 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hierarchical ordering in light-triggered additive manufacturing
Joël
Monti
a and
Eva
Blasco
 *abc
*abc
aInstitute of Nanotechnology, Karlsruhe Institute of Technology (KIT), Hermann-von-Helmoltz-Platz 1, 76128 Karlsruhe, Germany. E-mail: eva.blasco@kit.edu
bOrganisch-Chemisches Institut, University of Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg
cCentre for Advanced Materials, University of Heidelberg, Im Neuenheimer Feld 225, 69120 Heidelberg. E-mail: eva.blasco@oci.uni-heidelberg.de
First published on 13th October 2020
Abstract
Additive manufacturing (AM) emerged, in the last decades, as a promising manufacturing technique for the low-cost fabrication of personalised 3D objects. Recently, this technology has been increasingly utilised, both in everyday life and in industry. Among the numerous AM techniques available, light-triggered AM allows for great shape resolution and a smooth surface, while being a relatively fast process. The increasing interest given to AM naturally motivated the development of novel 3D printable materials in order to keep on widening the possibilities of the techniques. Herein, we present an overview of the materials having been recently reported in the field, for which the incorporation of hierarchical ordering was essential to access novel functionalities and properties. In particular, hybrid materials such as fibre- and nanocrystal-reinforced polymeric networks, liquid crystalline materials and metal–organic framework embedded printable materials along with their future prospects will be discussed.
1. Introduction
3D printing, or more generally additive manufacturing (AM), has gained, in the last years, an exponentially increasing popularity in everyday life.1 Indeed, this bottom-up, computer-aided approach of fabrication is one of the choices for the fast and cost-effective prototyping of custom pieces. Moreover, structures with complex geometries are, in specific cases, only accessible by AM. Hence, not only can AM provide a powerful prototyping platform for development and design, but also it opens new opportunities for the fabrication of novel 3D structures. The current use of AM across disparate disciplines including aerospace,2,3 automotive,4,5 and food industries,6 medicine,7 and others8 is a clear proof of the vast impact of these technologies in modern fabrication processes.Among all the materials utilised in AM, soft materials, and especially polymer-based inks, are part of the most promising printable materials due their excellent versatility and adaptability.9 Common AM techniques for polymeric materials include powder bed fusion, material and binder jetting, fused deposition modelling, direct ink writing and vat photo-polymerisation. In recent years, various comprehensive reviews highlighting the state of the art in the field have been published.10 When focussing on truly and defined three dimensional AM, stereolithography (SLA), digital light processing (DLP), and more recently, continuous liquid interface production (CLIP) as well as 3D laser lithography based on multi-photon polymerisation are examples of technologies directly based on photochemical processes.
First patented by Hull et al. in 1986,11 stereolithography (SLA) is known to be the original 3D-printing technology.12,13 Together with DLP, SLA is part of the more general class of “vat photopolymerisation processes”. These techniques produce, layer by layer, 3D objects by the photopolymerisation of a photoresist disposed within a vat. The difference among them relies on the light source used. While SLA uses a low cross-section laser source which is scanned pixel by pixel throughout each layer using galvanometers for laser deviation,14,15 DLP uses a digital light projector screen as the light source, allowing the printing of an entire layer at once.16 As a result, DLP is a faster process than SLA. Both techniques allow the fabrication of pieces with high definition and surface smoothness, hence not requiring mechanical post-processing of the surface.17 Typically, resolutions between 25 and 100 μm (depending on the technique and printer) can be achieved.9,18,19 Notwithstanding, they are facing several limitations: (i) the piece obtained straight after printing generally needs to be cured under UV-irradiation, in order to increase the crosslinking density, thus to enhance the material properties of the piece.20 (ii) Even after post-printing UV-curing, vat photopolymerisation processes generally produce brittle materials, with material properties that are not suitable for the construction of pieces purposed to endure strong mechanical stresses.20–22 (iii) Supporting structures have to be printed together with the targetted structure to ensure a good anchoring to the stage of the printing device.23 Those have to be mechanically removed to isolate the final piece; anchor points are then likely to still be visible at the end of the process. Considering these features, conventional vat photopolymerisation processes are particularly well-adapted to applications in which high shape-definition prevails over mechanical strength, such as prototyping or the fabrication of moulds.24–27
More recently, continuous liquid interface production (CLIP) has emerged as a promising AM technique. This approach invented by DeSimone28,29 exploits the oxygen inhibition noticeably observed for the free-radical polymerisation of acrylates, to continuously leave the cross-linked part dipped in the fluid photoresist.30–32 Thanks to oxygen-inhibition, the deepest layer of the photoresist in the vat always consists of a non-crosslinked material, allowing the photopolymerisation to occur, in upper layers, at the interface of the cross-linked polymer and the oxygen-rich fluid photoresist. Moreover, due to the continuous printing, CLIP limits the impact on the material properties of the inter-layer junctions, creating pieces which behave consistently in all directions, contrary to conventional AM techniques.33
Moving to the micro- and nanoregime, 3D laser lithography based on two-photon polymerisation enables sub-micron resolution. In this technique, the use of femtosecond lasers is essential for making two-photon absorption processes sufficiently probable. During the last years, 3D laser printing is being established as a suitable approach for the fabrication of functional 3D micro- and nanostructures.34–39
Light-triggered AM relies on the formation of stable polymeric networks from a liquid monomer mixture using a light source. In particular, three photocrosslinking strategies are being commonly used: (i) free-radical polymerisation of vinyl monomers and cationic polymerisation of epoxides; (ii) thiol–ene(yne) free-radical polymerisation and (iii) photodimerisation reactions.
Methacrylate-containing monomers (in the presence of a photoinitiator40–46) are, nowadays, the most broadly used inks in the context of light-triggered AM.47 One of the main reasons is the wide variety of mono- and multi-functional meth(acrylates) that can be directly purchased. Furthermore, for customised synthetic photoresists, functional (meth)acrylate derivatives are particularly popular as they can be easily introduced within a chemical structure via addition under basic conditions using (meth)acryloyl chloride,48–51 or via esterification52,53 using acrylic acid.
Cationic ring-opening of multifunctional epoxide resins containing a photoacid generator as a trigger of the photocrosslinking is also employed in 3D printing. Epoxide monomers have some advantages over (meth)acrylates, noticeably regarding the oxygen insensitivity of the cationic photocrosslinking of epoxides, the lower shrinkage and greater strength of the final materials.54–56 However, the less straightforward synthesis of monomers and the reduced popularity are important shortcomings of epoxy resins when compared to (meth)acrylates.57
Thiol–ene(yne) photopolymerisation has been extensively exploited in the field, too. In addition to having the kinetic and thermodynamic features associated with the free-radical polymerisation mechanism, it also depicts specific advantages:58 thiol–ene(yne) shows a decent insensitivity to oxygen quenching, a noticeably less prominent polymerisation-caused shrinking than the free-radical polymerisation of acrylates59–61 and involves non-toxic chemicals.62 As a result, the thiol–ene coupling is popular for the 3D printing of elastomers and bio-compatible elastomers or hydrogels; these materials are of great interest for tissue engineering, drug delivery technologies and in vivo processes.62–66 However, the final material usually exhibits a rather low crosslinking density.67 Besides, the elaboration, based on thiol–ene(yne) photopolymerisation, of materials with a higher cross-linking density has also been investigated.58–62,65,67–69
Photodimerisation reactions have also been successfully used either to fabricate films under UV-irradiation70–72 or for 3D printing. Unlike photopolymerisation reactions, the photoinduced dimerisation reaction does not require the presence of a photoinitiator since the reaction is self-initiated by the functional group. On the other hand, because dimerisation reactions do not proceed through a living propagation mechanism, photons are consumed in stoichiometric amounts. The [2 + 2] photodimerisation of coumarins (λmax ≈ 340 nm) is one of the most prominent examples of such systems.73–76 Interestingly, the photo-induced dimerisation of amino acids containing a photooxidisable group, such as Trp, His, Tyr, Cys and Met, has also been used for 3D-printing.77–84
The photocrosslinking methods described above lead to the formation of 3D printed polymeric structures composed of, in most cases, an undefined internal structure due to the lack of control at the molecular level during the polymerisation processes. However, having a look at active natural materials (muscles, tendons, proteins, etc.), all of them exhibit ordering from the molecular to microscale. This structural hierarchy plays a crucial role in their performance in terms of mechanical properties and/or actuation. Therefore, the introduction of ordering in new functional printable materials is of outstanding interest in the field of AM.
Herein, we will discuss recent developments in the 3D printing of polymeric materials with structural hierarchy and their applications. By increasing the extent of ordering (anisotropy), selected recent examples of 3D printable hybrid materials and self-assembled materials, (i) liquid–crystalline materials and (ii) metal–organic frameworks (MOFs), will be presented, Fig. 1. In addition, future prospects and viewpoints for the incorporation of hierarchical order in materials for light-triggered AM will be discussed.
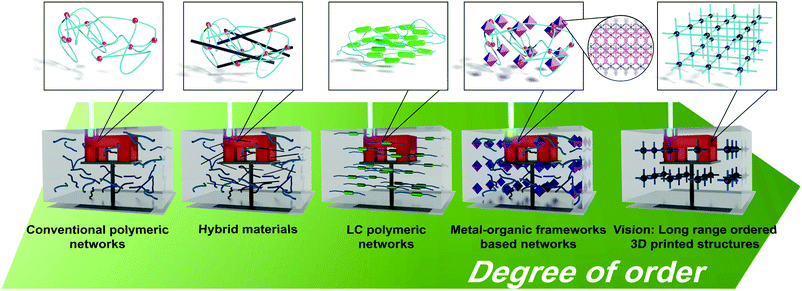 | ||
| Fig. 1 Schematic representation of 3D printable materials used in light-triggered AM. From left to right (increasing degree of order), conventional polymeric networks, hybrid materials, liquid crystalline polymeric networks, networks containing MOF particles and a visual representation of a perfectly ordered MOF obtained after photocrosslinking. Polymers and their precursors are drawn in light blue, crosslinking points are represented as red spheres, microfibers as black rods, the LC mesogens as light green rods, MOF particles as purple octahedra and metal centres of the MOF as black spheres. The structure of Cu-BTB MOF was computed using crystallographic data from ref. 85, ccdc deposition nbr 708312. | ||
2. Hybrid materials
The final brittleness of the printed structures produced by light-triggered AM using conventional polymer-based inks is undeniably one of the main challenges that should be faced to allow a wider spreading of light-triggered AM technologies for the manufacturing of mechanically strong pieces. As discussed above, a control of the internal structure enables the fabrication of structures exhibiting higher performances. Hybrid materials furnish a conceptually simple alternative to this issue.86Hybrid (composite) materials refer, for polymeric matrices, (i) to designs making use of functional moieties of at least two different types. In such systems and in the context of stereolithography, two different monomeric functional groups are introduced in the photoresist. While a photopolymerisable group is used to enable the 3D printing of the material, a second functionalisation, innocent during the photoprinting process, is activated during a post-printing curing (thermal treatment, pyrolysis, chemical treatment, etc.), and provides the final material its specific characteristics. Chemically, while the photoresponsive functionalisation usually consists in (meth)acrylate groups, a broad variety of compounds can be used in post-printing cured functionalisation. Indeed, it can for example consist of a functional monomeric group of a different type, leading, by the polymerisation of the second polymeric backbone during curing, to highly crosslinked and highly entangled dual polymeric networks,87 or can consist of a ceramic from which a glassy or metallic material can be recovered through post-printing curing.88–94 (ii) Hybrid polymeric materials can also refer to matrices finally containing at least two microphases of different types, as, for example, to mechanically strong fibres (such as glass fibres, carbon fibres, or nanocellulose) dispersed in a polymeric matrix,95–101 or to nanocrystalline moieties covalently embedded within the final structure of the polymeric material.
In this section, we focus on a hybrid system for which the property enhancement arises (i) from the embedding of highly ordered nanocrystalline phases which do not show any particular ordering on the macroscale and (ii) the alignment of microfibers on the macroscale. Also, we will present some hybrid materials which were successfully employed in the formulation of photoresists for light-triggered AM. For readers interested in further details about mechanically reinforced composite materials for AM from a broader point-of-view, we recommend other dedicated review works.35,99,100,102–104
2.1. Cellulose-based hybrid materials
Cellulose is of particular interest as it can be obtained from renewable sources and takes part in the development of green chemical processes. Cellulose is obviously known for its material reinforcement abilities, as it is in nature, among other compounds, responsible for the mechanical strength of wood and other vegetal tissues.Crystalline cellulose exists as cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs) or microfibrillated cellulose (MFC), with increasing particle dimensions, from (diameter × length) 100–101 nm × 102–103 nm for CNCs to 101–102 nm × μm for MFC.105,106 MFC and CNFs, most likely due to the bigger particle size, are only employed in the context of material extrusion AM, while research on cellulose-based inks for light-triggered AM usually employs CNCs.107,108 Cellulose-based photoresists consist of either of the photopolymerisable matrices containing additive CNCs, yielding crosslinked materials with embedded CNCs after photoprinting. In this case, the CNC particles interact with the surrounding matrix through non-covalent interactions. Also, they can consist of CNC cores, decorated with functional groups that are non-innocent with respect to photopolymerisation, yielding materials where CNCs are covalently introduced in the polymeric structure through photocrosslinking. Although more synthetically challenging, this last strategy leads to materials depicting greater mechanical, thermal and chemical resistance.
Palaganas et al.109 detailed the effect of the reinforcement of polyethylene glycol diacrylate (PEGDA) with CNCs (Fig. 2I), introduced as additives with loadings ranging from 0 wt% to 1.2 wt%. Crosslinked specimens were obtained by SLA with a 50 μm resolution, and characterised noticeably by their mechanical properties. As a general trend, it was found that increasing CNC loading gradually enhances the material properties of the composite up to a threshold at around 0.3 wt% loading. On the other hand, increasing the CNC loading to 0.5 wt%, 0.9 wt% and 1.2 wt% had, overall, a negative effect upon the materials properties. The optimal fracture energy obtained in the context of this study (ca. 3600 J m−2) was higher than that of natural cartilage (1102 ± 136 J m−2)110 or than that of a similar material printed by direct ink writing (DIW) (1500 J m−2).111
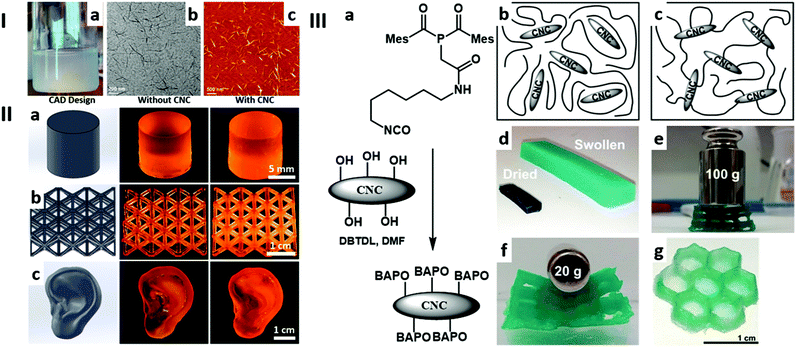 | ||
| Fig. 2 Examples of cellulose containing 3D printed materials. I: (a) CNCs redispersed in water. (b) TEM and (c) AFM images showing CNCs with diameters of 3 ± 1 nm and lengths of 246 ± 100 nm. Adapted from ref. 109 with permission from the American Chemical Society, Copyright © 2017. II: (a) A disk structure, (b) an octet-truss lattice structure, and (c) an ear model. In the first column are shown the SolidWorks designs; in the second column, the DLP 3D printed structures that are printed from the matrix resin without any CNC addition; and in the third column the corresponding 3D structures that are printed from 1 wt% CNCs incorporated into the matrix resin. Adapted from ref. 106 with permission from Springer Nature, Copyright © 2019. III: (a) Synthesis of CNC-BAPO by urethane bond formation, Mes = mesityl = 2,4,6-Me3C6H2. Schematic representation of the CNC/polymer composite after photopolymerisation for (b) neat CNCs and (d) CNC-BAPO. (d) Flat specimen generated from CNC-BAPO (7 wt%). (e) A dried cubic–lattice structure from CNC-BAPO (7 wt%) and (f) the same structure after swelling for 1 h. (g) A swollen hexagonal structure from CNC-BAPO (14 wt%). Adapted from ref. 112 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright © 2018. | ||
By using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PEGDA
1 PEGDA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3-diglyceroate diacrylate (DiGlyDA) matrix, Li et al.106 were able to obtain photoresists for SLA with suitable viscosity while having a CNC loading up to 5 wt%. UV-vis transmittance analysis highlighted that the presence of DiGlyDA enhances the dispersibility of CNCs in the matrix, by introducing a competing H-bonding species that limits the agglomeration of CNCs. However, although the loading threshold for optimal mechanical properties was higher in this report than in previous studies,109 the optimal material properties were still observed with CNC loadings lower than 1 wt%. The printed 3D structures investigated in this work are shown in Fig. 2II.
1,3-diglyceroate diacrylate (DiGlyDA) matrix, Li et al.106 were able to obtain photoresists for SLA with suitable viscosity while having a CNC loading up to 5 wt%. UV-vis transmittance analysis highlighted that the presence of DiGlyDA enhances the dispersibility of CNCs in the matrix, by introducing a competing H-bonding species that limits the agglomeration of CNCs. However, although the loading threshold for optimal mechanical properties was higher in this report than in previous studies,109 the optimal material properties were still observed with CNC loadings lower than 1 wt%. The printed 3D structures investigated in this work are shown in Fig. 2II.
Wang et al.112 used a different approach, where they functionalised the surface of CNCs with Bis(acyl)phosphane oxides (BAPOs) photoinitiating functional groups (Fig. 2IIIa). The decorated CNC (CNC-BAPO) cores were further able to initiate the photocrosslinking of polyethylene glycol methyl ether methacrylate (PEGMEM), leading to a hydrogel in which CNCs are covalently introduced in the polymeric matrix (Fig. 2IIIc). The effective inclusion of the CNC cores within the polymeric structure was supported by the insolubility of these materials in water, contrarily, the hydrogel containing neat CNCs as additives (Fig. 2IIIb) or the pure PEGMEM hydrogel totally disintegrated while sonication for 1 min after 1 d of exposure to an aqueous medium. The Young's modulus of the material was characterised as a function of CNC-BAPO loading, which showed a Young's modulus of 270 ± 10 MPa and 340 ± 25 MPa for CNC-BAPO loadings of 3.27 wt% and 6.14 wt%, respectively. In comparison, the pure PEGMEM hydrogel and the hydrogel with neat CNCs (2.54 wt%) showed Young's moduli of 130 ± 38 MPa and 272 ± 20 MPa, respectively. The materials showed reversible swelling capabilities of up to 1100%, with great shape-integrity. Finally, the studied hydrogels were successfully used as photoresists for DLP, without a significant loss in mechanical performances compared to the cast samples previously characterised, Fig. 2IIId–g.
Pure cellulose-based photoresists have also been transferred to two-photon 3D laser printing by Rothammer et al.113 In their work, the authors used methacrylated cellulose diacetate, as a solution in acetone (800 wt%), which was successfully printed at 780 nm with a resolution of 750 nm and feature size of 430 ± 40 nm in the lateral direction. This is, to the best of our knowledge, the only example of a cellulose-based photoresist for two-photon 3D laser printing.
2.2. Hybrid materials based on carbon nanotubes (CNTs)
Already observed and described from the 40s,114–116 CNTs are pictorially needle-shaped rolls of graphene-sheets, which can be produced as multiple wall carbon nanotubes (MWCNTs) or as single wall carbon nanotubes (SWCNTs). Due to their outstanding intrinsic properties, noticeably regarding their electrical and thermal conductivity as well as their tensile strength117 and light weight, carbon nanotubes (CNTs) are of remarkable interest for the development of novel materials.118 With AM fabricated CNT/polymer composite materials having unequalised potential for the aerospace industry,119 medical purposes,120 as 4D materials,121etc. Effectively, CNT/polymer composite materials address the issue of the brittleness of pieces produced by AM, but also allow the fabrication of polymeric materials with unprecedented electrical and thermal conductivity. Moreover, the introduction of anisotropy within such materials can lead to a directional amplification of these properties.CNT/polymer composites have been successfully 3D printed employing material extrusion AM techniques. Those offer several advantages for the fabrication of CNT/polymer composite materials; noticeably, (i) the setup is usually more simple than phototriggered AM. (ii) CNTs are easily aligned by the shearing force applied by the nozzle. (iii) The issue arising from the light-scattering of the CNTs does not have to be addressed, thus allowing greater CNT loadings. Notwithstanding, the homogeneous dispersion of CNTs within the polymer matrix becomes then limiting and usually does not permit loadings greater than ca. 5 wt%.122 In addition, CNTs tend to damage the extrusion nozzle.123 Light-triggered AM offers, as presented previously, the opportunity to fabricate pieces with better surface resolution and smoothness as well as the opportunity to fabricate pieces on the microscale. Hence, there is still an important potential of CNT composite materials in this context. To mention some results, the electrical conductivity of a PEGDA:PEGMEM matrix went from 2.00 10−11 S m−1 to 2.00 10−7 S m−1 for CNT loadings ranging from 0 wt% to 0.5 wt%, respectively.124 Furthermore, electrical conductivities up to 0.025 S m−1 were measured for a 0.3 wt% MWCNT/acrylate matrix composite material.125 SWCNT/polymer composite photoresists were also successfully implemented in two-photon 3D laser printing with a definition of ca. 200 nm,126 and were evidenced, by Raman spectroscopy, to be self-aligned during the printing process.127
In 2016, Xiong et al.128 performed an extensive study of MWCNT/polymer composite materials for two-photon 3D laser printing, Fig. 3Ia–c. Noticeably, the authors evidenced the self-alignment of MWCNTs under the influence of the laser beam irradiation. This alignment was proposed to arise from the strong electric field locally generated by the MWCNTs when irradiated with a high-intensity fs-laser beam.122 Thanks to the enhanced shape integrity and mechanical resistance provided by the alignment of the CNTs, large, complex and/or challenging 3D-structures could be fabricated (Fig. 3Ic and d). Finally, the good electrical conductivity of the CNT–polymer composite, 46.8 S m−1 with a 0.3 wt% MWCNT loading (much higher than values obtained in other reports129), motivated its use for the fabrication of micro-scale devices such as capacitors and resistors.
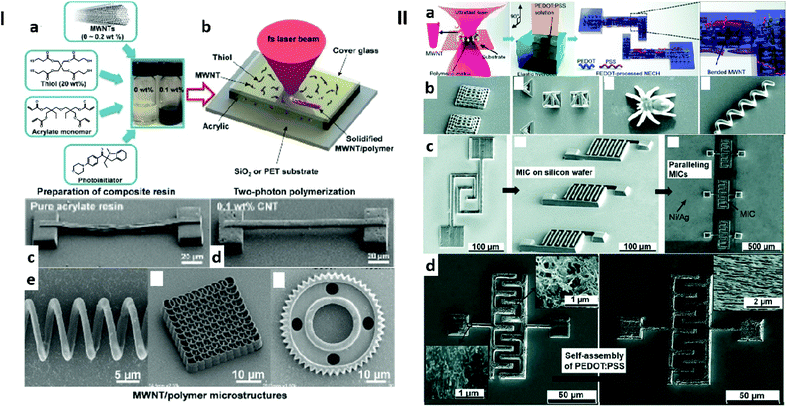 | ||
| Fig. 3 Examples of 3D printed hybrid materials. I: (a) Schematic representation of the experimental procedure of preparing CNT/polymer composite photoresists and (b) of their fabrication by two-photon 3D laser printing. (c) SEM images of some printed structures. From left to right, a microcoil inductor, a spiral-like photonic crystal and a microgear. (d and e) SEM images of a microbridge printed using a pure acrylic matrix and with the composite material containing MWCNTs (0.1 wt%), respectively. Adapted from ref. 128 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright © 2016. II: (a) Schematic representation of the stepwise fabrication containing: i) two-photon hydrogelation and ii) self-assembly of PEDOT:PSS (driven by π–π interaction and H-bonding). (b) SEM images of some printed structures. From left to right, a photonic crystal, tetrahedra and cubes (45 × 45 × 45 μm3), a spider and a spiral inductor. (c) SEM images of, from left to right, a common capacitor consisting in a single pair of electrodes, a micro interdigital capacitor, and 3 parallel micro interdigital capacitors with electrode connection on silicon. (d) SEM images of the micro interdigital capacitor before and after immersion in the PEDOT solution, evidencing the self-assembly of PEDOT. Adapted from ref. 134 with permission from the Royal Society of Chemistry, Copyright © 2019. | ||
Greenhall and Raeymaekers130 demonstrated that Ni-coated CNTs were successfully aligned using ultrasonic waves. In their work, the authors constructed a specific vat for SLA manufacturing purposes. By surrounding the 30.2 mm vat with eight ultrasound transducers disposed in an octagon, the fibrils were selectively aligned at 0°, 45°, 90° and −45°, using the facing transducers in pairs and achieving an alignment along the direction perpendicular to the wave propagation. The manufacturing setup reported in this work allowed, among others, the fabrication of strong Bouligand 3D structures. Moreover, the authors demonstrated the power of the directed self-assembly–SLA procedure to fabricate composite materials with enhanced conductive behaviour. This behaviour was characterised by computing the electrical resistance within a 1 mm section of the material along or across the director vector. The 59.7 ± 14.5 Ω and 112 ± 23.2 MΩ resistances computed from the I/V measurements along the two directions, respectively, shows that Ni-coated CNTs increase the conductivity of the material, and that individual micro-wires appear to be insulated one from another in a direction perpendicular to the alignment.
Poly(3,4-ethylenedioxythiophene) (PEDOT):polystyrene sulfonate (PSS) is a well-known polymeric semiconductor, used for example as the hole-transporting material in perovskite solar cells.131–133 Tao et al.134 utilised PEDOT:PSS to dope a 3D printable MWCNT/acrylated matrix composite ink. Two-photon 3D laser printing was used for gelation, achieving 3D structures with a 200 nm definition. A schematic representation of the composite material and images of the printed structures are proposed in Fig. 3II. The embedded MWCNTs were shown to provide the tertiary composite material with an increased stiffness (Young's modulus up to 900 MPa), while decreasing the elongation at break from ca. 200% at <0.05 wt% MWCNT loading to 130% at 0.15 wt% MWCNT loading and yielding a brittle material at higher loadings. Most importantly, MWCNTs had a positive effect on the electrical conductivity of the material (0.0007 S m−1 at 0.05 wt%, 0.12 S m−1 at 0.2 wt% and 0.8 S m−1 at 0.3 wt%), while the self-assembly of PEDOT further improved the electrical conductivity by an order of magnitude. Caused by the reactivity of PEDOT:PSS with polar solvents, the electrical conductivity of the crosslinked material could be tuned by putting it in contact with alcohols (methanol, ethanol, glycol and D-sorbitol), leading to potential application for alcohol sensing.
The investigation of the electrical conductivity of 3D printed PEDOT:PSS polymer composites proposed by Heo et al.135 highlighted the positive impact of the embedded PEDOT:PSS independently to CNTs, as the system described in this work did not make use of CNTs, unlike the system proposed by Tao et al.134 Once again, the presence of an alcohol, ethylene glycol (EG) here, leads to a drop in the electrical resistance of the material containing EG (662.0 ± 100.6 Ω sq−1) compared to the 0.91% PEDOT:PSS hydrogel (968 ± 245.1 Ω sq−1). This effect was again attributed, in accordance to previous works,136,137 to the re-alignment of the PEDOT:PSS fibres in the presence of EG.
Besides, though CNT/polymer composite materials are promising as printable functional materials, there is still room for improvement until they can fulfill the requirements for their broader implementation on an industrial scale.120 Importantly, the ability to obtain well-dispersed stable suspensions of the CNTs within the matrix is one of the main challenges limiting their reproducibility and application on larger scales.
3. Self-assembled materials
Self-assembly is globally present in natural systems (examples are proteins, DNA, protein expression, lipid membrane bilayers in cells, etc.) and is the trigger for the good functioning of these systems. The direct inclusion of such biomacromolecular systems within photoresists can open a way towards biocompatible photocrosslinked polymeric materials.138–141 For example, as reported by Zhao et al.,142 the selective self-assembly of DNA strands can be used for the design of printable hydrogels with a selective crosslinking density, having the biomacromolecular systems as a source of physical secondary crosslinking. Notably, such designs can provide the materials with pH, thermal and binder selectivity. Also, Kim et al.143 successfully reported a bioink from silk fibroin in DLP for tissue engineering applications. In particular, methacrylate functionalised fibroin was used to fabricate organ structures, such as heart, vessel, brain, trachea and ear with excellent structural stability and good biocompatibility.In another approach, biomimetically transposing the working concepts of these systems to synthetic polymeric materials is a promising pathway for the development of novel functional materials. Self-assembled synthetic materials such as amphiphilic block copolymers, liquid crystals or metallopolymers, among others, are potential candidates for this purpose.144–148
In the context of AM, out of the most noticeable self-assembled materials are liquid crystalline materials, and particularly nematic liquid crystals (NLCs). NLC mesophases are composed of stiff rod-shaped (calamitic) mesogens that align within the mesophases along a preferential director vector. Their ordering can be driven by phase segregation, by low-energy intermolecular interactions (such as hydrogen bonding, metal coordination, van der Waals forces or π–π interactions), by the limitation of voids in the bulk, or by the coexistence of the latter driving forces.149
An undeniable example of highly ordered self-assembled synthetic materials is metal–organic frameworks (MOFs). Effectively, MOFs’ crystalline units (metal/metallic cluster centre–organic linker) can have up to a three-dimensional extension. As will be detailed, this particular constitutive structure provides them with unique properties which can be transferred to polymeric materials by embedding MOFs in printable matrices.
In this section, we aim to describe recent examples where both self-assembly (bottom-up) and light-triggered AM (top-down) approaches have been merged, enabling the 3D printing of complex and functional structures exhibiting a clear hierarchical order. In particular, we will pay attention to printable liquid crystalline polymeric materials and MOF/polymer systems.
3.1. Liquid crystalline materials
First described by Finkelmann et al. in 1978,150,151 nematic liquid crystal (NLC) materials are well-known self-assembled materials exhibiting a relatively high degree of order.152–158 They are of great interest, as the averaged orientation along a director vector gives rise to intrinsic features such as optical properties,159–165 or macroscopic response to external stimuli.166–168 As NLCs are engendered by low-energy interactions, their alignment can be altered, generally by a temperature increase. The increasing randomness in the arrangement of the bulk commonly results in a spatial constriction along the formal director vector, as well as in an associated elongation along the directions normal to it. Most importantly, NLC materials can, after relaxation, recover their initial shape through the reappearance of a director vector. Acrylate-based NLC photoresists being commercially available supports their broad utilisation as a benchmark for the transfer of LC materials to light-triggered AM technologies.The initial alignment of NLC-based photoresists can be achieved using a previously printed template,169–171 along the rubbing direction,172 under the influence of an external magnetic field,173 or under the influence of an external electric field.174,175 Furthermore, crosslinking of the monomeric functional groups was shown to efficiently freeze the alignment observed in the photoresist. Visual evidence for the alignment of NLC mesophases along an external electric field was provided by Yoshida et al.176 In addition, this study evidenced that (i) the orientation of the NLC was frozen by crosslinking and (ii) the shape of the investigated platelets does not impact their preferred orientation under the influence of an external electric field.
Due to the importance of achieving a controlled orientation to fully exploit the potential of NLC materials, they are, in the context of AM, usually studied using two-photon 3D laser printing, DIW177 or mask lithography.178 In other words, AM techniques that do not require the photoresist to have a low viscosity (while this is an important requirement for SLA, DLP and CLIP). As a matter of fact, besides the notable reports of Ullett et al.,173,179 who reported the 3D printing of NLC materials by SLA using a highly specific setup, the 3D printing of NLC materials by conventional DLP or SLA technology remains inaccessible to date. In the later mentioned works, the alignment of NLC mesogens was achieved by applying an external magnetic field to a mini-vat, using a surrounding 0.32 T magnet. And an alignment of the NLC mesophases was indirectly demonstrated by attributing a directional anisotropy in the glassy state elastic modulus and the tensile strength to the anisotropically oriented nematic mesophases. Nevertheless, the complexity of the process (and its associated limitations) justifies the lack of popularity of SLA for the processing of NLC materials after this example.
Wiersma and co-workers intensively studied NCL microstructures printed by two-photon laser lithography from a photoresist formulated with a mono acrylated reactive mesogen, [4-(6-(acryloyloxy)hexyl)oxy)phenyl] 4-methoxybenzoate, 78 mol%; RM257 as the crosslinker, 4-(3-acryloyloxypropyloxy)-benzoesure 2-methyl-1,4-phenylester, 20 mol%; an azobenzene monoacrylate, (E)-6-((4-((2-cyano-4-nitrophenyl)diazenyl)phenyl)(ethyl)amino)hexyl acrylate, 1 mol%; and Irgacure 369, 1 mol%. The corresponding chemical structures are shown in Fig. 4Ia. In particular, the authors studied the mechanism for the observed swelling of the material during printing,180 the kinetics of the light-responsivity of the material181 and the effect of the medium on their spatial responsivity.182 The group also demonstrated the potential and the versatility of their material by proposing innovative designs for microfabricated devices. Among them, a photonic microhand with high wavelength-selectivity for folding and a light-fuelled microscopic walker are worth mentioning.183,184 In 2018,185 the group proposed the implementation of the same material for the construction of dual rigid/soft polymeric photonic circuits, tuneable by light. A 90° bend polymeric waveguide terminated by a grating coupler at both ends, as well as an anchoring structure for a whispering gallery mode resonator (WGMR) was constructed using a neutral photoresist. The light-responsive NLC material was used during a second printing step to provide the WGMR its light tunability. In the first design, the WGMR (free spectral range of 12.5 nm, neff = 1.52, and Qcalc = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was itself made out of the light-responsive material and was designed as a ring, while the second design consists in an Ip-Dip ring-shaped WGMR, topped with a NLC cylinder, which can alter the WGMR geometry by mechanical deformation under light irradiation, Fig. 4Ib and c. As the wavelength selectivity of WGMRs depends on their outer circumference (i.e. resonance will occur if the outer circumference is an integer multiple of the propagated wavelength), their wavelength selectivity could be tuned by varying the outer circumference of the WGMR. Hence, the authors could tune the wavelength selectivity of the proposed WGMR by up to −3.7 nm and +11.5 nm for the first and the second designs, respectively. E7,186,187 supplied by Merck®, is a NLC mixture that has been broadly used by the scientific community. Noticeably, the group of Elston174,175,188,189 investigated its use for 3D laser lithography. In the context of light-triggered AM, E7 is generally used together with one or several reactive mesogen(s), such as RM257 mentioned previously, responsible for crosslinking.190
000) was itself made out of the light-responsive material and was designed as a ring, while the second design consists in an Ip-Dip ring-shaped WGMR, topped with a NLC cylinder, which can alter the WGMR geometry by mechanical deformation under light irradiation, Fig. 4Ib and c. As the wavelength selectivity of WGMRs depends on their outer circumference (i.e. resonance will occur if the outer circumference is an integer multiple of the propagated wavelength), their wavelength selectivity could be tuned by varying the outer circumference of the WGMR. Hence, the authors could tune the wavelength selectivity of the proposed WGMR by up to −3.7 nm and +11.5 nm for the first and the second designs, respectively. E7,186,187 supplied by Merck®, is a NLC mixture that has been broadly used by the scientific community. Noticeably, the group of Elston174,175,188,189 investigated its use for 3D laser lithography. In the context of light-triggered AM, E7 is generally used together with one or several reactive mesogen(s), such as RM257 mentioned previously, responsible for crosslinking.190
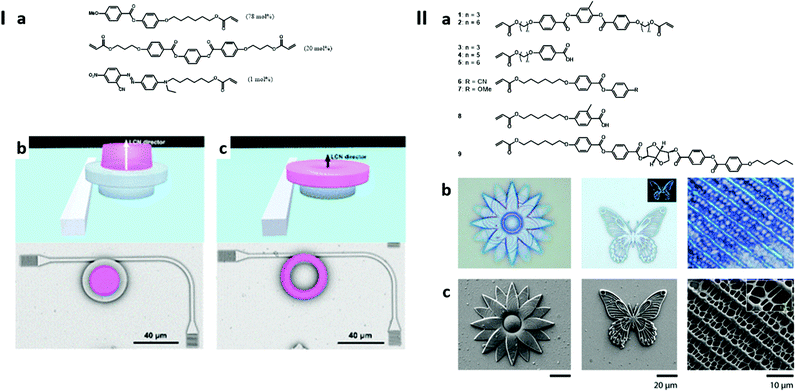 | ||
| Fig. 4 Examples of 3D printed liquid crystalline microstructures. I: (a) Chemical structures of the NLC compounds used in the formulation of the photoresist and the formulation ratio. (b) Rendering and SEM image (top view, false colour for the NLC element) for the design consisting of a WGMR out of IP-Dip, topped with a light-responsive actuator of NLC ink (second design). (c) Rendering and SEM image (top view, false colour for the NLC element) for the design consisting of a WGMR made out of the light-responsive NLC ink (first design). Adapted from ref. 185 with permission from the American Chemical Society, Copyright © 2018. II: (a) Chemical structures of the NLC compounds used in the formulation of the photoresist. (b) Optical microscopy and (c) SEM images of the printed structures. From left to right, a flower, a butterfly and the biomimetic pattern of the wing of the Papilio Paris butterfly. Adapted from ref. 191 with permission from the American Chemical Society, Copyright © 2020. | ||
Recently, Del Pozo et al.191 used a 9-component mixture of mono- and di-acrylated NLC mesogens which was processed by two-photon 3D laser printing with a minimum feature size of around 200 nm, (Fig. 4II). In the design of this system, the H-bonding participating in the stability of NLC mesophases could be broken by basic treatment. The obtained photonic material thereafter depicts a colour change and swelling in the presence of humidity. Indeed, the basic treatment leads to the formation of a hygroscopic polymer, allowing the uptake of water vapour. Moreover, temperature treatment was shown to reversibly recover the initially non-hydrated state of the material. While total dehydration was observed from 70 °C, the reversibility of the swelling was successful over ten cycles under 75% relative humidity (RH) with temperatures varying between 25 °C (12–18% expansion) and 20 °C (25–30% expansion). Correspondingly, a slight colour change was observed in the same temperature range at 75% RH, while a clear light-blue to bright-green colour sweep was observed from 18 °C to 16 °C at 75% RH.
In 2019, Chen et al.192 reported a novel strategy for the preparation of light-responsive materials, whose photoresponsivity was not based on the common azobenzene moiety.193 Instead, the authors used a nanocomposite photoresist composed of gold nanoparticles (3 wt%) embedded in a C6BP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RM257 (88.2
RM257 (88.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.8 mol%) matrix. In this system, the near infrared light irradiation of the printed structures engenders a local temperature increase caused by light-harvesting by the gold nanoparticles. The temperature increase finally induces a phase transition of the LC in the nematic state toward the isotropic state. As a result, the authors observed a spatial constriction along the director vector and a 20% length elongation perpendicular to the director vector. The process showed good reversibility as a relative 20% loss in elongation was observed over 300 cycles.
9.8 mol%) matrix. In this system, the near infrared light irradiation of the printed structures engenders a local temperature increase caused by light-harvesting by the gold nanoparticles. The temperature increase finally induces a phase transition of the LC in the nematic state toward the isotropic state. As a result, the authors observed a spatial constriction along the director vector and a 20% length elongation perpendicular to the director vector. The process showed good reversibility as a relative 20% loss in elongation was observed over 300 cycles.
Although the majority of the publications in the field make use of commercial compounds in their formulations, some groups reported novel reactive mesogens in the past years. Sungur et al.172 synthesised a novel monoacrylated reactive mesogen. Rectangular films were fabricated through a one-photon process, depicting a temperature-induced constriction along the director vector and elongation in the perpendicular direction.
3.2. Metal organic framework based networks
As mentioned previously, MOFs represent one of the most advanced examples of ordering achievable by self-assembly. MOFs are 1D- to 3D-extended crystalline compounds conventionally formed by the self-assembly of a metallic centre (usually a d-metal or a metallic cluster) and organic linkers. Out of many more factors their unique porosity and optical, adsorption, catalytic and magnetic properties explain the research interest they are attracting.194–198 Adapting such compounds to allow their processing by light-triggered AM has the potential to allow the fabrication of active materials of controlled macroscopic shape.Magdassi and coworkers199 furnished, to the best of our knowledge, the single example of the embedding of MOF crystals in a polymer matrix by 3D printing. In this work, the authors dispersed 10 wt% copper benzene-1,3,5-tricarboxylate (Cu-BTC) MOF in a 2-phenoxyethyl acrylate:PEGDA photopolymerisable matrix, which was successfully printed into a flexible hydrogel by DLP, Fig. 5. Post-printing X-ray diffraction analysis supported the stability of the crystalline structure of Cu-BTC through photopolymerisation. The analysis of adsorptive capabilities, for the adsorption of methylene blue (MB), showed that the adsorptive capabilities of the printed structure were strongly dependent on their 3D design, as solely surface MOF particles apparently demonstrated adsorption activity. The comparison with pure Cu-BTC demonstrated the superior adsorptive capabilities of the latter (0.75 mg g−1) with respect to the MOF/polymer composite (0.6 mg g−1) over their exposure to an aqueous solution of MB for less than 2 h. Notwithstanding, longer exposure times highlighted the greater performances of the MOF/polymer composite. Most likely, this arises from an increased stability, provided by the surrounding polymeric matrix, of the MOF–polymer composite in aqueous media compared to the pure MOF.
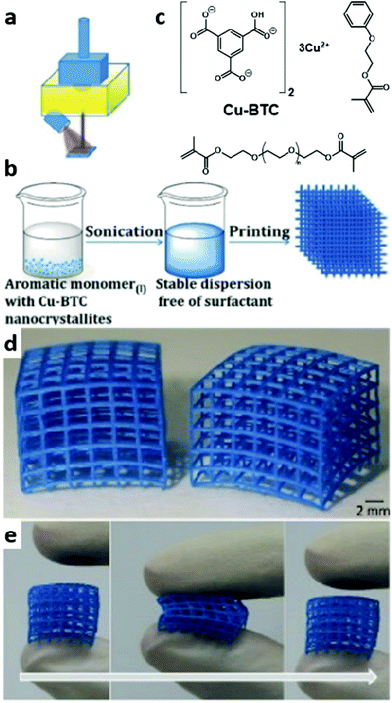 | ||
| Fig. 5 (a) Representation of the DLP fabrication process. (b) Fabrication process of the photoresist containing an embedded Cu-BTC MOF. From left to right, the Cu-BTC crystals are dispersed in the matrix, the photoinitiator is added and the photoresist is printed by DLP. (c) Chemical structures of Cu-BTC, the monomer and the crosslinker. (d) 3D-printed cubic nets of the MOF–polymer composite. (e) Demonstration of the flexibility of the 3D-printed cubic nets. From left to right before, during and after pressing. The time axis is denoted by a grey arrow. Adapted from ref. 199 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Copyright © 2017. | ||
The same group used [Cu(TAcO)2(H2O)(4,4′-bipy)]n·2H2O, a 1D metal–organic coordination polymer, together with dipropylene glycol diacrylate and diethylene glycol methyl ether, to fabricate, by DLP, 3D printed structures capable of moisture sensing.200 Effectively, the reversible substitution of the water ligand by a solvent molecule led to a colour change from blue to purple after substitution. The original is recovered after several minutes when exposed to ambient atmosphere. The material could then be used for moisture sensing in organic solvents, a colour change being indicative of an anhydrous solvent.
The group also developed a porous (surface area of 53.32 ± 23.00 m2 g−1 and pore size of 35.90 ± 24.80 Å) material 3D printed by DLP.201 For this purpose, the authors made use of Ni2+ complexes carrying acrylamide ligands. The analysis of the crosslinked materials evidenced that the coordination sphere of the Ni2+ metal centres remained unchanged through photopolymerisation. Although the strategy being used here does not permit the synthesis of perfectly ordered metal–organic extended compounds, the achievement of the processing of a metal–organic extended structure by DLP using a well-defined methacrylated Ni-complex is an important step forward.
The treated examples demonstrated that the activity of the MOF can be retained when MOF particles are dispersed in a photopolymerisable matrix and further processed. Although a slight decrease in activity can be expected, one can also expect an increase in chemical resistance of the embedded MOF particles. These studies open the road to the transfer of more diverse MOF species to light-triggered AM.
4. Outlook and future perspectives
In this perspective, we present an overview of the state of the art of 3D printable materials for light-triggered AM, exhibiting different degrees of ordering, in particular, hybrid materials containing CNTs or crystalline nanostructures as additives for the enhancement of the properties of printable polymers matrices, particularly regarding their mechanical, thermal and chemical resistance as well as their conductivity. Despite the great progress that has been made in the 3D printing of hybrid materials using light during the last years, the preparation of well-dispersed inks including higher loads of the additive within the polymeric matrix and its upscaling at competitive costs are the major challenges of the field. Also, examples of 3D printed structures from self-assembled materials based on liquid crystals and MOFs were discussed. Specifically, photoreactive inks based on liquid crystals have successfully been employed for the fabrication of functional devices such as light-tunable WGMRs and of 3D dynamic microstructures showing response to different stimuli, such as temperature, light or humidity. However, further efforts to improve these systems are still necessary. For example, the incorporation of novel stimuli-responsive mesogens (so far, the number of liquid crystalline monomers employed in 3D printing is very limited) and the control of their alignment in any desired direction would allow the preparation of 3D structures exhibiting a complex actuation, which are currently unattainable using conventional inks. Furthermore, MOF-based materials promisingly show the retention of the properties of the embedded MOF over an AM process and a good step towards the achievement of long-range ordered printed structures. In addition, the MOF/polymer composites were shown to have an enhanced chemical resistance compared to the pure MOF.In summary, it was demonstrated that light-triggered AM is a versatile fabrication technique that not only enables a facilitated fabrication of personalised pieces, with complicated structures, or of structures that cannot be fabricated otherwise, but also that the limit of printable materials is far from being reached. By choosing pre-ordered materials as a guideline, novel functional structures with enhanced properties become possible. Indeed, hierarchically ordered materials can offer multiple opportunities in the emerging field of 4D printing for the fabrication of stimuli-responsive and chemically active materials. Furthermore, as already mentioned the variety of self-assembling materials currently being investigated in the context of light-triggered AM remains fairly narrow from a chemical point-of-view. We are though confident that, considering the extensive variety of self-assembled materials in nature (e.g. DNA, proteins, etc.) and functional polymers (e.g. amphiphilic block copolymers), many of these materials could be successfully transferred to light-triggered AM.
We believe that merging the bottom-up molecular control of self-assembled materials with the versatility of 3D printing techniques has good prospects and will enable new opportunities for the design and realisation of 3D structures, whose properties can be controlled on demand. Thus, further efforts on the development of new polymeric printable materials exhibiting hierarchical order are essential and will have a great impact in the future of the field.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge funding from the Helmholtz program “Science and Technology of Nanosystems” (STN) and from the Deutsche Forchungksgemeinschaft (DFG, German research Foundation) under Germany's Excellence Strategy via the Excellence Cluster 3D Matter Made to Order (EXC-2082 - 390761711), and by the Carl Zeiss Foundation through the Carl-Zeiss-Focus@HEiKA.References
- E. E. Petersen and J. Pearce, Technologies, 2017, 5, 7 CrossRef.
- R. Liu, Z. Wang, T. Sparks, F. Liou and J. Newkirk, in Laser Additive Manufacturing, ed. M. Brandt, Woodhead Publishing, 2017, pp. 351–371 Search PubMed.
- B. Lyons, Bridge, 2012, 42, 13–19 Search PubMed.
- M. Delic and D. R. Eyers, Int. J. Prod. Econ., 2020, 228, 107689 CrossRef.
- J. Patalas-Maliszewska, M. Topczak and S. Kłos, Appl. Sci., 2020, 10, 735 CrossRef.
- N. Nachal, J. A. Moses, P. Karthik and C. Anandharamakrishnan, Food Eng. Rev., 2019, 11, 123–141 CrossRef CAS.
- K. Qiu, G. Haghiashtiani and M. C. McAlpine, Annu. Rev. Anal. Chem., 2018, 11, 287–306 CrossRef.
- J. Gardan, Int. J. Prod. Res., 2016, 54, 3118–3132 CrossRef.
- S. C. Ligon, R. Liska, J. R. Stampfl, M. Gurr and R. Mülhaupt, Chem. Rev., 2017, 117, 10212–10290 CrossRef CAS.
- I. Gibson, D. Rosen and B. Stucker, Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing, Springer-Verlag, New York, 2015 Search PubMed.
- C. W. Hull, US4575330A, 1986.
- T. D. Ngo, A. Kashani, G. Imbalzano, K. T. Nguyen and D. Hui, Composites, Part B, 2018, 143, 172–196 CrossRef CAS.
- M. Vaezi, H. Seitz and S. Yang, Int. J. Adv. Manuf. Technol., 2013, 67, 1721–1754 CrossRef.
- X. Luo, J. Li and M. Lucas, presented in part at the Laser 3D Manufacturing IV, 2017.
- H. Jigang, Q. Qin, W. Jie and F. Hui, Int. J. Mater., Mech. Manuf., 2018, 6, 332–336 Search PubMed.
- T. Finnes, J. Undergrad. Res., 2015, 13, 3 Search PubMed.
- N. Fang, C. Sun and X. Zhang, Appl. Phys. A, 2004, 79, 1839–1842 CrossRef CAS.
- B. Msallem, N. Sharma, S. Cao, F. S. Halbeisen, H.-F. Zeilhofer and F. M. Thieringer, J. Clin. Med., 2020, 9, 817 CrossRef.
- L. Yao, P. Hu, Z. Wu, W. Liu, Q. Lv, Z. Nie and H. Zhengdi, presented in part at the Journal of Physics: Conference Series, 2020.
- S. Mansour, M. Gilbert and R. Hague, Mater. Sci. Eng., A, 2007, 447, 277–284 CrossRef.
- J. Z. Manapat, Q. Chen, P. Ye and R. C. Advincula, Macromol. Mater. Eng., 2017, 302, 1600553 CrossRef.
- X. Wang, M. Jiang, Z. Zhou, J. Gou and D. Hui, Composites, Part B, 2017, 110, 442–458 CrossRef CAS.
- M. A. Matos, A. M. A. Rocha and A. I. Pereira, Int. J. Adv. Manuf. Technol., 2020, 1–13 Search PubMed.
- D. Miedzińska, E. Małek and A. Popławski, Appl. Comput. Sci., 2019, 15, 16–26 Search PubMed.
- D. Miedzińska, R. Gieleta and E. Małek, Mech. Mater., 2020, 141, 103245 CrossRef.
- S. Razavi Bazaz, N. Kashaninejad, S. Azadi, K. Patel, M. Asadnia, D. Jin and M. Ebrahimi Warkiani, Adv. Mater. Technol., 2019, 4, 1900425 CrossRef CAS.
- Y. Yang, H. Li, Y. Xu, Y. Dong, W. Shan and J. Shen, Int. J. Pharm., 2019, 562, 66–75 CrossRef CAS.
- J. R. Tumbleston, D. Shirvanyants, N. Ermoshkin, R. Janusziewicz, A. R. Johnson, D. Kelly, K. Chen, R. Pinschmidt, J. P. Rolland, A. Ermoshkin, E. T. Samulski and J. M. DeSimone, Science, 2015, 347, 1349 CrossRef CAS.
- J. M. DeSimone, A. Ermoshkin, N. Ermoshkin and E. T. Samulski, US10016938B2, 2018.
- J. Balli, S. Kumpaty and V. Anewenter, presented in part at the ASME 2017 International Mechanical Engineering Congress and Exposition, Tampa, Florida, USA, November 3–9, 2017.
- J. R. Tumbleston, D. Shirvanyants, N. Ermoshkin, R. Janusziewicz, A. R. Johnson, D. Kelly, K. Chen, R. Pinschmidt, J. P. Rolland and A. Ermoshkin, Science, 2015, 347, 1349–1352 CrossRef CAS.
- R. Janusziewicz, J. R. Tumbleston, A. L. Quintanilla, S. J. Mecham and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11703–11708 CrossRef CAS.
- J. W. Stansbury and M. J. Idacavage, Dent. Mater., 2016, 32, 54–64 CrossRef CAS.
- C. A. Spiegel, M. Hippler, A. Münchinger, M. Bastmeyer, C. Barner-Kowollik, M. Wegener and E. Blasco, Adv. Funct. Mater., 2019, 1907615 Search PubMed.
- M. Carlotti and V. Mattoli, Small, 2019, 15, 1902687 CrossRef.
- H. B. Sun and S. Kawata, in Nmr - 3d Analysis - Photopolymerization, Springer, 2004, vol. 170, pp. 169–273 Search PubMed.
- Y. L. Zhang, Q. D. Chen, H. Xia and H. B. Sun, Nano Today, 2010, 5, 435–448 CrossRef CAS.
- S. D. Gittard and R. Narayan, Expert Rev. Med. Devices, 2010, 7, 343–356 CrossRef.
- J. Fischer and M. Wegener, Laser Photonics Rev., 2013, 7, 22–44 CrossRef CAS.
- W. Zapka, Handbook of Industrial Inkjet Printing: A Full System Approach, John Wiley & Sons, 2017 Search PubMed.
- C. S. Sheppard and V. R. Kamath, Polym. Eng. Sci., 1979, 19, 597–606 CrossRef CAS.
- H. Hageman, in Photopolymerisation and photoimaging science and technology, Springer, 1989, pp. 1–53 Search PubMed.
- S. Jockusch, M. S. Landis, B. Freiermuth and N. J. Turro, Macromolecules, 2001, 34, 1619–1626 CrossRef CAS.
- K. Dietliker, P. Murer, R. Hüsler and T. Jung, US9199934B2, 2015.
- S. Jockusch and N. J. Turro, J. Am. Chem. Soc., 1998, 120, 11773–11777 CrossRef CAS.
- M. Haas, J. Radebner, A. Eibel, G. Gescheidt and H. Stueger, Chem. – Eur. J., 2018, 24, 8258–8267 CrossRef CAS.
- D. Bird, E. Caravaca, J. Laquidara, K. Luhmann and N. M. Ravindra, presented in part at the TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings, Cham, 2019.
- K. C. Wong, E. Ng, W. T. Wong and P. Chiu, Chem. – Eur. J., 2016, 22, 3709–3712 CrossRef CAS.
- J. H. Ding and D. L. Gin, Chem. Mater., 2000, 12, 22–24 CrossRef CAS.
- E. Maron, J. H. Swisher, J. J. Haven, T. Y. Meyer, T. Junkers and H. G. Börner, Angew. Chem., Int. Ed., 2019, 58, 10747–10751 CrossRef CAS.
- N. Zabarjad Shiraz, E. Enferad, A. Monfared and M. A. Mojarrad, ISRN Polym. Sci., 2013, 280897 Search PubMed.
- G. Jyoti, A. Keshav and J. Anandkumar, Int. J. Chem. React. Eng., 2016, 14, 571–578 CAS.
- G. P. Monnier, P. Ciceron, C. M. Leroy and C. Bourrousse, US20170355660A1, 2019.
- S. Juodkazis, V. Mizeikis, K. K. Seet, M. Miwa and H. Misawa, Nanotechnology, 2005, 16, 846 CrossRef CAS.
- V. Mizeikis, K. K. Seet, S. Juodkazis and H. Misawa, Opt. Lett., 2004, 29, 2061–2063 CrossRef.
- J. M. McCracken, V. P. Tondiglia, A. D. Auguste, N. P. Godman, B. R. Donovan, B. N. Bagnall, H. E. Fowler, C. M. Baxter, V. Matavulj and J. D. Berrigan, Adv. Funct. Mater., 2019, 29, 1903761 CrossRef.
- P. J. Bártolo and I. Gibson, Stereolithography: Materials, Processes and Applications, Springer US, Boston, MA, 2011 Search PubMed.
- S. K. Reddy, N. B. Cramer, M. Kalvaitas, T. Y. Lee and C. N. Bowman, Aust. J. Chem., 2006, 59, 586–593 CrossRef CAS.
- E. Blasco, M. Wegener and C. Barner-Kowollik, Adv. Mater., 2017, 29, 1604005 CrossRef.
- T. Y. Lee, J. Carioscia, Z. Smith and C. N. Bowman, Macromolecules, 2007, 40, 1473–1479 CrossRef CAS.
- J. W. Chan, J. Shin, C. E. Hoyle, C. N. Bowman and A. B. Lowe, Macromolecules, 2010, 43, 4937–4942 CrossRef CAS.
- A. Oesterreicher, S. Ayalur-Karunakaran, A. Moser, F. H. Mostegel, M. Edler, P. Kaschnitz, G. Pinter, G. Trimmel, S. Schlögl and T. Griesser, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 3484–3494 CrossRef CAS.
- H. Leonards, S. Engelhardt, A. Hoffmann, L. Pongratz, S. Schriever, J. Bläsius, M. M. Wehner and A. Gillner, presented in part at the Laser 3D Manufacturing II, 2015.
- S. Baudis, F. Nehl, S. C. Ligon, A. Nigisch, H. Bergmeister, D. Bernhard, J. Stampfl and R. Liska, Biomed. Mater., 2011, 6, 055003 CrossRef.
- A. Oesterreicher, J. Wiener, M. Roth, A. Moser, R. Gmeiner, M. Edler, G. Pinter and T. Griesser, Polym. Chem., 2016, 7, 5169–5180 RSC.
- G.-H. Wu and S.-H. Hsu, J. Med. Biol. Eng., 2015, 35, 285–292 CrossRef.
- B. D. Fairbanks, T. F. Scott, C. J. Kloxin, K. S. Anseth and C. N. Bowman, Macromolecules, 2009, 42, 211–217 CrossRef CAS.
- A. B. Lowe, Polymer, 2014, 55, 5517–5549 CrossRef CAS.
- A. F. Senyurt, H. Wei, C. E. Hoyle, S. G. Piland and T. E. Gould, Macromolecules, 2007, 40, 4901–4909 CrossRef CAS.
- A. Concellón, A. P. Schenning, P. Romero, M. Marcos and J. L. Serrano, Macromolecules, 2018, 51, 2349–2358 CrossRef.
- E. Sato, S. Nagai and A. Matsumoto, Prog. Org. Coat., 2013, 76, 1747–1751 CrossRef CAS.
- J. He, Y. Zhao and Y. Zhao, Soft Matter, 2009, 5, 308–310 RSC.
- S. R. Govindarajan, Y. Xu, J. P. Swanson, T. Jain, Y. Lu, J.-W. Choi and A. Joy, Macromolecules, 2016, 49, 2429–2437 CrossRef CAS.
- Q. Liu, T. Jain, C. Peng, F. Peng, A. Narayanan and A. Joy, Macromolecules, 2020, 53, 3690–3699 CrossRef CAS.
- V. T. Widyaya, E. K. Riga, C. Müller and K. Lienkamp, Macromolecules, 2018, 51, 1409–1417 CrossRef CAS.
- C. P. Kabb, C. S. O'Bryan, C. C. Deng, T. E. Angelini and B. S. Sumerlin, ACS Appl. Mater. Interfaces, 2018, 10, 16793–16801 CrossRef CAS.
- Y. L. Sun, W. F. Dong, R. Z. Yang, X. Meng, L. Zhang, Q. D. Chen and H. B. Sun, Angew. Chem., Int. Ed., 2012, 51, 1558–1562 CrossRef CAS.
- Y.-L. Sun, Z.-S. Hou, S.-M. Sun, B.-Y. Zheng, J.-F. Ku, W.-F. Dong, Q.-D. Chen and H.-B. Sun, Sci. Rep., 2015, 5, 12852 CrossRef CAS.
- N. Igarashi, S. Onoue and Y. Tsuda, Anal. Chem., 2007, 23, 943–948 CAS.
- B. Kaehr and J. B. Shear, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8850–8854 CrossRef CAS.
- B. Kaehr, R. Allen, D. J. Javier, J. Currie and J. B. Shear, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16104–16108 CrossRef CAS.
- B. Narupai and A. Nelson, ACS Macro Lett., 2020, 9, 627–638 CrossRef CAS.
- A. K. Nguyen and R. J. Narayan, Mater. Today, 2017, 20, 314–322 CrossRef CAS.
- S. Derakhshanfar, R. Mbeleck, K. Xu, X. Zhang, W. Zhong and M. Xing, Bioact. Mater., 2018, 3, 144–156 CrossRef.
- B. Mu, F. Li and K. S. Walton, Chem. Commun., 2009, 2493–2495 RSC.
- G. W. Ehrenstein and J. Kabelka, in Ullmann's Encyclopedia of Industrial Chemistry, 2000, pp. 453–469 Search PubMed.
- X. Kuang, Z. Zhao, K. Chen, D. Fang, G. Kang and H. J. Qi, Macromol. Rapid Commun., 2018, 39, 1700809 CrossRef.
- E. Shukrun, I. Cooperstein and S. Magdassi, Adv. Sci., 2018, 5, 1800061 CrossRef.
- I. Cooperstein, E. Shukrun, O. Press, A. Kamyshny and S. Magdassi, ACS Appl. Mater. Interfaces, 2018, 10, 18879–18885 CrossRef CAS.
- G. Fang, H. Cao, L. Cao and X. Duan, Adv. Mater. Technol., 2018, 3, 1700271 CrossRef.
- M. Layani, X. Wang and S. Magdassi, Adv. Mater., 2018, 30, 1706344 CrossRef.
- S. Shukla, X. Vidal, E. P. Furlani, M. T. Swihart, K.-T. Kim, Y.-K. Yoon, A. Urbas and P. N. Prasad, ACS Nano, 2011, 5, 1947–1957 CrossRef CAS.
- E. Blasco, J. Müller, P. Müller, V. Trouillet, M. Schön, T. Scherer, C. Barner-Kowollik and M. Wegener, Adv. Mater., 2016, 28, 3592–3595 CrossRef CAS.
- Z. B. Sun, X. Z. Dong, W. Q. Chen, S. Nakanishi, X. M. Duan and S. Kawata, Adv. Mater., 2008, 20, 914–919 CrossRef CAS.
- X. Feng, Z. Yang, S. Chmely, Q. Wang, S. Wang and Y. Xie, Carbohydr. Polym., 2017, 169, 272–281 CrossRef CAS.
- D. Karalekas, Mater. Des., 2003, 24, 665–670 CrossRef CAS.
- K. K. Chawla, Composite materials: science and engineering, Springer Science & Business Media, 2012 Search PubMed.
- P. Parandoush and D. Lin, Compos. Struct., 2017, 182, 36–53 CrossRef.
- J. J. Fallon, S. H. McKnight and M. J. Bortner, Addit. Manuf., 2019, 100810 CAS.
- Y. Li, Z. Feng, L. Huang, K. Essa, E. Bilotti, H. Zhang, T. Peijs and L. Hao, Composites, Part A, 2019, 105483 CrossRef CAS.
- Y. Sano, R. Matsuzaki, M. Ueda, A. Todoroki and Y. Hirano, Addit. Manuf., 2018, 24, 521–527 CAS.
- Z. Spitalsky, D. Tasis, K. Papagelis and C. Galiotis, Prog. Polym. Sci., 2010, 35, 357–401 CrossRef CAS.
- N. van de Werken, H. Tekinalp, P. Khanbolouki, S. Ozcan, A. Williams and M. Tehrani, Addit. Manuf., 2020, 31, 100962 CAS.
- S. Singh, S. Ramakrishna and R. Singh, J. Manuf. Process., 2017, 25, 185–200 CrossRef.
- A. Peterson, I. östergren, A. Lotsari, A. Venkatesh, J. Thunberg, A. Ström, R. Rojas, M. Andersson, L. A. Berglund and A. Boldizar, Biomacromolecules, 2019, 20, 3924–3932 CrossRef CAS.
- V. C.-F. Li, X. Kuang, A. Mulyadi, C. M. Hamel, Y. Deng and H. J. Qi, Cellulose, 2019, 26, 3973–3985 CrossRef CAS.
- Q. Q. Wang, Q. Yao, J. Liu, J. Z. Sun, Q. Q. Zhu and H. L. Chen, Cellulose, 2019, 26, 7585–7617 CrossRef CAS.
- Q. Q. Wang, J. Z. Sun, Q. Yao, C. C. Ji, J. Liu and Q. Q. Zhu, Cellulose, 2018, 25, 4275–4301 CrossRef CAS.
- N. B. Palaganas, J. D. Mangadlao, A. C. C. de Leon, J. O. Palaganas, K. D. Pangilinan, Y. J. Lee and R. C. Advincula, ACS Appl. Mater. Interfaces, 2017, 9, 34314–34324 CrossRef CAS.
- N. K. Simha and J. L. Lewis, presented in part at the 2003 Summer Bioengineering Conference, Key Biscayne, FL, USA, 2003.
- S. Hong, D. Sycks, H. F. Chan, S. Lin, G. P. Lopez, F. Guilak, K. W. Leong and X. Zhao, Adv. Mater., 2015, 27, 4035–4040 CrossRef CAS.
- J. Wang, A. Chiappone, I. Roppolo, F. Shao, E. Fantino, M. Lorusso, D. Rentsch, K. Dietliker, C. F. Pirri and H. Grützmacher, Angew. Chem., Int. Ed., 2018, 57, 2353–2356 CrossRef CAS.
- M. Rothammer, M.-C. Heep, G. von Freymann and C. Zollfrank, Cellulose, 2018, 25, 6031–6039 CrossRef CAS.
- R. Iley and H. Riley, J. Chem. Soc., 1948, 1362–1366 RSC.
- R. Bacon, J. Appl. Phys., 1960, 31, 283–290 CrossRef.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- D. Young, N. Wetmore and M. Czabaj, Addit. Manuf., 2018, 22, 508–515 CAS.
- B. Brenken, E. Barocio, A. Favaloro, V. Kunc and R. B. Pipes, Addit. Manuf., 2018, 21, 1–16 CAS.
- C. Shemelya, A. De La Rosa, A. R. Torrado, K. Yu, J. Domanowski, P. J. Bonacuse, R. E. Martin, M. Juhasz, F. Hurwitz and R. B. Wicker, Addit. Manuf., 2017, 16, 186–196 CAS.
- A. Schmitz, J. Eng. Mater. Technol., 2020, 142, 024501 CrossRef CAS.
- A. Mitchell, U. Lafont, M. Hołyńska and C. Semprimoschnig, Addit. Manuf., 2018, 24, 606–626 CAS.
- Y. Liu, W. Xiong, L. J. Jiang, Y. Zhou, D. Li, L. Jiang, J.-F. Silvain and Y. Lu, Laser-directed 3D assembly of carbon nanotubes using two-photon polymerization (Conference Presentation), SPIE, 2017 Search PubMed.
- S. Ghoshal, Fibers, 2017, 5, 40 CrossRef.
- G. Gonzalez, A. Chiappone, I. Roppolo, E. Fantino, V. Bertana, F. Perrucci, L. Scaltrito, F. Pirri and M. Sangermano, Polymer, 2017, 109, 246–253 CrossRef CAS.
- Q. Mu, L. Wang, C. K. Dunn, X. Kuang, F. Duan, Z. Zhang, H. J. Qi and T. Wang, Addit. Manuf., 2017, 18, 74–83 CAS.
- S. Ushiba, S. Shoji, P. Kuray, K. Masui, J. Kono and S. Kawata, presented in part at the Advanced Fabrication Technologies for Micro/Nano Optics and Photonics VI, 2013.
- S. Ushiba, S. Shoji, K. Masui, P. Kuray, J. Kono and S. Kawata, Carbon, 2013, 59, 283–288 CrossRef CAS.
- W. Xiong, Y. Liu, L. J. Jiang, Y. S. Zhou, D. W. Li, L. Jiang, J.-F. Silvain and Y. F. Lu, Adv. Mater., 2016, 28, 2002–2009 CrossRef CAS.
- U. Staudinger, G. Zyla, B. Krause, A. Janke, D. Fischer, C. Esen, B. Voit and A. Ostendorf, Microelectron. Eng., 2017, 179, 48–55 CrossRef CAS.
- J. Greenhall and B. Raeymaekers, Adv. Mater. Technol., 2017, 2, 1700122 CrossRef.
- S. Schumann, A. Elschner, A. Sautter, W. Lövenich, R. Sauer, J. Sütterlin and N. Kausch-Busies, US20200013559A1, 2019.
- K. M. Reza, A. Gurung, B. Bahrami, S. Mabrouk, H. Elbohy, R. Pathak, K. Chen, A. H. Chowdhury, M. T. Rahman and S. Letourneau, J. Energy Chem., 2020, 44, 41–50 CrossRef.
- H. Dong, E. Zheng, Z. Niu, X. Zhang, Y.-Y. Lin, P. Jain and Q. Yu, ACS Appl. Mater. Interfaces, 2020, 12, 17571–17582 CrossRef CAS.
- Y. Tao, C. Wei, J. Liu, C. Deng, S. Cai and W. Xiong, Nanoscale, 2019, 11, 9176–9184 RSC.
- D. N. Heo, S.-J. Lee, R. Timsina, X. Qiu, N. J. Castro and L. G. Zhang, Mater. Sci. Eng., C, 2019, 99, 582–590 CrossRef CAS.
- J. Y. Oh, M. Shin, J. B. Lee, J.-H. Ahn, H. K. Baik and U. Jeong, ACS Appl. Mater. Interfaces, 2014, 6, 6954–6961 CrossRef CAS.
- J. Nevrela, M. Micjan, M. Novota, S. Kovacova, M. Pavuk, P. Juhasz, J. Kovac Jr., J. Jakabovic and M. Weis, J. Polym. Sci., Part B: Polym. Phys., 2015, 53, 1139–1146 CrossRef CAS.
- F. P. W. Melchels, M. A. N. Domingos, T. J. Klein, J. Malda, P. J. Bartolo and D. W. Hutmacher, Prog. Polym. Sci., 2012, 37, 1079–1104 CrossRef CAS.
- H. N. Chia and B. M. Wu, J. Biol. Eng., 2015, 9, 4 CrossRef.
- E. Mancha Sánchez, J. C. Gómez-Blanco, E. López Nieto, J. G. Casado, A. Macías-García, M. A. Díaz Díez, J. P. Carrasco-Amador, D. Torrejón Martín, F. M. Sánchez-Margallo and J. B. Pagador, Front. Bioeng. Biotechnol., 2020, 8, 776 CrossRef.
- E. Sanchez-Rexach, T. G. Johnston, C. Jehanno, H. Sardon and A. Nelson, Chem. Mater., 2020, 32, 7105–7119 CrossRef CAS.
- Z. Zhao, C. Wang, H. Yan and Y. Liu, Adv. Funct. Mater., 2019, 29, 1905911 CrossRef CAS.
- S. H. Kim, Y. K. Yeon, J. M. Lee, J. R. Chao, Y. J. Lee, Y. B. Seo, M. T. Sultan, O. J. Lee, J. S. Lee, S.-I. Yoon, I.-S. Hong, G. Khang, S. J. Lee, J. J. Yoo and C. H. Park, Nat. Commun., 2018, 9, 1620 CrossRef.
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30, 267–277 CrossRef CAS.
- S. O. Kim, H. H. Solak, M. P. Stoykovich, N. J. Ferrier, J. J. de Pablo and P. F. Nealey, Nature, 2003, 424, 411–414 CrossRef CAS.
- G. R. Whittell and I. Manners, Adv. Mater., 2007, 19, 3439–3468 CrossRef CAS.
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176–188 CrossRef CAS.
- J.-C. Eloi, L. Chabanne, G. R. Whittell and I. Manners, Mater. Today, 2008, 11, 28–36 CrossRef CAS.
- C. Y. Huang, in Current Trends of Optics and Photonics, ed. C. C. Lee, 2015, vol. 129, pp. 263–271 Search PubMed.
- H. Finkelmann, M. Happ, M. Portugal and H. Ringsdorf, Makromol. Chem., 1978, 179, 2541–2544 CrossRef CAS.
- H. Finkelmann, H. Ringsdorf and J. H. Wendorff, Makromol. Chem., 1978, 179, 273–276 CrossRef CAS.
- D. J. Broer, G. N. Mol and G. Challa, Makromol. Chem., 1991, 192, 59–74 CrossRef CAS.
- D. J. Broer, R. A. Hikmet and G. Challa, Makromol. Chem., 1989, 190, 3201–3215 CrossRef CAS.
- D. J. Broer, J. Boven, G. N. Mol and G. Challa, Makromol. Chem., 1989, 190, 2255–2268 CrossRef CAS.
- D. J. Broer and G. N. Mol, Polym. Eng. Sci., 1991, 31, 625–631 CrossRef CAS.
- J. Schultz, J. Bhatt, R. Chartoff, R. Pogue and J. Ullett, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 1183–1190 CrossRef CAS.
- J. Schultz and R. Chartoff, Polymer, 1998, 39, 319–325 CrossRef CAS.
- J. Schultz, R. Chartoff and J. Ullett, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 1081–1089 CrossRef CAS.
- J. Li, C.-H. Wen, S. Gauza, R. Lu and S.-T. Wu, J. Disp. Technol., 2005, 1, 51 CrossRef CAS.
- J. Li, S.-T. Wu, S. Brugioni, R. Meucci and S. Faetti, J. Appl. Phys., 2005, 97, 073501 CrossRef.
- T. T. Alkeskjold, J. Lægsgaard, A. Bjarklev, D. S. Hermann, J. Broeng, J. Li, S. Gauza and S.-T. Wu, Appl. Opt., 2006, 45, 2261–2264 CrossRef CAS.
- H. Chen, R. Zhu, J. Zhu and S.-T. Wu, Liq. Cryst., 2015, 42, 1738–1742 CrossRef CAS.
- Z. He, Y.-H. Lee, D. Chanda and S.-T. Wu, Opt. Express, 2018, 26, 21184–21193 CrossRef CAS.
- K. Tanaka, Y. Yahagi, H. Fujisawa, T. Gotou, K. Murai, K. Mitsuhata and K. Nakamura, US10120206, 2018.
- Y. P. Huang, in Current Trends of Optics and Photonics, ed. C. C. Lee, Springer-Verlag Berlin, Berlin, 2015, vol. 129, pp. 309–319 Search PubMed.
- S. Yang and Y. Liu, Ultrasonics, 2018, 88, 193–206 CrossRef CAS.
- T. J. White, A. D. Zhao, S. A. Cazzell, T. J. Bunning, T. Kosa, L. Sukhomlinova, T. J. Smith and B. Taheri, J. Mater. Chem., 2012, 22, 5751–5757 RSC.
- M. Giese, J. C. De Witt, K. E. Shopsowitz, A. P. Manning, R. Y. Dong, C. A. Michal, W. Y. Hamad and M. J. MacLachlan, ACS Appl. Mater. Interfaces, 2013, 5, 6854–6859 CrossRef CAS.
- H. Zeng, P. Wasylczyk, C. Parmeggiani, D. Martella and D. S. Wiersma, J. Visualized Exp., 2016, e53744 Search PubMed.
- H. Zeng, P. Wasylczyk, G. Cerretti, D. Martella, C. Parmeggiani and D. S. Wiersma, Appl. Phys. Lett., 2015, 106, 111902 CrossRef.
- M. Fleisch, S. Gao, D. Bošnjaković, X. Zhang, R. Rupp and I. Drevenšek-Olenik, Liq. Cryst., 2019, 46, 2075–2084 CrossRef CAS.
- E. Sungur, M.-H. Li, G. Taupier, A. Boeglin, M. Romeo, S. Méry, P. Keller and K. D. Dorkenoo, Opt. Express, 2007, 15, 6784–6789 CrossRef CAS.
- J. S. Ullett, T. Benson-Tolle, J. W. Schultz and R. Chartoff, Mater. Des., 1999, 20, 91–97 CrossRef CAS.
- C. C. Tartan, P. S. Salter, M. J. Booth, S. M. Morris and S. J. Elston, J. Appl. Phys., 2016, 119, 183106 CrossRef.
- C. Tartan, P. Salter, T. Wilkinson, M. Booth, S. Morris and S. Elston, RSC Adv., 2017, 7, 507–511 RSC.
- H. Yoshida, G. Nakazawa, K. Tagashira and M. Ozaki, Soft Matter, 2012, 8, 11323–11327 RSC.
- L. Li, Q. Lin, M. Tang, A. J. Duncan and C. Ke, Chem. – Eur. J., 2019, 25, 10768–10781 CrossRef CAS.
- D. Liu and D. J. Broer, Langmuir, 2014, 30, 13499–13509 CrossRef CAS.
- J. Ullett, J. Schultz and R. Chartoff, Rapid Prototyping J., 2000, 6, 8–17 CrossRef.
- H. Zeng, D. Martella, P. Wasylczyk, G. Cerretti, J. C. G. Lavocat, C. H. Ho, C. Parmeggiani and D. S. Wiersma, Adv. Mater., 2014, 26, 2319–2322 CrossRef CAS.
- D. Martella, D. Antonioli, S. Nocentini, D. Wiersma, G. Galli, M. Laus and C. Parmeggiani, RSC Adv., 2017, 7, 19940–19947 RSC.
- S. Nocentini, D. Martella, C. Parmeggiani, S. Zanotto and D. S. Wiersma, Adv. Opt. Mater., 2018, 6, 1800167 CrossRef.
- H. Zeng, P. Wasylczyk, C. Parmeggiani, D. Martella, M. Burresi and D. S. Wiersma, Adv. Mater., 2015, 27, 3883–3887 CrossRef CAS.
- D. Martella, S. Nocentini, D. Nuzhdin, C. Parmeggiani and D. S. Wiersma, Adv. Mater., 2017, 29, 1704047 CrossRef.
- S. Nocentini, F. Riboli, M. Burresi, D. Martella, C. Parmeggiani and D. S. Wiersma, ACS Photonics, 2018, 5, 3222–3230 CrossRef.
- A. Mouquinho, M. Saavedra, A. Maiau, K. Petrova, M. T. Barros, J. Figueirinhas and J. Sotomayor, Mol. Cryst. Liq. Cryst., 2011, 542, 132–140 CAS.
- J. Cooper, Waters Corporation, Application Note 720004814en, 2013 Search PubMed.
- J. J. Sandford O'Neill, P. S. Salter, M. J. Booth, S. J. Elston and S. M. Morris, Nat. Commun., 2020, 11, 2203 CrossRef.
- C. C. Tartan, J. J. Sandford, O'Neill, P. S. Salter, J. Aplinc, M. J. Booth, M. Ravnik, S. M. Morris and S. J. Elston, Adv. Opt. Mater., 2018, 6, 1800515 CrossRef.
- B. Akdeniz and E. Bukusoglu, Langmuir, 2019, 35, 13126–13134 CrossRef CAS.
- M. Del Pozo, C. Delaney, C. W. Bastiaansen, D. Diamond, A. P. Schenning and L. Florea, ACS Nano, 2020, 14, 9832–9839 CrossRef CAS.
- L. Chen, Y. Dong, C.-Y. Tang, L. Zhong, W.-C. Law, G. C. Tsui, Y. Yang and X. Xie, ACS Appl. Mater. Interfaces, 2019, 11, 19541–19553 CrossRef CAS.
- E. Descrovi, F. Pirani, V. P. Rajamanickam, S. Licheri and C. Liberale, J. Mater. Chem. C, 2018, 6, 10428–10434 RSC.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef.
- G. Ferey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- O. Halevi, J. M. Tan, P. S. Lee and S. Magdassi, Adv. Sustainable Syst., 2018, 2, 1700150 CrossRef.
- N. Maldonado, V. G. Vegas, O. Halevi, J. I. Martínez, P. S. Lee, S. Magdassi, M. T. Wharmby, A. E. Platero-Prats, C. Moreno and F. Zamora, Adv. Funct. Mater., 2019, 29, 1808424 CrossRef.
- O. Halevi, J. Chen, G. Thangavel, S. A. Morris, T. B. Uliel, Y. R. Tischler, P. S. Lee and S. Magdassi, RSC Adv., 2020, 10, 14812–14817 RSC.
| This journal is © The Royal Society of Chemistry 2020 |
