Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exposure medium and particle ageing moderate the toxicological effects of nanomaterials to Daphnia magna over multiple generations: a case for standard test review?†
Laura-Jayne A.
Ellis
 *,
Eugenia
Valsami-Jones
*,
Eugenia
Valsami-Jones
 and
Iseult
Lynch
and
Iseult
Lynch
 *
*
University of Birmingham, College of life and Environmental Sciences, Birmingham B15 2TT, UK. E-mail: L.A.Ellis@bham.ac.uk; I.lynch@bham.ac.uk
First published on 20th March 2020
Abstract
Pristine engineered nanomaterials (NMs) entering the aquatic environment become ‘aged’ during their lifetime via chemical, physical and/or biological process. Therefore, traditional ecotoxicology tests which were designed for soluble chemicals prior to the emergence of NMs, use pristine NMs and salt-only media which are not representative of realistic NM exposure scenarios. Exposure medium and NM ageing moderation of NM toxicity were explored using Daphnia magna multigenerational studies aiming to determine whether the daphnids adapted to continuous exposure and/or if parent-only exposure resulted in epigenetic effects in subsequent generations. Daphnids were continuously, or parent-only, exposed to pristine and aged titanium dioxide (TiO2) and silver (Ag) NMs, in a standard high hardness culture media and synthetic European Class V lowland water. Pristine NMs in the standard culture medium had the most severe toxic consequences, and displayed negative effects in two generations post exposure. NMs aged in the class V water had fewer overall toxic consequences on growth and longevity across all generations in both continuous and parent-only exposure scenarios. Overall, in the absence of environmentally relevant media and aged NMs, standardised Daphnia tests currently overestimate NM toxicity, and fail to consider potential impacts in subsequent generations. The results demonstrate the importance of updating standard testing to reflect scientific advances and increase stakeholder trust in regulation.
Environmental significanceEnvironmental nanomaterial (NM) transformations can be diverse and the role of environmental conditions will strongly influence these processes. The majority of NMs ecotoxicology tests don't use environmentally transformed NMs in environmentally representative waters. Natural water standards containing natural organic matter (NOM) are far more representative for realistic exposure conditions than traditional toxicology test and culture media outlined in such protocols. To identify the effect of exposure media and NM ageing, Daphnia magna were exposed to a variety of pristine and 6 month aged titanium dioxide (TiO2) and silver (Ag) NMs in a standard Daphnia culture medium and a European class V lowland natural water standard. Pristine NMs had less toxic consequences in environmentally realistic medium compared to the standard culture medium, suggesting that the presence of NOM alters both the NM physico-chemistry and the interactions with Daphnia that are persistent across multiple generations. Furthermore, the aged NMs exposed to the Daphnia in the class V water had fewer overall toxic consequences on growth, mortality and reproduction including of generations F1–F3, compared to both pristine and aged NMs in the standard Daphnia culture medium. Thus, standardised Daphnia tests following the OECD protocols overestimate NMs toxicity, which can be resolved through ageing of the NMs in the medium and/or use of representative natural water compositions. Assessment of reproductive success of offspring of exposed daphnids is recommended, as surprising effects of pristine NMs were determined that could not be predicted from the F0 data. |
Introduction
The Organisation for Economic Co-operation and Development (OECD) recommends a defined synthetic media for the harmonised test guidelines (TGs) of chemicals in acute and chronic ecotoxicity of Daphnia magna immobilization and reproduction tests. The TGs recommended not to include any additives (such as soil extracts) to avoid inconsistencies in the characterisation of complex media between laboratories. Therefore, the recommended media for standardized toxicity testing of Daphnia are the Elendt M7/M4 and the high hardness combo media (HH combo).1,2 Other commonly used synthetic waters include those advised by the Environmental Protection Agency (EPA).3 Natural water usage is not discouraged for regulatory tests as long as they are well characterized and meet the validity criteria for testing and culturing Daphnia (pH, hardness, heavy metal analysis and the total organic carbon (TOC) levels do not exceed 2 mg L−1).2 Thus, the goal of standard testing is to facilitate comparison of chemicals under identical conditions for ranking of toxicity, rather than to provide environmental realism. While this works well for soluble chemicals, the highly reactive surfaces of NMs mean that the current tests are not optimal for NMs whose properties (and toxicity) are typically determined by their surroundings, a feature referred to as having extrinsic properties as well as intrinsic (unchanging) ones.4,5 Indeed it is widely acknowledged that “the influence of the dispersion medium on facilitating the existence of single particles or agglomerates is important” and should be considered in revision of TGs for ecotoxicity evaluation of NMs, as noted by.6 In their review of the OECD's ongoing work on revising TGs for NMs to facilitate mutual acceptability of data. Thus, the enormous surface reactivity of NMs and their tendency to interact with themselves or surrounding biomolecules to form a corona, suggests that lack of dispersing agent in the OECD Daphnia tests compromises the ability of the tests to rank NMs toxicity.Significant work is underway to assess the suitability of regulatory tests designed for (soluble) chemicals for use with nanomaterials (NMs) whose enormous reactive surface area makes them more challenging to assess. It is well known that media composition affects NM physicochemical properties7,8 and their toxicity,9 thus it is essential to consider more environmentally relevant waters for NMs ecotoxicology studies10,11 as well as assessment of appropriately transformed NMs under relevant conditions. Hammes, Gallego-Urrea12 classified European water types (class I–VI) based on their chemical properties (pH, ionic strength, composition and natural organic matter (NOM) content) for prediction of the stability of NMs. Application of these water classes for ecotoxicology assessments would provide more representative conditions and allow comparison of differently stabilized and transformed NM variants based on core speciation, acquisition of a NOM or other biomolecule corona and other environmental transformations. Environmental exposure assessment should consider older and/or transformed NMs in addition to the pristine (often highly reactive) forms. In the absence of realistic media and environmental ageing, the data is neither predictive or reflective of real NM exposure scenarios nor appropriate for risk assessments.13
We demonstrate here that the current use of pristine NMs in simple synthetic media significantly overestimates the NM hazards and risks, and that these impacts persist over multiple generations, whether the offspring are themselves also exposed or are removed from the exposure conditions and allowed to recover. While multigenerational impacts to daphnids are not currently considered in REACH and other regulatory regimes, understanding of the mechanisms of action of NMs including longer term and multigenerational effects are essential for future grouping, read-across and in silico predictions, as well as for population and ecosystems level understanding of the longer-term implications of exposure to NMs. Revisions to the harmonised TGs for NMs are underway currently (via the OECD) and data such as that reported here may drive further changes and adaptions in the longer term, including consideration of multi-generational effects especially in light of the drive to reduce animal testing and the need for alternative test systems. Indeed, the need to consider environmental coronas and feeding of the organisms during the acute Daphnia immobilisation TGs (OECD 202) have recently been suggested as adaptions needed for NMs testing.14
Few studies to date have investigated the long term effects of ‘aged/transformed’ NMs and those that have, concentrate efforts on NMs exposed to simple media between 24–48 hours and up to 21 days.15,16 Although both are necessary, they do not capture the long-term ageing of NMs, nor consider the effects of parental exposure to subsequent generations, whether in sustained multigeneration exposure or parental only exposure effects. The present study compares the toxicity of a panel of pristine and 6 month aged titanium dioxide (TiO2) and silver (Ag) NMs, where the NM ageing and exposure took place in both a standard Daphnia culture media (HH combo) and a synthetic class V European lowland water containing NOM.12 Effect concentrations, determined from acute 48 hour immobilization tests, were exposed to daphnids over 28 days (F0) with the F1–F3 generations either continuously exposed (Fexp), or removed for recovery in NM-free media (Frec).
Experimental
Materials
Commercially available chemicals, solvents and humic acids (HA) were purchased from Sigma-Aldrich (Dorset, UK) and were of analytical reagent grade. Ultrapure water (UPW) with a maximum resistivity of 18.2 MΩ cm−1 was used throughout the experiments.Media and representative waters
Experiments were conducted in Daphnia high hardness combo media (HH combo)17 and in a synthetic lowland class V artificial water;12 compositions of both are given in the ESI† Tables SI.1, SI.1A and SI.1B. The HH combo media represents an average hard water standard found in the environment without any natural organic matter (NOM)17 and is commonly used for the culturing of Daphnia. The class V lowland water has high alkalinity, high ionic strength and 4.6 mg L−1 NOM, and is typical of waters found in the southern UK, Poland, Greece, France, the Balearic countries and the Iberian Peninsula.12Nanomaterials and characterization
The NMs used in this study include PVP-coated and uncoated TiO2 NMs (both supplied by Promethean Particles Ltd.), Ag2S PVP coated Ag (AppNano Ltd, Spain), uncoated (bare) Ag (Promethean Particles Ltd.) and PVP coated Ag (Amepox Ltd, Poland) (see section 1.2 ESI†). Dynamic light scattering (DLS) was used to measure the hydrodynamic size of the pristine and aged NMs, using a Malvern Nanosizer 5000 instrument. Transmission electron microscopy (TEM) analysis (Table 1, Fig. 1 and 5) were performed using a JEOL 1200EX 80 kV and a JEOL 1400Ex 80 kV Max system. Samples were prepared for TEM by depositing a 10 μL drop of the NM suspension onto a 300 mesh carbon-coated copper TEM grid (Agar Scientific, UK). TEM primary particle sizes were determined by counting at least 100 NMs.| Identifier | Pristine TEM individual particle size (nm) | Aged in HH combo TEM individual size (nm) | Aged in class V TEM individual sizea (nm) | Pristine DLS particle size (nm) | Pristine PDI | Aged in HH combo DLS sizea (nm) | PDI of NMs aged in HH combo | Aged in class V river water DLS sizea (nm) | PDI of NMs aged in class V water | Surface coatingb |
|---|---|---|---|---|---|---|---|---|---|---|
| Note: The TEM sizes are reported as the individual NM sizes. PDI = polydispersity index. a Aged in the respective media for 6 months. b According to manufacturer. | ||||||||||
| TiO2-Uncoated | 9 ± 2 | 11 ± 3 | 11 ± 3 | 207 ± 11 | 0.5 | 305 ± 139 | 0.8 | 291 ± 149 | 1 | Bare |
| TiO2-PVP coated | 9 ± 2 | 10 ± 3 | 10 ± 2 | 311 ± 43 | 0.4 | 305 ± 176 | 0.8 | 409 ± 289 | 0.9 | PVP10 |
| Ag2S | 44 ± 14 | 39 ± 15 | 45 ± 15 | 299 ± 6 | 0.4 | 145 ± 2 | 0.3 | 171 ± 22 | 0.3 | PVP10 |
| Ag PVP | 18 ± 11 | 38 ± 19 | 105 ± 102 | 260 ± 180 | 0.3 | 129 ± 22 | 0.4 | 129 ± 141 | 0.7 | PVP10 |
| Ag uncoated | 61 ± 36 | 36 ± 16 | 95 ± 111 | 120 ± 30.5 | 0.5 | 7363 ± 1054 | 0.9 | 1423 ± 545 | 0.8 | Bare |
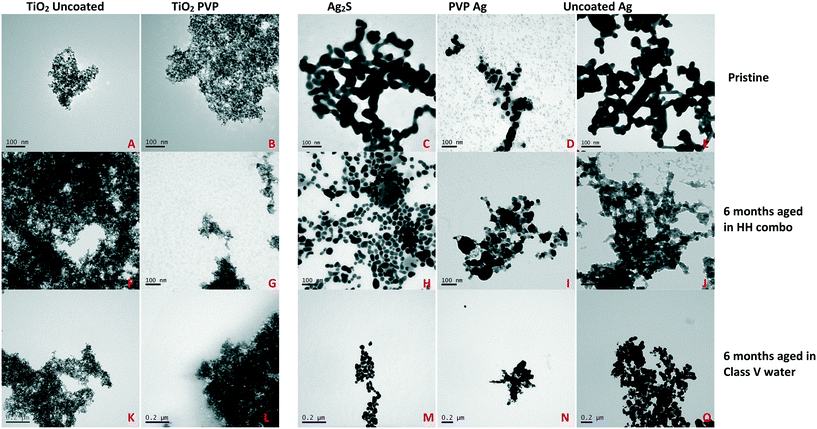 | ||
| Fig. 1 Characterization of the NMs in the two media before and after 6 months ageing. A: Pristine uncoated TiO2 in ultrapure water (UPW), B: pristine PVP TiO2 in UPW, C: pristine Ag2S in UPW, D: pristine PVP Ag in UPW, E: pristine uncoated Ag in UPW, F: aged uncoated TiO2 in HH combo media, G: aged PVP TiO2 in HH combo media, H: aged Ag2S in HH combo media, I: aged PVP Ag in HH combo media, J: aged uncoated Ag in HH combo media, K: aged uncoated TiO2 in class V water, L: aged PVP TiO2 in class V water. M: Aged Ag2S in class V water, N: aged PVP Ag in class V water and O: aged uncoated Ag in class V water. The pristine and aged TiO2 dispersions in each of the media were visibly aggregated when imaged with TEM, although little difference was observed between the pristine and aged TiO2 NM primary NM sizes. Ageing the Ag NMs in the two media had least effect on the Ag2S NMs, whereas the uncoated Ag was the most unstable leading to hetero-agglomeration (Table 1). | ||
Nanomaterial ageing
NMs were chemically aged by exposing stock solutions of 1000 mg L−1 to the HH combo and class V water for at least 6 months prior to Daphnia exposure (stored at 4 °C in darkness during the ageing). Aged samples were re-characterised by DLS and TEM (Table 1 and Fig. 1). Ageing and transformations of NMs is considered to reduce their reactivity and toxicity, and often results in sulfidation, phosphidation, chloride binding or other changes to the surface chemistry depending on the salts present in the media, and/or adsorption of biomolecules from their surroundings.18–20Daphnia maintenance, culturing and exposure conditions
The Daphnia magna Bham2 strain originated from the University of Reading.21Daphnia magna Straus (Clone Type 5 – IRCHA) were originally obtained from the Water Research Centre (WRc), Medmenham, UK. Initial stocks of daphnids were maintained using pools of third brood Bham2 strain (genetically identical) and were kept in a 20 °C temperature-controlled environment with 12 hour light and dark cycles. Daphnids were cultured in HH combo media and synthetic class V lowland water using only third broods to create successive generations. Media was refreshed weekly to ensure healthy maintenance and cultures were fed Cholorella vulgaris algae daily (0.5 mg carbon for days 0–5 and 0.75 mg carbon from day 5).We understand there is a difference between using environmental concentrations and ECs. Our justification for using effect concentrations in this study is due to regulation and environmental risks being assessed by characterizing the overall effects in biological receptors. For this reason, pilot studies (using 10 daphnids/250 mL medium in three replicates) were conducted to identify any issues with study design and/or the NM concentrations selected, as described in section 1.2 of the ESI.† The final selected concentrations for the multigenerational reproductive studies were EC30 values for the Ag NPs (20 μg L−1 of PVP Ag, 20 μg L−1 of uncoated Ag and 100 μg L−1 of A2gS, respectively (Fig. SI.1B†)) and EC5 values for the TiO2 NMs (5 mg L−1 for each of the TiO2 NMs).
Daphnia survival and growth
Daphnids were checked daily for survival, egg production and neonate release. Neonates of the third broods were used to set up the following generations (unless otherwise stated). Measurements of body length were taken every 3 days from release in accordance with the cycles of moulting of the carapace (between days 3–24).22 Body lengths were measured from the apex of the helmet to the base of the apical spine using a Nikon (Japan) stereomicroscope, model SMZ800 Digital Sight fitted with a D5-Fi2 camera, using NIS Elements software.Multigenerational study design
The goal of the multigenerational study was to assess in parallel the impacts of continuous exposure to the NMs in order to determine if there was any adaption to the exposure or a progressively severe response, versus the impacts of parent only exposure to determine if there were any epigenetic effects passed from exposed mothers to offspring grown under NM-free conditions, i.e. the recovery generations. Thus, the third brood neonates from the NM exposed F0 parent generations were split with half continuously exposed to the same NM and the other half grown in medium only, as shown schematically in Fig. SI.2.†The F0 generation consisted of a total of 10 daphnids/250 mL in three replicates exposed to the EC30 (Ag NMs) and EC5 (TiO2) concentrations for 1 month. The third broods (F1) from the F0 generation were removed within 24 hours of birth and then split into two groups: one group were kept exposed to the same EC concentration of NMs to produce a continuously exposed group (Fexp) over four successive generations (F0, F1exp, F2exp and F3exp) (see section 1.4 ESI†). The second group were removed from the parental F0 exposure and maintained in the relevant media only (NM free) for the three subsequent generations to produce the recovery sets (F1rec, F2rec and F3rec) (Fig. SI.2†). The medium (with or without NMs for the exposed and recovery experiments) was refreshed once per week. The F1–3 generations were always made from the third brood of the previous generation (unless otherwise stated, where 3rd brood numbers were very low), and neonates were removed from the exposures within 24 hours of birth for set up of the next generation.
Sample preparation for determination of NM (bio)accumulation (TEM)
The TEM cross sections of the F0 generations after 7 days exposure were prepared by the Centre for Electron Microscopy at the University of Birmingham (UK). Briefly whole Daphnia (neonates <24 h) were euthanised and fixed immediately in a 2.5% glutaraldehyde in a 0.1 M phosphate buffer suspension. Daphnids were dehydrated in ethanol and embedded in epoxy resin before sectioning using a ultramicrotome with a diamond knife. Images were visualized using JEOL 1200EX 80 kV and JEOL 1400EX 80 kV microscopes.Accumulation in daphnids and metal concentrations
At day 7, pools of 10 F0 daphnids were euthanized (using liquid nitrogen) and mechanically homogenised in 2% nitric acid (HNO3) using a Precellys 24 instrument (Bertin Technologies) with 2 cycles of 30 s at a 6000 pulse speed. Samples (in triplicate) were analysed for their (bio)accumulated concentration (accumulation in the gut and potential bioaccumulation inside the tissue) using inductively coupled plasma mass spectrometry (ICP-MS) (Nexion 300X instrument, Perkin Elmer). Samples were dissolved in 2% nitric acid and ran alongside an online germanium internal standard.After the first 7 days of exposure only, when the media was refreshed, samples of the old media containing NMs were taken for single particle-ICP-MS (NexION 300D, Perkin Elmer) in order to quantify the dissolved and NM concentrations. Operating conditions were optimized to produce maximum Ag/Ti intensity using Ag/Ti NM and ionic standards. Instrument calibration was achieved by analysis of a blank and 3 dissolved Ag/Ti solutions ranging from 0 to 1 μg L−1. Sample flow rates were measured prior to analysis. Transport efficiencies were determined using three standards of citrate stabilized gold NMs with diameters of 20, 40 and 80 nm. Time resolved signals were acquired using the Syngistix nano application module in the NexION software. All concentrations and particle diameters were determined from triplicate samples. To ensure no deviations of instrumental drift over time, Ag/Ti dissolved calibration check standard was also ran in between sampling. Experimental procedures were followed according to the Perkin Elmer application notes.23
Statistical analysis
All experiments were repeated in triplicate, and the data was recorded as the mean with standard deviation. For the growth studies, a student's unpaired t-test for unequal variance was used to observe the significant difference between the control and all exposed/recovery generations. In all analyses, a p-value < 0.05 was considered to be statistically significant (ESI:† Appendix 1 Tables AP.1–5).The linear model rate (using the slope coefficient to denote the rate of change) of daphnid growth between each population for their age versus time was analysed in RStudio using log10 transformations. A positive correlation number shows an incline in the rate of growth and the further from 0 the slope is, the steeper the fits though the data. Therefore, all exposed and recovery generations were compared to the control slope coefficients to denote the rate of growth. ESI:† (Appendix 1 Tables AP.6–7).
Results and discussion
Characterization of NMs pre- and post-ageing
Differently surface coated Ag and TiO2 NMs were specifically selected, since both materials are routinely used in health and fitness products, and have the most potential for widespread environmental release.24,25 The NMs were pre-characterised using TEM and DLS for both the pristine and post 6 months aged NMs in the standard Daphnia culture media (HH combo) and synthetic class V water. The results are presented in Table 1 and Fig. 1. The individual reported TEM sizes for the aged TiO2 were no different to the pristine dispersions. However, increased sizes and agglomeration were observed for all aged Ag NMs.It is important to note that TEM only measures a very small fraction of the sample, and in order to be able to size the individual particles it is necessary to focus on areas that are not so highly aggregated. While the TEM images (Fig. 1) clearly show aggregation between the different sets of particles, in many cases the aggregates were so large it was impossible to fit them into a single image to allow for sizing. For this reason, only the individual (primary) particle sizes are reported from the TEM data. The DLS reports the z-average size, which by definition, is an average over all sizes detected and as scattering scales 1/D6 (where D is the particle diameter) it skews the size towards larger particles. Thus, the absolute numbers are not the main message from Table 1, but rather changes in the numbers as a result of the ageing processes in the different media are the important feature.
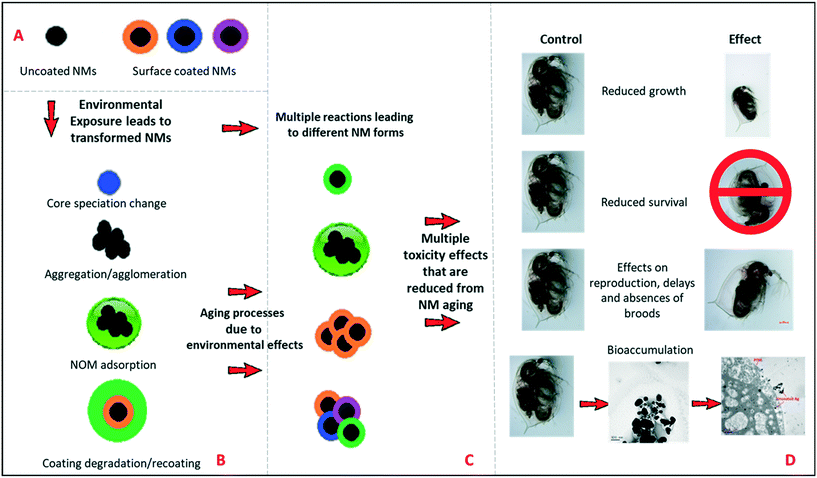
Nanomaterial environmental transformations and their possible effects
It is important for risk assessment and regulation to consider the types of transformations that NMs can undergo in the environment, and the impacts of these on NMs reactivity and toxicity. Fig. 2(A and B) represents schematically the possible environmental transformations of pristine NMs, such as TiO2 and Ag NMs, including altered surface speciation, acquisition of an environmental corona, or agglomeration, all of which reduce the surface reactivity and potential for oxidative dissolution (in the case of Ag NMs). Similarly, long-term ageing of the NMs in simple salt media lacking NOM, has also been found to passivate NMs surface chemistry (Fig. 2C) as demonstrated in this work, leading to dramatically reduced toxicity and impacts on Daphnia compared to the pristine NMs (Fig. 2D). Such transformations have previously been grouped into chemical, physical, biological (i.e. in the presence of microbes) and macromolecule binding, by Lowry et al.18 The fact that purely salt-based medium can result in reduced toxicity of the NMs is an interesting finding, and correlates with the observed changes in agglomeration and polydispersity (TiO2 and uncoated Ag NMs, as per Table 1).Moreover, initial pristine NM that are released into the environment is also an important aspect to consider, for this reason the pristine NM comparisons were conducted in both waters, to represent such interactions. The likelihood of contact between the pristine NMs with the Daphna, or any other aquatic species would be negotiable due to rapid transformation, which would occur almost instantaneously due to the high surface reactivity of the NMs with the surrounding environment. Rapid transformation has also been heavily demonstrated in the literature in a variety of different media using a range of different surface coated NMs. For example multiple studies have focused on determining the physicochemical changes undergone by Ag NPs during and after their use,26 assessments of their stability in various test media,7,27 fate/transformations28 and bioavailability.29
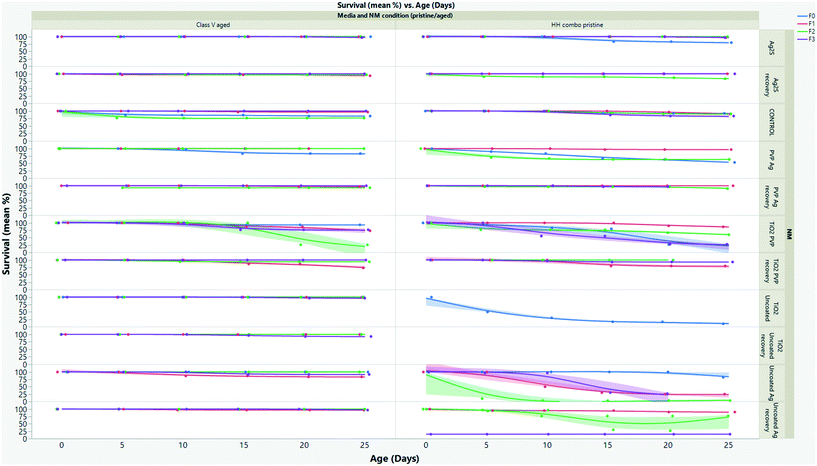
Longevity
The effect of pollutants on life history traits such as survival (Fig. 3 and SI.3†), growth (Fig. 4 and SI.4†) and reproduction (Fig. SI.3†), provides sensitive information for ecological stress and chemical toxicity.30 The results from the multigenerational study show that the NM composition (TiO2versus Ag), age (pristine/aged), surface coating and water condition (without/with NOM) significantly affected survival in each of the continuously exposed generations (Fig. 3 and SI.3†). By day 25 the F0 parent generation cultured in HH combo medium exposed to pristine Ag NMs had overall survivals of 73% (uncoated Ag), 53% (PVP Ag) and 83% (Ag2S) (Fig. 3A). The PVP Ag NMs were more tolerated in the subsequent generations with survivals of 97% (F1exp) and 63% (F2exp) by day 25, suggesting some adaption to the continuous exposure, although there were no surviving populations in the F3exp generations, whereas the F1–3exp generations continuously exposed to the uncoated Ag NMs had much lower total survivals of 25% (F1exp), 3% (F2exp) and 27% (F3exp) by day 25 (Fig. 3A).The F0 generation exposed to the pristine uncoated TiO2 NMs in the HH combo media were also extremely sensitive. After 5 days, only 50% survival was observed, which decreased to 17% by day 11 (Fig. 3A). Due to the delay between egg clutch production and the time taken to release the fifth brood, the F0 generational study continued for a total of 36 days (compared to between 25–28 days for controls). Due to poor neonate numbers produced by F0 daphnids exposed to uncoated pristine TiO2 NMs, the F1 generations were taken from the fourth broods (rather than the third broods), and within 24 hours post birth the F1exp generation had 100% mortality. Neonates removed from exposure to uncoated pristine TiO2 NMs in the F1rec generation survived for only 16 days before 100% mortality was observed. Due to reduced longevity, there were not enough individuals (for statistical significance) in the subsequent broods to produce the F2–3 generations thereafter. Reduced longevity and delays between broods were also observed for daphnids exposed to the pristine PVP TiO2 in the HH combo medium, with a total population survival 27% at day 25 and 24% by day 34. The F1 generations were also taken from the fourth broods due to poor neonate numbers in the third. The F1exp generation continued for 31 days and had survival of 70% (by day 31), showing some adaptation to the exposure conditions (Fig. 3A). Those in the F2exp and F3exp populations had 60% (day 29) and 50% (day 28) survival evidencing increased sensitivity to the exposures as the subsequent generations continue.
Explanations for the high mortality observed in the pristine NM exposure studies must be related to differences in exposure conditions between acute and chronic exposure studies. Food is added during the chronic multigenerational studies which may enhance the uptake of the NMs by ensuring a constant passage of material through the organism's guts31,32 Feeding is not recommended in the OECD acute immobilization TG,1 although it has been suggested that this is not ideal for NM exposures to filter feeding organisms since the EC can significantly change in chronic studies in the presence of food and NOM.31,32 Zhu33 and Das34 suggested that the presence of food during chronic exposure tests reduces the retention of TiO2 by accelerating excretion, thus lowering toxicity. This appears not to be true with the present study since there were higher mortalities in the presence of food (chronic exposures) than in its absence (acute exposures). It is possible that the TiO2 NMs can adsorb to the food sources accelerating their ingestion/retention and become trapped in the gut (Fig. 5B), leading to increased uptake and localized toxicity as observed by Nasser and Lynch.14,35
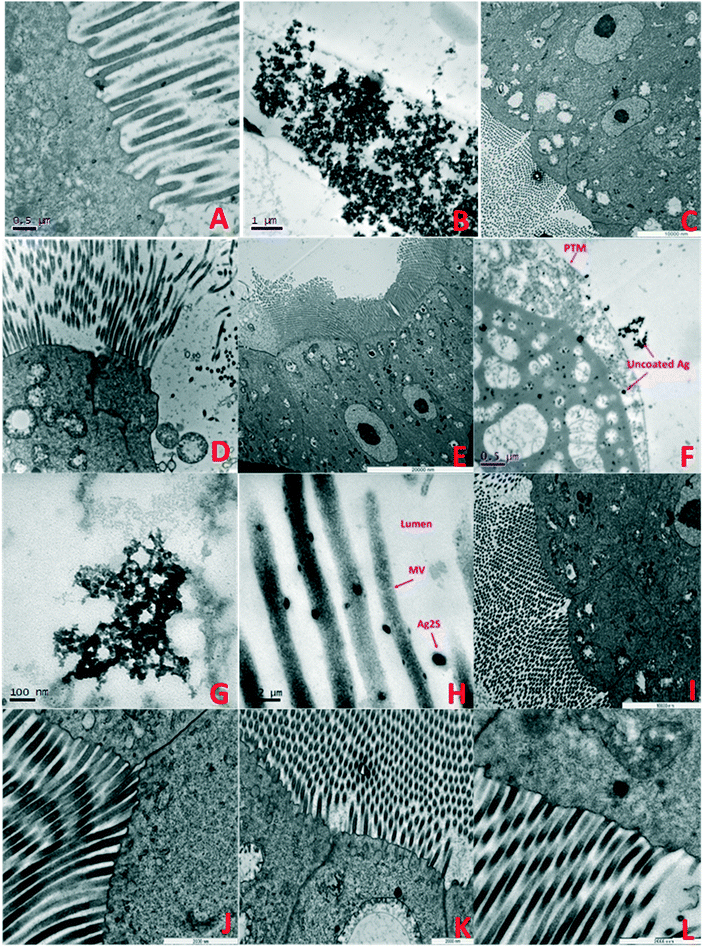 | ||
| Fig. 5 TEM images of F0 generation daphnid guts showing accumulation and impacts of NMs after 7 days exposure: A) F0 control: B) pristine PVP TiO2 in HH combo; showing accumulated TiO2 NMs in the gut lumen contained by the PTM, C) aged PVP TiO2 NMs in HH combo media, ordered cellular structures, evidence of lipofuscin and AV, D) pristine PVP TiO2 NMs in class V water showing losses of structural membranes, enlarged mitochondria and disorganisation of the MV, E) aged PVP TiO2 NMs in class V water showing a regular structured cell alignment, with evidence of enlarged mitochondria and AV, F) pristine uncoated Ag NMs in HH combo interacting with the cell, with lipid like cytoplasmic inclusions and large vacuole structures. Such interactions which may have disturbed metabolism and chemical transformations48 which is consistent with reduced growth and delayed reproduction (Fig. SI.3†), G) pristine PVP Ag NMs in HH combo, H) pristine Ag2S NMs in HH combo, I) aged PVP Ag NMs in HH combo, J) aged Ag2S Ag NMs in HH combo, K) aged uncoated Ag NMs in class V, showing disorganization to the mitochondria, appearing lysed with empty internal spaces and disorganization of their cristae, and L) aged Ag2S NMs in class V. Key: mitochondria (M), cell junctions (CJ), autophagy vacuoles (AV) nucleus (N), nucleolus (n), microvilli (MV), apical membrane (AM) and the peritrophic membrane (PTM). | ||
The initial uptake via food sources may explain the high population mortality in the F0 generations, leading to interrupted metabolic pathways, cellular signalling, and enzyme function.36 Zhu33 also reported from acute studies, that daphnids exposed to 50 and 100 mg L−1 uncoated TiO2 NMs (21 nm) had between 10 and 20% immobilization with no significant mortality. However, in chronic exposures (3–21 days) to 0.1 mg L−1 uncoated TiO2, the mortality increased significantly ranging from 13–100%, in strong agreement with our study. Chronic 21 day exposures by Kim et al.36 further observed 90% mortality at concentrations of 2.5 mg L−1 uncoated TiO2. Those experiments were conducted in moderately hard water (pH 7.6) using uncoated TiO2 (40 nm). The original 48 hour EC50 was 4.5 mg L−1 whereas the EC50 values for 7 and 14 day exposures were 2.7 and 1.9 mg L−1, respectively, thus, as with our pilot acute studies (ESI,† section 1.3), the EC30 values significantly underestimated the toxicity in the chronic exposures.
We hypothesize that the toxic effects were mediated from the direct exposure of accumulated uncoated TiO2 as shown in Fig. 5B, leading to ROS production in vivo. We further consider that the uptake of TiO2 with food leads to accumulation in the gut. Heavily agglomerated TiO2 has been shown to disturb chemical transformations in the digestive tract, and the accumulation can interfere with food intake.37 Therefore, as a result the accumulation causes localized toxic effects when the daphnids fail to excrete the TiO2, which would not have been observed in the initial OECD acute range finding tests.
When the NMs were chemically aged in the HH combo media, the survival across the exposed generations was significantly increased when compared to the pristine NM exposures in HH combo media (Fig. SI.3B†), showing that NM aging even in simple salt medium reduces their toxicity. The F0 generations exposed to the aged Ag NMs, had overall increased survival at day 25 at 97% (uncoated Ag), 97% (PVP Ag) and 90% (Ag2S). Survival of daphnids continuously exposed to the aged uncoated Ag was 86% (F1exp), 97% (F2exp) and 77% (F3exp) between days 25–30. For the aged uncoated TiO2 exposures (Fig. SI.3B†), the F0 generation had 97% survival, and successive F1–3 generations (continuously exposed) had total survivorships of 100% (F1exp), 93% (F2exp) and 70% (F3exp) at day 25. Survival was also increased for populations exposed to aged PVP TiO2 NMs, (F0 had 90% survival at day 25) when compared to the pristine NM exposures in HH combo medium. The F1–3rec/exp generations had ≥90% survival by day 25, showing that the parental exposure had very little effect on the subsequent non-exposed generations for the aged NMs. In all cases, the increased survival of exposure generations shows positive adaptations to continuous exposure and of recovery generations suggests that parental exposure to the aged NMs does not significantly affect fitness or survival of subsequent unexposed generations, thus further indicating that ageing the NMs greatly reduced the toxic effects compared to the pristine (highly reactive) NMs.
To mimic realistic environmental conditions, the pristine and aged NM exposures were repeated in synthetic class V European lowland water (Table SI.1†), in order to assess the media composition effects on NM toxicity. Significant differences were observed in the daphnid survival in the class V water compared to the HH combo medium studies (Fig. 3 and SI.3B†). The survival of the F0–F3 generations in the controls was ≥96% which was notably higher than those in the HH combo medium (although those were within the OECD guidelines for control survival), suggesting that more realistic medium supports healthier daphnids. Daphnids exposed to pristine uncoated Ag in the class V water were more sensitive to toxic effects (Fig. SI.3C†) compared to the pristine NM exposures in the HH combo medium. Reduced survival was observed for each of the exposed generations, with 10% (F0), 40% (F1exp) 7% (F2exp) and 13% (F3exp) survival at day 25. These findings are consistent with previous research, which also observed increased toxicity in the presence of pristine NMs in the presence of NOM,38 or a secreted biomolecule corona25 which was hypothesised to be a result of NM agglomeration and the texture of the corona layer resulting in the particles appearing more like the daphnids typical food source of algae.
The recovery generations had survivals of 63% (F1rec), 93% (F2rec), and 13% (F3rec) between days 25–28 showing that maternal exposure to pristine uncoated Ag in class V media had some epigenetic consequences with effects from maternal exposure continuing into the subsequent recovery generations. On the other hand, the pristine PVP Ag and the uncoated TiO2 NMs exposures evidenced significantly increased survival in the class V exposures (compared to the HH combo), showing surface coating and media specific differences. Daphnids exposed to the pristine uncoated TiO2 in the class V media were able to produce successive offspring showing positive outcomes using the same pristine NMs in realistic waters. The survivorship of the F0–3exp generations was 86% (F0), 67 (F1exp) 41% (F2exp) and 83% (F3exp) and for the recovery generations was between 90–100% between days 25–30.
Based on the HH combo results, high survival for the daphnid generations was expected for those exposed to pristine PVP TiO2 NMs (Fig. SI.3C†). Intriguingly, only 50% survived (F0) the duration of the study (until day 28), and both the exposed and recovery F2 generations did not produce sufficient neonate numbers in their third broods to produce the F3 generations. Thus the PVP–NOM interactions and resulting corona may have led to increased uptake and retention, and thus toxicity. Interestingly, the uncoated Ag NMs under the same conditions also had increased toxicity which in this case may be linked to NOM-enhanced dissolution (Table SI.4†). The adsorption process of NOM to the NM surface is not fully clear (given the qualitative difference between the NOM and extracted humic substances used in many studies), and the mechanisms of toxicity between the uncoated Ag and TiO2 NMs in both water types is observably different. The difference is perhaps linked to the surface chemistry and stability issues with Ag, which is known to produce ionic Ag from dissolution in vivo39,40 and in different media.7
The toxicity of the pristine PVP TiO2 and Ag exposures was high in the class V water. To rule out the possibility of this being PVP surface coating toxicity resulting from the PVP being displaced by NOM, studies exposing only PVP (at concentrations equal to those coating the NMs) to the daphnids for the same length of time were conducted, resulting in no significant differences compared to control populations (Fig. SI.3B and D and SI.4B and SI.4B–D†). Apart from the aged PVP Ag which showed some toxicity, most of the aged NM exposures in the class V media had little to no mortality in the multigenerational exposures (Fig. 3). These results emphasize that NM ageing/transformation and environmentally suitable media are key for realistic exposure scenarios when addressing the potential hazards of NMs, and that more work is needed to really understand these time-resolved transformations and their consequences for toxicity. Previous studies have highlighted that NMs that were thought to be stable when coated in PVP7 are not when exposed in realistic waters, as evidenced also by the differences in survival for daphnids exposed to pristine TiO2 PVP exposures in the two different media. Furthermore, feeding the animals in the acute immobilization tests may avoid underestimation of the acute EC50 from the initial range finding studies.
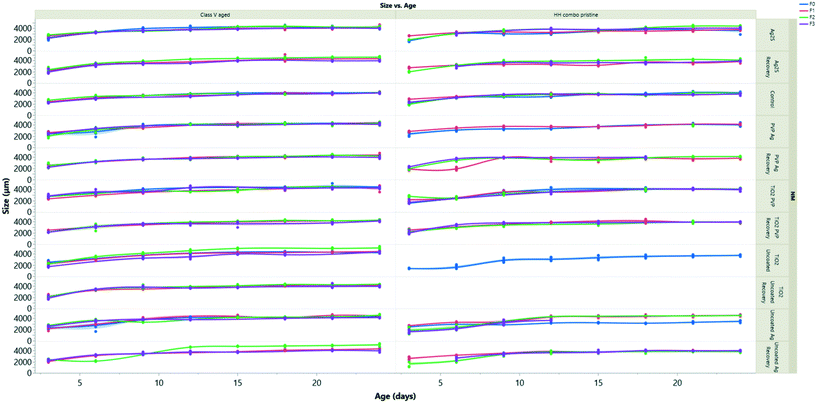
Growth effects
The growth measurements over time for all generations are presented in Fig. 4 following exposure to pristine/aged Ag and TiO2 NMs in both water conditions. log10 transformations were used to create linear models to assess the rate of daphnid growth over time (using the equation of the line of best fit to determine the slope) determined as the coefficient of change (Appendix 1: Table AP.7†) as shown in Constantinou, Sullivan.41 NMs are considered to be toxic if there is a reduction in the coefficient relative to the control groups, which had values between 0.008–0.009.When compared to the control populations, the daphnids exposed to pristine uncoated Ag NMs (F0) in the HH combo medium were on average 5% larger than the controls after 24 hours but were significantly smaller (p < 0.05) from day 9 onwards (Fig. 4A). The rate of growth (log10 transformation of the slope) was 0.011, evidencing the accelerated growth up to day 9. The F1–3exp populations had values closer to 0 (0.003–0.006) evidencing the decrease in size and the fact that they grew slower than the controls. The F1–3rec also had variances in their growth rates (0.003–0.0110) compared to the controls, despite being removed from the exposure scenario, showing epigenetic traits from maternal exposure stress. Similarly, exposures to pristine uncoated TiO2 NMs in the HH combo medium under the same conditions also significantly inhibited the growth of the daphnids (Appendix 1: Table AP.6†) (Fig. 4). The F0 populations grew slowly (0.006 Appendix 1: Table AP.6†) relative to the control populations (0.008 Appendix 1: Table AP.6) and were on average 48% smaller by comparison on day 6 of the exposure.
The inhibition in growth over the exposure period correlates well with the survival data and the delays between broods observed (Fig. SI.5 ESI†) and suggest that the effects may be due to negative impacts on the feeding behaviour. As previously discussed, food quality has an influence on life history traits such as growth,42 and maternal feeding has been documented to affect offspring growth and reproduction.43 It should be noted that there were large green (algae) and white (TiO2) aggregates at the bottom of each of the beakers (observed for all TiO2 exposure conditions), which was also observed in studies by Bundschuh, Vogt,44 who also observed size reductions of juvenile daphnids exposed to TiO2 NMs. The sedimentation/hetero-aggregation of the alage-TiO2 complexes suggests that the TiO2 NMs reduced the maternal (F0) food availability and likely also food intake. Zhu33 reported exposures to uncoated TiO2 NMs reduced the feeding and filtration behaviour of daphnids, resulting in inhibited growth and reproduction, as observed in the present study. Despite visual sedimentation of the NMs with algae, our results agree with the previous findings and suggest that TiO2 NMs ingested with food enhances the internal concentration (ESI† Table SI.4) and associated toxicity compared to NM exposure in the absence of food.
Daphnids (F0) exposed to pristine PVP TiO2 in HH combo medium (Fig. 4) between 24 hours and day 6, were significantly (23%, p = 0.004 and 0.0002) smaller than the control populations. After day 9, there was no significant difference between the growth of the control and daphnids exposed to the pristine PVP TiO2 NMs, indicating that NM-exposed juveniles do reach full size, but more slowly, which is confirmed by the growth correlation coefficient value of 0.006 compared to the control of 0.008 (Appendix 1 Table AP.6†). The F1–3exp generations were also, on average, significantly (p < 0.02) smaller and grew slowly compared to the controls, whereas the F1–2rec generations were comparable with the control populations indicating no impacts on growth or growth rate from material exposure to pristine PVP TiO2 NMs in HH combo medium in subsequent generations.
Comparing the pristine and aged NM exposures under the same conditions, daphnids exposed to the aged Ag and TiO2 NMs had less variance and less significant size differences between each of the different coated particles compared to the pristine NMs (Fig. SI.4B†). The most significant differences were observed at day 3 where the NM exposed daphnids (F0) were up to 11% (uncoated Ag), 18% (PVP Ag) and 4% (Ag2S) smaller than the controls and grew more slowly (Appendix 1: Table AP 7†). Interestingly, daphnids (F0) exposed to the uncoated TiO2 NMs aged in HH combo media (Fig. SI.4B†) exhibited accelerated growth (up to day 15), where they were on average 10–33% larger than the control populations which was evidenced by increased growth (Appendix 1 Table AP.6†). Accelerated growth has been documented to occur under increased stress situations.45 In most cases the F1–3exp generations were significantly smaller than the controls, with the most extreme being 35% smaller in the F3exp population, for the aged uncoated TiO2, which also grew more slowly in HH combo media (0.006: Appendix 1 Table AP.6†). The decline in growth in the multigenerational exposures may be due to an energy trade-off whereby growth is sacrificed to support basic survival under toxic conditions.46 In all recovery populations (F1–F3rec), the sizes and growth rates (ESI† Appendix 1 Table AP.6) were comparable with the controls, showing regeneration after the F0 maternal exposure.
The F0 generation exposed to the aged PVP TiO2 NMs were also larger than the control populations in HH combo media. The F1–3exp generations had comparable growth rates to the controls with corresponding log10 transformation coefficients of 0.008 (ESI† Appendix 1 Table AP.6). To exclude the possibility of the PVP surface coating affecting growth, measurements were made to daphnids exposed only to the PVP coating in both the HH combo and class V media (Fig. SI.4B† and 4D), showing little to no differences from day 6.
The pristine NM exposure (TiO2 and Ag NMs) in the class V water, had a similar reduced daphnid size effect which was also observed in the pristine NM exposures in the HH combo media. However, despite reduced size effects fewer toxic consequences were observed when exposed to the pristine uncoated TiO2 NMs. Unlike the uncoated TiO2 in the HH combo, the F0 generation in class V water were able to produce successive F1–3 generations.
For all conditions (TiO2 and Ag exposures), the successive F1–3exp generations were on average always smaller than the controls and grew slower (Appendix 1: Tables AP.6 and AP.7†). The daphnids exposed to the aged NMs in the class V water had even less variance between their body sizes compared to the controls. Therefore, the combination of both NM ageing and realistic exposure media show fewer toxic consequences to the daphnids when compared to the pristine exposures in the HH combo media.
(Bio)accumulation
Understanding the life cycle and interactions of NMs with biological matter has an important role in nanosafety and regulation. Although individual organ distributions of the TiO2 and Ag NMs were not identified, the average (bio)accumulation concentration of Ag and Ti per daphnid in the F0 generation was determined (ESI† Tables SI.2–SI.5) 7 days after initial exposure. Measurements further include the average NM size, NM concentration (particles per mL) and dissolved metal concentrations (μg L−1) in solution after the 7 days. In all cases, the behaviours of the NMs between the pristine/aged and different waters were surface coating and media specific. The internalized concentrations were always lower in the aged NMs than the pristine exposures. In the pristine HH combo medium, the highest retained concentration of total Ag was in the pristine Ag2S exposures at 0.716 μg L−1 (F0), compared to 0.174 μg L−1 (F0) after exposure to aged Ag2S NMs. Interestingly, the aged uncoated Ag NMs had higher concentrations of total Ag compared to the pristine exposures (Table SI.2†), although less toxic effects (growth/mortality) were observed, due to the reduction of ionic Ag arising from NOM stabilization of the NMs. The internalized concentration of pristine uncoated Ti was on average 4.279 μg L−1 per exposed daphnid in the HH combo medium. This was particularly high when compared with the internalized concentrations identified in the daphnids exposed to aged uncoated TiO2, which ranged between 1.078–1.754 μg L−1 under the same conditions. The pristine uncoated and PVP TiO2 NMs in class V river water also had high accumulation of Ti compared to the aged TiO2 NMs (Table SI.5†).TEM cross sections revealed localised accumulation in the daphnid guts of all NMs. Pristine PVP TiO2 can be seen along the peritrophic membrane (PTM) Fig. 5B showing disorganisation of the gut microvilli (MV) and the presence of autophagic vacuoles (AV). Autophagic vacuoles contribute to homeostasis by degrading damaged organelles, proteins and lipid droplets, and are involved in development and growth regulation. In response to stress, AV have been linked to survival mechanisms during short terms starvation, by degrading nonessential components from which the cells can continue to sustain nutrients for biosynthetic processes.47 This may be true for the conditions observed in our experiments, since the food source intake may have been disrupted by TiO2 and Ag NM accumulation in the gut and/or the agglomeration and precipitation of NM-algal complexes noted previously.
Conclusion
Clear differences between the toxicological consequences of pristine and aged TiO2 and Ag NMs were observed in the two different media conditions – salt only (HH combo) or NOM-containing (class V) water. Pristine NMs exposed in the standard culture media had the most toxic consequences to survivorship and growth and the highest (bio)accumulation, and furthermore displayed negative effects in daphnids two recovery generations post the parental exposure. The F0 generations exposed to the pristine uncoated TiO2 NMs in the HH combo medium were the most sensitive to chronic exposure, resulting in decreased longevity, reduced growth and dramatically inhibited reproductive success. For example, the survival of the F1 generations from parents exposed to pristine TiO2 NMs in HH combo was less than 24 hours in the continuously exposed group, and just 16 days in the recovery generation. Since the reproductive TG as currently designed (OECD 211) does not look at the survival of the F1 generation, nor its ability to reproduce, these longer-term effects of chronic exposure to NMs are completely missed. The poor survival of the F1 recovery generation, where the daphnid neonates were removed from exposure within 24 hours of birth and subsequently cultured in medium free of NMs, suggests that the effects are sufficiently dramatic as to warrant further investigation. The results warrant serious consideration of whether multiple generations should be incorporated into the reproductive assay since collapse of F1 populations would have dramatic effects on food-chains and the ecosystems services provided by crustaceans. The evidence of epigenetic effects is also interesting in the context of developing alternatives to animal testing.NM ageing in the salt-only HH combo medium reduced the toxic consequences for both the exposed and recovery generations, whereas the NMs aged in the NOM-containing class V water had fewest overall toxic consequences on growth, longevity and survivorship, reproduction, and (bio)accumulation across all exposed generations, with no observable differences between the recovery generations post parental exposure and the controls. The results suggest a mediating role of the ionic strength and NOM in the realistic exposure medium. In addition, particle ageing reduces the toxicity via transformations to less reactive surfaces, through interactions with salts or with NOM. Analysis of the toxicity data provides very strong evidence that the pristine NMs are more toxic compared to their aged counter parts, with the NMs being especially toxic in the HH combo medium.
Current harmonised TG for Daphnia acute and chronic toxicity tests have herein been demonstrated to overestimate NM toxicity to the F0 generation but also to completely ignore the potentially even stronger toxic effects on subsequent generations arising from parental and/or continuous exposure to NMs. The results show the importance of updating harmonised TG documents to reflect scientific advances and increase stakeholder trust in regulation. Based on the data presented here, standardised Daphnia tests following the OECD TGs greatly overestimate the toxicity of pristine NMs in the absence of an environmentally relevant media, and ignore potential epigenetic and/or cumulative effects in subsequent generations. When making a regulatory assessment it is necessary to obtain a complete understanding of NMs toxicity, which requires comparison of the differences between the pristine and transformed/aged NMs under a range of media conditions that span the range of transformations/extrinsic properties of the NMs in order to facilitate toxicity ranking. The fact that the media conditions are closer to ‘real’ or environmentally relevant form of these materials that would actually be encountered by target organisms, is an additional benefit, but is not the central goal. Longer term considerations and test revisions would ideally also ensure that the offspring are themselves able to survive and reproduce in order not to cause catastrophic population collapse. Further revision of harmonised TGs are to consider multi-generational effects is likely to be required as further weight of evidence is accumulated.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded via a NERC highlight topic grant (NE/N006569/1), with additional support from EU H2020 grant NanoSolveIT (Grant Agreement No. 814572). The authors acknowledge access to the following University of Birmingham facilities: the Daphnia Research and the Environmental Genomics facilities, the Proteomics Facility and the school of Materials and Metallurgy. Technical support and assistance from Paul Stanley and Theresa Morris with the TEM histology preparations is also acknowledged.References
- OECD, Guideline for testing of chemicals. Daphnia sp., Acute Immobilisation Test 202, Adpoted April 2004., 2004 Search PubMed.
- OECD, OECD Guidline for the testing of chemicals. Test No. 211: Daphnia magna Reproduction Test, OECD Publishing, 2012 Search PubMed.
- US–EPA, Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, EPA/600/4–90/027F, 1993 Search PubMed.
- I. Lynch, C. Weiss and E. Valsami-Jones, A strategy for grouping of nanomaterials based on key physico-chemical descriptors as a basis for safer-by-design NMs, Nano Today, 2014, 9(3), 266–270 CrossRef CAS.
- J. Rose, M. Auffan, O. Proux, V. Nivière and J.-Y. Bottero, Physicochemical properties of nanoparticles in relation with toxicity, Encyclopedia of Nanotechnology, 2012, p. 2085 Search PubMed.
- K. Rasmussen, H. Rauscher, P. Kearns, M. González and J. R. Sintes, Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data, Regul. Toxicol. Pharmacol., 2019, 104, 74–83 CrossRef PubMed.
- M. Tejamaya, I. Römer, R. C. Merrifield and J. R. Lead, Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media, Environ. Sci. Technol., 2012, 46(13), 7011–7017 CrossRef CAS PubMed.
- I. Römer, T. A. White, M. Baalousha, K. Chipman, M. R. Viant and J. R. Lead, Aggregation and dispersion of silver nanoparticles in exposure media for aquatic toxicity tests, J. Chromatogr. A, 2011, 1218(27), 4226–4233 CrossRef PubMed.
- C. Loureiro, B. B. Castro, J. L. Pereira and F. Gonçalves, Performance of standard media in toxicological assessments with Daphnia magna: chelators and ionic composition versus metal toxicity, Ecotoxicology, 2011, 20(1), 139–148 CrossRef CAS PubMed.
- R. Y. Prasad, K. Wallace, K. M. Daniel, A. H. Tennant, R. M. Zucker and J. Strickland, et al. Effect of treatment media on the agglomeration of titanium dioxide nanoparticles: impact on genotoxicity, cellular interaction, and cell cycle, ACS Nano, 2013, 7(3), 1929–1942 CrossRef CAS PubMed.
- M.-H. Shen, X.-X. Zhou, X.-Y. Yang, J.-B. Chao, R. Liu and J.-F. Liu, Exposure medium: key in identifying free Ag+ as the exclusive species of silver nanoparticles with acute toxicity to Daphnia magna, Sci. Rep., 2015, 5, 9674 CrossRef CAS PubMed.
- J. Hammes, J. A. Gallego-Urrea and M. Hassellöv, Geographically distributed classification of surface water chemical parameters influencing fate and behavior of nanoparticles and colloid facilitated contaminant transport, Water Res., 2013, 47(14), 5350–5361 CrossRef CAS PubMed.
- M. Markiewicz, J. Kumirska, I. Lynch, M. Matzke, J. Köser and S. Bemowsky, et al. Changing environments and biomolecule coronas: consequences and challenges for the design of environmentally acceptable engineered nanoparticles, Green Chem., 2018, 20(18), 4133–4168 RSC.
- F. Nasser and I. Lynch, Updating traditional regulatory tests for use with novel materials: Nanomaterial toxicity testing with Daphnia magna, Saf. Sci., 2019, 118, 497–504 CrossRef.
- A. D'Agata, S. Fasulo, L. J. Dallas, A. S. Fisher, M. Maisano and J. W. Readman, et al. Enhanced toxicity of ‘bulk'titanium dioxide compared to ‘fresh'and ‘aged'nano-TiO2 in marine mussels (Mytilus galloprovincialis), Nanotoxicology, 2014, 8(5), 549–558 CrossRef PubMed.
- N. Chouhan, R. Ameta and R. K. Meena, Biogenic silver nanoparticles from Trachyspermum ammi (Ajwain) seeds extract for catalytic reduction of p-nitrophenol to p-aminophenol in excess of NaBH4, J. Mol. Liq., 2017, 230, 74–84 CrossRef CAS.
- S. S. Kilham, D. A. Kreeger, S. G. Lynn, C. E. Goulden and L. Herrera, COMBO: a defined freshwater culture medium for algae and zooplankton, Hydrobiologia, 1998, 377(1–3), 147–159 CrossRef CAS.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46(13), 6893–6899 CrossRef CAS PubMed.
- D. M. Mitrano, S. Motellier, S. Clavaguera and B. Nowack, Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products, Environ. Int., 2015, 77, 132–147 CrossRef CAS PubMed.
- S. M. Briffa, I. Lynch, V. Trouillet, M. Bruns, D. Hapiuk and E. Valsami-Jones, Thermal transformations of manufactured nanomaterials as a proposed proxy for ageing, Environ. Sci.: Nano, 2018, 5(7), 1618–1627 RSC.
- L.-H. Heckmann, R. Connon, T. H. Hutchinson, S. J. Maund, R. M. Sibly and A. Callaghan, Expression of target and reference genes in Daphnia magna exposed to ibuprofen, BMC Genomics, 2006, 7(1), 175 CrossRef PubMed.
- D. Ebert, Ecology, epidemiology, and evolution of parasitism in Daphnia, National Library of Medicine, 2005 Search PubMed.
- J. Shipp, C. Stephan and C. Shelton, Analysis of CeO2 Chemical Mechanical Planarization Slurries Using SP-ICP-MS, 2017.
- F. Gottschalk, T. Sun and B. Nowack, Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies, Environ. Pollut., 2013, 181, 287–300 CrossRef CAS PubMed.
- B. Nowack, M. Baalousha, N. Bornhöft, Q. Chaudhry, G. Cornelis and J. Cotterill, et al. Progress towards the validation of modeled environmental concentrations of engineered nanomaterials by analytical measurements, Environ. Sci.: Nano, 2015, 2(5), 421–428 RSC.
- S. Pakrashi, C. Tan and W. X. Wang, Bioaccumulation-based silver nanoparticle toxicity in Daphnia magna and maternal impacts, Environ. Toxicol. Chem., 2017, 36(12), 3359–3366 CrossRef CAS PubMed.
- A. M. E. Badawy, T. P. Luxton, R. G. Silva, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions, Environ. Sci. Technol., 2010, 44(4), 1260–1266 CrossRef PubMed.
- G. V. Lowry, B. P. Espinasse, A. R. Badireddy, C. J. Richardson, B. C. Reinsch and L. D. Bryant, et al. Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland, Environ. Sci. Technol., 2012, 46(13), 7027–7036 CrossRef CAS PubMed.
- K. Park, E.-J. Park, I. K. Chun, K. Choi, S. H. Lee and J. Yoon, et al. Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats, Arch. Pharmacal Res., 2011, 34(1), 153–158 CrossRef CAS PubMed.
- E. Besseling, B. Wang, M. Lürling and A. A. Koelmans, Nanoplastic affects growth of S. obliquus and reproduction of D. magna, Environ. Sci. Technol., 2014, 48(20), 12336–12343 CrossRef CAS PubMed.
- H. J. Allen, C. A. Impellitteri, D. A. Macke, J. L. Heckman, H. C. Poynton and J. M. Lazorchak, et al. Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna, Environ. Toxicol. Chem., 2010, 29(12), 2742–2750 CrossRef PubMed.
- I. Blinova, J. Niskanen, P. Kajankari, L. Kanarbik, A. Käkinen and H. Tenhu, et al. Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus, Environ. Sci. Pollut. Res., 2013, 20(5), 3456–3463 CrossRef CAS PubMed.
- X. Zhu, Y. Chang and Y. Chen, Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna, Chemosphere, 2010, 78(3), 209–215 CrossRef CAS PubMed.
- P. X. Das and A. Marguerite, Metcalfe, Chris D. Toxicity of silver and titanium dioxide nanoparticle suspensions to the aquatic invertebrate, Daphnia magna, Bull. Environ. Contam. Toxicol., 2013, 91(1), 76–82 CrossRef CAS PubMed.
- F. Nasser and I. Lynch, Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna, J. Proteomics, 2016, 137, 45–51 CrossRef CAS PubMed.
- K.-T. Kim, S. J. Klaine and S. D. Kim, Acute and chronic response of daphnia magna exposed to TiO2 nanoparticles in agitation system, Bull. Environ. Contam. Toxicol., 2014, 93(4), 456–460 CrossRef CAS PubMed.
- D. Kwon, H. W. Nho and T. H. Yoon, Transmission electron microscopy and scanning transmission X-ray microscopy studies on the bioaccumulation and tissue level absorption of TiO2 nanoparticles in Daphnia magna, J. Nanosci. Nanotechnol., 2015, 15(6), 4229–4238 CrossRef CAS PubMed.
- F. Seitz, S. Lüderwald, R. R. Rosenfeldt, R. Schulz and M. Bundschuh, Aging of TiO2 nanoparticles transiently increases their toxicity to the pelagic microcrustacean Daphnia magna, PLoS One, 2015, 10(5), e0126021 CrossRef PubMed.
- J. Hou, Y. Zhou, C. Wang, S. Li and X. Wang, Toxic effects and molecular mechanism of different types of silver nanoparticles to the aquatic crustacean Daphnia magna, Environ. Sci. Technol., 2017, 51(21), 12868–12878 CrossRef CAS PubMed.
- Y. Hu, X. Chen, K. Yang and D. Lin, Distinct toxicity of silver nanoparticles and silver nitrate to Daphnia magna in M4 medium and surface water, Sci. Total Environ., 2018, 618, 838–846 CrossRef CAS PubMed.
- J. Constantinou, J. Sullivan and L. Mirbahai, Ageing differently: Sex-dependent ageing rates in Daphnia magna, Exp. Gerontol., 2019, 121, 33–45 CrossRef CAS PubMed.
- G. Abrusán, P. Fink and W. Lampert, Biochemical limitation of resting egg production in Daphnia, Limnol. Oceanogr., 2007, 52(4), 1724–1728 CrossRef.
- J. S. Garbutt and T. J. Little, Maternal food quantity affects offspring feeding rate in Daphnia magna, Biol. Lett., 2014, 10(7), 20140356 CrossRef PubMed.
- M. Bundschuh, R. Vogt, F. Seitz, R. R. Rosenfeldt and R. Schulz, Do titanium dioxide nanoparticles induce food depletion for filter feeding organisms? A case study with Daphnia magna, Environ. Pollut., 2016, 214, 840–846 CrossRef CAS PubMed.
- R. Smolders, M. Baillieul and R. Blust, Relationship between the energy status of Daphnia magna and its sensitivity to environmental stress, Aquat. Toxicol., 2005, 73(2), 155–170 CrossRef CAS PubMed.
- A. Mikulski, P. Bernatowicz, M. Grzesiuk, M. Kloc and J. Pijanowska, Differential levels of stress proteins (HSPs) in male and female Daphnia magna in response to thermal stress: a consequence of sex-related behavioral differences?, J. Chem. Ecol., 2011, 37(7), 670–676 CrossRef CAS PubMed.
- E.-L. Eskelinen, Maturation of autophagic vacuoles in mammalian cells, Autophagy, 2005, 1(1), 1–10 CrossRef CAS PubMed.
- D. Kwon, H. W. Nho and T. H. Yoon, X-ray and electron microscopy studies on the biodistribution and biomodification of iron oxide nanoparticles in Daphnia magna, Colloids Surf., B, 2014, 122, 384–389 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en00049c |
| This journal is © The Royal Society of Chemistry 2020 |