Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of quaternary centres by single electron reduction and alkylation of alkylsulfones†‡
Masakazu
Nambo
 *a,
Yasuyo
Tahara
a,
Jacky C.-H.
Yim
a,
Daisuke
Yokogawa
*a,
Yasuyo
Tahara
a,
Jacky C.-H.
Yim
a,
Daisuke
Yokogawa
 b and
Cathleen M.
Crudden
b and
Cathleen M.
Crudden
 *ac
*ac
aInstitute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Chikusa, Nagoya, Aichi 464-8601, Japan. E-mail: mnambo@itbm.nagoya-u.ac.jp
bGraduate School of Arts and Science, The University of Tokyo, Komaba, Meguro-ku, Tokyo, 153-8902, Japan
cDepartment of Chemistry, Queen's University, Chernoff Hall, Kingston, Ontario K7L 3N6, Canada. E-mail: cruddenc@chem.queensu.ca
First published on 4th February 2021
Abstract
A new method for the generation of tertiary radicals through single electron reduction of alkylsulfones promoted by Zn and 1,10-phenanthroline has been developed. These radicals could be employed in the Giese reaction, affording structurally diverse quaternary products in good yields. With the high modularity and functional group compatibility of sulfones, the utility of this method was demonstrated by intramolecular and iterative reactions to give complex structures. The radical generation process was investigated by control experiments and theoretical calculations.
Introduction
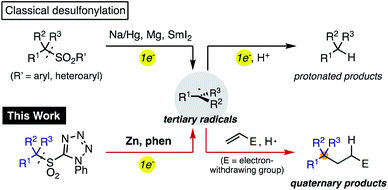
Organosulfones are versatile intermediates in organic synthesis because of the ease with which they permit facile structural modification through α-functionalization or conjugate addition.1 Due to the inherent stability of sulfonyl groups, strong reducing agents such as Na amalgam and Mg are generally required for their removal (Scheme 1).2 However, a stepwise alkylative desulfonylation process would be much more valuable. In this process, single electron transfer to the sulfonyl group by a reductant would fragment into a sulfinic anion and a sp3-carbon radical, which could be trapped with organic electrophiles rather than merely reduced and protonated. Thus, a controlled single electron reduction would have the potential to establish a new method for the generation of carbon radicals in organic synthesis and expand the utility of sulfones.3Recently, organosulfones have attracted considerable attention in cross-coupling reactions as a new class of electrophiles.4 Our group and others have developed several transition-metal catalyzed cross-coupling reactions of functionalized aromatic and benzylic sulfones via carbon–sulfonyl (C–SO2) bond activation.5 Substituents on the sulfonyl group were found to provide a powerful new avenue for controlling reactivity. For example, strongly electron-withdrawing substituents such as 3,5-bis(trifluoromethyl)phenyl5b,d,e and trifluoromethyl groups5f,g not only facilitate α-functionalization, but also promote C–SO2 bond activation, enabling the rapid synthesis of multiply-arylated structures. In Baran's Ni-catalyzed radical cross-coupling of primary and secondary alkylsulfones with arylzinc reagents,4d tetrazolyl group7 as the sulfone substituent was critical to enable the desired reaction. Hu,6a and others6b–d reported a radical fluoroalkylations using fluoroalkylsulfones, and Gouverneur and Davis recently established a photocatalytic radical modification of proteins.6e
Building on these reports, we envisioned a new method for generating reactive alkyl radicals by careful selection of a suitable sulfonyl substituent8 and reducing agent. Herein we report a simple method for the conversion of tertiary alkylsulfones to tertiary alkyl radicals, and their alkylation to generate quaternary carbon-centers. This reaction proceeds efficiently using readily available Zn powder with 1,10-phenanthroline (phen) as a new single electron reducing agent. The key reducing species generated by Zn and phen was investigated by control experiments and theoretical calculations.
Results and discussion
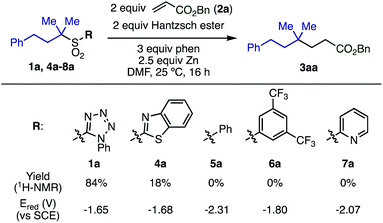
We began our investigation by studying the Giese reaction9 of 3-phenyl-1,1-dimethylpropyl-1-phenyltetrazolyl sulfone 1a with benzyl acrylate 2a as a model reaction at room temperature (Table 1). Optimized conditions include the use of Hantzsch ester as a proton source in DMF, and the combination of Zn powder (particle size <10 μm, 2.5 equiv.) with phen (3 equiv.), giving the quaternary product 3aa in 82% isolated yield (entry 1).| Entry | Deviations from above | Yieldb [%] |
|---|---|---|
| a Conditions: 1a (0.1 mmol), 2a (2 equiv.), Hantzsch ester (2 equiv.), phen (3 equiv.), Zn (particle size < 10 μm, 2.5 equiv.), DMF (0.2 M), 25 °C, 16 h. b Yields were determined by 1H NMR using anisole as an internal standard. Isolated yield is shown in parentheses (0.2 mmol scale). | ||
| 1 | None | 84(82) |
| 2 | No Zn | 0 |
| 3 | No phen | 0 |
| 4 | Zn pre-activated by TMSCl, no phen | 0 |
| 5 | Ricke Zn, no phen | 0 |
| 6 | Zn (particle size 75–150 μm) | 0 |
| 7 | Mg or Mn instead of Zn | 0 |
| 8 | 1.5 equiv. Hantzsch ester | 67 |
| 9 | DMAP or bpy instead of phen | 0 |
| 10 | Terpy instead of phen | 25 |
| 11 | 2 equiv. phen | 61 |
| 12 | 50 °C | 77 |
| 13 | 2 equiv. LiCl | 58 |
| 14 | 10 mol% NiCl2, 2 equiv. MgCl2 | 59 |
|
||
Both Zn and phen were essential for this reaction (entries 2 and 3). Even the use of Zn activated by TMSCl10 or Rieke zinc11 showed no reactivity in the absence of phen, indicating that the ligand does more than simply activate the Zn surface. Using Zn with a larger particle size (75–150 μm) or other metals, such as Mg and Mn, also in the presence of phen, shut down the reaction (entries 4–6). The use of 1.5 equiv. Hantzsch ester decreased the yield of product (entry 8). Other N-heteroarene additives including 4-dimethylaminopyridine (DMAP), 2,2′-bipyridyl (bpy), and 2,2′:6′,2′′-terpyridine (terpy) instead of phen also inhibited product formation (entries 9 and 10). Reducing the amount of phen to 2 equiv. resulted in a decreased yield (entry 11). Product yield was not improved by increasing the reaction temperature (entry 12). Other additives that have been employed in similar transformations were examined12 but did not show any beneficial effect in this reaction (entries 13 and 14).
The substituent on the sulfonyl group was found to be a key factor for radical generation (Scheme 2). 2-Benzothiazolyl sulfone (4a) was converted into 3aa, albeit in poor yield. Other sulfones with substituents such as phenyl (5a), 3,5-bis(trifluoromethyl)phenyl (6a), and 2-pyridyl (7a) were completely unreactive. The first reduction potentials (Ered) of sulfones were measured to evaluate the ease of single electron reduction, and were shown to correlate with reactivity. Specifically, sulfones 1a and 4a were the easiest to reduce, and the only two to show any product under the conditions examined.
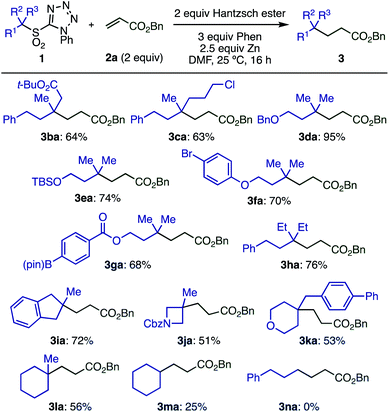
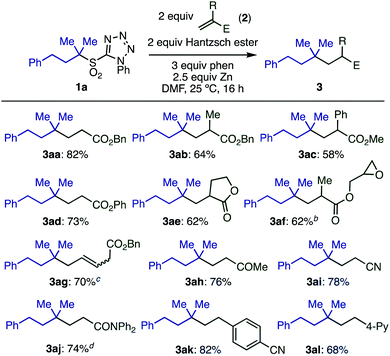
With optimized conditions in hand, we investigated the substrate scope of electron-deficient olefins 2 (Scheme 3). A variety of acrylates having substituents in the 2-position (2a–2e) gave the desired products in good yields. Interestingly, the sensitive oxirane moiety in acrylate 2f was compatible with the reaction conditions, and conjugated olefin 2g gave the product from selective reaction at the terminal position. Other electron-deficient olefins, including vinyl ketone 2h and acrylonitrile 2i, reacted smoothly. Although acrylamide 2j was less reactive, the yield of 3aj could be improved to 74% by running the reaction at 50 °C.
Electron-poor styrene derivatives (2k, 2l) were also viable substrates, giving products 3ak and 3al in good yields. Simple styrenes without electron withdrawing groups or acrylate derivatives with steric hindrance at the beta-position were not reactive. For full details see ESI Table S6.‡
When the more complex allyl sulfone 2m bearing an ester moiety was reacted with excess 1a, the doubly alkylated product 3ma was formed through radical addition and then subsequent elimination of the sulfonyl group followed by the Giese reaction (Scheme 4).
Next, the scope of tertiary sulfones 1 was evaluated (Scheme 5). These substrates were readily prepared by α-alkylation of primary or secondary sulfones with high functional group compatibility. Acyclic sulfones bearing various functional groups including ester, chloride, benzyloxy, silyloxy, bromoaryl, and boryl groups (1b–1g) were well tolerated, which is important for further transformations. Sterically hindered radicals generated from sulfone 1h gave the desired product (3ha) without loss of yield. Cyclic tertiary sulfones with different ring sizes (1i–1l) were also suitable substrates, however, secondary sulfones gave decreased yields of the desired product (3ma) due to the formation of a by-product derived from the addition of sulfinic radical to the olefin. Primary sulfone 1n showed no conversion under optimized conditions, likely because the generation of a primary radical is unfavorable.
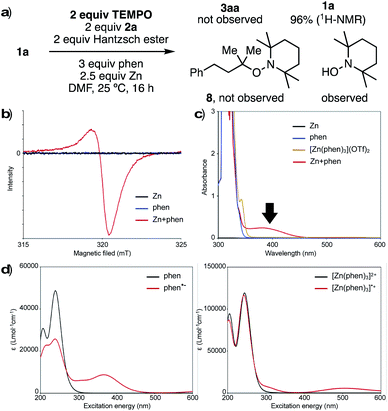
To investigate the nature of the key reducing agent for radical generation, we performed several control experiments. When the reaction of 1a with 2a was conducted under standard conditions in the presence of 2,2,6,6-tetramethylpiperidine-1-oxy (TEMPO), neither product 3a nor TEMPO-adduct 8 was observed, and 1a was recovered quantitatively (Fig. 1a). In addition, 1-hydroxy-2,2,6,6-tetramethylpiperidine (TEMPO-OH) was detected by GCMS and LCMS analysis. This suggests that generated reductant has radical character, and the single electron transfer process can be inhibited before desulfonylation. When Zn and phen were stirred in DMF at room temperature, a red/purple-colored mixture was obtained. This mixture was analyzed by an electron paramagnetic resonance (EPR), and an EPR signal was observed (g = 2.0017), clearly indicating that a radical species is generated from Zn and phen (Fig. 1b). One reasonable hypothesis is that Zn reduces phen to form reactive phen radical anion (phen˙−)13 and [Zn(phen)3]˙+ species.
UV-Vis spectroscopic analysis of the mixture of Zn and phen showed a broad absorption band from 350 nm to 460 nm, which was not observed in the spectra for either phen or [Zn(phen)3](OTf)2 alone (Fig. 1c). To understand the origin of this absorption, simulation of UV-Vis absorption spectra was carried out by TD-DFT calculations (Fig. 1d). The predicted spectra of both phen and [Zn(phen)3]2+ care characterized by signals below 350 nm (black lines, Fig. 1d right and left). On the other hand, the proposed radical species, phen˙− and [Zn(phen)3]˙+, are predicted to absorb at a longer wavelength (red lines, Fig. 1d right and left). In particular, the absorption band of phen˙− (Fig. 1d, left) is similar to the experimentally obtained spectra from a mixture from Zn and phen, supporting the proposed generation of radical species as key reducing agents.
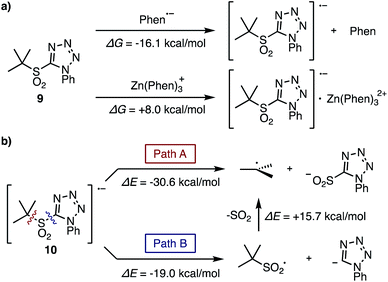
The fragmentation process of the tertiary sulfone was also evaluated by theoretical studies. For the first single electron reduction of t-butyl sulfone 9, both phen˙− and [Zn(phen)3]˙+ are possible reducing agents based on the Gibbs free energies for their generation at room temperature (ΔG, −16.1, +8.0 kcal mol−1, respectively, Fig. 2a). In addition, the Lei group reported the first reduction potential of phen in DMF to be −2.06 V.13 With an experimentally determined sulfone reduction potential of −1.65 V, (Scheme 2) a thermodynamically downhill reduction by phen˙− is completely reasonable.
Considering the fragmentation process of sulfone radical 10, two pathways are possible (Fig. 2b): direct formation of the requisite t-butyl radical along with the formation of tetrazolylsulfinic anion (Path A), or fragmentation to generate the tetrazolyl anion along with the t-butylsulfonyl radical, which subsequently fragments into the t-butyl radical and SO2 (Path B). The bond dissociation energies (ΔE) of these two pathways were calculated to be −30.6 and −19.0 kcal mol−1 respectively, therefore Path A should be favorable. Moreover, the addition of in situ generated tertiary alkylsulfonyl radical to electron-deficient olefins was reported by Wu et al.14 and Ley et al.,7c but this product was never observed under our reaction conditions. Thus, Path B cannot be ruled out in this stage, but Path A is more plausible for the generation of radical species.
Finally, substrates in which the olefin moieties were introduced by α-alkylation of secondary sulfones can be employed in an intramolecular Giese reaction, giving interesting spiro compounds (Scheme 6a) and demonstrating the high modularity and functional group compatibility of the transformation. A variety of spiro compounds (12) with different ring sizes were prepared in good to excellent yields by varying the alkyl tether lengths.
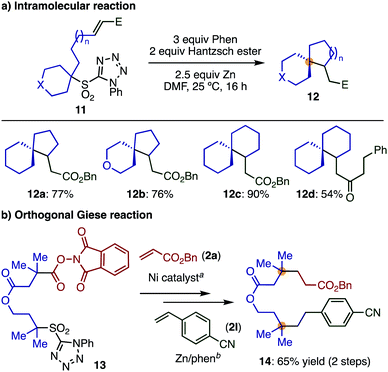 | ||
| Scheme 6 Synthetic applications. Reaction conditions: (a) 2a, Ni(CIO4)2·6H2O, Zn, LiCl, MeCN, 25 °C. (b) 2l, Hantzsch ester, phen, Zn, DMF, 25 °C. | ||
Moreover, we were interested in whether the sulfone employed in our transformation would be sufficiently differentiated from Baran's widely employed redox-active ester,12 enabling an orthogonal Giese reaction. As a first step, we showed that tertiary sulfone 1a was completely inactive under Ni-catalyzed decarbonylative reaction condition as developed by Baran et al. for cross coupling8c (see ESI‡). Then, we prepared compound 13, bearing a sulfone and a redox-active ester, and reacted this species sequentially with two different electron-deficient olefins. This simple sequence of reactions afforded compound 14, bearing two quaternary centers in reasonable yield (Scheme 6b). This transformation highlights how our method can be used complementarily with other methods, resulting in facile routes to highly functionalized products with multiple quaternary centers.
Conclusions
We have developed a new and simple protocol for the generation of tertiary radicals through reductive desulfonylation of alkylsulfones by Zn and phen, enabling the Giese reaction to form various quaternary products. Control experiments and theoretical calculations illustrate the importance of the substituents on sulfonyl group and the nature of reducing agents to control the radical generation process. Most importantly, we have demonstrated that the sulfone, which has been regarded as an electrophile, can also be used to generate nucleophilic radicals simply by the modification of sulfonyl group. This umpolung approach will allow the expansion of the utility of organosulfones in chemical transformations using radical species. Further studies to develop new bond forming reactions through reductive activation of sulfones are currently ongoing in our laboratory.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by KAKENHI from JSPS (26810056 to M. N.). M. N. thanks the support from Tatematsu Foundation. We thank Prof. Kunio Awaga and Dr Yoshiaki Shuku for assistance with the EPR analysis, and Shimadzu corporation for the measurement of ICP-MS analysis. JSPS and NU are acknowledged for funding of this research through The World Premier International Research Center Initiative (WPI) program. The Natural Sciences and Engineering Research Council of Canada (NSERC) is thanked for Discovery Grant funding to CMC.Notes and references
- (a) B. M. Trost, Bull. Chem. Soc. Jpn., 1988, 61, 107–124 CrossRef CAS; (b) A. Costa, C. Nájera and J. M. Sansano, Synlett, 2001, 12, 1881–1884 CrossRef; (c) D. Ravelli, S. Montanaro, M. Zema, M. Fagnoni and A. Albini, Adv. Synth. Catal., 2011, 353, 3295–3300 CrossRef CAS; (d) B. Zheng, T. Jia and P. J. Walsh, Org. Lett., 2013, 15, 1690–1693 CrossRef CAS; (e) B. M. Trost and C. A. Kalnmals, Chem. Eur. J., 2019, 25, 11193–11213 CrossRef CAS.
- Reviews, see: (a) D. A. Alonso and C. Nájera, Desulfonylation Reactions, Org. React., 2009, 72(2), 367–656 Search PubMed; recent examples, see: (b) Y. Zhao, C. Ni, F. Jiang, B. Gao, X. Shen and J. Hu, ACS Catal., 2013, 3, 631–634 CrossRef CAS; (c) D.-T. Yang, Q.-Y. Meng, J.-J. Zhong, M. Xiang, Q. Liu and L.-Z. Wu, Eur. J. Org. Chem., 2013, 33, 7528–7532 CrossRef; (d) Y.-M. Su, Y. Hou, F. Yin, Y.-M. Xu, Y. Li, X. Zheng and Z.-S. Wang, Org. Lett., 2014, 16, 2958–2961 CrossRef CAS; (e) J. Gui, Q. Zhou, C.-M. Pan, Y. Yabe, A. C. Burns, M. R. Collins, M. A. Ornelas, Y. Ishihara and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 4853–4856 CrossRef CAS; (f) K. Inanaga, T. Fukuyama, M. Kubota, Y. Komatsu, H. Chiba, A. Kayano and K. Tagami, Org. Lett., 2015, 17, 3158–3161 CrossRef CAS; (g) H. Watanabe, M. Takemoto, K. Adachi, Y. Okuda, A. Dakegata, T. Fukuyama, I. Ryu, K. Wakamatsu and A. Orita, Chem. Lett., 2020, 49, 409–412 CrossRef CAS; (h) Y. Jiang, W.-Y. Fang, K. P. Rakesh and H.-L. Qin, Org. Chem. Front., 2020, 7, 1696–1702 RSC.
- For selected recent reviews in radical functionalization, see: (a) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2016, 55, 58–102 CrossRef CAS; (b) H. Yi, G. Zhang, H. Wang, Z. Huang, J. Wang, A. K. Singh and A. Lei, Chem. Rev., 2017, 117, 9016–9085 CrossRef CAS; (c) S. Roslin and L. R. Odell, Eur. J. Org. Chem., 2017, 1993–2007 CrossRef CAS; (d) H. Matsubara, T. Kawamoto, T. Fukuyama and I. Ryu, Acc. Chem. Res., 2018, 51, 2023–2035 CrossRef CAS; (e) K. Duan, X. Yan, Y. Liu and Z. Li, Adv. Synth. Catal., 2018, 360, 2781–2795 CrossRef CAS.
- (a) J. C. Wu, L. B. Gong, Y. Xia, R. J. Song, Y. X. Xie and J. H. Li, Angew. Chem., Int. Ed., 2012, 51, 9909–9913 CrossRef CAS; (b) S. E. Denmark and A. J. Cresswell, J. Org. Chem., 2013, 78, 12593–12628 CrossRef CAS; (c) T. Markovic, P. R. D. Murray, B. N. Rocke, A. Shavnya, D. C. Blakemore and M. C. Willis, J. Am. Chem. Soc., 2018, 140, 15916–15923 CrossRef CAS; (d) R. R. Merchant, J. T. Edwards, T. Qin, M. M. Kruszyk, C. Bi, G. Che, D.-H. Bao, W. Qiao, L. Sun, M. R. Collins, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, P. Nuhant and P. S. Baran, Science, 2018, 360, 75–80 CrossRef CAS; (e) J. M. E. Hughes and P. S. Fier, Org. Lett., 2019, 21, 5650–5654 CrossRef CAS; (f) P. Chatelain, A. Sau, C. N. Rowley and J. Moran, Angew. Chem., Int. Ed., 2019, 58, 14959–14963 CrossRef CAS; (g) J. I. Fukuda, K. Nogi and H. Yorimitsu, Org. Lett., 2019, 21, 8987–8991 CrossRef CAS; (h) L. Gong, H. B. Sun, L. F. Deng, X. Zhang, J. Liu, S. Yang and D. Niu, J. Am. Chem. Soc., 2019, 141, 7680–7686 CrossRef CAS.
- (a) M. Nambo and C. M. Crudden, Angew. Chem., Int. Ed., 2014, 53, 742–746 CrossRef CAS; (b) M. Nambo, E. C. Keske, J. P. G. Rygus, J. C. H. Yim and C. M. Crudden, ACS Catal., 2017, 7, 1108–1112 CrossRef CAS; (c) Z. T. Ariki, Y. Maekawa, M. Nambo and C. M. Crudden, J. Am. Chem. Soc., 2018, 140, 78–81 CrossRef CAS; (d) M. Nambo, Y. Tahara, J. C. H. Yim and C. M. Crudden, Chem. Eur. J., 2019, 25, 1923–1926 CrossRef CAS; (e) J. C. H. Yim, M. Nambo, Y. Tahara and C. M. Crudden, Chem. Lett., 2019, 48, 975–977 CrossRef CAS; (f) M. Nambo, J. C. H. Yim, L. B. O. Freitas, Y. Tahara, Z. T. Ariki, Y. Maekawa, D. Yokogawa and C. M. Crudden, Nat. Commun., 2019, 10, 4528 CrossRef; (g) Y. Maekawa, M. Nambo, D. Yokogawa and C. M. Crudden, J. Am. Chem. Soc., 2020, 142, 15667–15672 CrossRef CAS.
- (a) J. Rong, L. Deng, P. Tan, C. Ni, Y. Gu and J. Hu, Angew. Chem., Int. Ed., 2016, 55, 2743–2747 CrossRef CAS; (b) W. Fu, X. Han, M. Zhu, C. Xu, Z. Wang, B. Ji, X. Q. Hao and M. P. Song, Chem. Commun., 2016, 52, 13413–13416 RSC; (c) G. Zou and X. Wang, Org. Biomol. Chem., 2017, 15, 8748–8754 RSC; (d) H. Sun, Y. Jiang, Y. S. Yang, Y. Y. Li, L. Li, W. X. Wang, T. Feng, Z. H. Li and J. K. Liu, Org. Biomol. Chem., 2019, 17, 6629–6638 RSC; (e) B. Josephson, C. Fehl, P. G. Isenegger, S. Nadal, T. H. Wright, A. W. J. Poh, B. J. Bower, A. M. Giltrap, L. Chen, C. Batchelor-McAuley, G. Roper, O. Arisa, J. B. I. Sap, A. Kawamura, A. J. Baldwin, S. Mohammed, R. G. Compton, V. Gouverneur and B. G. Davis, Nature, 2020, 585, 530–537 CrossRef CAS.
- For recent examples in catalytic transformations using tetrazolyl sulfones as substrates see: (a) J. C. Lo, D. Kim, C. M. Pan, J. T. Edwards, Y. Yabe, J. H. Gui, T. Qin, S. Gutierrez, J. Giacoboni, M. W. Smith, P. L. Holland and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 2484–2503 CrossRef CAS; (b) K. Ohmatsu, T. Nakashima, M. Sato and T. Ooi, Nat. Commun., 2019, 10, 2706 CrossRef; (c) Y. Chen, N. McNamara, O. May, T. Pillaiyar, D. C. Blakemore and S. V. Ley, Org. Lett., 2020, 22, 5746–5748 CrossRef CAS.
- For selected examples in radical-mediated functionalization using alkyl allyl sulfone, see: (a) S. Kim and C. J. Lim, Angew. Chem., Int. Ed., 2002, 41, 3265–3267 CrossRef CAS; (b) S. Kim, S. Kim, N. Otsuka and I. Ryu, Angew. Chem., Int. Ed., 2005, 44, 6183–6186 CrossRef CAS; (c) N. Charrier and S. Z. Zard, Angew. Chem., Int. Ed., 2008, 47, 9443–9446 CrossRef CAS; (d) Y. Xia, L. Wang and A. Studer, Angew. Chem., Int. Ed., 2018, 57, 12940–12944 CrossRef CAS; (e) Y. Xia and A. Studer, Angew. Chem., Int. Ed., 2019, 58, 9836–9840 CrossRef CAS.
- For selected examples, see: (a) G. L. Lackner, K. W. Quasdorf and L. E. Overman, J. Am. Chem. Soc., 2013, 135, 15342–15345 CrossRef CAS; (b) M. Nagatomo, D. Kamimura, Y. Matsui, K. Masuda and M. Inoue, Chem. Sci., 2015, 6, 2765–2769 RSC; (c) T. Qin, L. R. Malins, J. T. Edwards, R. R. Merchant, A. J. Novak, J. Z. Zhong, R. B. Mills, M. Yan, C. Yuan, M. D. Eastgate and P. S. Baran, Angew. Chem., Int. Ed., 2017, 56, 260–265 CrossRef CAS; (d) X. Wu, W. Hao, K. Y. Ye, B. Jiang, G. Pombar, Z. Song and S. Lin, J. Am. Chem. Soc., 2018, 140, 14836–14843 CrossRef CAS; (e) B. Schweitzer-Chaput, M. A. Horwitz, E. de Pedro Beato and P. Melchiorre, Nat. Chem., 2019, 11, 129–135 CrossRef CAS.
- (a) G. Picotin and P. Miginiac, J. Org. Chem., 1987, 52, 4796–4798 CrossRef CAS; (b) M. C. P. Yeh, H. G. Chen and P. Knochel, Org. Synth., 1992, 70, 195 CrossRef CAS.
- (a) L. Zhu, R. M. Wehmeyer and R. D. Rieke, J. Org. Chem., 1991, 56, 1445–1453 CrossRef CAS; (b) S. Kudret, J. D'Haen, L. Lutsen, D. Vanderzande and W. Maes, Adv. Synth. Catal., 2013, 355, 569–575 CrossRef CAS.
- (a) Y. Yu, H. Chen, Q. Qian, K. Yao and H. Gong, Tetrahedron, 2018, 74, 5651–5658 CrossRef CAS; (b) Y. Ye, H. Chen, J. L. Sessler and H. Gong, J. Am. Chem. Soc., 2019, 141, 820–824 CrossRef CAS.
- H. Yi, A. Jutand and A. W. Lei, Chem. Commun., 2015, 51, 545–548 RSC.
- T. Liu, Y. Li, L. Lai, J. Cheng, J. Sun and J. Wu, Org. Lett., 2018, 20, 3605–3608 CrossRef CAS.
Footnotes |
| † This paper is dedicated to the memory of Professor Victor Snieckus. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc00133g |
| This journal is © The Royal Society of Chemistry 2021 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: