A bioinspired bifunctional catalyst: an amphiphilic organometallic catalyst for ring-closing metathesis forming liquid droplets in aqueous media†
Miki
Mori
a,
Hiroka
Sugai
 b,
Kohei
Sato‡
b,
Kohei
Sato‡
 a,
Asuki
Okada
c,
Takashi
Matsuo
a,
Asuki
Okada
c,
Takashi
Matsuo
 c and
Kazushi
Kinbara
c and
Kazushi
Kinbara
 *ab
*ab
aSchool of Life Science and Technology, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8501, Japan. E-mail: kinbara.k.aa@m.titech.ac.jp
bResearch Center for Autonomous Systems Materialogy (ASMat), Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8501, Japan
cDivision of Materials Science, Nara Institute of Science and Technology, 8916-5 Takayama-cho, Ikoma 630-0192, Japan
First published on 26th June 2024
Abstract
Inspired by phase-separated biopolymers with enzymatic activity, we developed an amphiphilic catalyst consisting of alternating hydrophilic oligo(ethylene glycol) and hydrophobic aromatic units bearing a Hoveyda–Grubbs catalyst center (MAHGII). MAHGII served as both a droplet-forming scaffold and a catalyst for ring-closing metathesis reactions, providing a new biomimetic system that promotes organic reactions in an aqueous environment.
Creating life-like functions from abiotic molecules is a crucial issue for constructively understanding complicated living systems1,2 or developing bioinspired technologies.3,4 Among various life-like functions,5 compartmentalization and chemical reactions therein are especially fundamental principles that underlie the spatiotemporal regulation of molecular processes in life.6,7 Encouraged by recent increasing attention on membrane-less organelles in the cells,8–10 phase-separated condensates of biopolymers have become widely recognized as one of the bioinspired compartments. Many in vitro experiments have demonstrated that their liquid-like nature allows selective incorporation/exclusion between droplets and surrounding solutions,11–13 and highly condensed environments in droplets contribute to an acceleration of the chemical reactions.14–16 Recent studies have also reported that several proteins with enzymatic activity, such as the RNA helicase LAF-117 and Ddx4,18 double as a phase-separated scaffold, showing the sophisticated catalytic reaction field in nature.
In this context, although there have been numerous instances of phase-separated droplets composed of synthetic polymers exhibiting low critical solution temperatures (LCST),19 the potential of synthetic catalysts within such droplets remains unexplored, despite the various strategies that have been proposed for using water as a solvent in catalytic organic reactions,20,21 including the development of water-soluble homogeneous precatalysts,22–27 on-water synthesis,28,29 micellar catalysis,30–37 utilization of organic cosolvents,33,38,39 and bio-catalysis.40,41 In particular, a single synthetic compound, that can act as both a catalyst and a reaction field like the phase-separated droplets in an aqueous environment, has not yet been achieved.
Herein, we introduce a new concept of artificial catalytic reaction field in an aqueous environment: organometallic reactions by a liquid droplet-forming transition metal catalyst. Inspired by a natural catalytic reaction field consisting of a rigid enzymatic domain and flexible intrinsically disordered regions, such as LAF-117 and Ddx4,18 we designed a compound with a rigid transition metal catalytic unit and flexible hydrophilic polymer moieties. We synthesized a multiblock amphiphilic Hoveyda–Grubbs 2nd generation catalyst (MAHGII, Fig. 1) which is composed of hydrophilic octa(ethylene glycol) chains and hydrophobic aromatic units with a catalytic moiety at the center. The amphiphilic poly(ethylene glycol) (PEG)-based structure was adopted from its flexible conformation, and also from its droplet-forming property in aqueous media demonstrated in our previous report.42 Hoveyda–Grubbs 2nd generation catalysts are known as air- and water-tolerant catalysts for olefin metathesis reactions,43,44 which could be an ideal model in this study.
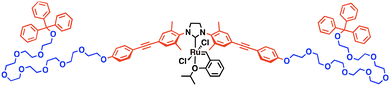 | ||
| Fig. 1 Molecular structure of MAHGII. Red, blue, and black indicate the hydrophobic, hydrophilic, and catalytic units, respectively. | ||
The multiblock N-heterocyclic carbene (NHC) ligand precursor was synthesized following the previously reported procedures by our group.45 The complexation of the NHC ligand precursor with Grubbs 1st generation catalyst was carried out under basic conditions, followed by ligand exchange with 2-isopropoxystyrene to afford the Hoveyda-type 2nd generation catalytic unit. All the newly synthesized compounds were characterized by 1H and 13C NMR spectroscopy and high-resolution ESI-TOF-MS spectrometry (Fig. S1–S9, ESI†).
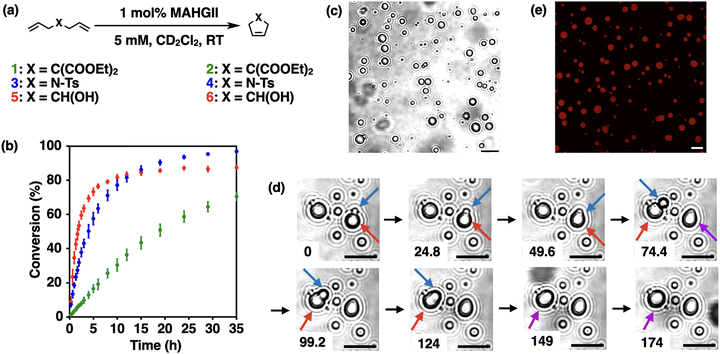
First, we investigated the catalytic activity of MAHGII in an organic solution. Ring-closing metathesis (RCM) reactions were carried out in CD2Cl2 with three substrates: diethyl diallylmalonate (1), N,N-diallyl-4-methylbenzenesulfonamide (3) and 1,6-heptadien-4-ol (5), which gave the cyclized products 2, 4, and 6, respectively (Fig. 2a). Reactions were performed at room temperature under open air at a substrate concentration of 5 mM with 1 mol% catalyst loading. The conversions were calculated based on 1H NMR spectroscopy over 35 h. The RCM reaction of 1 proceeded slowest and gave the lowest conversion of 70% among the three substrates examined (Fig. 2b, green dots). In contrast, the reaction proceeded fastest for 5, which reached its plateau around 15 h with the conversion of 86% (Fig. 2b, red dots). The RCM of 3 was initiated slower compared to 5; however, it gave the highest conversion of 96% after 35 h (Fig. 2b, blue dots). These trends of the kinetic profile followed those observed for the commercially available Hoveyda–Grubbs 2nd generation catalyst (HGII) under the same reaction conditions (Fig. S10, ESI†). Although the NHC unit includes ethyne units, the ene-yne-type metathesis product was not observed in each case.
Having confirmed that MAHGII with the amphiphilic moieties kept similar catalytic activity with HGII, we then examined the capability of MAHGII to form liquid droplets in aqueous environments. A DMF solution of MAHGII was added to a 100 mM KCl aq. under stirring at room temperature. Here, a 100 mM KCl aq. was chosen as a solvent to stabilize the catalytic Ru center, where the equilibrium of the Ru complex is shifted to the chloride-coordinated catalytically active state rather than the hydroxide-coordinated inactive state.46,47 As shown in Fig. 2c, micrometer-sized spherical droplets were observed by phase contrast microscopy. Importantly, these droplets could coalesce over time (Fig. 2d), showing the liquid-like nature of the MAHGII droplets. In addition, the capability of the MAHGII droplets to accumulate other molecules within themselves was investigated by using Nile Red, a hydrophobic dye. Confocal laser scanning microscopy (CLSM) showed the localization of the red emission due to Nile Red within the MAHGII droplets (Fig. 2e). The gray values of the Nile Red emission across the droplet showed the mostly constant values (Fig. S11, ESI†), indicating the uniform distribution of Nile Red in the droplet. These results suggested that the MAHGII droplets could accumulate hydrophobic molecules.
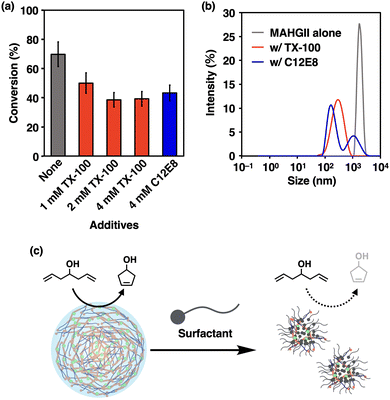
Since the MAHGII droplets were shown to act as hydrophobic containers in an aqueous environment, the catalytic activity of MAHGII droplets was investigated for the RCM reaction. 5 was chosen as a model substrate because it allows direct measurement of the reaction progress by 1H NMR spectroscopy in aqueous environments. The reaction mixtures were prepared by dispersing the DMF solution of MAHGII in 100 mM KCl D2O solution, followed by the addition of DMF solution of 5, so that the final concentrations of MAHGII and 5 were 200 μM and 5 mM, respectively. Note that 5 did not disrupt the MAHGII droplets under these concentrations (Fig. S12, ESI†). The dynamic light scattering (DLS) measurement (Fig. S13, ESI†) also indicated that 5 formed tiny droplets with hydrodynamic diameter DH of 440 nm, which disappeared in the presence of MAHGII, while the size of the MAHGII droplets (DH = 1.8 μm) remained unchanged. This result indicated that MAHGII droplets accommodated 5. Then, the reaction mixtures were heated at 50 °C for 4 h under open air without stirring, which was cooled down to room temperature for 1H NMR measurement to evaluate the conversion.48 The conversion of the RCM reaction catalyzed by MAHGII droplets reached 70% (Fig. 3a, gray bar), whereas that by the commercially available HGII under the same conditions was only 16% (Fig. S14, ESI†). Since the RCM reaction carried out in an organic solution (in CDCl3) under the same reaction conditions proceeded quantitatively (Fig. S15, ESI†), it is noteworthy that MAHGII maintained high catalytic activity compared to HGII in an aqueous medium.
Furthermore, we examined the importance of the droplet formation on the catalytic activity. Here, we chose two neutral surfactants, Triton X-100 (TX-100) and octa(ethylene glycol) monododecyl ether (C12E8). Prior to performing the RCM reaction of 5 in the presence of these surfactants, we confirmed the prevention of droplet formation using DLS. MAHGII alone gave a monodisperse size distribution with DH of 1.8 μm (Fig. 3b, gray line). In contrast, MAHGII in the presence of TX-100 and C12E8 gave a monodisperse size distribution with DH of 315 nm and polydisperse size distribution with DH of 193 nm and 1.2 μm, respectively (Fig. 3b, red and blue lines). These results suggested that the MAHGII droplets were disrupted in the presence of surfactants. Then we carried out the RCM reaction in the presence of various concentrations of surfactants, while those of 5 and MAHGII were fixed to 5 mM and 200 μM, respectively. In the presence of 1 mM TX-100, the conversion dropped to 50%. Upon increasing the TX-100 concentration to 2 mM and 4 mM, the drop in the conversion had likely reached a plateau, giving the conversion of 39% for both concentrations (Fig. 3a, red bars). Similarly, the conversion dropped to 43% in the presence of 4 mM C12E8 (Fig. 3a, blue bars). In sharp contrast to these results, the catalytic activity of commercially available HGII enhanced to 50–58% in the presence of the surfactants (Fig. S14, ESI†) while those without any surfactant reached only 16% as mentioned above. Altogether, the disruption of the MAHGII droplets by the surfactants led to the decrease of the catalytic activity of MAHGII, suggesting that the droplets were indeed providing efficient environments for the RCM reaction in the aqueous medium (Fig. 3c).
In conclusion, inspired by the phase-separated biopolymers in nature, we designed and synthesized a multiblock amphiphilic Hoveyda–Grubbs 2nd generation catalyst (MAHGII), bearing both rigid catalytic active and flexible droplet-inducing moieties. NMR spectroscopic and microscopic experiments demonstrated that the MAHGII droplets successfully catalyzed the RCM reactions in aqueous environments. In some cellular systems, the acceleration of reactions induced by the colocalization of the catalyst with its substrate within the droplets has been observed. Examples include the mRNA processing reaction within the histone locus body49 and the pre-mRNA splicing reaction within Cajal bodies in zebrafish.50MAHGII successfully demonstrated the expansion of similar systems to an abiotic catalytic reaction, specifically the RCM reaction. Furthermore, the use of water as a solvent in organic synthesis has massive advantages over the organic solvents not only for biological applications but also for green chemistry.51,52 Therefore, we believe that this study could potentially be recognized as one of the new concepts for biomimetic organometallic catalysis in an aqueous environment, which contributes to the development of environmentally friendly organic synthetic procedures.
Miki Mori: investigation; funding acquisition; writing – original draft. Hiroka Sugai: investigation; writing – original draft; writing – review & editing. Kohei Sato: Project administration; funding acquisition; writing – review & editing. Asuki Okada: investigation. Takashi Matsuo: resources; writing – review & editing. Kazushi Kinbara: conceptualization; supervision; resources; funding acquisition; project administration; writing – review & editing.
The authors thank Suzukakedai Materials Analysis Division, Open Facility, Tokyo Institute of Technology for ESI-TOF-MS spectrometry and NMR spectroscopy, Prof. M. Takinoue, Tokyo Institute of Technology, for CLSM, and Rei Hamaguchi for experimental support. This work was supported by a MEXT/JSPS Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Engine” (JP18H05418, JP18H05419, and JP23H05418 to KK), Grant-in-Aid for Challenging Research (Pioneering) (JP23K17363 to KK), Grant-in-Aid for Scientific Research (B) (JP23H02080 to KS), Grant-in-Aid for Challenging Research (Exploratory) (JP23K17973 to KS), Grant-in-Aid for Transformative Research Areas “Molecular Cybernetics” (JP23H04408 to KS), Grant-in-Aid for JSPS Fellows (JP22J14625 to MM), and JST SPRING (JPMJSP2106 to MM). MM also thanks the Leave a Nest Grant in•cube award.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Mann, Acc. Chem. Res., 2012, 45, 2131–2141 CrossRef CAS PubMed.
- W. Jiang, Z. Wu, Z. Gao, M. Wan, M. Zhou, C. Mao and J. Shen, ACS Nano, 2022, 16, 15705–15733 CrossRef CAS PubMed.
- A. Levin, T. A. Hakala, L. Schnaider, G. J. L. Bernardes, E. Gazit and T. P. J. Knowles, Nat. Rev. Chem., 2020, 4, 615–634 CrossRef CAS.
- X. Wang, X. Liu and X. Huang, Adv. Mater., 2020, 32, e2001436 CrossRef PubMed.
- N. A. Yewdall, A. F. Mason and J. C. M. van Hest, Interf. Focus, 2018, 8, 20180023 CrossRef PubMed.
- M. J. York-Duran, M. Godoy-Gallardo, C. Labay, A. J. Urquhart, T. L. Andresen and L. Hosta-Rigau, Colloids Surf., B, 2017, 152, 199–213 CrossRef CAS PubMed.
- P.-A. Monnard and P. Walde, Life, 2015, 5, 1239–1263 CrossRef CAS PubMed.
- A. S. Lyon, W. B. Peeples and M. K. Rosen, Nat. Rev. Mol. Cell Biol., 2021, 22, 215–235 CrossRef CAS PubMed.
- D. L. J. Lafontaine, J. A. Riback, R. Bascetin and C. P. Brangwynne, Nat. Rev. Mol. Cell Biol., 2021, 22, 165–182 CrossRef CAS PubMed.
- S. Alberti and A. A. Hyman, Nat. Rev. Mol. Cell Biol., 2021, 22, 196–213 CrossRef CAS PubMed.
- T.-Y. Dora Tang, C. Rohaida Che Hak, A. J. Thompson, M. K. Kuimova, D. S. Williams, A. W. Perriman and S. Mann, Nat. Chem., 2014, 6, 527–533 CrossRef CAS PubMed.
- T. Lu and E. Spruijt, J. Am. Chem. Soc., 2020, 142, 2905–2914 CrossRef CAS PubMed.
- I. A. Klein, A. Boija, L. K. Afeyan, S. W. Hawken, M. Fan, A. Dall’Agnese, O. Oksuz, J. E. Henninger, K. Shrinivas, B. R. Sabari, I. Sagi, V. E. Clark, J. M. Platt, M. Kar, P. M. McCall, A. V. Zamudio, J. C. Manteiga, E. L. Coffey, C. H. Li, N. M. Hannett, Y. E. Guo, T.-M. Decker, T. I. Lee, T. Zhang, J.-K. Weng, D. J. Taatjes, A. Chakraborty, P. A. Sharp, Y. T. Chang, A. A. Hyman, N. S. Gray and R. A. Young, Science, 2020, 368, 1386–1392 CrossRef CAS PubMed.
- R. R. Poudyal, R. M. Guth-Metzler, A. J. Veenis, E. A. Frankel, C. D. Keating and P. C. Bevilacqua, Nat. Commun., 2019, 10, 490 CrossRef CAS PubMed.
- R. R. Poudyal, C. D. Keating and P. C. Bevilacqua, ACS Chem. Biol., 2019, 14, 1243–1248 CrossRef CAS PubMed.
- M. Abbas, W. P. Lipiński, K. K. Nakashima, W. T. S. Huck and E. Spruijt, Nat. Chem., 2021, 13, 1046–1054 CrossRef CAS PubMed.
- S. Elbaum-Garfinkle, Y. Kim, K. Szczepaniak, C. C.-H. Chen, C. R. Eckmann, S. Myong and C. P. Brangwynne, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 7189–7194 CrossRef CAS PubMed.
- T. J. Nott, E. Petsalaki, P. Farber, D. Jervis, E. Fussner, A. Plochowietz, T. D. Craggs, D. P. Bazett-Jones, T. Pawson, J. D. Forman-Kay and A. J. Baldwin, Mol. Cell, 2015, 57, 936–947 CrossRef CAS PubMed.
- Y. Yuan, K. Raheja, N. B. Milbrandt, S. Beilharz, S. Tene, S. Oshabaheebwa, U. A. Gurkan, A. C. S. Samia and M. Karayilan, RSC Appl. Polym., 2023, 1, 158–189 RSC.
- U. M. Lindström, Chem. Rev., 2002, 102, 2751–2772 CrossRef PubMed.
- C.-J. Li, Chem. Rev., 2005, 105, 3095–3165 CrossRef CAS PubMed.
- T. J. Ahmed, L. N. Zakharov and D. R. Tyler, Organometallics, 2007, 26, 5179–5187 CrossRef CAS.
- J. P. Jordan and R. H. Grubbs, Angew. Chem., Int. Ed., 2007, 46, 5152–5155 CrossRef CAS PubMed.
- K. Skowerski, G. Szczepaniak, C. Wierzbicka, L. Gułajski, M. Bieniek and K. Grela, Catal. Sci. Technol., 2012, 2, 2424–2427 RSC.
- Z. J. Wang, W. R. Jackson and A. J. Robinson, Green Chem., 2015, 17, 3407–3414 RSC.
- M. Finn, J. A. Ridenour, J. Heltzel, C. Cahill and A. Voutchkova-Kostal, Organometallics, 2018, 37, 1400–1409 CrossRef CAS.
- I. Nicolas, P. L. Maux and G. Simonneaux, Tetrahedron Lett., 2008, 49, 5793–5795 CrossRef CAS.
- S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS PubMed.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS PubMed.
- T. Dwars, E. Paetzold and G. Oehme, Angew. Chem., Int. Ed., 2005, 44, 7174–7199 CrossRef CAS PubMed.
- B. H. Lipshutz, G. T. Aguinaldo, S. Ghorai and K. Voigtritter, Org. Lett., 2008, 10, 1325–1328 CrossRef CAS PubMed.
- G. La Sorella, G. Strukul and A. Scarso, Green Chem., 2015, 17, 644–683 RSC.
- C. M. Gabriel, N. R. Lee, F. Bigorne, P. Klumphu, M. Parmentier, F. Gallou and B. H. Lipshutz, Org. Lett., 2017, 19, 194–197 CrossRef CAS PubMed.
- B. H. Lipshutz, S. Ghorai and M. Cortes-Clerget, Chem. – Eur. J., 2018, 24, 6672–6695 CrossRef CAS PubMed.
- M. Cortes-Clerget, N. Akporji, J. Zhou, F. Gao, P. Guo, M. Parmentier, F. Gallou, J.-Y. Berthon and B. H. Lipshutz, Nat. Commun., 2019, 10, 2169 CrossRef PubMed.
- B. H. Lipshutz and S. Ghorai, Org. Lett., 2009, 11, 705–708 CrossRef CAS PubMed.
- B. H. Lipshutz and S. Ghorai, Tetrahedron, 2010, 66, 1057–1063 CrossRef CAS.
- S. J. Connon, M. Rivard, M. Zaja and S. Blechert, Adv. Synth. Catal., 2003, 345, 572–575 CrossRef CAS.
- R. P. Megens and G. Roelfes, Org. Biomol. Chem., 2010, 8, 1387–1393 RSC.
- T. K. Hyster, L. Knörr, T. R. Ward and T. Rovis, Science, 2012, 338, 500–503 CrossRef CAS PubMed.
- F. Schwizer, Y. Okamoto, T. Heinisch, Y. Gu, M. M. Pellizzoni, V. Lebrun, R. Reuter, V. Köhler, J. C. Lewis and T. R. Ward, Chem. Rev., 2018, 118, 142–231 CrossRef CAS PubMed.
- S. Kawasaki, T. Muraoka, H. Obara, T. Ishii, T. Hamada and K. Kinbara, Chem. – Asian J., 2014, 9, 2778–2788 CrossRef CAS PubMed.
- M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953–956 CrossRef CAS PubMed.
- S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168–8179 CrossRef CAS.
- M. Mori, K. Sato, T. Ekimoto, S. Okumura, M. Ikeguchi, K. V. Tabata, H. Noji and K. Kinbara, Chem. – Asian J., 2021, 16, 147–157 CrossRef CAS PubMed.
- T. Matsuo, T. Yoshida, A. Fujii, K. Kawahara and S. Hirota, Organometallics, 2013, 32, 5313–5319 CrossRef CAS.
- J. C. Foster, M. C. Grocott, L. A. Arkinstall, S. Varlas, M. J. Redding, S. M. Grayson and R. K. O’Reilly, J. Am. Chem. Soc., 2020, 142, 13878–13885 CrossRef CAS PubMed.
- The real-time monitoring of the RCM reaction using 1H NMR spectroscopy turned out to be difficult due to the heterogeneity of the reaction media.
- D. C. Tatomer, E. Terzo, K. P. Curry, H. Salzler, I. Sabath, G. Zapotoczny, D. J. McKay, Z. Dominski, W. F. Marzluff and R. J. Duronio, J. Cell Biol., 2016, 213, 557–570 CrossRef CAS PubMed.
- M. Strzelecka, S. Trowitzsch, G. Weber, R. Lührmann, A. C. Oates and K. M. Neugebauer, Nat. Struct. Mol. Biol., 2010, 17, 403–409 CrossRef CAS PubMed.
- J. C. Jewett and C. R. Bertozzi, Chem. Soc. Rev., 2010, 39, 1272–1279 RSC.
- M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc01117a |
| ‡ Present address: Department of Chemistry, School of Science, Kwansei Gakuin University, 1 Gakuen Uegahara, Sanda-Shi, Hyogo 669-1330, Japan. |
| This journal is © The Royal Society of Chemistry 2024 |