manufacturing one large-dimension singular unit membrane. This is different from the stacking concept, which offers the simplicity of manufacturing smaller dimension membranes. The stacking design may offer flexibility to perform the recycling of multiple spent LIB sources or different types of spent LIB concurrently. The target output may need to be considered before choosing and implementing any envisioned design in a real-scale setup.
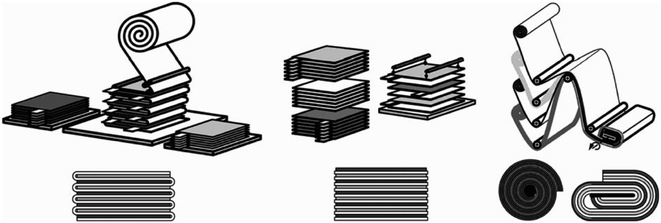 | ||
| Fig. 12 ESC designs in LIBs.136 Included are the folded (left), stacked (middle) and winding (right) design, primarily to confront the stress and bending issues in the separator. | ||
Techno-economic assessment (TEA) and life-cycle analysis (LCA) of recycling techniques
Techno-economic analysis (TEA) and life-cycle analysis (LCA) are two vital components requiring that require equal attention to the technical parts of the electrochemical recycling technique. TEA is a metric to evaluate the economic viability of a process through profit and cost analyses, while the LCA assesses the environmental performance using suitable analyses that include parameters like GHG emissions and global warming potential (GWP).138–141 Within the thriving industry of spent Li-ion battery (LIB) recycling, both components play crucial role in assisting industry stakeholders making informed decisions regarding the adoption of recycling techniques. The studies outlined in Table 8 focus exclusively on TEA and LCA studies performed for pyrometallurgical and hydrometallurgical technologies; these assessments serve as pivotal factors facilitating the global industrial-scale adoption of these techniques.| Recycling technique | Reagent cost (USD) | Energy cost (USD) | Revenue (USD) | Profit (USD) | Ref. |
|---|---|---|---|---|---|
| Recovery of 1 ton of spent LiMn2O4 | |||||
| Pyrometallurgy | — | 559.05 | 1687.14 | 1128.09 | 143 |
| Hydrometallurgy | — | 387.53 | 1237.78 | 850.25 | 144 |
| Hydrometallurgy | 659.28 | 415.5 | 1303.10 | 228.32 | 81 |
| Electrochemical | 442.43 | 392.00 | 2260.85 | 1426.42 | 17 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Recovery of 1 ton of spent LiFePO4 | |||||
| Hydrometallurgy | 275.70 | 250.1 | 1272.2 | 510.8 | 145 |
| Hydrometallurgy | 274.22 | 106.50 | 1329.90 | 949.18 | 15 |
| Hydrometallurgy | 858.00 | 77.88 | 1901.13 | 965.25 | 15 |
| Electrochemical | — | 416.26 | 1566.65 | 1150.39 | 15 |
Checking the scalability of the recycling technology necessitates TEA to assess the aspect of economic viability. Table 8 below provides the preliminary economic analysis conducted from studies using the electrochemical recycling method for 1 ton of spent LiMn2O4 (LMO) and spent LiFePO4 (LFP) cathodes. Pyrometallurgy and hydrometallurgy are also included in the table. The emphasis here is on indicating that the electrochemical recycling method is a more economical option than pyro- and hydrometallurgy. The table presents the preliminary cost analysis involving the raw material costs: reagents, operation cost, energy, revenue, and net profit of the recycling technologies. In this defined scope of techno-economic assessment, one could come to conclude the economic viability of the electrochemical method from the revenue and profit gained. The reagent cost and energy cost for the electrochemical method are both the lowest among the recycling methods. The energy cost for hydrometallurgy may be interpreted as lower than that of the electrochemical method considering the much lower temperature required during operation (<150 °C) to facilitate the leaching procedure compared to the electrochemical method. Nonetheless, it has the great drawback of enormous reagent cost. Despite encompassing the metallurgical and electrochemical method in the perspective, the inaccessibility of economic analysis extending to other cathode chemistries, including ternary cathodes such as Li/Ni/Mn/Co (NMC) and Li/Ni/Co/Al (NCA), is another failing in the economic viability forecast of the recycling methods. The study of Lander et al. highlighted the economic feasibility of NMC and NCA recycling via hydrometallurgy in the United States (U.S.), United Kingdom (U.K.), and China.142 This is not the case for LFP and LMO recycling, which have diminished net benefits due to emissions and constrained recycling profit.142 Despite contributing to a simple judgement on the profitability of recycling different types of cathode chemistries, the findings lack inclusion of the electrochemical recycling technique. Hence, any conclusions made may not be translated into transparent decision-making aimed at choosing most economically viable recycling method. The presence of this gap in the current research is a call for action to researchers to supply insights on the economic analysis of each recycling study carried out as early as at the laboratory scale. This valuable perspective could dictate the commercialisation potential of conventional and developing recycling techniques.
Even if it is only at a preliminary stage, the availability of economic analysis provides stakeholders with information on the risks and potential of the commercialisation of this technology, along with the tangible and intangible returns. Addition to this preliminary analysis is needed for an advanced and comprehensive TEA and LCA analysis that covers a broader scope involving the electrochemical recycling technique. Displayed in Table 9 are the sets of studies performed with a focus on the various extensive aspects of TEA and LCA. Accessing this information could take time, but once completed, the data would provide a bigger picture of the economic and environmental performance of existing and developing technologies. For Table 8, the detailed calculations of the numerical data can be accessed in the ESI, Tables S1–S4.†
| Recycling techniques | Key focus | Ref. |
|---|---|---|
| Hydrometallurgy | Key enhancement for hydrometallurgical recycling through modified pretreatment methods | 146 |
| Pyrometallurgy | Evaluate profitability of pyrometallurgical recycling for various cathode chemistries in spent LIBs | 147 |
| Hydrometallurgy | Assess cost and environmental impacts of producing cathode active material for LIB through hydrometallurgical recycling of spent LIBs | 148 |
| Pyro- and hydrometallurgy | Economic value and environmental benefit of the metallurgical recycling of spent LIBs | 149 |
| Hydrometallurgy | Assess environmental impacts of hydrometallurgical recycling for two Li-ion traction batteries via life-cycle analysis | 150 |
| Pre-treatment, pyro- and hydrometallurgy | Evaluating the environmental and economic impacts of prevalent recycling methods through detailed analysis of energy and material flows at the process unit level, considering both physical and chemical aspects | 151 |
| Pyro- and hydrometallurgy | Economic, environmental, and geospatial analysis of future LIB recycling in United Kingdom (UK) | 152 |
| Pre-treatment, pyro- and hydrometallurgy | Examining the economic aspects of Li-ion battery (LIB) recycling involving evaluation of the pros and cons of recycling, as well as investigation of the factors that impact the cost and economic viability of battery disposal | 153 |
| Pyro- and hydrometallurgy | Techno-economic cost model for electric vehicle battery recycling | 142 |
A direct approach for establishing TEA and LCA analysis is to perform them locally. This should minimise the intricacies in obtaining data and samples, which is complex when considering international or cross-continent data and samples. Furthermore, the findings could precisely reflect local returns and costs. Tables 10 and 11 present data from a study that briefly covers the TEA and LCA aspects in the sustainability analysis of battery material supply chains specifically in Canada.148 The study conducted in Canada considers the circularity of the battery materials, including Li, via a hydrometallurgy recycling approach. It should be noted that the study's methodology involved translating bench-scale hydrometallurgy process parameters into a conventional hydrometallurgy plant with an annual processing capacity of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 tons of black mass. This simulation emerges as a pivotal element, providing the study with concrete findings regarding the advantages of sustaining the circularity of battery materials through the recycling approach.
560 tons of black mass. This simulation emerges as a pivotal element, providing the study with concrete findings regarding the advantages of sustaining the circularity of battery materials through the recycling approach.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 tons per year148
560 tons per year148
| Description | Canadian dollars per year |
|---|---|
| Reagents | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 153 |
| Shipping and disposal | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 325![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384 384 |
| Labour | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041 041![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607 607 |
| Other | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 872 872![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 877 877 |
| Contingency | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 749![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734 734 |
| Total | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249 249![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 755 755 |
| Battery precursor cathode active materials (CAM) | Recycling Processing (kg CO2e per kg CAM) | Mining, beneficiation, extraction, and refining | Emission reduction |
|---|---|---|---|
| Li2CO3—brine | 0.8810 | 1.3500 | −35% |
| Li2CO3—ore | 8.7300 | −90% |
The study's key revelation underscores that the production of Li-ion battery (LIB) cathode active materials via recycling incurs costs that are 48% and 54% lower in overall expenses and environmental impacts, respectively. These findings derived from the TEA and LCA analyses serve as valuable guidelines and benchmarks for the development of the electrochemical technique, shedding light on the potential benefits of adopting similar recycling approaches in the realm of battery material production.
Scrutiny using TEA and LCA analyses are the gears bridging the transformation and rational adoption of technology that includes the emerging electrochemical Li recycling technique. The purpose of TEA implementation is wide-ranging, encompassing the evaluation of economic factors, payback period, internal rate of return, return on investment, discounted cash flow rate of return, capital cost, general costs, profit or revenue, economic potential, overall economic feasibility, process factors, efficiency of operation and environmental factors.138,154–163 LCA covers a broad spectrum of environmental performance parameters, including greenhouse gas emissions, global warming potential, human toxicity potential, terrestrial ecotoxicity potential, ozone layer depletion potential, water consumption, freshwater ecotoxicity, and land use.141,164,165 In addition to informed decision-making, the variety of scopes covered in the assessments put all stakeholders in the know about the key issues to be resolved for successful scale-up of a particular technology.
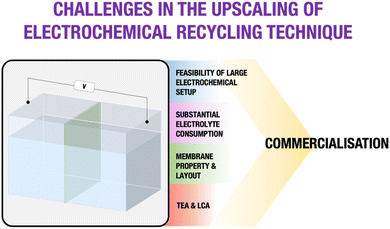
A thorough technological and economic evaluation driven by meticulous data analyses underscores the imperative of accumulating a substantial dataset for the electrochemical method. Addressing challenges, including the availability of large electrochemical setups, strategic planning for significant electrolyte consumption, and customization of the membrane and layout, is crucial for gathering precise data and facilitating a comprehensive TEA. This detailed dataset could contribute to valid LCA and TEA assessments. The primary focus should be directed towards deriving definite insights into the technological profitability and environmental performance. Obtaining clear results in these areas is crucial for determining the feasibility of implementing the emerging electrochemical technique on an industrial scale. Fig. 14 serves as a visual representation, briefly highlighting the key metrics essential for addressing challenges in electrochemical recycling techniques and thereby facilitating a transparent pathway towards successful commercialization.
Future directions: lithium circularity
The essence of this review is to underscore the paramount importance of synergy among experts and professionals from diverse fields in preparation for the upscaling of the electrochemical recycling method. Envisioning collaborative efforts from professionals such as process engineers, chemical engineers, techno-economists, and experts from recycling and electrochemical-related industries, the objective is to successfully implement the strategies outlined in this review and effectively address the prominent issues at hand. In the review, it has been identified that the endeavour to expand electrochemical recycling encompasses several challenges. Establishing a feasible setup for broad application is imperative for this emerging technique. The likely compensation of membrane features in a large-scale application poses a challenge that demands an appropriate counter-strategy from both academia and industry. Additionally, a solution for efficient membrane layout design to allow it to retain its properties while considering process efficiency is required. It is crucial to emphasize the significance of techno-economic analysis (TEA) in analysing the economic feasibility of the electrochemical technique.In addition to the technical hurdles inherent in developing efficient electrochemical recycling methods, the battery recycling landscape in general faces significant wider challenges that include the battery materials, battery design, recycling setup and economic feasibility. It is well known that the extraction and sourcing of critical materials indicate the substantial importance of recycling. If not coupled with management of hazardous components and sufficient resources, the battery recycling effort will fail.
The aspects of battery performance and architectures must evolve to meet the demands of emerging technologies. Optimisation of the composition of new batteries and utilisation of novel materials contributing to facile recycling efforts are areas that require exploration. At present, research on post-LIB is a burgeoning field. The development of metal-based batteries such as magnesium metal batteries, which are safer when being recycled, is ongoing. Being relatively stable in the atmosphere with a high melting point and boiling point, the handling of spent magnesium metal batteries would guarantee safer operation. There is also the option of zinc-based batteries, which are being investigated as a better alternative to LIB with greater safety compared to Li containing a metal that is easier to recycle and less toxic than Li. By reviewing and choosing possible candidates to replace the current LIB technology, a facile and safer recycling method could be established. The wider compromises in each choice of material are well understood, but the vision of positioning recycling as one of the priorities should not be neglected. In addition to the choice of materials for battery production, another interesting topic is research into choosing materials or reagents that could translate into successful or efficient recycling outputs. With recycling technologies like the electrochemical method, the choice of membrane materials is broad, which offers many options and at the same time presents the puzzle of choosing a particular option that is profitable.
Additionally, eliminating obstacles to easy recycling represents an aspect of battery design. Academic and industry should instil the idea of designing batteries with a sustainable production–recycling concept. This concept could be considered at the early stages of designing the battery packaging. With the current design of LIB opting for physical fastenings like clips and welds, the strategy of opting for an easy-to-remove adhesive in LIB packaging could speed the disassembly and avoid the need to subject the battery to shredding, preserving the purity of the battery. A standardised design battery pack design could immensely improve the rate of recycling. The current situation of varying cell packs of LIB from brand to brand and from device to device truly complicates the recycling process. This would require imposing strong regulations and policy, giving a clear indication of our commitment to the sustainability of the existing technology. The emphasis on developing batteries with recyclability in mind from the outset would mitigate end-of-life challenges effectively. In addition to battery design considerations, tailoring the recycling technology could also be an additional initiative that could be executed. As discussed earlier, the layout of the ESC in the electrochemical recycling technique could imitate the concept used in LIB systems. The efforts made to further validate the feasibility and efficiency of this layout would have a noteworthy influence on the recycling industry.
Moreover, economic feasibility has emerged as a central factor determining the scalability of battery recycling initiatives. The cost-effectiveness of recycling methods, particularly electrochemical techniques, is paramount for their widespread adoption and long-term sustainability. In general, it is believed that recycling is much more expensive than mining the materials. This paramount issue could cause existing and potential recycling practitioners and investors to deviate from the goal of sustainability. Economic instruments like rebates and subsidiaries from local and international parties could alleviate the costs that may be the deciding factor influencing the efficiency and rapid development of the recycling industry.
It is overwhelming to look at each of the multifaceted challenges. There should be a pressing call for a joint effort among diverse stakeholders, including the industry players, consumers, and government. There is an obligation to consult and engage with professionals from various disciplines, including process engineering, chemical engineering, economics, and the recycling industry, to cultivate the development of an all-inclusive solution. By aligning these efforts with the ultimate Sustainable Development Goals (SDG) and of Net-Zero Emission policies, the battery industry can spearhead innovation in recycling technologies and facilitate the transition to a circular economy.
Conflicts of interest
The authors declare that they possess no conflict of interest.Acknowledgements
The authors wish to express their gratitude to PETRONAS Research Sdn. Bhd. for funding the research project entitled ‘Resource Circularity of Lithium-Ion Batteries Waste’ (UTVSB/CP/P.20221001006). Special thanks are also extended to Universiti Teknologi MARA (UiTM) Shah Alam, Malaysia; International Islamic University Malaysia (IIUM); Universiti Putra Malaysia (UPM); and Universiti Teknologi Malaysia (UTM) for their collaboration and support throughout the research.References
- J. Ma, J. Wang, K. Jia, Z. Liang, G. Ji, H. Ji, Y. Zhu, W. Chen, H. Cheng and G. Zhou, et al., Subtractive transformation of cathode materials in spent Li-ion batteries to a low-cobalt 5 V-class cathode material, Nat. Commun., 2024, 15, 1046 CrossRef CAS PubMed.
- X. Wu, J. Ma, Z. Xuan, Z. Guangmin and L. Zheng, Progress, Key Issues, and Future Prospects for Li-Ion Battery Recycling, Global Challenges, 2022, 6, 6 CrossRef PubMed.
- Statista, Electric Vehicles – Worldwide, 2023 Search PubMed.
- Z. J. Baum, R. E. Bird, X. Yu and J. Ma, Lithium-Ion Battery Recycling–Overview of Techniques and Trends, ACS Energy Lett., 2022, 7, 712–719 CrossRef CAS.
- M. Stone, As Electric Vehicles Take Off, We’ll Need to Recycle Their Batteries, National Geographic, 2021, https://www.nationalgeographic.com/environment/article/electric-vehicles-take-off-recycling-ev-batteries Search PubMed.
- M. Kaya, State-of-the-art lithium-ion battery recycling technologies, Circ. Econ., 2022, 1, 100015 Search PubMed.
- V. Srivastava, V. Rantala, P. Mehdipour, T. Kauppinen, S. Tuomikoski, A. Heponiemi, H. Runtti, P. Tynjälä, G. S. D. Reis and U. Lassi, et al., A comprehensive review of the reclamation of resources from spent lithium-ion batteries, Chem. Eng. J., 2023, 474, 145822 CrossRef CAS.
- L. Terborg, S. Weber, F. Blaske, S. Passerini, M. Winter, U. Karst and S. Nowak, Investigation of thermal aging and hydrolysis mechanisms in commercial lithium ion battery electrolyte, J. Power Sources, 2013, 242, 832–837 CrossRef CAS.
- M. Grützke, V. Kraft, B. Hoffmann, S. Klamor, J. Diekmann, A. Kwade, M. Winter and S. Nowak, et al., Aging investigations of a lithium-ion battery electrolyte from a field-tested hybrid electric vehicle, J. Power Sources, 2015, 273, 83–88 CrossRef.
- Y. Li, J. B. Richardson, R. M. Bricka, X. Niu, H. Yang, L. Li and A. Jimenez, Leaching of heavy metals from E-waste in simulated landfill columns, Waste Manage., 2009, 29, 2147–2150 CrossRef CAS PubMed.
- H. Bae and Y. Kim, Technologies of lithium recycling from waste lithium ion batteries: A review, Mater. Adv., 2021, 2, 3234–3250, 10.1039/d1ma00216c.
- K. Giza, B. Pospiech and J. Gęga, Future Technologies for Recycling Spent Lithium-Ion Batteries (LIBs) from Electric Vehicles—Overview of Latest Trends and Challenges, Energies, 2023, 16, 5777 CrossRef CAS.
- TES-AMM, The Difference between Hydrometallurgy and Pyrometallurgy, TES-AMM, 2023 Search PubMed.
- H. Bae, S. M. Hwang, I. Seo and Y. Kim, Electrochemical Lithium Recycling System toward Renewable and Sustainable Energy Technologies, J. Electrochem. Soc., 2016, 163, E199–E205 CrossRef CAS.
- Z. Li, L. He, Y. Zhu and C. Yang, A Green and Cost-Effective Method for Production of LiOH from Spent LiFePO 4, ACS Sustain. Chem. Eng., 2020, 8, 15915–15926 CrossRef CAS.
- Z. Li, D. Liu, J. Xiong, L. He, Z. Zhao and D. Wang, Selective recovery of lithium and iron phosphate/carbon from spent lithium iron phosphate cathode material by anionic membrane slurry electrolysis, Waste Manage., 2020, 107, 1–8 CrossRef CAS PubMed.
- Z. Li, L. He, Z. Zhao, D. Wang and W. Xu, Recovery of Lithium and Manganese from Scrap LiMn 2 O 4 by Slurry Electrolysis, ACS Sustain. Chem. Eng., 2019, 7, 16738–16746 CrossRef CAS.
- J. B. Goodenough and K. S. Park, The Li-ion rechargeable battery: A perspective, J. Am. Chem. Soc., 2013, 135, 1167–1176, DOI:10.1021/ja3091438.
- Y. Guo, Y. Liu, J. Guan, Q. Chen, X. Sun, N. Liu, L. Zhang, X. Zhang, X. Lou and Y. Li, Global Trend for Waste Lithium-Ion Battery Recycling from 1984 to 2021: A Bibliometric Analysis, Minerals, 2022, 12, 1514 CrossRef CAS.
- Y. Huang and J. Li, Key Challenges for Grid-Scale Lithium-Ion Battery Energy Storage, Adv. Energy Mater., 2022, 12 Search PubMed.
- U.S. Geological Survey, Mineral Commodity Summaries-Lithium, 2023 Search PubMed.
- W. B. Jaskula, Mineral Commodity Summaries: Lithium, U.S. Geological Survey, 2024 Search PubMed.
- J. Wang, K. Jia, J. Ma, Z. Liang, Z. Zhuang, Y. Zhao, B. Li, G. Zhou and H. Cheng, Sustainable upcycling of spent LiCoO2 to an ultra-stable battery cathode at high voltage, Nat. Sustain., 2023, 6, 797–805 CrossRef.
- X. Wu, G. Ji, J. Wang, G. Zhou and Z. Liang, Toward Sustainable All Solid-State Li–Metal Batteries: Perspectives on Battery Technology and Recycling Processes, Adv. Mater., 2023, 35 Search PubMed.
- M. Bruno and S. Fiore, Material Flow Analysis of Lithium-Ion Battery Recycling in Europe: Environmental and Economic Implications, Batteries, 2023, 9, 231 CrossRef CAS.
- G. Khodadadmahmoudi, K. Javdan Tabar, A. H. Homayouni and S. Chehreh Chelgani, Recycling spent lithium batteries – an overview of pretreatment flowsheet development based on metallurgical factors, Environ. Sci. Technol., 2023, 12, 3 Search PubMed.
- S. Kim, J. Bang, J. Yoo, Y. Shin, J. Bae, J. Jeong, K. Kim, P. Dong and K. Kwan, A comprehensive review on the pretreatment process in lithium-ion battery recycling, J. Clean. Prod., 2021, 294, 126329 CrossRef CAS.
- Y. Yang, G. Huang, S. Xu, Y. He and X. Liu, Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries, Hydrometallurgy, 2016, 165, 390–396 CrossRef CAS.
- L. Verdugo, L. Zhang, K. Saito, W. Bruckard, J. Menacho and A. Hoadley, Flotation behavior of the most common electrode materials in lithium ion batteries, Sep. Purif. Technol., 2022, 301, 121885 CrossRef CAS.
- X. Yu, W. Li, V. Gupta, H. Gao, D. Tran, S. Sarwar and Z. Chen, Current Challenges in Efficient Lithium-Ion Batteries’ Recycling: A Perspective, Global Challenges, 2022, 2200099, DOI:10.1002/gch2.202200099.
- A. Mondal, Y. Fu, W. Gao and C. C. Mi, Pretreatment of Lithium Ion Batteries for Safe Recycling with High-Temperature Discharging Approach, Batteries, 2024, 10, 37 CrossRef CAS.
- J. Shaw-Stewart, A. Alvarez-Reguera, A. Greszta, J. Marco, M. Masood, R. Sommerville and E. Kendrick, Aqueous solution discharge of cylindrical lithium-ion cells, Sustainable Mater. Technol., 2019, 22, e00110 CrossRef CAS.
- T. Zhang, et al., Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries, J. Power Sources, 2013, 240, 766–771 CrossRef CAS.
- G. Salazar and M. N. Russi-Vigoya, Technology Readiness Level as the Foundation of Human Readiness Level, Ergon. Des. Q. Hum. Factors Appl., 2021, 29, 25–29 Search PubMed.
- R. Wagner-Wenz, et al., Recycling routes of lithium-ion batteries: A critical review of the development status, the process performance, and life-cycle environmental impacts, MRS Energy Sustain., 2022, 10, 1–34 CrossRef.
- F. I. Bahrudin, N. Daud, I. Harun, M. F. Aizamddin, S. N. A. Shaffee and M. M. Mahat, A Malaysian Perspective on Lithium-Ion Batteries Recycling, Proceedings of the 15th International Green Energy Conference, 2023, vol. 1, pp. 39–52 Search PubMed.
- R. Singh, Introduction, in Applied Welding Engineering, Elsevier, 2020, pp. 3–5, DOI:10.1016/B978-0-12-821348-3.00001-X.
- M. Zhou, B. Li, J. Li and Z. Xu, Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge, ACS ES&T Eng., 2021, 1, 1369–1382 Search PubMed.
- B. Makuza, Q. Tian, X. Guo, K. Chattopadhyay and D. Yu, Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review, J. Power Sources, 2021, 491, 229622 CrossRef CAS.
- G. Zhang, Z. Du, Y. He, H. Wang, W. Xie and T. Zhang, A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries, Sustainability, 2019, 11, 2363 CrossRef CAS.
- J. Shi, M. Chen, Y. Li, H. Eric, L. Klemettinen, M. Lundström, P. Taskinen and A. Jokilaakso, Sulfation Roasting Mechanism for Spent Lithium-Ion Battery Metal Oxides Under SO2-O2-Ar Atmosphere, JOM, 2019, 71, 4473–4482 CrossRef CAS.
- G. Pavoski et al., Nanotechnology and recycling, remanufacturing, and reusing battery, in Nano Technology for Battery Recycling, Remanufacturing, and Reusing, Elsevier, 2022, pp. 53–78, DOI:10.1016/B978-0-323-91134-4.00022-4.
- ELIBAMA, European Li-Ion Battery Advanced Manufacturing for Electric Vehicles (Elibama): Li-Ion Batteries Recycling, 2014 Search PubMed.
- S. Windisch-Kern, A. Holzer, C. Ponak and H. Raupenstrauch, Pyrometallurgical Lithium-Ion-Battery Recycling: Approach to Limiting Lithium Slagging with the InduRed Reactor Concept, Processes, 2021, 9, 84 CrossRef CAS.
- C. Liu, J. Lin, H. Cao, Y. Zhang and Z. Sun, Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review, J. Clean. Prod., 2019, 228, 801–813 CrossRef CAS.
- H. Pinegar and Y. R. Smith, Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes, J. Sustain. Metall., 2019, 5, 402–416 CrossRef.
- W. Lv, Z. Wang, H. Cao, Y. Sun, Y. Zhang and Z. Sun, A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries, ACS Sustain. Chem. Eng., 2018, 6, 1504–1521 CrossRef CAS.
- Umicore, Our Recycling Process, Our Recycling Process, Umicore Group, 2019 Search PubMed.
- V. Velázquez-Martínez, R. Santasalo-Aarnio and S. Guerrero, A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective, Batteries, 2019, 5, 68 CrossRef.
- T. Georgi-Maschler, B. Friedrich, R. Weyhe, H. Heegn and M. Rutz, Development of a recycling process for Li-ion batteries, J. Power Sources, 2012, 207, 173–182 CrossRef CAS.
- P. van der Werf, MHSW Processor Audit Report, 2011 Search PubMed.
- V. Velázquez-Martínez, R. Santasalo-Aarnio and S. Guerrero, A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective, Batteries, 2019, 5, 68 CrossRef.
- Accurec Recycling GmbH, Managing Metal Resources from Batteries, Accurec Recycling GmbH, 2020 Search PubMed.
- R. Sojka, Accurec Invests and Signs Long Term Contracts, Accurec, 2017 Search PubMed.
- B. D. H. Knights & F. Saloojee. Lithium Battery Recycling, 2015 Search PubMed.
- Y. Haga, K. Saito & K. Hatano, in Waste Lithium-Ion Battery Recycling in JX Nippon Mining & Metals Corporation, 2018, pp. 143–147, DOI:10.1007/978-3-319-72131-6_12.
- M. Assefi, S. Maroufi, Y. Yamauchi and V. Sahajwalla, Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview, Curr. Opin. Green Sustainable Chem., 2020, 24, 26–31 CrossRef.
- Y. Jie, S. Yang, Y. Li, D. Zhao, Y. Lai and Y. Chen, Oxidizing Roasting Behavior and Leaching Performance for the Recovery of Spent LiFePO4 Batteries, Minerals, 2020, 10, 949 CrossRef CAS.
- K. Duncan, Lithium Ion Battery Recycling Technology 2015 Current State and Future Prospects, 2015 Search PubMed.
- S. Wang, Y. Tian, X. Zhang, B. Yang, F. Wang, B. Xu, D. Liang and L. Wang, A Review of Processes and Technologies for the Recycling of Spent Lithium-ion Batteries, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 782, 022025 CrossRef CAS.
- V. M. Leal, J. S. Ribeiro, E. L. D. Coelho and M. B. J. G. Freitas, Recycling of spent lithium-ion batteries as a sustainable solution to obtain raw materials for different applications, J. Energy Chem., 2023, 79, 118–134 CrossRef CAS.
- W. Yu, Y. Guo, S. Xu, Y. Yang, Y. Zhao and J. Zhang, Comprehensive recycling of lithium-ion batteries: Fundamentals, pretreatment, and perspectives, Energy Storage Mater., 2023, 54, 172–220 CrossRef.
- Y. Jian, Z. Zongliang, Z. Gang, J. Liangxing, L. Fangyang, J. Ming and L. Yanqing, Process study of chloride roasting and water leaching for the extraction of valuable metals from spent lithium-ion batteries, Hydrometallurgy, 2021, 203, 105638 CrossRef CAS.
- L. Zhang, L. Fu, W. Qin, Y. He, H. Liu and H. Hu, Enhancing the ionic conductivity and mechanical properties of PEO-based solid electrolytes through thermal pre-stretching treatment, Phys. Chem. Chem. Phys., 2023, 25, 18297–18309 RSC.
- X. Zeng, J. Li and N. Singh, Recycling of Spent Lithium-Ion Battery: A Critical Review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 1129–1165 CrossRef CAS.
- W. Mrozik, M. A. Rajaeifar, O. Heidrich and P. Christensen, Environmental impacts, pollution sources and pathways of spent lithium-ion batteries, Energy Environ. Sci., 2021, 14, 6099–6121 RSC.
- M. Mohr, J. F. Peters, M. Baumann and M. Weil, Toward a cell-chemistry specific life cycle assessment of lithium-ion battery recycling processes, J. Ind. Ecol., 2020, 24, 1310–1322 CrossRef CAS.
- M. A. Rajaeifar, M. Raungei, B. Steunbing, A. Hartwell, P. A. Anderson and O. Heidrich, Life cycle assessment of lithium-ion battery recycling using pyrometallurgical technologies, J. Ind. Ecol., 2021, 25, 1560–1571 CrossRef CAS.
- P. A. Christensen, P. A. Anderson, G. D. J. Harper, S. M. Lambert, W. Mrozik, M. A. Rajaeifar, M. S Wise and O. Heidrich, Risk management over the life cycle of lithium-ion batteries in electric vehicles, Renew. Sustain. Energy Rev., 2021, 148, 111240 CrossRef.
- C. Pan and Y. Shen, Pyrometallurgical recycling of spent lithium-ion batteries from conventional roasting to synergistic pyrolysis with organic wastes, J. Energy Chem., 2023, 85, 547–561 CrossRef CAS.
- J.-P. Harvey, W. Courchesne, M. D. Vo, K. Oishi, C. Robelin, U. Mahue, P. Leclerc and A. Al-Haiek, Greener reactants, renewable energies and environmental impact mitigation strategies in pyrometallurgical processes: A review, MRS Energy Sustain., 2022, 9, 212–247 CrossRef PubMed.
- Z. Dobó, T. Dinh and T. Kulcsár, A review on recycling of spent lithium-ion batteries, Energy Rep., 2023, 9, 6362–6395 CrossRef.
- L. F. Zhou, D. Yang, T. Du, H. Gong and W. B. Luo, The Current Process for the Recycling of Spent Lithium Ion Batteries, Front. Chem., 2020, 8, 578044 CrossRef CAS PubMed.
- A. B. Botelho Junior, S. Stopic, B. Friedrich, J. A. S. Tenório and D. C. R. Espinosa, Cobalt Recovery from Li-Ion Battery Recycling: A Critical Review, Metals, 2021, 11, 1999 CrossRef CAS.
- L. Sun and K. Qiu, Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries, J. Hazard. Mater., 2011, 194, 378–384 CrossRef CAS PubMed.
- F. Pagnanelli, E. Moscardini, G. Granata, S. Cerebelli, L. Agosta, A. Fieramosca and L. Toro, Acid reducing leaching of cathodic powder from spent lithium ion batteries: Glucose oxidative pathways and particle area evolution, J. Ind. Eng. Chem., 2014, 20, 3201–3207 CrossRef CAS.
- L.-P. He, S.-Y. Sun, X.-F. Song and J.-G. Yu, Leaching process for recovering valuable metals from the LiNi 1/3 Co 1/3 Mn 1/3 O 2 cathode of lithium-ion batteries, Waste Manage., 2017, 64, 171–181 CrossRef CAS PubMed.
- M. Joulié, R. Laucournet and E. Billy, Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries, J. Power Sources, 2014, 247, 551–555 CrossRef.
- R. Zheng, L. Zhao, W. Wang, Y. Liu, Q. Ma, D. Mu, R. Li and C. Dai, Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method, RSC Adv., 2016, 6, 43613–43625 RSC.
- K. Tanong, L. Coudert, M. Chartier, G. Mercier and J.-F. Blais, Study of the factors influencing the metals solubilisation from a mixture of waste batteries by response surface methodology, Environ. Technol., 2017, 38, 3167–3179 CrossRef CAS PubMed.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching, Chem. Eng. J., 2015, 281, 418–427 CrossRef CAS.
- L. Li, J. Ge, F. Wu, R. Chen, S. Chen and B. Wu, Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant, J. Hazard. Mater., 2010, 176, 288–293 CrossRef CAS PubMed.
- G. P. Nayaka, J. Manjanna, K. V. Pai, R. Vadavi, S. J. Kenny and V. S. Tripathi, Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids, Hydrometallurgy, 2015, 151, 73–77 CrossRef CAS.
- L. Yao, Y. Feng and G. Xi, A new method for the synthesis of LiNi 1/3 Co 1/3 Mn 1/3 O 2 from waste lithium ion batteries, RSC Adv., 2015, 5, 44107–44114 RSC.
- W. Gao, J. Song, H. Cao, X. Lin, X. Zhang, X. Zheng, Y. Zhang and Z. Sun, Selective recovery of valuable metals from spent lithium-ion batteries – Process development and kinetics evaluation, J. Clean. Prod., 2018, 178, 833–845 CrossRef CAS.
- X. Chen, B. Fan, L. Xu, T. Zhou and J. Kong, An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries, J. Clean. Prod., 2016, 112, 3562–3570 CrossRef CAS.
- L.-P. He, S.-Y. Sun, Y.-Y. Mu, X.-F. Song and J.-G. Yu, Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using l -Tartaric Acid as a Leachant, ACS Sustain. Chem. Eng., 2017, 5, 714–721 CrossRef CAS.
- H. Wang, K. Huang, Y. Zhang, X. Chen, W. Jin, S. Zheng, Y. Zhang and P. Li, Recovery of Lithium, Nickel, and Cobalt from Spent Lithium-Ion Battery Powders by Selective Ammonia Leaching and an Adsorption Separation System, ACS Sustain. Chem. Eng., 2017, 5, 11489–11495 CrossRef CAS.
- D. A. Ferreira, L. M. Z. Prados, D. Majuste and M. B. Mansur, Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries, J. Power Sources, 2009, 187, 238–246 CrossRef CAS.
- C. Wu, B. Li, C. Yuan, S. Ni and L. Li, Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching, Waste Manage., 2019, 93, 153–161 CrossRef CAS PubMed.
- Y. Chen, N. Liu, F. Hu, L. Ye, Y. Xi and S. Yang, Thermal treatment and ammoniacal leaching for the recovery of valuable metals from spent lithium-ion batteries, Waste Manage., 2018, 75, 469–476 CrossRef CAS PubMed.
- G. Zeng, X. Deng, S. Luo, X. Luo and J. Zou, A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries, J. Hazard. Mater., 2012, 199–200, 164–169 CrossRef CAS PubMed.
- G. Zeng, S. Luo, X. Deng, L. Li and C. Au, Influence of silver ions on bioleaching of cobalt from spent lithium batteries, Miner. Eng., 2013, 49, 40–44 CrossRef CAS.
- D. Mishra, D.-J. Kim, D. E. Ralph, J.-G. Ahn and Y.-H. Rhee, Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans, Waste Manage., 2008, 28, 333–338 CrossRef CAS PubMed.
- J. C.-Y. Jung, P.-C. Sui and J. Zhang, A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments, J. Energy Storage, 2021, 35, 102217 CrossRef.
- M. Chen, X. Ma, B. Chen, R. Arsenault, P. Karlson, N. Simon and Y. Wang, Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries, Joule, 2019, 3, 2622–2646 CrossRef CAS.
- N. M. Asl, S. S. Cheah, J. Salim and Y. Kim, Lithium-liquid battery: Harvesting lithium from waste Li-ion batteries and discharging with water, RSC Adv., 2012, 2, 6094–6100 RSC.
- X. Li, S. Liu, J. Yang, Z. He, J. Zheng and Y. Li, Electrochemical methods contribute to the recycling and regeneration path of lithium-ion batteries, Energy Storage Mater., 2023, 55, 606–630 CrossRef.
- Z. Li, L. He, Z. Zhao, D. Wang and W. Xu, Recovery of Lithium and Manganese from Scrap LiMn 2 O 4 by Slurry Electrolysis, ACS Sustain. Chem. Eng., 2019, 7, 16738–16746 CrossRef CAS.
- T. Yang, Y. Lu, L. Li, D. Ge, H. Yang, W. Leng, H. Zhou, X. Han, N. Schmidt, M. Ellis and Z. Li, An Effective Relithiation Process for Recycling Lithium-Ion Battery Cathode Materials, Adv. Sustainable Syst., 2020, 4 CAS.
- J. Wang, J. Lv, M. Zhang, M. Tang, Q. Lu, Y. Qin, Y. Lu and B. Yu, Recycling lithium cobalt oxide from its spent batteries: An electrochemical approach combining extraction and synthesis, J. Hazard. Mater., 2021, 405, 124211 CrossRef CAS PubMed.
- L. Li, R. Chen, F. Sun, F. Wu and J. Liu, Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process, Hydrometallurgy, 2011, 108, 220–225 CrossRef CAS.
- B. Zhang, X. Qu, J. Qu, X. Chen, H. Xie, P. Xing, D. Wang and H. Yin, A paired electrolysis approach for recycling spent lithium iron phosphate batteries in an undivided molten salt cell, Green Chem., 2020, 22, 8633–8641 RSC.
- L. Zhang, Z. Xu and Z. He, Electrochemical Relithiation for Direct Regeneration of LiCoO 2 Materials from Spent Lithium-Ion Battery Electrodes, ACS Sustain. Chem. Eng., 2020, 8, 11596–11605 CrossRef CAS.
- S. Li, X. Wu, Y. Jiang, T. Zhou, Y. Zhao and X. Chen, Novel electrochemically driven and internal circulation process for valuable metals recycling from spent lithium-ion batteries, Waste Manage., 2021, 136, 18–27 CrossRef CAS PubMed.
- Y. Zhang, Q. Meng, P. Dong, J. Duan and Y. Lin, Use of grape seed as reductant for leaching of cobalt from spent lithium-ion batteries, J. Ind. Eng. Chem., 2018, 66, 86–93 CrossRef CAS.
- R. Sojka, Q. Pan & L. Billmann, Comparative Study of Li-Ion Battery Recycling Processes, 2020 Search PubMed.
- Evonik, Lithium from Electric Vehicle Batteries: Moving towards Better Recycling, 2022, https://corporate.evonik.com/en/media/press-releases/corporate/lithium-from-electric-vehicle-batteries-moving-towards-better-recycling-177408.html Search PubMed.
- C. Hampel, Evonik Makes Headway in Battery Recycling Processes, 2022, https://www.electrive.com/2022/09/17/evonik-makes-headway-in-battery-recycling-processes/ Search PubMed.
- M. Z. M. Halizan, I. Harun, F. I. Bahrudin, N. Daud, M. A. Kasri, A. Hassim, N. N. Maliaman, N. A. Rahman, M. F. Aizamddin, S. N. A. Shaffee and M. M. Mahat, A Technical Review on the Implementation of Lithium-Ion Batteries Waste Recycling Methods, Proceedings of the 15th International Energy Conference, 2024, vol. 1, pp. 21–37 Search PubMed.
- N. T. Beukes and J. Badenhorst, Copper electrowinning: theoretical and practical design, J. South. Afr. Inst. Min. Metall., 2009 Search PubMed.
- S. Rajoria, M. Vashishtha and V. K. Sangal, Treatment of electroplating industry wastewater: a review on the various techniques, Environ. Sci. Pollut. Res., 2022, 29, 72196–72246 CrossRef CAS PubMed.
- Malaysia Department of Environment, Environmental Quality (Industrial Effluent) Regulation, Environmental Quality Act 1974, 2009, pp. 4010–4059 Search PubMed.
- Official Journal of the European Union, Regulation (Eu) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse, The European Parliament And The Council Of The European Union, 2020 Search PubMed.
- Minister of Natural Resources and Environment, Environmental Quality (Prescribed Activities) (Environmental Impact Assessment) Order 2015. (Malaysia Federal Legislation, 2015 Search PubMed.
- H. Liu, et al., China Industrial Wastewater Policy Overview and Opportunities for EU SMEs in Qingdao & Chengdu, 2019 Search PubMed.
- The Law Revision Commission, Environmental Protection and Management Act 1999. (Legislation Division of the Attorney-General’s Chambers of Singapore, 1999 Search PubMed.
- Korea Legislation Research Institute, Article 18 (Treatment of Industrial Wastes), Wastes Control Act, 2019 Search PubMed.
- World Bank, What is the Blue Economy?, 2017 Search PubMed.
- M. Yashiro, Sustainable Blue Economy Approach to National Development Planning, 2023 Search PubMed.
- N. Yazie, D. Worku, N. G. Habtu and A. Alemayehu, Development of polymer blend electrolytes for battery systems: recent progress, challenges, and future outlook, Mater. Renew. Sustain. Energy, 2023, 12, 73–94 CrossRef.
- S. Kitajima, S. Ryu, J. Ku, S. Kim, Y. Park and D. Im, Methodology for enhancing the ionic conductivity of superionic halogen-rich argyrodites for all-solid-state lithium batteries, Mater. Today Commun., 2021, 28, 102727 CrossRef CAS.
- S. Arwish, R. Manzoor, K. H. Khan, S. M. Syah, I. Ahmad and H. Hussain, Nanocomposite Solid Polymer Electrolytes with Polymer Blend (PVDF-HFP/Pluronic) as Matrix and GO as Nanofiller: Preparation, Structural Characterization, and Lithium-Ion Conductivity Analysis, Macromol. Chem. Phys., 2023, 224, 2300169 CrossRef CAS.
- M. F. Aizamddin, S. N. A. Shaffee, M. Z. M. Halizan, S. A. Shafiee, A. S. M. Sabere, Z. M. Sofian, N. Daud, F. I. Bahrudin, I. Harun, N. A. Rahman, M. M. Mahat, Utilizing Membrane Technologies in Advancing the Recycling of Spent Lithium-Ion Batteries Using Green Electrochemical Method – A Review, Materials Research Proceedings, 2023, pp. 170–191 Search PubMed.
- X. Zhu, K. Wang, Y. Xu, G. Zhang, S. Li, C. Li, X. Zhang, X. Sun, X. Ge and Y. Ma, Strategies to Boost Ionic Conductivity and Interface Compatibility of Inorganic - Organic Solid Composite Electrolytes, Energy Storage Mater., 2021, 36, 291–308 CrossRef.
- K. Leš and C. S. Jordan, Ionic conductivity enhancement in solid polymer electrolytes by electrochemical: In situ formation of an interpenetrating network, RSC Adv., 2020, 10, 41296–41304 RSC.
- L. Chen, Y. Li, S.-P. Li, L.-Z. Fan, C.-W. Nan and J. B. Goodenough, PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”, Nano Energy, 2018, 46, 176–184 CrossRef CAS.
- L. Zhu, J. Li, Y. Jia, P. Zhu, M. Jing, S. Yao, X. Shen, S. Li and F. Tu, Toward high performance solid-state lithium-ion battery with a promising PEO/PPC blend solid polymer electrolyte, Int. J. Energy Res., 2020, 44, 10168–10178 CrossRef CAS.
- C. Wang, H. Xie, W. Ping, J. Dai, G. Feng, Y. Yao, S. He, J. Weaver, H. Wang, K. Gaskell and L. Hu, A general, highly efficient, high temperature thermal pulse toward high performance solid state electrolyte, Energy Storage Mater., 2019, 17, 234–241 CrossRef.
- Z. Osman, K. B. Md Isa, A. Ahmad and L. Othman, A comparative study of lithium and sodium salts in PAN-based ion conducting polymer electrolytes, Ionics, 2010, 16, 431–435 CrossRef CAS.
- Y. Zheng, X. Li and C. Y. Li, A novel de-coupling solid polymer electrolyte via semi-interpenetrating network for lithium metal battery, Energy Storage Mater., 2020, 29, 42–51 CrossRef.
- Y. An, X. Han, Y. Liu, A. Azhar, J. Na, A. K. Nanjundan, S. Wang, J. Yu and Y. Yamauchi, Progress in Solid Polymer Electrolytes for Lithium-Ion Batteries and Beyond, Small, 2022, 18 Search PubMed.
- R. Yang, G. Yu, Z. Wu, T. Lu, T. Hu, F. Liu and H. Zhao, Aging of lithium-ion battery separators during battery cycling, J. Energy Storage, 2023, 63, 107107 CrossRef.
- X. Zhang, J. Zhu and E. Sahraei, Degradation of battery separators under charge–discharge cycles, RSC Adv., 2017, 7, 56099–56107 RSC.
- A. Rheinfeld, J. Sturm, A. Frank, S. Kosch, S. V. Erhard and A. Jossen, Impact of Cell Size and Format on External Short Circuit Behavior of Lithium-Ion Cells at Varying Cooling Conditions: Modeling and Simulation, J. Electrochem. Soc., 2020, 167, 013511 CrossRef.
- S. C. Mun and J. H. Won, Manufacturing Processes of Microporous Polyolefin Separators for Lithium-Ion Batteries and Correlations between Mechanical and Physical Properties, Crystals, 2021, 11, 1013 CrossRef CAS.
- A. Schilling, J. Schmitt, F. Dietrich and K. Dröder, Analyzing Bending Stresses on Lithium-Ion Battery Cathodes induced by the Assembly Process, Energy Technol., 2016, 4, 1502–1508 CrossRef CAS.
- S. Y. W. Chai, F. J. F. Phang, L. S. Yeo, L. H. Ngu and B. S. How, Future era of techno-economic analysis: Insights from review, Front. Sustain., 2022, 3 Search PubMed.
- Z. Barahmand and M. S. Eikeland, Techno-Economic and Life Cycle Cost Analysis through the Lens of Uncertainty: A Scoping Review, Sustainability, 2022, 14, 12191 CrossRef CAS.
- E. Yoo, U. Lee, J. C. Kelly and M. Wang, Life-cycle analysis of battery metal recycling with lithium recovery from a spent lithium-ion battery, Resour. Conserv. Recycl., 2023, 196, 107040 CrossRef CAS.
- E. Kallitsis, A. Korre and G. H. Kelsall, Life cycle assessment of recycling options for automotive Li-ion battery packs, J. Clean. Prod., 2022, 371, 133636 CrossRef CAS.
- L. Lander, T. Cleaver, M. A. Rajaeifar, V. Nguyen-Tien, R. J. R. Elliott, O. Heidrich, E. Kendrick, J. S. Edge and G. Offer, Financial viability of electric vehicle lithium-ion battery recycling, iScience, 2021, 24, 102787 CrossRef PubMed.
- J. Xiao, J. Li and Z. Xu, Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy, J. Hazard. Mater., 2017, 338, 124–131 CrossRef CAS PubMed.
- J. Kondás, J. Jandová and M. Nemeckova, Processing of spent Li/MnO2 batteries to obtain Li2CO3, Hydrometallurgy, 2006, 84, 247–249 CrossRef.
- J. Zhang, J. Hu, Y. Liu, Q. Jing, C. Yang, Y. Chen and C. Wang, Sustainable and Facile Method for the Selective Recovery of Lithium from Cathode Scrap of Spent LiFePO4 Batteries, ACS Sustain. Chem. Eng., 2019, 7, 5626–5631 CrossRef CAS.
- D. Thompson, C. Hyde, J. M. Hartley, A. P. Abbott, P. A. Anderson and G. D. J. Harper, To shred or not to shred: A comparative techno-economic assessment of lithium ion battery hydrometallurgical recycling retaining value and improving circularity in LIB supply chains, Resour. Conserv. Recycl., 2021, 175, 105741 CrossRef CAS.
- L. Reinhart, D. Vrucak, R. Woeste, H. Lucas, E. Rombach, B. Friedrich and P. Letmathe, Pyrometallurgical recycling of different lithium-ion battery cell systems: Economic and technical analysis, J. Clean. Prod., 2023, 416, 137834 CrossRef CAS.
- G. Gonzales-Calienes, M. Kannangara and F. Bensebaa, Economic and Environmental Viability of Lithium-Ion Battery Recycling—Case Study in Two Canadian Regions with Different Energy Mixes, Batteries, 2023, 9, 375 CrossRef CAS.
- G. Ji, J. Wang, Z. Liang, K. Jia, J. Ma, Z. Zhuang, G. Zhou and H.-M. Cheng, Direct regeneration of degraded lithium-ion battery cathodes with a multifunctional organic lithium salt, Nat. Commun., 2023, 14, 584 CrossRef CAS PubMed.
- S. Jiang, H. Hua, L. Zhang, X. Liu, H. Wu and Z. Yuan, Environmental impacts of hydrometallurgical recycling and reusing for manufacturing of lithium-ion traction batteries in China, Sci. Total Environ., 2022, 811, 152224 CrossRef CAS PubMed.
- S. Blömeke, C. Scheller, F. Cerdas, C. Thies, R. Hachenberger, M. Gonter, C. Herrmann and T. S. Spengler, Material and energy flow analysis for environmental and economic impact assessment of industrial recycling routes for lithium-ion traction batteries, J. Clean. Prod., 2022, 377, 134344 CrossRef.
- V. Nguyen-Tien, Q. Dai, G. D. J. Harper, P. A. Anderson and R. J. R. Elliott, Optimising the geospatial configuration of a future lithium ion battery recycling industry in the transition to electric vehicles and a circular economy, Appl. Energy, 2022, 321, 119230 CrossRef CAS.
- M. C. C. Lima, L. P. Pontes, A. S. M. Vasconcelos, W. de Araujo Silva Junior and K. Wu, Economic Aspects for Recycling of Used Lithium-Ion Batteries from Electric Vehicles, Energies, 2022, 15, 2203 CrossRef CAS.
- P. Lubello, F. Papi, A. Bianchini and C. Carcasci, Considerations on the impact of battery ageing estimation in the optimal sizing of solar home battery systems, J. Clean. Prod., 2021, 329, 129753 CrossRef.
- A. Datas, A. Ramos and C. del Cañizo, Techno-economic analysis of solar PV power-to-heat-to-power storage and trigeneration in the residential sector, Appl. Energy, 2019, 256, 113935 CrossRef.
- Ö. Gönül, A. C. Duman, B. Barutçu and Ö. Güler, Techno-economic analysis of PV systems with manually adjustable tilt mechanisms, Eng. Sci. Technol. Int. J., 2022, 35, 101116 Search PubMed.
- M. Wiatrowski, B. C. Klein, R. W. Davis, C. Quiroz-Arita, E. C. D. Tan, R. W. Hunt and R. E. Davis, Techno-economic assessment for the production of algal fuels and value-added products: opportunities for high-protein microalgae conversion, Biotechnol. Biofuels Bioprod., 2022, 15, 8 CrossRef CAS PubMed.
- G. Bagnato and A. Sanna, Process and Techno-Economic Analysis for Fuel and Chemical Production by Hydrodeoxygenation of Bio-Oil, Catalysts, 2019, 9, 1021 CrossRef CAS.
- L. J. F. Comidy, M. D. Staples and S. R. H. Barrett, Technical, economic, and environmental assessment of liquid fuel production on aircraft carriers, Appl. Energy, 2019, 256, 113810 CrossRef CAS.
- L. Kong, J. Zhao, J. Li, R. Lou and Y. Zhang, Evaluating energy efficiency improvement of pulp and paper production: Case study from factory level, J. Clean. Prod., 2020, 277, 124018 CrossRef.
- A. E. Samani, J. D. M. De Kooning, C. A. Urbina Blanco and L. Vandevelde, Flexible operation strategy for formic acid synthesis providing frequency containment reserve in smart grids, Int. J. Electr. Power Energy Syst., 2022, 139, 107969 CrossRef.
- S. Bock, B. Stoppacher, K. Malli, M. Lammer and V. Hacker, Techno-economic analysis of fixed-bed chemical looping for decentralized, fuel-cell-grade hydrogen production coupled with a 3 MWth biogas digester, Energy Convers. Manag., 2021, 250, 114801 CrossRef CAS.
- M. Shawky Ismail, W. M. El-Maghlany and M. Elhelw, Utilizing the solar ice storage system in improving the energy, exergy, economic and environmental assessment of conventional air conditioning system, Alex. Eng. J., 2022, 61, 8149–8160 CrossRef.
- A. Boyden, V. K. Soo and M. Doolan, The Environmental Impacts of Recycling Portable Lithium-Ion Batteries, Procedia CIRP, 2016, 48, 188–193 CrossRef.
- M. Iturrondobeitia, C. Vallejo, M. Berroci, O. Akizu-Gardoki, R. Minguez and E. Lizundia, Environmental Impact Assessment of LiNi1/3Mn1/3Co1/3O2 Hydrometallurgical Cathode Recycling from Spent Lithium-Ion Batteries, ACS Sustain. Chem. Eng., 2022, 10, 9798–9810 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra00972j |
| This journal is © The Royal Society of Chemistry 2024 |