A shish-kebab-like supramolecular polymer and its light-responsive self-assembly into nanofibers†
ChuanShuang
Chen
,
Pei
Huang
,
Hui
Pan
,
Meiwei
Qi
,
Qingsong
Xu
,
Haojie
Dai
,
Yi
Ji
,
Yuling
Wang
,
Chunyang
Yu
 and
YongFeng
Zhou
and
YongFeng
Zhou
 *
*
School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China. E-mail: yfzhou@sjtu.edu.cn
First published on 8th December 2020
Abstract
This work reports on the synthesis and light-responsive self-assembly of a shish-kebab-like supramolecular polymer (SKSP). The SKSP has a linear alternating sequence structure and can self-assemble into nanofibers in water. In addition, the fibers can transform into spherical micelles under UV light due to the trans-to-cis isomerization of the azobenzene groups.
In contrast to traditional polymers, the monomers or polymer segments in supramolecular polymers (SPs) are connected by directional and reversible noncovalent interactions, including electrostatic interactions, hydrogen bonds, host–guest recognition, van der Waals forces, coordination, π–π stacking, etc.1–6 In some aspects, SPs have shown intrinsic outstanding properties, such as a highly dynamic molecular structure, abundant stimuli-responsiveness and self-adjusting or self-healing characteristics, and the advantage of facile preparation over the traditional covalent polymers (CPs). As a result, SPs are very promising in applications such as optoelectronics, biomedical or self-healing materials.2,7–13 However, despite the progress, the topological structures of SPs are still insufficient when compared with a large number of CPs. Most of the reported SPs have only a linear structure, and the SPs with topological structures such as graft, block, hyperbranched, dendritic, star-shaped, and network structures are quite limited.14–21 It is still highly challenging to develop new SPs with unique topological structures.
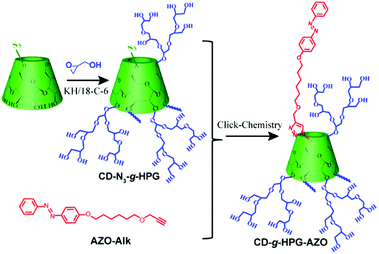
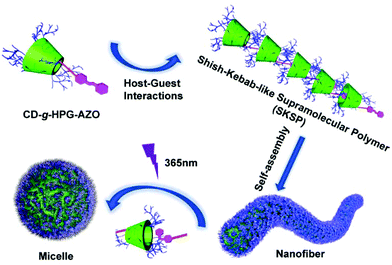
A shish-kebab-like (SK) structure is a typical structure in polymer crystals, nanohybrids, small molecule assemblies and some inorganic nanomaterials.22–29 However, to our knowledge, there are few reports on SPs with a SK structure, mainly due to the difficulty in synthesis. In our previous work, we reported linear-branched, Janus, dumbbell-like and dandelion-like SPs and studied their self-assembly behaviours.14–16,18 On the basis of these works, herein, we report the construction of a light-responsive shish-kebab-like supramolecular polymer (SKSP). As shown in Scheme 1, we first synthesized a functional molecule of β-cyclodextrin (CD) grafted with hyperbranched glycidyl ether (HPG) on the surface and one azobenzene (AZO) group at the end (CD-g-HPG-AZO). Then the SKSP was prepared through the noncovalent host–guest interaction between the CD and AZO groups of CD-g-HPG-AZOs (Scheme 2). It was found that such an amphiphilic SKSP could self-assemble into nanofibers in water, which transformed into micelles under UV light due to the trans-to-cis isomerization of the AZO groups (Scheme 2).
The synthesis of CD-g-HPG-AZO was based on the click-chemistry reaction between the alkynyl group of AZO-Alk and the azide group of CD-N3-g-HPG (Scheme 1).30 AZO-Alk was prepared in two steps. In the first step, 4-hydroxyazobenzene and 6-bromohexanol were reacted under the catalysis of potassium carbonate to give 6-hydroxyoxo-4-azobenzene (AZO-g-OH). Then, AZO-g-OH was reacted with bromopropyne under the catalysis of potassium hydroxide and a crown ether to form 6-(propynyloxy)hexyloxy-4-azobenzene (AZO-Alk) (Fig. S1–S6, ESI†). CD-N3-g-HPG (Mn, GPC = 1800 Da, Mn, NMR = 5000 Da, DPn = 58, Mn/Mw = 1.4) was synthesized by an anionic ring-opening multibranching polymerization method reported by Frey, using CD-N3 as a multi-hydroxyl initiator together with the slow addition of the glycidol monomer, and the detailed synthesis procedure was also modified based on our previous work (Fig. S7–S11, ESI†).15,16,31–33 The detailed synthesis and characterization of final CD-g-HPG-AZO are summarized in the ESI (Scheme S1, Fig. S12 and 13, ESI†).
The host–guest complexation between the hydrophobic AZO group and CD-g-HPG of CD-g-HPG-AZOs was characterized by 2D-NOESY 1H NMR spectroscopy (Fig. S14†). The intermolecular correlations between the H3 and H5 protons in the inner cave of CDs and the protons of AZO groups provide direct evidence to support the complexation of CD-g-HPG and the AZO group of CD-g-HPG-AZOs through the specific AZO/CD host–guest interactions. In addition, 1H NMR titration experiments were carried out to explore the host–guest complexation between CD and AZO groups by the sequential addition of CD-g-HPG into the sodium 4-phenylazophenol (AZO-ONa)/D2O solution.15,34 The proton signals of AZO-ONa shifted to a low field with increasing concentration of CD-g-HPG (Fig S15a, ESI†), which indicated the formation of the inclusion complex between CD-g-HPG and AZO-ONa through CD/AZO specific host–guest recognition. According to the Benesi–Hildebrand equation, the average binding constant (Kc) between CD-g-HPG and AZO-ONa was calculated to be 1412 M−1 (Fig S15b†), which was smaller than the typical Kc between β-CD and AZO (∼104), mainly because the graft HPG weakened the interaction between CD and AZO groups.35
The supramolecular polymerization process was further investigated by diffusion ordered spectroscopy (DOSY) NMR.36 The H3 and H5 proton signals of CD-g-HPG-AZOs varied in different solutions (Fig. S16a, ESI†). The diffusion coefficient (D) of CD-g-HPG-AZOs in DMF-d7 (4.5 × 10−10 m2 s−1) was higher than that in the D2O/DMF-d7 (10 v% D2O) mixed solvent (2.9 × 10−10 m2 s−1), indicative of a larger hydrodynamic radius formed when D2O was added to trigger the host–guest interactions between CD-g-HPG-AZOs (Fig. S16b, ESI†). According to a previous report,37 the degree of supramolecular polymerization of CD-g-HPG-AZOs was estimated roughly to be 4. The detailed characterization and calculations are presented in the ESI (Fig. S16, ESI†).
In addition, dynamic light scattering (DLS) measurement was also used to track the supramolecular polymerization process. Firstly, CD-g-HPG-AZOs were dissolved in DMF, and then water was added dropwise into the solution to induce host–guest recognition to form supramolecular polymers. In the DLS measurement, the samples with different water contents had unimodal size distributions (Fig. 1a) and the number-average hydrodynamic diameters (Dh) increased with the water content (Fig. 1b). There seemed to be three stages in the Dhversus water content plot (Fig. 1b). In the first stage (water content: from 0% to 5%), the Dh was hardly changed and remained at 3.5 nm due to the good solubility of CD-g-HPG-AZOs in the water/DMF cosolvent. In other words, CD-g-HPG-AZOs were maintained as unimolecular micelles in this stage. In the second stage (water content: from 5% to 10%), a gradual increase of the Dh from 3.5 nm to 17.7 nm was observed, indicating the growth of SKSPs driven by the AZO/CD host–guest interactions.
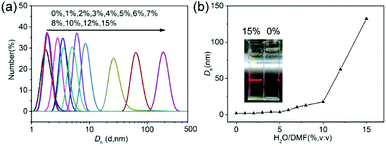 | ||
| Fig. 1 Complexation and self-assembly of SKSPs. (a) DLS curves of CD-g-HPG-AZOs at various contents of H2O in DMF. (b) Dependence of Dh on the water content for CD-g-HPG-AZOs. | ||
Interestingly, with the further addition of water, the self-assembly of SKSPs occurred. As shown in Fig. 1b, with an increase of the water content from 10% to 15%, the Dh of aggregates in solution increased sharply from 17.7 nm to 132.4 nm, indicating the formation of supramolecular structures in this third stage. As a further support, the solution turned turbid showing the Tyndall effect, which also proved the formation of large aggregates (inset in Fig. 1b). To obtain stable self-assemblies, the CD-g-HPG-AZO solution with a water–DMF volume ratio of 15% (v/v) was dialyzed against water to remove DMF (MWCO: 3500 Da), and a final solution with a polymer concentration of 0.1 mg mL−1 was obtained. The self-assemblies of SKSPs were characterized by TEM, AFM and SEM measurements (Fig. 2). The TEM image in Fig. 2a shows that the self-assemblies are nanofibers. Interestingly, in the enlarged figure (Fig. 2b), it can be seen that nanofibers have a hierarchical stacking structure. The nanofibers were further characterized by AFM and SEM (Fig. 2c and d), which further confirmed the nanofiber morphology in TEM. The stacking structure in the nanofibers was also proved by AFM (inset in Fig. 2c). Unfortunately, we still do not understand the molecular packing mechanism in these nanofibers. Both the synchrotron small angle X-ray scattering and wide-angle X-ray diffraction measurements on the nanofiber aqueous solutions were performed; however, no useful information was obtained.
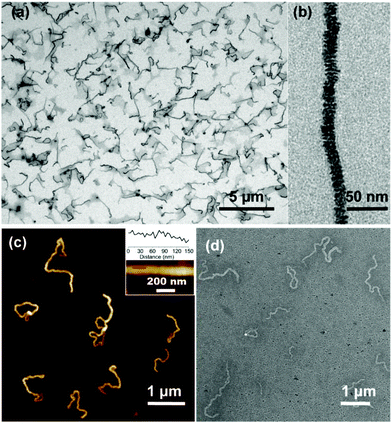 | ||
| Fig. 2 Characterization of nanofibers. (a and b) The TEM images of the nanofibers. (c) The AFM image of the nanofibers. (d) The SEM image of the nanofibers. | ||
In addition, the competitive guest molecule 1-adamantanamine hydrochloride (AD), which has a stronger binding ability than AZO, was added to the nanofiber solution (Fig. S17a, ESI†). The Dh of the assemblies became smaller and the PDI of the assemblies became larger according to the DLS measurement (Fig. S17b, ESI†). Besides, the TEM images showed that the assemblies transformed into small-sized nanoparticles or irregular fragments after adding AD molecules (Fig. S17c, ESI†). These results verified that the CD-g-HPG-AZO nanofibers could be destroyed by the stronger and more competitive AD/CD host–guest recognition, which provided further evidence to support that the formation of supramolecular polymers was driven by CD/AZO interaction.
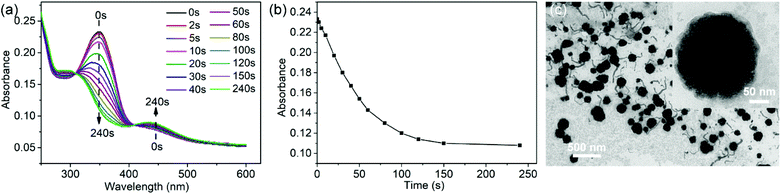
Under UV light irradiation, the nanofibers could further undergo the disassembly process. Only trans-AZO can be inserted into the cavity of β-CD to form a host–guest complex, while cis-AZO cannot.38,39 Thus, the photoisomerization of AZO groups will lead to the disassembly of nanofibers. As expected, with the increase of UV (365 nm) irradiation time, the peak of trans-AZO (λ = 350 nm) gradually weakened until almost disappeared, while the peak of cis-AZO (λ = 450 nm) gradually increased (Fig. 3a and b), which triggered the decomposition of SKSPs due to the disassociation of CD/AZO complexation. As a result, after irradiation with UV light, the nanofibers gradually transformed into spherical micelles. As shown in Fig. 3c, the TEM image displays some intermediates between nanofibers and nanospheres, and some nanofibers were attached with nanospheres, indicating the occurrence of nanofiber-to-nanosphere transition, which has seldom been observed in the self-assembly of supramolecular polymers. We had thought to use visible light to trigger the reversible transition from nanospheres to nanofibers. Unfortunately, it did not work, although the cis-to-trans transition of AZO groups did occur during the time (Fig. S18, ESI†). The irreversible morphology transformation from nanofibers to nanospheres indicates that the nanofiber is supposed to be kinetically trapped and the its formation relies on the pathway of preparation.
In conclusion, a shish-kebab-like supramolecular polymer was prepared through the noncovalent host–guest interaction. The obtained supramolecular polymers can self-assemble into nanofibers in water, which can transform into spherical micelles under irradiation of UV light. Such a new supramolecular polymer structure as well as the unique self-assembly behaviour will expand the category of supramolecular polymers, and might find application in smart supramolecular materials with dynamic morphology transitions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21890730, 21890733 and 51773115) and the Program for Basic Research of Shanghai Science and Technology Commission (19JC1410400).Notes and references
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071–4098 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687–5754 CrossRef CAS PubMed.
- S. Dong, B. Zheng, F. Wang and F. Huang, Acc. Chem. Res., 2014, 47, 1982–1994 CrossRef CAS PubMed.
- X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC.
- L. Yang, X. Tan, Z. Wang and X. Zhang, Chem. Rev., 2015, 115, 7196–7239 CrossRef CAS PubMed.
- K. Peng, I. Tomatsu and A. Kros, Chem. Commun., 2010, 46, 4094–4096 RSC.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
- R. Dong, Y. Su, S. Yu, Y. Zhou, Y. Lu and X. Zhu, Chem. Commun., 2013, 49, 9845–9847 RSC.
- J. Fox, J. J. Wie, B. W. Greenland, S. Burattini, W. Hayes, H. M. Colquhoun, M. E. Mackay and S. J. Rowan, J. Am. Chem. Soc., 2012, 134, 5362–5368 CrossRef CAS PubMed.
- X. Zhang, L. Wang, J. Xu, D. Chen, L. Shi, Y. Zhou and Z. Shen, Acta Polym. Sin., 2019, 50, 973–987 CAS.
- P. Chen, Y. Zhou and J. Yang, Chem. Commun., 2017, 53, 1144–1147 RSC.
- J. Yang, J. I. Song, Q. Song, J. Y. Rho, E. D. H. Mansfield, S. C. L. Hall, M. Sambrook, F. Huang and S. Perrier, Angew. Chem., 2020, 59, 8860–8863 CrossRef CAS PubMed.
- D. Zhang, Y. Fan, H. Li, K. Li, Y. Yao, Y. Zhou and D. Yan, RSC Adv., 2015, 5, 47762–47765 RSC.
- W. Tao, Y. Liu, B. Jiang, S. Yu, W. Huang, Y. Zhou and D. Yan, J. Am. Chem. Soc., 2012, 134, 762–764 CrossRef CAS PubMed.
- Y. Liu, C. Yu, H. Jin, B. Jiang, X. Zhu, Y. Zhou, Z. Lu and D. Yan, J. Am. Chem. Soc., 2013, 135, 4765–4770 CrossRef CAS PubMed.
- G. Yu, X. Zhao, J. Zhou, Z. Mao, X. Huang, Z. Wang, B. Hua, Y. Liu, F. Zhang, Z. He, O. Jacobson, C. Gao, W. Wang, C. Yu, X. Zhu, F. Huang and X. Chen, J. Am. Chem. Soc., 2018, 140, 8005–8019 CrossRef CAS PubMed.
- D. Zhang, Y. Liu, Y. Fan, C. Yu, Y. Zheng, H. Jin, L. Fu, Y. Zhou and D. Yan, Adv. Funct. Mater., 2016, 26, 7652–7661 CrossRef CAS.
- X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362–369 CrossRef CAS PubMed.
- A. Harada, Y. Takashima and H. Yamaguchi, Chem. Soc. Rev., 2009, 38, 875–882 RSC.
- L. Voorhaar and R. Hoogenboom, Chem. Soc. Rev., 2016, 45, 4013–4031 RSC.
- S. Kimata, T. Sakurai, Y. Nozue, T. Kasahara, N. Yamaguchi, T. Karino, M. Shibayama and J. A. Kornfield, Science, 2007, 316, 1014–1017 CrossRef CAS PubMed.
- J. K. Hobbs and M. J. Miles, Macromolecules, 2001, 34, 353–355 CrossRef CAS.
- N. Ning, F. Luo, B. Pan, Q. Zhang, K. Wang and Q. Fu, Macromolecules, 2007, 40, 8533–8536 CrossRef CAS.
- C. Y. Li, L. Li, W. Cai, S. L. Kodjie and K. K. Tenneti, Adv. Mater., 2005, 17, 1198–1202 CrossRef CAS.
- J. Yang, C. Wang, K. Wang, Q. Zhang, F. Chen, R. Du and Q. Fu, Macromolecules, 2009, 42, 7016–7023 CrossRef CAS.
- H. Jiang, Y. Jiang, J. Han, L. Zhang and M. Liu, Angew. Chem., Int. Ed., 2019, 58, 785–790 CrossRef CAS PubMed.
- J. Hu, A. Liu, H. Jin, D. Ma, D. Yin, P. Ling, S. Wang, Z. Lin and J. Wang, J. Am. Chem. Soc., 2015, 137, 11004–11010 CrossRef CAS PubMed.
- M. Qi, Y. Liu and Y. Zhou, Acta Chim. Sin., 2020, 78, 528–533 CrossRef.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed.
- D. Wilms, F. Wurm, J. Nieberle, P. Böhm, U. Kemmer-Jonas and H. Frey, Macromolecules, 2009, 42, 3230–3236 CrossRef CAS.
- A. Sunder, R. Hanselmann, H. Frey and R. Mülhaupt, Macromolecules, 1999, 32, 4240–4246 CrossRef CAS.
- D. Wilms, S.-E. Stiriba and H. Frey, Acc. Chem. Res., 2010, 43, 129–141 CrossRef CAS PubMed.
- K. Ohga, Y. Takashima, H. Takahashi, Y. Kawaguchi, H. Yamaguchi and A. Harada, Macromolecules, 2005, 38, 5897–5904 CrossRef CAS.
- M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875–1918 CrossRef CAS PubMed.
- E. O. Stejskal and J. E. Tanner, J. Chem. Phys., 1965, 42, 288–292 CrossRef CAS.
- T. F. Al-Azemi and M. Vinodh, Polym. Chem., 2020, 11, 3305–3312 RSC.
- R. J. Dong, B. S. Zhu, Y. F. Zhou, D. Y. Yan and X. Y. Zhu, Polym. Chem., 2013, 4, 912–915 RSC.
- X. Liao, G. Chen, X. Liu, W. Chen, F. Chen and M. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4409–4413 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0py01396j |
| This journal is © The Royal Society of Chemistry 2021 |