Functional zwitterionic biomaterials for administration of insulin
Xingyu
Chen
 * and
Dongqiong
Yang
* and
Dongqiong
Yang
College of Medicine, Southwest Jiaotong University, Chengdu 610031, China. E-mail: chenxy@swjtu.edu.cn
First published on 7th August 2020
Abstract
Insulin administration is necessary for patients with type 1 diabetes and advanced type 2 diabetes. However, there are many drawbacks associated with it, such as hypoglycemia and loss of insulin activity. Zwitterions with antifouling, nonthrombogenic, and cell-compatible properties have attracted wide scientific interest, particularly in biomedical applications. This review focuses on the application of functional zwitterionic materials for a variety of modes of insulin administration including controlled insulin release systems, improving insulin activity, and encapsulation of islet cells. In particular, the relationships between the function of zwitterionic materials and the administration of insulin are discussed in detail. Finally, the challenges and future of zwitterionic materials in the administration of insulin are summarized.
1. Introduction
Diabetes is a chronic metabolic disease characterized by increased production of glucose in the liver and its reduced clearance into muscle and fat, leading to abnormal accumulation of glucose in the blood, which is caused by inadequate levels of insulin.1 It has become one of the most challenging health problems in the world, with a dramatic increase in the number of diabetic patients.2,3 Approximately 425 million people suffer from diabetes mellitus globally according to 2017 reports, and this number is likely to rise to 625 million by 2045.4 Diabetes is commonly caused either by the failure of the pancreas to produce insulin (type 1 diabetes) or by a defect in the body's response to insulin (type 2 diabetes).5,6The general method for treating type 1 and advanced type 2 diabetes requires frequent subcutaneous injection or continuous infusion of exogenous insulin combined with monitoring of blood glucose.7 However, this treatment has many drawbacks such as hypoglycemia, insulin denaturation, and aggregation, resulting in decreased insulin activity while using of insulin pumps.8 Efforts have been made to develop new routes of insulin administration, such as controlled insulin delivery, modification of insulin molecules to enhance bioactivity, and artificial pancreas for insulin production.
Zwitterion is a type of electrically neutral material that contains both cations and anions in their molecular structure.9 According to their charge distribution, zwitterionic polymers are divided into two types. One is where a single monomer has both positive and negative charges, such as poly (2-methacryloyloxyethyl phosphorylcholine) (PMPC), poly(sulfobetaine) (PSB), poly(carboxybetaine) (PCB) and poly[2-(methacryloyloxy)ethyl choline phosphate] (PMCP). The second type is the zwitterionic electrolyte polymer where the same amount of positive and negative charge are carried by different monomers.10,11 In this review, we focus on the properties and applications of zwitterionic polymers formed by monomers with both positive and negative charges. A large number of studies have shown that zwitterionic polymers are polar and can combine with water molecules to form a hydration layer through strong ionic solvation. This can block the adhesion of proteins, platelets, and other biomolecules, and bacteria to the surface effectively, and improve the blood compatibility of materials.12–15 The hydration layer also confers excellent antifouling properties to these coating.14 Many reviews have summarized these properties of zwitterions.16–19 Because of these properties, zwitterionic polymers are widely used as effective substitutes for polyethylene glycol (PEG) polymers in non-fouling applications. Choline phosphate (CP) contains amino and phosphate groups in the reverse order of phosphate choline (PC) headgroups of the cell membrane. Thus, among these zwitterions, PMCP is different from the others because it can interact with cells through a unique CP–PC interaction, and this may have potential applications in the field of tissue engineering.20–26
In this review, we first describe the administration of insulin as a therapy for diabetes, and later, the role of zwitterions in preventing protein aggregation and reducing of foreign body reaction (FBR) are highlighted. Next, the role of zwitterionic biomaterials in insulin administration is summarized. Finally, the challenges and outlooks in this area are discussed. The keynote of this review is schematically illustrated in Fig. 1.
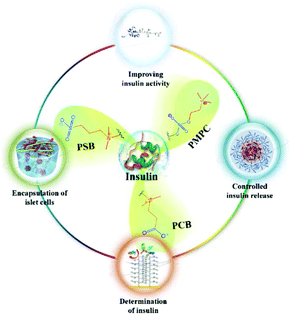 | ||
| Fig. 1 Schematic illustration of the biomedical applications of zwitterionic biomaterials in administration of insulin. | ||
2. Insulin administration in diabetes therapy
2.1 Controlled release systems for insulin
Controlling insulin release as a direct response to the blood glucose levels, allows continuous administration of insulin, forming a feedback control and closed-loop insulin release system.27–29 Glucose-sensitive insulin delivery systems, which can respond to changes in the blood glucose levels, have received increasing attention in recent decades and has a promising future in anti-diabetic therapy. Glucose-responsive systems, such as glucose oxidase (GOD), lectin, and phenylboronic acid-modified systems, have been investigated.30 The glucose oxidase-modified material was widely used in the glucose-sensitive drug delivery system.31,32 The immobilization of GOD can endow pH-sensitivity to glucose-sensitive materials because GOD converts glucose to gluconic acid, lowering the pH which changes the pH-sensitive materials.33–37 In our previous work, we fabricated glucose-sensitive multilayer films consisting of positively charged poly[2-(dimethylamino)ethyl methacrylate] (star PDMAEMA) and negatively charged insulin and GOD using a layer-by-layer method.34 Owing to the GOD in the films and the pH-sensitivity of star PDMAEMA, the films facilitate the on–off regulation of insulin release.To reduce the inconvenience and pain of subcutaneous needle injection, microneedles as a new attractive carrier were introduced for transdermal delivery of insulin for modulating glucose levels noninvasively in diabetes patients.38–40 For example, Jiang and co-workers fabricated a series of composite microneedles for transdermal delivery of insulin, using materials such as alginate and maltose, alginate and hyaluronate, calcium sulfate and gelatin, and gelatin and hydroxyapatite.41–44 In diabetic rats, these composite microneedles show good mechanical properties and excellent biocompatibility, and the released insulin from biodegradable composite microneedles had clear and effective hypoglycemic effects compared to the subcutaneous injections. To modulate insulin secretion from pancreatic cells, Gu et al. developed various types of glucose-sensitive microneedles.45–50 Some of these contain responsive particles, such as hypoxia-sensitive hyaluronic acid vesicles, H2O2-responsive polymeric vesicles, and H2O2-responsive PVA-TSPBA gels, and these microneedles can quickly release insulin in response to enhanced glucose.45–47 In addition, some new varieties of glucose-responsive insulin delivery microneedles, such as self-administrable powder-carrying microneedles, microneedle carrying pancreatic cells that can control the insulin secretion externally, triggered by the internal hyperglycemic state, and microneedles based on an interaction between glucose derivative-modified insulin and glucose transporters on red blood cells, have been developed to better mimic the function of pancreatic cells.50–52
2.2 Modified insulin with enhanced bioactivity
Insulin is usually administered through injections and pumps. These pumps eliminate the need for daily injections and provide an accurate amount of insulin. However, use of insulin pumps as a common method is hampered by protein aggregation and denaturation, resulting in loss of effectiveness of insulin over time, leading to insufficient dose. In addition, insulin aggregation can trigger a potentially dangerous immune response.53–55 Thus, it is a challenge to administer insulin while suppressing its aggregation and maintaining its activity.To address this issue, various small compounds, polymers, and metal ions were used to inhibit insulin aggregation.54,56–61 Small molecules like eugenol and epinephrine can effectively inhibit thermally-induced insulin aggregation, and maintain insulin activity to a considerable extent at 1 mM concentration.54 Covalently linking the proteins with poly (ethylene glycol) (PEG) is the most widely used polymer conjugation technique to improve the stability of proteins.62,63 A cationic polyelectrolyte, poly (ethylene glycol)-b-poly (L-histidine)\(PEG-polyHis) can effectively reduce insulin aggregation at the interface during the microencapsulation process.60 However, PEGylation does not necessarily improve the stability of proteins under different environmental pressure during storage and transportation. Many other polymers have also been used to enhance the stability of insulin. For example, trehalose, a non-reducing disaccharide formed by α,α-1,1-linked glucose units, is known to stabilize proteins, through vitrification, water replacement, and/or water entrapment.64,65 Addition of a trehalose glycopolymer by covalent conjugation can prevent thermal or agitation-induced aggregation of insulin.66,67 Further, a well-defined insulin-trehalose glycopolymer conjugate was polymerized from a site-specific initiator to stabilize insulin to heat and to improve its bioactivity.68 Furthermore, a series of zwitterion and trehalose-substituted degradable polymers were prepared to stabilize the therapeutic insulin against activity loss due to aggregation and extend its half-lives.56 These studies indicated that the activity of insulin can be effectively retained by molecular modification.
2.3 Artificial pancreas
Using of insulin in the treatment of diabetes has made great progress in the past few decades. Injection or infusion of exogenous insulin remains the primary treatment option for blood glucose control in patients with type 1 and advanced type 2 diabetes. However, insulin administration is cumbersome, often needs patient compliance and cannot completely prevent the side effects of diabetes. To overcome the drawbacks of direct insulin treatments, like aggregation and loss of activity of insulin, and to provide new alternates in type I diabetes therapy, encapsulation of living insulin-producing islet cells has been developed.69–73 Encapsulating biomaterials allow the permeation of nutrients, oxygen, glucose, insulin, and waste products, but block immunocompetent cells, antibodies, and complements.Alginate is a non-toxic structural polysaccharide with low immunogenicity, and is often used to encapsulate islets.70,74,75 For example, Zhang et al. fabricated a Alg/PEI hydrogel with balanced charged, which enables highly efficient encapsulation of islets.73 After intraperitoneal transplantation into streptozotocin (STZ)-induced T1DM C57BL/6J mice, the encapsulated islets were able to maintain their glucose-responsiveness and insulin-production. The hydrogel can achieve blood glucose correction within 2 days and stably maintain normoglycemia for at least 150 days. Moreover, Anderson and coworkers encapsulated rat pancreatic islet cells, and beta cells derived from human embryonic stem cells with modified alginate microspheres and transplanted into STZ-induced diabetic C57BL/6 mice, which can achieve glycemic correction without immunosuppression for about 6 months.74,76 A collagen-based cryogel with oxygen-generating bioscaffold containing calcium peroxide was also used to carry pancreatic islets.71 The oxygen-generating bioscaffold can provide a biostable and biocompatible 3D microenvironment for islets and facilitate their survival and function.
3. Properties of zwitterionic biomaterials
3.1 Anti-protein aggregation
Protein aggregation and denaturation are among the most serious concerns in the field of protein biopharmaceutics. Protein aggregation is usually accompanied by the formation of amyloid-like fibrils,77 which cause a wide range of diseases such as Alzheimer's disease, type II diabetes, Parkinson's disease, and prion disease.78–80 Therefore, inhibiting the formation of fibrils is of great significance for the development of protein biopharmaceuticals. A number of compounds, such as cyclodextrin, arginine, and proline, have been developed to prevent protein aggregation.81 However, these compounds were not efficient enough to be used in therapy. Thus there is a great need to develop new inhibitors of protein aggregation.Zwitterionic polymers in mixed charged state, exhibit properties similar to proteins,82 making them particularly interesting in this regard.8,83,84 Rajan et al. first reported a zwitterionic polymer polysulfobetaine (poly SPB) acted as a molecular shield to prevent collisions between protein that can inhibit protein aggregation.84 It was significantly more efficient than the previously described inhibitors of lysozyme aggregation. The solubility and enzymatic efficiency of lysozyme were retained even at higher temperatures in the presence of poly SPB, inhibiting its aggregation.84 Furthermore, they synthesized zwitterionic polymer-based nanogels to suppress the thermal aggregation of lysozyme with high efficiency. When heated in the presence of these nanogles, lysozyme retained its enzymatic activity and higher order structures, without forming amyloid fibrils.85 Zwitterionic polymers are also used to increase the stability of proteins without sacrificing their binding affinity or bioactivity. Jiang's group conjugated PCB to protein to improve the stability in a manner similar to PEGylation.86 Moreover, the conjugation maintains or may even improve the binding affinity, thus enhancing protein substrate hydrophobic interactions. This approach opens a new avenue for the development of protein therapeutics by avoiding the need to compromise between stability and affinity.
3.2 Low immunogenicity
Recent studies have found that zwitterionic biomaterials can reduce the FBR in vivo due to their superior ability to resist nonspecific protein adsorption, thereby avoid recognition by macrophages.90,103 Hydrogels prepared from zwitterions effectively suppress inflammatory reactions.87–89 For example, ultra-low-fouling zwitterionic hydrogels, poly (carboxybetaine methacrylate) (PCBMA) hydrogels prepared from a carboxybetaine monomer and a carboxybetaine cross-linker, can resist the formation of a capsule for at least 3 months after subcutaneous implantation in mice.90 Compared with poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogels using HEMA monomers and PEGX cross-linkers, the PCBMA hydrogels elicited less inflammation, and this weaker inflammatory response may be because of the superior ability of these hydrogels to resist nonspecific protein adsorption, thus avoiding recognition by macrophages. Catheters coated with a hydrogel containing the zwitterionic moiety sulfobetaine significantly reduced both protein adsorption in vitro and FBR in vivo.91 Zwitterionic PCB hydrogels were coated on neural interface implanted devices polyimide substrates, which could effectively minimize the FBR because macrophages were not activated after hydrogel coating.92 Wu et al. prepared ultra-low-fouling zwitterionic sulfated poly(sulfobetaine methacrylate) (polySBMA) hydrogels and applied them to full-thickness cutaneous wounds in mice.93 The SBMA hydrogels decreased the inflammatory response and effectively promoted wound healing and tissue regeneration in mice. In order to reduce the FBR of implantable materials, Leigh et al. developed a simple method to simultaneously photograft pSBMA and pCBMA zwitterionic hydrogels to a poly(dimethylsiloxane) (PDMS) surface.94 The zwitterion hydrogel-coated PDMS surface reduced fibrinogen adsorption by 90%, and fibroblast adhesion by more than 20-fold. These results indicated that zwitterion coating has a potential to drastically reduce the fibrotic response to implanted biomaterials.Because of the anti-fouling effect of zwitterions, functional biomaterial surfaces coated with them have low immunogenicity and long life.95–97 Coating the implants with a zwitterionic layer is a potential approach to mitigate the FBR. Golabchi et al. developed a coating composed of zwitterionic PSB and polydopamine (PDA) for neural probes.98 The PDA-PSB coating can significantly reduce protein adsorption and fibroblast adhesion in vitro, and also can suppress the acute inflammatory response in the brain tissue after implantation in the mouse brain. Zwitterinoic copolymers consisting of sulfobetaine methacrylate (SB) and 2-aminoethyl methacrylate (AE) modified PDMS substrates demonstrated a significant reduction in capsule thickness associated with the FBR.99 A report by Plegue et al. showed that zwitterion modified PDMS surface to protected the blood-contacting materials from the natural FBR.100 They modified the PDMS surface with SBSi, to provide the maximum shelf life stability, best platelet resistance, and best stability under low shear rates.100 A thiol-containing PMPC zwitterionic copolymer was used to coat the surface of alginate microspheres to protect them form the surface-mediated fibrotic reaction.101 Implantation of the these modified alginate microspheres into the IP space of C57BL/6J mice showed that PMCP-modified microspheres had little cellular deposition after 14 days in vivo, while significant fibrosis was seen on the unmodified alginate microspheres. In a further research, a zwitterionic polymer coating on the continuous glucose monitors (CGMs) improved their function by eliminating the FBR and sensor noise.102 At the early stages of sensor implantation, inflammation was significantly reduced by the PMPC zwitterionic coating. In addition, zwitterionic PCB-coated nanoparticles induce extremely low antibody response as compared to PEG-modified nanoparticles in vivo.103 Immunoglobulin M (IgM) was produced initially in response to PEG-nanoparticles, and later immunoglobulin G (IgG) was also produced, after repeated injections. However, the PCB nanoparticles prevented the production of poly-specific antibodies because the extremely low fouling of PCB coating shielded the nanoparticles from the immune response (Fig. 2). In our previous work, we also found that the zwitterionic PMCP-modified PCL surface induced weaker inflammatory responses than the unmodified PCL film.24
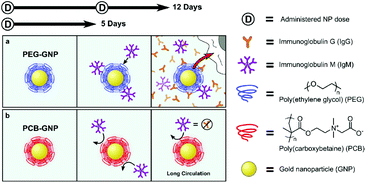 | ||
| Fig. 2 Schematic illustrations of the sequence of events after PEG-nanoparticle (a) and PCB-nanoparticle (b) enter the blood stream. Reprinted with permission from ref. 100 Copyright 2014, Elsevier. | ||
4. Zwitterionic biomaterials in insulin administration
4.1 Drug delivery system
In recent years, a variety of glucose-sensitive drug delivery systems have been developed to treat diabetes.104 Micelle, which has a hydrophilic shell connecting the biological environment, and a hydrophobic core that acts as drug reservoir, is one type of effective platform for glucose-sensitive insulin delivery, due to its high sensitivity to glucose and a long circulation stability.105–107 For instance, zwitterionic dialdehyde starch-based micelles with glucose-responsive behavior have been developed to control insulin release.108 Zwitterionic sulfobetaine (SB) was used as the hydrophilic shell to reduce macrophage response, and 3-aminophenylboronic acid (APBA) was used as a glucose-responsive group with the dialdehyde starch (DAS) backbones (SB-DAS-APBA) (Fig. 3). Insulin released from the nanocarriers was sensitive to various glucose concentrations, and the release rate was rapid at a concentration of 3 mg mL−1 at pH 7.4. Meanwhile, PEGylated micelles, with monomethyl ether (mPEG) as the hydrophilic shell in place of zwitterionic SB, were also synthesized to compare the macrophage response. However, the PEGylated and zwitterion coated micelleswere recognized by macrophages through different processes. Immune cells clearly responded to PEGylated micelles, resulting in macrophage activation in the blood microenvironment, wheraeas, zwitterionic micelles, due to the SB chain, elicited poor response from immune cells, resulting in unresponsive macrophages. This study indicated that zwitterionic micelles SB-DAS-APBA not only had the insulin delivery characteristics with glucose response, but also reduced the activation macrophages.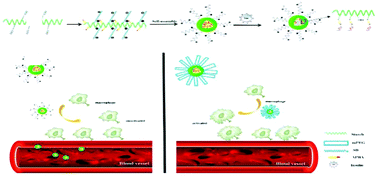 | ||
| Fig. 3 (a) The preparation of insulin-loaded SB-DAS-APBA micelles. (b) Different endocytotic response for zwitterionic and PEGylated micelles in contact with immune cells in the blood vessel. Reprinted with permission from ref. 104 Copyright 2018, Elsevier. | ||
Wang et al. constructed a unique zwitterionic Janus dendrimer (JD) system, designed to integrate the protein binding domains in the core, and protein repelling domain on the surface of nanocarriers.109 The core of the dendron combined the charged and hydrophobic moieties via multivalent and synergistic interactions, and the zwitterionic peripheries on the surface of dendrons endowed the system with a hydrophilic and antifouling surface, which effectively prevented the adsorption and exchange of proteins in the biological environment, and reduced the premature release of proteins (Fig. 4). The insulin loaded in zwitterionic JD (GPC-JD) elicited the best hypoglycemic response when subcutaneously injected into the fasted mice, and the effect was maintained up to 4 h. Pharmacological availability of insulin in insulin-GPC-JD was 160% in comparison to the free insulin injection. These results indicated that the bioactivity of insulin was significantly enhanced after loading it in the zwitterionic JD nanocarrier.
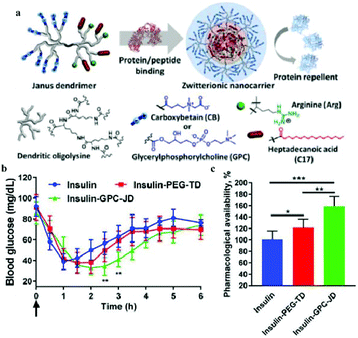 | ||
| Fig. 4 (a) Structure of zwitterionic Janus dendrimer with protein binding and antifouling properties. (b) Blood glucose response of normal mice after subcutaneous injection with free insulin and insulin-loaded nanoparticles. (c) Pharmacological availability of insulin formulations for blood sugar control (0–6 h). Reprinted with permission from ref. 105 Copyright 2019, Elsevier. | ||
Based on the characteristics of the zwitterion used, zwitterionic hydrogels with anti-fouling properties and good biocompatibility are also fabricated for insulin release. Biomimetic polymer phosphorylcholine (PC) and biocompatible poly(propylene glycol) (PPG) were used to prepare a zwitterionic thermogel (PC-PPG-PC), which had unique multiple sol–gel transitions as the temperature increased.110 Based on the low protein adsorption and the broad gel window properties, the zwitterionic gel proved to be useful for sustained release of insulin. Bhuchar et al. reported a zwitterionic nanogel with stable thermoresponsive and acid-degradable, cross-linked cores, and a nanogel fabricated with hydrophilic shell of PMCP, and a hydrophobic core composed of poly(methoxydiethylene glycol methacrylat) (poly(MeODEGM)), and poly(2-amino-ethyl methacrylamide hydrochloride) (poly(AEMA)) (Fig. 5).111 The hydrophilic and zwitterionic poly (MPC) shell determined the solubility and stability of the nanogels, and poly(AEMA) provided cationic character to the nanogel core, which facilitated the encapsulation of anionic insulin. The thermoresponsive and degradable cross-linked cores swelled and shrank with the change in temperature, which helped control the encapsulation and release of insulin.
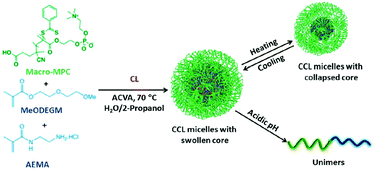 | ||
| Fig. 5 Schematic illustration of zwitterionic nanogel with thermoresponsive and degradable cores. Reprinted with permission from ref. 107 Copyright 2012, American Chemical Society. | ||
Nam et al. reported spontaneously forming hydrogels induced by two different molecular polymers with MPC moieties, e.g. water-soluble polymer poly(MPC-co-methacrylic aid [MA]) (PMA) with carboxylic acid groups and poly(MPC-co-n-butyl methacrylate [BMA]) (PMB) with hydrophobic groups.112–114 The PMA/PMB polymer hydrogels were formed by physical cross-linking through hydrophobic interactions and hydrogen bonding, and the hydrogel dissociated with the change in pH caused by the ionization of carboxylic acid groups.115 This pH-sensitive hydrogel system was used to load insulin using the hydrophobic domain in PMB, and it released the insulin load in the pH range prevalent in the small intestine and be suppressed under acidic conditions.112 In addition, insulin release could be regulated by changes in the molecular weight of the polymers, the ratio of PMA and PMB and the polymer concentration.114 In brief, the PMPC hydrogel can be used as an oral insulin delivery system, and the insulin delivery process can be controlled by adjusting the composition of the hydrogel.
A biocompatible polymer alloy membrane for implantable artificial pancreas was composed of a zwitterionic MPC polymer and segmented polyurethane (SPU).116 The MPC polymer poly(MPC-co-2-ethyl-hexyl methacrylate) (PMEH) was dissolved in the same solvent as SPU to prepare the SPU/PMEH alloy membrane by the solvent evaporation method. This membrane can resist the adherence of fibroblasts to its surface because of the antifouling property of zwitterionic MPC units. Because of its excellent biocompatibility, mechanical properties, and permeability for glucose and insulin, it was used for the fabrication of an implantable insulin pump.117 By controlling the domain structure of PMEH, the insulin permeation pathway was formed in the SPU/PMEH alloy membrane. The permeability of insulin through the polymer alloy membrane was synchronized with the pressure applied to the membrane (Fig. 6). Under pressure, insulin permeability was enhanced, and the reproducibility of the insulin permeation could be maintained for several periods and a constant level of insulin permeation could be maintained by changing the pressure gradient. Thus, the polymer alloy membrane can be applied as a valve in an insulin pump to control the insulin release. Based on this insulin pump design, Uchiyama et al. reported the use of a H2O2 degradable MPC hydrogel in an implantable insulin pump device.118 The PMCP hydrogel was degraded by H2O2, which was produced by enzymatic reaction between glucose oxidase and glucose, and the degradation was proportionate to the concentration of H2O2. This system can be used as an insulin release device synchronized with glucose concentration by using this enzymatic reaction.
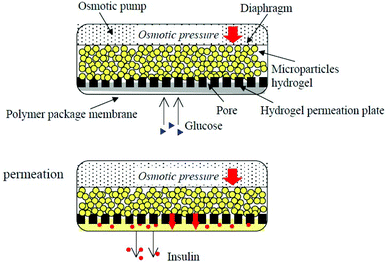 | ||
| Fig. 6 Insulin release controlled by an implantable insulin pump under pressure. Reprinted with permission from ref. 113 Copyright 2002, Elsevier. | ||
Diabetes is often accompanied by many complications. Vascular diabetes is one of the most serious ones and it results in high morbidity.119 In this case, synergistic therapy that can simultaneously load multiple drugs using drug delivery platforms for diabetes and associated potential complications may have a better therapeutic effect. Micelle-hydrogel composites combine easily, have stability based on micelles, significantly improve different drug domains, allowing a variety of drugs carried by the gel.120,121 Wen et al. have prepared chitosan/zwitterionic derivatives (CS/SB-DAS-VPBA) micelle-hydrogel synergistic therapy system, in which insulin was loaded into the micelle and nattokinase, as a thrombolytic agent, was loaded in hydrogel matrix to treat the complications of vascular diabetes (Fig. 7).122 Drug release studies indicated that the release of the two drugs was relatively independent, and the synergistic therapy system allowed continuous release of insulin and rapid release of nattokinase for improving the therapeutic effect of diabetes and reducing vascular complications.
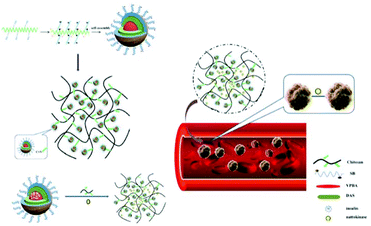 | ||
| Fig. 7 Schematic illustration of chitosan/zwitterionic dialdehyde starch derivative micelle-hydrogel synergistic therapy system. Reprinted with permission from ref. 118 Copyright 2019, Elsevier. | ||
4.2 Improving insulin activity
Modifying insulin with small compounds, polymers, or metal ions could enhance its pharmacokinetics (PK) and retain bioactivity.4,54,56 The most common strategy is to use PEG to conjugate insulin, called PEGylation.123 However, conjugating PEG or other polymers with proteins significantly affects the bioactivity of proteins because the conventional covalent coupling attaches the polymer chains near the active sites of the protein or on its surfaces groups.124 For example, Lispro, a PEGlated insulin developed by Eli Lily, is needed at high molar concentrations to achieve the level of glucose lowering effect of unconjugated insulin in the in vitro insulin receptor binding studies.125Compared with PEG, zwitterion is superhydrophilic, and when conjugated to a protein, it does not interfere with the hydrophobic–hydrophobic interactions and substrate–binding site interactions.86 Xie et al. conjugated PCB to insulin through simple conventional coupling chemistry, which improved the PK of insulin without compromising its bioactivity.126Fig. 8 shows that the in vitro bioactivity of PEG-insulin decreased significantly, while no such decrease was observed in the zwitterionic PCB-insulin. When PCB-insulin and PEG-insulin were injected into diabetic mice, the pharmacological activity of PCB-insulin was about 124.3%, and that of PEG-insulin was about 97.5% of that of native insulin, and the PCB-insulin showed significantly greater blood glucose lowering ability than insulin. Matsumura et al. synthesized a hydrophobic zwitterionic polymer poly-sulfobetaine (poly-SPB), which was more efficient in suppressing insulin aggregation, and significantly inhibited aggregation and fibrillation of insulin even at very low polymer concentrations and long incubation periods.8 In addition, while being incubated with poly-SPB, insulin could completely preserve its secondary structure, even making the denatured insulin refold. This is because the poly-SPB can interact with the hydrophobic domains of insulin, preventing aggregation and facilitating refolding. This group further studied the ability of poly-SPBs with different chain lengths to inhibit insulin aggregation and reversible addition fragmentation chain transfer (RAFT) agents to notice the change in the inhibition patterns of these polymers.83 Thioflavin T (ThT) fluorescence assay was used to analyze the effect of sulfobetaine polymers on insulin fibrillation.127 ThT shows specific binding to amyloid fibrils, which enhanced fluorescenece.128 Their results indicated that sulfobetaines are highly efficient in inhibiting insulin aggregation, and a change in molecular weight and hydrophobicity of polymers caused a greater stabilization of insulin.
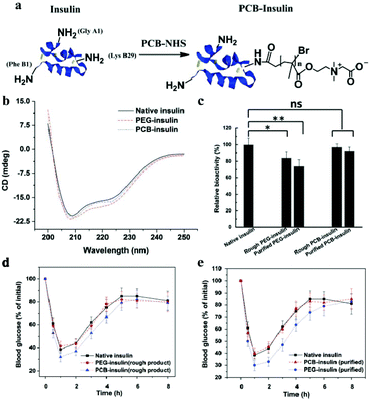 | ||
| Fig. 8 (a) Schematic illustration of PCB conjugation with insulin. (b) Circular dichroism spectra of native insulin, PEG-insulin, and PCB-insulin, respectively. (c) The bioactivity of native insulin, rough PEG-insulin, purified PEG-insulin, rough PCB-insulin, and purified PCB-insulin, respectively. (d) Before and (e) after purifying insulin conjugate products. Reprinted with permission from ref. 122 Copyright 2017, John Wiley and Sons. | ||
Zwitterionic molecules have been used to explore amphiphilic surfactants to inhibit insulin fibrillation.129,130 Wang et al. have studied the effect of two amphiphilic surfactants (1,2-dihexanoyl-sn-glycero-3-phosphocholine (di-C6-PC) and 1,2-diheptanoyl-sn-glycero-3-phosphocholine (di-C7-PC)) on the in vitro fibrillation process of bovine insulin at pH 2.0 and 55 °C.130 Their results indicated that di-C6-PC and di-C7-PC decreased the β-sheet formation and aggregation, and insulin fibrillation may be inhibited by both surfactants in a dose-dependent fashion. The best inhibition of fibril formation was observed when insulin was incubated with 4 mM di-C7-PC.
To trap insulin in biomaterials without aggregation and to control its release in the native form are very important in designing effective insulin therapy. A zwitterionic 2-methacryloyloxyethyl phosphorylcholine (MPC) nanogel was used to build blocks and multiple cross-linkers for fabricating hybrid hydrogels with an ability to trap insulin without aggregations and release in its native form.131 Hybrid hydrogels with chaperone-like activity were considered promising as a versatile technique for insulin reservoirs.
Insulin may lose activity during transportation due to mechanical agitation, and studies have shown that insulin degrades rapidly when shaken.132 Thus, the degradable polymers with adjustable degradation rate on their backbone can be used to attenuate this process by stabilizing proteins against aggregation.133 Maynard et al. have reported a series of zwitterionic and trehalose side-chain polymers with tunable degradability, added to insulin solution in a transportation simulation environment at thermal and mechanical stress (37 °C, 250 rpm).56 These polymers were able to maintain the stability of insulin, and prevent the aggregation and consequent loss of activity, showing their potential as degradable excipients in insulin formulation.
4.3 Encapsulation system for islet cells
Encapsulation of insulin-producing pancreatic islet cells provides additional options for the treatment of type 1 diabetes. However, immune reactions, such as FBR lead to fibrosis, which cuts off the diffusion of nutrients to the encapsulated cells, causing cell death.70 Thus, in spite of their side effects, immunosuppressive drugs need to be used to inhibit immune reactions and prolong graft functioning.134 To promote cell transplantation therapy that does not need immunosuppressive drugs,75,135 non-fouling and biocompatible zwitterions are applied to biomaterials used to encapsulate islets. Multilayered nanocoating, applied in a layer-by-layer technique, was developed for the encapsulation of islets or β-cell spheroids. Insulin-producing pancreatic β-cell spheroids were encapsulated in chitosan/alginate multilayers, and a layer of phosphorylcholine-modified chondroitin-4-sulfate was deposited on the outermost layer, to shield the large molecules from the antigen-presenting cells (Fig. 9).136 After encapsulation, the coating did not damage the cell structure, and the cell viability and their metabolic functions were retained. In addition, insulin secretion from encapsulated pseudoislets indicated that encapsulation did not compromise the ability of MIN6 cells to respond to stimuli of changes in the extracellular concentration of glucose stimuli. To improve the survival and function of islets, spheroids were formed by co-culturing MIN6 β-cells with mesenchymal stem cells (MSCs). Inclusion of a small number of MSCs in the culture enhanced the structural and morphologic stability, improved insulin secretion and conferred immunosuppressive and antiapoptotic properties.137 These nanofilm islet encapsulation provide the necessary features for efficient transplantation as a therapy for type 1 diabetes.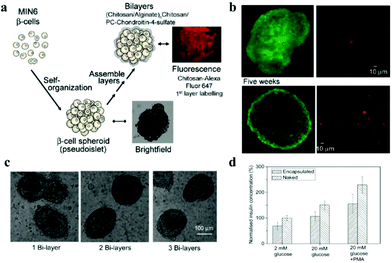 | ||
| Fig. 9 (a) Schematic illustration showing formation of pseudoislets from MIN6 cells with LBL nanofilm encapsulation. (b) Live/dead MIN6 cells encapsulated within chitosan/alginate multilayers after 2 and 5 weeks post-encapsulation maintained in tissue culture. (c) Light microscopy images showing capsule stability in culture medium. (d) Insulin release from the naked and encapsulated MIN6 pseudoislets in response to different glucose concentration stimuli. Reprinted with permission from ref. 132 Copyright 2010, American Chemical Society. | ||
Although hydrogel materials have elicited extensive interest for pancreatic islet encapsulation, FBR leads to formation of a dense, avascular collagenous capsule around the implanted hydrogels. This capsule compromises the diffusion of oxygen and nutrients, leading to hypoxic death, finally resulting in the implant failure. For biomedical application, PEG is widely used as an antifouling material in hydrogels. Even though the biocompatibility of PEG hydrogels is sufficient in many cases, sometimes it can induce FBR. Zwitterionic hydrogels as antifouling materials, have a better ability to aboid FBR.90 A new class of triazole-zwitterionic hydrogels with excellent mechanical robustness and biocompatibility were used for islet encapsulation and transplantation, which induced less fibrosis and more blood vessel formation upon subcutaneous implantation in vivo compare with a poly (2-hydroxyethyl methacrylate) hydrogel control. These zwitterionic hydrogels demonstrated diabetes correction up to 1 month in mice from the subcutaneous site of implantation (Fig. 10).89 The intraperitoneal glucose tolerance test indicated that the mice form the P(TR-SB) hydrogel group could restore normoglycemia within 90 min, which was similar to the non-diabetic mice, and the glucose-stimulated insulin secretion assay performed on the islets encapsulated in P(TR-SB) hydrogels were responsive to glucose change and secreted insulin upon stimulation with glucose, indicating their viability and function.
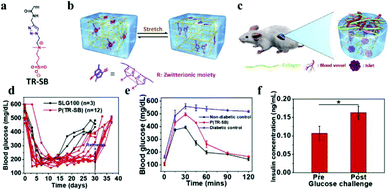 | ||
| Fig. 10 (a) Chemical structure of TR-SB monomers. (b) Schematic illustration showing the π–π stacking between the triazole rings. (c) Schematic illustration of the low level of fibrosis and high degree of vascularization around the robust P(TR-SB) hydrogel for islet encapsulation. (d) Blood glucose concentrations for SLG100 hydrogels and P(TR-SB) hydrogels. (e) Intraperitoneal glucose tolerance test. (f) Glucose challenge stimulated insulin secretion of islets in P(TR-SB) hydrogels. Reprinted with permission from ref. 86 Copyright 2020, Elsevier. | ||
Liu et al. reported a group of zwitterion-modified alginates, SB-alginate and CB-alginate, which can reduce the FBR against implanted alginate microcapsules in mice, dogs, and pigs.138 Ziwitterion-modified alginate microcapsules, viz., SB-SLG20 were examined 180 days after implantation in C57BL/6J mice. These microcapsules were free of cellular deposition, whereas unmodified alginate microcapsules (SLG-20) showed strong cellular overgrowth (Fig. 11). They also used SB-alginates to demonstrate improved outcome of islet encapsulation in diabetic mice model (Fig. 11). Most diabetic mice transplanted with islets encapsulated in SB-SLG20 microcapsules maintained to normoglycemia during a long-term 200-day transplantation. In the intraperitoneal glucose tolerance test (IPGTT) mice from the SB-SLG20 group returned to normoglycemia as quickly as the non-diabetic mice did, even 200 days after transplantation. However, the blood glucose levels of the mice from the unmodified alginate group could not restore the blood sugar to normal range after 150 min, which was similar to diabetic mice with no islet transplantation. In addition, the glucose-stimulated insulin secretion test, performed on the retrieved SB-SLG20 microcapsules, showed that encapsulated islets responded to glucose and secreted insulin, further supporting normal islet function.
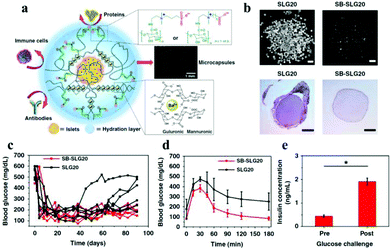 | ||
| Fig. 11 (a) Schematic illustration of zwitterion-modified alginate microcapsules for islet encapsulation. (b) Images of microcapsules and the HE stained histological sections 180 days after their implantation in vivo. (c) Blood glucose concentrations of mice; (c) intraperitoneal glucose tolerance test; (d) glucose-stimulated insulin secretion by islets in SB-SLG20 microcapsules. Reprinted with permission from ref. 134 Copyright 2019, Springer Nature. | ||
Pancreatic β-cells need optimum cell–cell and cell–matrix interactions to survive and function in vitro.139 To meet this demand, Perugini et al. modified the surface of the succinylated chitosan-based beads (NSC) with zwitterionic carboxy-betaine (CB) moieties to regulate the cellular hydration state and promote the formation of β-cell spheroids.140 Their results indicated that NSC coated with CB at a concentration of 0.3 mg mL−1, encouraged pancreatic cells to proliferate and form spheroids of about 80 μm. These spheroids exhibited high cell viability and enhanced insulin expression secretion.
Membrane materials are also used to encapsulate islets. For instance, a compound membrane made of phosphorylcholine-containing polymer and cellulose acetate to encapsulate islets, achieved rapid insulin production and diffusion across the membrane in response to glucose challenges.141
4.4 Others
There are many other zwitterionic functional biomaterials for insulin administration or assist in insulin therapy. For instance, a water-soluble zwitterionic phospholipid-like polymer, poly(2-methacryloyloxyethyl phosphorylcholine-co-n stearyl methacrylate) (PMC18) with a hydrophilic and a hydrophobic region, stimulated insulin release from RINm5G rat insulinoma cells.142 PMC18 had an n/m ratio (the ratio of the hydrophilic to the hydrophobic region) of 80/20, and it showed the most potent insulin release-stimulating activity.The determination of insulin present in the blood is of great value in the diagnosis of diabetes mellitus. Various approaches have been employed for the determining the amount of insulin, including immunoassays, bioassays, electrochemistry, surface plasma resonance, chromatography, and flow injection analysis.143–146 A sensitive and non-fouling electrical insulin biosensor was fabricated by immobilizing anti-insulin antibody on a zwitterionic PCBMA interface, and chemisorbed on a gold electrode surface (Fig. 12).147 For this, cysteamine gold electrode was modified by CBMA monomers containing carboxyl groups with EDC and NHS. Then, the connected monomers were photopolymerized from monomer solution to form the zwitterionic PCBMA polymer interface, for chemical adsorption. Finally, anti-insulin antibodies were coupled to the carboxyl groups of PCBMA, forming a selective antifouling receptor interface for insulin. The zwitterionic PCBMA provided excellent antifouling property, and the anti-insulin antibody had the specific binding sites for insulin. The interfaces are highly sensitive to insulin and are completely free from interference, even in undiluted serum.
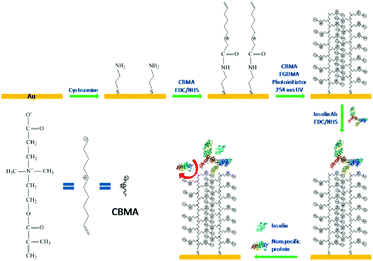 | ||
| Fig. 12 Schematic illustration of the fabrication of insulin receptive interface and its antifouling and selective insulin-binding properties. Reprinted with permission from ref. 143 Copyright 2013, American Chemical Society. | ||
Due to reduced insulin sensitivity in peripheral tissues, insulin therapy was found to be ineffective for some of patients with diabetic.148 Therefore, other forms of diabetic therapy were developed to regulate blood glucose level in such patients with severe insulin resistance.149 Xiao et al. developed a sugar-breathing glycopolymerssomes for regulating the levels of glucose. The glycopolymersomes can bind and store the glucose from surrounding solution when the glucose concentration is higher and optimum and release it when the concentration is lower than necessary.149 Recently, a new antidiabetic treatment strategy using an injectable glucose-responsive zwitterionic nanogel was developed to regulate blood glucose in diabetic rats.150 The glucose-responsive nanogles were prepared by copolymerization of zwitterionic sulfobetaine methacrylate (SBMA) and 4-acrylamido-3-fluorophenylboronic acid (AFBA). AFBA can interact with glucose molecules directly and tune to enable glucose storage and release at high and low blood glucose concentrations, respectively. At hyperglycemia, the nanogels absorb glucose molecules, and swell. During hypoglycemia, they shrink to release stored glucose. In vivo studies have shown that the in type I diabetic rats, glucose-sensitive nanogels can regulate blood level up to 6 h after being injected.
An intelligent composite microneedle (cMN) patch composed of methacrylated hyaluronic acid (MeHA) microneedle array with embedded glucose-responsive zwitterionic microgels was developed to prevent hypoglycemia, associated with intensive insulin therapy.151 The zwitterionic microgels were prepared using zwitterionic PSB, 4-acrylamido-3-fluorophenylboronic acid (AFBA), and cationic carboxybetaine (CB) (Fig. 13). In the design of microgels, the zwitterionic PSB was used to stabilize encapsulated glucagon, CB facilitated glucagon loading, and AFBA provided glucose-responsiveness in the physiological environments. Then, a mixture of loaded microgel and PMeHA was introduced into silicone MN molds to prepare cMN patch. During hypoglycemia, AFBA units formed bis-complexation with glucose and generated secondary crosslinking between polymer chains, resulting in substantial microgel shrinkage. By this, the loaded glucagon was squeezed out of the microgels and the cMN patch could release glucagon rapidly. However, at hyperglycemia or euglycemia, the AFBA units formed mono-complexation with glucose leading to microgel swelling, and glucagon was released the relatively slowly from the cMN patch (Fig. 13). Based on the functions of the cMN patch, the treatment of hypoglycemia can shift from intervention to prevention, reducing the risk of insulin therapy.
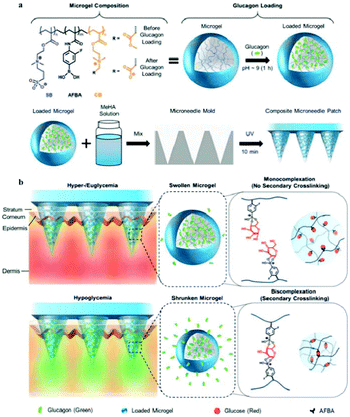 | ||
| Fig. 13 (a) Schematic illustration of the glucose-sensitive composite microneedle patch based on zwitterionic nanogel. (b) Schematic of hypoglycemia-triggered delivery of glucagon. Reprinted with permission from ref. 147 Copyright 2019, John Wiley and Sons. | ||
5. Concluding remarks and outlook
Zwitterionic materials are promising next-generation biomaterials for a wide range of biomedical engineering applications owing to their excellent biocompatibility, through avoiding nonspecific protein adsorption, increasing the stability of proteins without losing their bioactivity, and inducing no immunological response in vivo. In addition, PMCP materials have the characteristics of promoting cell adhesion and preventing protein adsorption, which may be used for tissue engineering materials with anti-fouling feature. Thus, the future direction for this field is to develop new zwitterionic materials through naturally generated ones, and to tune the structure and functions of these to adjust their properties for diverse applications.As diabetes is a growing global public health problem, new technologies and methods for insulin administration have to be developed, and modification of insulin need to be designed to suppress its aggregation and improve its activity. Zwitterionic materials have advantages in administration of insulin. In controlled insulin release systems, and encapsulation islet cells, zwitterionic materials contribute antifouling function and reduce immune response to improve the biocompatibility of the materials. Zwitterionic materials have been used to increase the stability of insulin without sacrificing its binding affinity or its bioactivity, to prevent its aggregation and inhibit the induction of fibrosis. Although considerable progress has been made in increasing the sensitivity, durability, and biocompatibility of insulin, the current approaches need to be further improved to make them suitable for the clinical setting. First, as it is still difficult to rapidly release insulin in response to blood glucose under physiological conditions, mimicking the ability of normal islet cells in complex and dynamic environments has become a research hotspot in this field. Second, it is still a challenge to release insulin consistently and stably in response to blood glucose levels. Third, precise control of insulin release is not yet achieved. Further optimization of biocompatibility, reduction of inflammation and the ensuing non-toxic side effects after long-term use are also issues of concern while selecting functional biomaterials. As for zwitterionic biomaterials, scaling up the manufacturing process, i.e., improving the productivity to make their application viable in clinical settings remain to be achieved. All these demands will certainly promote the development of new methods and techniques for insulin administration.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51803173), and Fundamental Research Funds for the Central Universities (2682019CX64).References
- P. G. N. Sarwar, S. R. Kondapally Seshasai, R. Gobin and S. Kaptoge, Lancet, 2010, 375, 2215–2222 CrossRef.
- G. R. Sarah, H. Wild, A. Green, R. Sicree and H. King, Diabetes Care, 2004, 27, 1047–1053 CrossRef PubMed.
- J. E. Shaw, R. A. Sicree and P. Z. Zimmet, Diabetes Res. Clin. Pract., 2010, 87, 4–14 CrossRef CAS PubMed.
- J. Wang, Z. Wang, J. Yu, Y. Zhang, Y. Zeng and Z. Gu, Biomater. Sci., 2019, 7, 4508–4513 RSC.
- M. A. Atkinson and G. S. Eisenbarth, Lancet, 2001, 358, 221–229 CrossRef CAS.
- M. Stumvoll, B. J. Goldstein and T. W. van Haeften, Lancet, 2005, 365, 1333–1346 CrossRef CAS.
- D. R. Owens, B. Zinman and G. B. Bolli, Lancet, 2001, 358, 739–746 CrossRef CAS.
- R. Rajan, Y. Suzuki and K. Matsumura, Macromol. Biosci., 2018, 18, e1800016 CrossRef PubMed.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177–4189 CrossRef CAS PubMed.
- Q. Shao and S. Jiang, J. Phys. Chem. B, 2013, 117, 1357–1366 CrossRef CAS PubMed.
- G. C. Matthew, T. Bernards, Z. Zhang, S. Chen and S. Jiang, Macromolecules, 2008, 41, 4216–4219 CrossRef.
- W. Lin, J. Zhang, Z. Wang and S. Chen, Acta Biomater., 2011, 7, 2053–2059 CrossRef CAS PubMed.
- Y. Chang, Y.-J. Shih, C.-J. Lai, H.-H. Kung and S. Jiang, Adv. Funct. Mater., 2013, 23, 1100–1110 CrossRef CAS.
- G. Cheng, Z. Zhang, S. Chen, J. D. Bryers and S. Jiang, Biomaterials, 2007, 28, 4192–4199 CrossRef CAS PubMed.
- Y. Xiao, M. Wang, L. Lin, L. Du, M. Shen and X. Shi, Mater. Chem. Front., 2018, 2, 891–900 RSC.
- L. Mi and S. Jiang, Angew. Chem., Int. Ed., 2014, 53, 1746–1754 CrossRef CAS PubMed.
- S. Lowe, N. M. O'Brien-Simpson and L. A. Connal, Polym. Chem., 2015, 6, 198–212 RSC.
- L. D. Blackman, P. A. Gunatillake, P. Cass and K. E. S. Locock, Chem. Soc. Rev., 2019, 48, 757–770 RSC.
- S. Jiang and Z. Cao, Adv. Mater., 2010, 22, 920–932 CrossRef CAS PubMed.
- X. Yu, Z. Liu, J. Janzen, I. Chafeeva, S. Horte, W. Chen, R. K. Kainthan, J. N. Kizhakkedathu and D. E. Brooks, Nat. Mater., 2012, 11, 468–476 CrossRef CAS PubMed.
- X. Chen, T. Chen, Z. Lin, X. Li, W. Wu and J. Li, Chem. Commun., 2015, 51, 487–490 RSC.
- X. Chen, H. Shang, S. Cao, H. Tan and J. Li, RSC Adv., 2015, 5, 76216–76220 RSC.
- X. Chen, M. Yang, B. Liu, Z. Li, H. Tan and J. Li, Langmuir, 2017, 33, 8295–8301 CrossRef CAS PubMed.
- X. Chen, Z. Lin, Y. Feng, H. Tan, X. Xu, J. Luo and J. Li, Small, 2019, 15, 1903784 CrossRef CAS PubMed.
- X. Chen and J. Li, Mater. Chem. Front., 2020, 4, 750–774 RSC.
- F. Huang and J. Li, J. Bioresour. Bioprod., 2018, 3, 3–8 CAS.
- Q. Wu, L. Wang, H. Yu, J. Wang and Z. Chen, Chem. Rev., 2011, 111, 7855–7875 CrossRef CAS PubMed.
- X. Cao, A. Halder, Y. Tang, C. Hou, H. Wang, J. Ø. Duus and Q. Chi, Mater. Chem. Front., 2018, 2, 1944–1986 RSC.
- M. Zhu, J. Tang, W. Wei and S. Li, Mater. Chem. Front., 2020, 4, 1105–1149 RSC.
- J. Xie, A. Li and J. Li, Macromol. Rapid Commun., 2017, 38, 1700413 CrossRef PubMed.
- K. Wang, K. Amin, Z. An, Z. Cai, H. Chen, H. Chen, Y. Dong, X. Feng, W. Fu, J. Gu, Y. Han, D. Hu, R. Hu, D. Huang, F. Huang, F. Huang, Y. Huang, J. Jin, X. Jin, Q. Li, T. Li, Z. Li, Z. Li, J. Liu, J. Liu, S. Liu, H. Peng, A. Qin, X. Qing, Y. Shen, J. Shi, X. Sun, B. Tong, B. Wang, H. Wang, L. Wang, S. Wang, Z. Wei, T. Xie, C. Xu, H. Xu, Z.-K. Xu, B. Yang, Y. Yu, X. Zeng, X. Zhan, G. Zhang, J. Zhang, M. Q. Zhang, X.-Z. Zhang, X. Zhang, Y. Zhang, Y. Zhang, C. Zhao, W. Zhao, Y. Zhou, Z. Zhou, J. Zhu, X. Zhu and B. Z. Tang, Mater. Chem. Front., 2020, 4, 1803–1915 RSC.
- M. Fu, C. Zhang, Y. Dai, X. Li, M. Pan, W. Huang, H. Qian and L. Ge, Biomater. Sci., 2018, 6, 1480–1491 RSC.
- N. Kashyap, B. Viswanad, G. Sharma, V. Bhardwaj, P. Ramarao and M. N. Ravi Kumar, Biomaterials, 2007, 28, 2051–2060 CrossRef CAS PubMed.
- X. Chen, W. Wu, Z. Guo, J. Xin and J. Li, Biomaterials, 2011, 32, 1759–1766 CrossRef CAS PubMed.
- X. Chen, J. Luo, W. Wu, H. Tan, F. Xu and J. Li, Acta Biomater., 2012, 8, 4380–4388 CrossRef CAS PubMed.
- J. Luo, S. Cao, X. Chen, S. Liu, H. Tan, W. Wu and J. Li, Biomaterials, 2012, 33, 8733–8742 CrossRef CAS PubMed.
- Y. Pan, J. Wang, P. Cai and H. Xiao, Int. J. Biol. Macromol., 2018, 118, 132–140 CrossRef CAS PubMed.
- X. Jin, D. D. Zhu, B. Z. Chen, M. Ashfaq and X. D. Guo, Adv. Drug Delivery Rev., 2018, 127, 119–137 CrossRef CAS PubMed.
- J. Wang, Z. Wang, J. Yu, A. R. Kahkoska, J. B. Buse and Z. Gu, Adv. Mater., 2020, 32, e1902004 CrossRef PubMed.
- G. Chen, J. Yu and Z. Gu, J. Diabetes Sci. Technol., 2019, 13, 41–48 CrossRef PubMed.
- Y. Zhang, G. Jiang, W. Yu, D. Liu and B. Xu, Mater. Sci. Eng., C, 2018, 85, 18–26 CrossRef CAS PubMed.
- W. Yu, G. Jiang, Y. Zhang, D. Liu, B. Xu and J. Zhou, Mater. Sci. Eng., C, 2017, 80, 187–196 CrossRef CAS PubMed.
- W. Yu, G. Jiang, D. Liu, L. Li, H. Chen, Y. Liu, Q. Huang, Z. Tong, J. Ao and X. Kong, Mater. Sci. Eng., C, 2017, 71, 725–734 CrossRef CAS PubMed.
- W. Yu, G. Jiang, D. Liu, L. Li, Z. Tong, J. Yao and X. Kong, Mater. Sci. Eng., C, 2017, 73, 425–428 CrossRef CAS PubMed.
- J. Yu, Y. Zhang, Y. Ye, R. DiSanto, W. Sun, D. Ranson, F. S. Ligler, J. B. Buse and Z. Gu, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8260–8265 CrossRef CAS PubMed.
- X. Hu, J. Yu, C. Qian, Y. Lu, A. R. Kahkoska, Z. Xie, X. Jing, J. B. Buse and Z. Gu, ACS Nano, 2017, 11, 613–620 CrossRef CAS PubMed.
- J. Wang, Y. Ye, J. Yu, A. R. Kahkoska, X. Zhang, C. Wang, W. Sun, R. D. Corder, Z. Chen, S. A. Khan, J. B. Buse and Z. Gu, ACS Nano, 2018, 12, 2466–2473 CrossRef CAS PubMed.
- J. Wang, J. Yu, Y. Zhang, X. Zhang, A. R. Kahkoska, G. Chen, Z. Wang, W. Sun, L. Cai, Z. Chen, C. Qian, Q. Shen, A. Khademhosseini, J. B. Buse and Z. Gu, Sci. Adv., 2019, 5, eaaw4357 CrossRef CAS PubMed.
- J. Yu, J. Wang, Y. Zhang, G. Chen, W. Mao, Y. Ye, A. R. Kahkoska, J. B. Buse, R. Langer and Z. Gu, Nat. Biomed. Eng., 2020, 4, 499–506 CrossRef CAS PubMed.
- Y. Ye, J. Yu, C. Wang, N. Y. Nguyen, G. M. Walker, J. B. Buse and Z. Gu, Adv. Mater., 2016, 28, 3115–3121 CrossRef CAS PubMed.
- S. Kim, H. Yang, J. Eum, Y. Ma, S. F. Lahiji and H. Jung, Biomaterials, 2020, 232, 119733 CrossRef CAS PubMed.
- C. Wang, Y. Ye, W. Sun, J. Yu, J. Wang, D. S. Lawrence, J. B. Buse and Z. Gu, Adv. Mater., 2017, 29, 1606617 CrossRef PubMed.
- C. J. Roberts, Trends Biotechnol., 2014, 32, 372–380 CrossRef CAS PubMed.
- S. Haghighi-Poodeh, L. Navidpour, P. Yaghmaei and A. Ebrahim-Habibi, Biochem. Biophys. Res. Commun., 2019, 518, 362–367 CrossRef CAS PubMed.
- K. D. Ratanji, J. P. Derrick, R. J. Dearman and I. Kimber, J. Immunotoxicol., 2014, 11, 99–109 CrossRef CAS PubMed.
- E. M. Pelegri-O'Day, A. Bhattacharya, N. Theopold, J. H. Ko and H. D. Maynard, Biomacromolecules, 2020, 21, 2147–2154 CrossRef PubMed.
- M. Levy-Sakin, M. Shreberk, Y. Daniel and E. Gazit, Islets, 2009, 1, 210–215 CrossRef PubMed.
- Y. Porat, A. Abramowitz and E. Gazit, Chem. Biol. Drug Des., 2006, 67, 27–37 CrossRef CAS PubMed.
- B. Cheng, H. Gong, H. Xiao, R. B. Petersen, L. Zheng and K. Huang, Biochim. Biophys. Acta, 2013, 1830, 4860–4871 CrossRef CAS PubMed.
- A. Taluja and Y. H. Bae, Mol. Pharm., 2007, 4, 561–570 CrossRef CAS PubMed.
- W. Wang, Z. Zhao, A. Zhou, Y. Liu, Y. Chen, M. Lin, G. Chen, C. Ding and J. Li, J. Bioresour. Bioprod., 2017, 2, 132–141 Search PubMed.
- S. N. S. Alconcel, A. S. Baas and H. D. Maynard, Polym. Chem., 2011, 2, 1442 RSC.
- E. M. Pelegri-O'Day, E. W. Lin and H. D. Maynard, J. Am. Chem. Soc., 2014, 136, 14323–14332 CrossRef PubMed.
- N. Teramoto, N. D. Sachinvala and M. Shibata, Molecules, 2008, 13, 1773–1816 CrossRef CAS PubMed.
- R. D. Lins, C. S. Pereira and P. H. Hunenberger, Proteins: Struct., Funct., Bioinf., 2004, 177–186 CrossRef CAS PubMed.
- Y. Liu, J. Lee, K. M. Mansfield, J. H. Ko, S. Sallam, C. Wesdemiotis and H. D. Maynard, Bioconjugate Chem., 2017, 28, 836–845 CrossRef CAS PubMed.
- M. S. Messina, J. H. Ko, Z. Yang, M. J. Strouse, K. N. Houk and H. D. Maynard, Polym. Chem., 2017, 8, 4781–4788 RSC.
- K. M. Mansfield and H. D. Maynard, ACS Macro Lett., 2018, 7, 324–329 CrossRef CAS PubMed.
- J. T. Wilson and E. L. Chaikof, Adv. Drug Delivery Rev., 2008, 60, 124–145 CrossRef CAS PubMed.
- B. L. Strand, A. E. Coron and G. Skjak-Braek, Stem Cells Transl. Med., 2017, 6, 1053–1058 CrossRef CAS PubMed.
- M. Razavi, R. Primavera, B. D. Kevadiya, J. Wang, P. Buchwald and A. S. Thakor, Adv. Funct. Mater., 2020, 1902463 CrossRef CAS.
- A. Schaschkow, S. Sigrist, C. Mura, J. Barthes, N. E. Vrana, E. Czuba, F. Lemaire, R. Neidl, C. Dissaux, A. Lejay, P. Lavalle, C. Bruant-Rodier, K. Bouzakri, M. Pinget and E. Maillard, Acta Biomater., 2020, 102, 259–272 CrossRef CAS PubMed.
- J. Zhang, Y. Zhu, J. Song, T. Xu, J. Yang, Y. Du and L. Zhang, Adv. Funct. Mater., 2019, 29, 1900140 CrossRef.
- O. Veiseh, J. C. Doloff, M. Ma, A. J. Vegas, H. H. Tam, A. R. Bader, J. Li, E. Langan, J. Wyckoff, W. S. Loo, S. Jhunjhunwala, A. Chiu, S. Siebert, K. Tang, J. Hollister-Lock, S. Aresta-Dasilva, M. Bochenek, J. Mendoza-Elias, Y. Wang, M. Qi, D. M. Lavin, M. Chen, N. Dholakia, R. Thakrar, I. Lacik, G. C. Weir, J. Oberholzer, D. L. Greiner, R. Langer and D. G. Anderson, Nat. Mater., 2015, 14, 643–651 CrossRef CAS PubMed.
- D. Dufrane, R. M. Goebbels and P. Gianello, Transplantation, 2010, 90, 1054–1062 CrossRef PubMed.
- A. J. Vegas, O. Veiseh, M. Gurtler, J. R. Millman, F. W. Pagliuca, A. R. Bader, J. C. Doloff, J. Li, M. Chen, K. Olejnik, H. H. Tam, S. Jhunjhunwala, E. Langan, S. Aresta-Dasilva, S. Gandham, J. J. Mcgarrigle, M. A. Bochenek, J. Hollister-Lock, J. Oberholzer, D. L. Greiner, G. C. Weir, D. A. Melton, R. Langer and D. G. Anderson, Nat. Med., 2016, 22, 306–311 CrossRef CAS PubMed.
- C. M. Dobson, Semin. Cell Dev. Biol., 2004, 15, 3–16 CrossRef CAS PubMed.
- C. M. Dobson, Nature, 2003, 426, 884–890 CrossRef CAS PubMed.
- K. H. Y. Xing, Mech. Ageing Dev., 2002, 123, 1625–1636 CrossRef PubMed.
- D. Eisenberg and M. Jucker, Cell, 2012, 148, 1188–1203 CrossRef CAS PubMed.
- W. Wang, Int. J. Pharm., 2005, 289, 1–30 CrossRef CAS PubMed.
- M. Bernards and Y. He, J. Biomater. Sci., Polym. Ed., 2014, 25, 1479–1488 CrossRef CAS PubMed.
- N. Sharma, R. Rajan, S. Makhaik and K. Matsumura, ACS Omega, 2019, 4, 12186–12193 CrossRef CAS PubMed.
- R. Rajan and K. Matsumura, J. Mater. Chem. B, 2015, 3, 5683–5689 RSC.
- R. Rajan and K. Matsumura, Sci. Rep., 2017, 7, 45777 CrossRef CAS PubMed.
- A. J. Keefe and S. Jiang, Nat. Chem., 2011, 4, 59–63 CrossRef PubMed.
- Y. Zhang, S. Liu, T. Li, L. Zhang, U. Azhar, J. Ma, C. Zhai, C. Zong and S. Zhang, Carbohydr. Polym., 2020, 236, 116021 CrossRef CAS PubMed.
- L. E. Jansen, L. D. Amer, E. Y. Chen, T. V. Nguyen, L. S. Saleh, T. Emrick, W. F. Liu, S. J. Bryant and S. R. Peyton, Biomacromolecules, 2018, 19, 2880–2888 CrossRef CAS PubMed.
- Q. Liu, A. Chiu, L. Wang, D. An, W. Li, E. Y. Chen, Y. Zhang, Y. Pardo, S. P. McDonough, L. Liu, W. F. Liu, J. Chen and M. Ma, Biomaterials, 2020, 230, 119640 CrossRef CAS PubMed.
- L. Zhang, Z. Cao, T. Bai, L. Carr, J. R. Ella-Menye, C. Irvin, B. D. Ratner and S. Jiang, Nat. Biotechnol., 2013, 31, 553–556 CrossRef CAS PubMed.
- Y. Yong, M. Qiao, A. Chiu, S. Fuchs, Q. Liu, Y. Pardo, R. Worobo, Z. Liu and M. Ma, Langmuir, 2019, 35, 1927–1934 CrossRef CAS PubMed.
- D. Trel'ova, A. R. Salgarella, L. Ricotti, G. Giudetti, A. Cutrone, P. Sramkova, A. Zahoranova, D. Chorvat Jr., D. Hasko, C. Canale, S. Micera, J. Kronek, A. Menciassi and I. Lacik, Langmuir, 2019, 35, 1085–1099 CrossRef PubMed.
- J. Wu, Z. Xiao, A. Chen, H. He, C. He, X. Shuai, X. Li, S. Chen, Y. Zhang, B. Ren, J. Zheng and J. Xiao, Acta Biomater., 2018, 71, 293–305 CrossRef CAS PubMed.
- B. L. Leigh, E. Cheng, L. Xu, A. Derk, M. R. Hansen and C. A. Guymon, Langmuir, 2019, 35, 1100–1110 CrossRef CAS PubMed.
- J. Xu, J. Xu, H. Moon, H. O. Sintim and H. Lee, ACS Appl. Polym. Mater., 2020, 2, 528–536 CrossRef CAS PubMed.
- R. Yang, J. Xu, G. Ozaydin-Ince, S. Y. Wong and K. K. Gleason, Chem. Mater., 2011, 23, 1263–1272 CrossRef CAS.
- O. Veiseh and A. J. Vegas, Adv. Drug Delivery Rev., 2019, 144, 148–161 CrossRef CAS PubMed.
- A. Golabchi, B. Wu, B. Cao, C. J. Bettinger and X. T. Cui, Biomaterials, 2019, 225, 119519 CrossRef CAS PubMed.
- W.-H. Chen, T.-Y. Liao, H. Thissen and W.-B. Tsai, ACS Biomater. Sci. Eng., 2019, 5, 6454–6462 CrossRef CAS.
- T. J. Plegue, K. M. Kovach, A. J. Thompson and J. A. Potkay, Langmuir, 2018, 34, 492–502 CrossRef CAS PubMed.
- V. Yesilyurt, O. Veiseh, J. C. Doloff, J. Li, S. Bose, X. Xie, A. R. Bader, M. Chen, M. J. Webber, A. J. Vegas, R. Langer and D. G. Anderson, Adv. Healthcare Mater., 2017, 6, 1601091 CrossRef PubMed.
- X. Xie, J. C. Doloff, V. Yesilyurt, A. Sadraei, J. J. McGarrigle, M. Omami, O. Veiseh, S. Farah, D. Isa, S. Ghani, I. Joshi, A. Vegas, J. Li, W. Wang, A. Bader, H. H. Tam, J. Tao, H. J. Chen, B. Yang, K. A. Williamson, J. Oberholzer, R. Langer and D. G. Anderson, Nat. Biomed. Eng., 2018, 2, 894–906 CrossRef CAS PubMed.
- W. Yang, S. Liu, T. Bai, A. J. Keefe, L. Zhang, J.-R. Ella-Menye, Y. Li and S. Jiang, Nano Today, 2014, 9, 10–16 CrossRef CAS.
- L. Zhao, C. Xiao, L. Wang, G. Gai and J. Ding, Chem. Commun., 2016, 52, 7633–7652 RSC.
- H. Yang, X. Sun, G. Liu, R. Ma, Z. Li, Y. An and L. Shi, Soft Matter, 2013, 9, 8589 RSC.
- M. Qi and Y. Zhou, Mater. Chem. Front., 2019, 3, 1994–2009 RSC.
- X. L. Chinomso, M. Ewulonu, M. Wu and H. Yong, J. Bioresour. Bioprod., 2019, 4, 3–10 Search PubMed.
- N. Wen, S. Lü, C. Gao, X. Xu, X. Bai, C. Wu, P. Ning and M. Liu, Chem. Eng. J., 2018, 335, 52–62 CrossRef CAS.
- L. Wang, C. Shi, X. Wang, D. Guo, T. M. Duncan and J. Luo, Biomaterials, 2019, 215, 119233 CrossRef CAS PubMed.
- Y. Ko du, M. Patel, B. K. Jung, J. H. Park and B. Jeong, Biomacromolecules, 2015, 16, 3853–3862 CrossRef PubMed.
- N. Bhuchar, R. Sunasee, K. Ishihara, T. Thundat and R. Narain, Bioconjugate Chem., 2012, 23, 75–83 CrossRef CAS.
- K. W. Nam, J. Watanabe and K. Ishihara, J. Biomater. Sci., Polym. Ed., 2002, 13, 1259–1269 CrossRef CAS.
- K. Nam, J. Watanabe and K. Ishihara, Int. J. Pharm., 2004, 275, 259–269 CrossRef CAS PubMed.
- K. Nam, J. Watanabe and K. Ishihara, Eur. J. Pharm. Sci., 2004, 23, 261–270 CrossRef CAS PubMed.
- K. Ishihara, H. Oda and T. Konno, Biomaterials, 2020, 230, 119628 CrossRef CAS.
- J. W. Tomoaki Uchiyama and K. Ishihara, J. Membr. Sci., 2002, 208, 39–48 CrossRef.
- J. W. Tomoaki Uchiyama and K. Ishihara, J. Membr. Sci., 2002, 210, 423–431 CrossRef.
- T. Uchiyama, Y. Kiritoshi, J. Watanabe and K. Ishihara, Biomaterials, 2003, 24, 5183–5190 CrossRef CAS PubMed.
- R. J. Wright and B. M. Frier, Diabetes Metab. Res. Rev., 2008, 24, 353–363 CrossRef CAS PubMed.
- Y. Wen, F. Li, C. Li, Y. Yin and J. Li, J. Mater. Chem. B, 2017, 5, 961–971 RSC.
- M. Patel, T. Kaneko and K. Matsumura, J. Mater. Chem. B, 2017, 5, 3488–3497 RSC.
- N. Wen, S. Lu, X. Xu, P. Ning, Z. Wang, Z. Zhang, C. Gao, Y. Liu and M. Liu, Mater. Sci. Eng., C, 2019, 100, 94–103 CrossRef CAS PubMed.
- S. Jevsevar, M. Kunstelj and V. G. Porekar, Biotechnol. J., 2010, 5, 113–128 CrossRef CAS PubMed.
- F. M. Veronese, Biomaterials, 2001, 22, 405–417 CrossRef CAS PubMed.
- T. M. Caparrotta and M. Evans, Diabetes, Obes. Metab., 2014, 16, 388–395 CrossRef CAS PubMed.
- J. Xie, Y. Lu, W. Wang, H. Zhu, Z. Wang and Z. Cao, Adv. Healthcare Mater., 2017, 6, 1601428 CrossRef PubMed.
- R. Khurana, C. Coleman, C. Ionescu-Zanetti, S. A. Carter, V. Krishna, R. K. Grover, R. Roy and S. Singh, J. Struct. Biol., 2005, 151, 229–238 CrossRef CAS PubMed.
- M. Biancalana and S. Koide, Biochim. Biophys. Acta, 2010, 1804, 1405–1412 CrossRef CAS PubMed.
- S. S. Wang, Y. T. Chen and S. W. Chou, Biochim. Biophys. Acta, 2005, 1741, 307–313 CrossRef CAS PubMed.
- S. S. Wang, K. N. Liu and T. C. Han, Biochim. Biophys. Acta, 2010, 1802, 519–530 CrossRef CAS PubMed.
- T. E. Nobuyuki Morimoto, Y. Iwasaki and K. Akiyoshi, Biomacromolecules, 2005, 6, 1829–1834 CrossRef PubMed.
- J. B. F. Alexis Oliva and M. Llabrés, Int. J. Pharm., 1996, 143, 163–170 CrossRef.
- T. Steinbach and F. R. Wurm, Biomacromolecules, 2016, 17, 3338–3346 CrossRef CAS PubMed.
- Y. Teramura and H. Iwata, Adv. Drug Delivery Rev., 2010, 62, 827–840 CrossRef CAS PubMed.
- J. D. W. Jaime, A. Giraldo and C. L. Stabler, J. Diabetes Sci. Technol., 2010, 4, 1238–1247 CrossRef PubMed.
- B. L. Zheng-Liang Zhi, P. M. Jones and J. C. Pickup, Biomacromolecules, 2010, 11, 610–616 CrossRef PubMed.
- Z.-L. Z. Tasneem Bhaiji and J. C. Pickup, J. Biomed. Mater. Res., Part A, 2012, 100, 1628–1636 CrossRef PubMed.
- Q. Liu, A. Chiu, L. H. Wang, D. An, M. Zhong, A. M. Smink, B. J. de Haan, P. de Vos, K. Keane, A. Vegge, E. Y. Chen, W. Song, W. F. Liu, J. Flanders, C. Rescan, L. G. Grunnet, X. Wang and M. Ma, Nat. Commun., 2019, 10, 5262 CrossRef PubMed.
- D. B. K. John, C. Stendahl and S. I. Stupp, Cell Transplant., 2009, 18, 1–12 Search PubMed.
- V. Perugini, M. Best, S. Kumar, A. L. Guildford, A. J. Bone, W. M. Macfarlane, M. Santin and G. J. Phillips, J. Mater. Sci.: Mater. Med., 2017, 29, 15 CrossRef PubMed.
- Y. Yang, S. Zhang, G. Jones, N. Morgan and A. J. El Haj, Artif. Cells, Blood Substitutes, Immobilization Biotechnol., 2004, 32, 91–104 CrossRef CAS PubMed.
- S. Maruyama, J. Hu, A. Yamanaka and T. Ichimura, Biosci., Biotechnol., Biochem., 2004, 68, 2197–2200 CrossRef CAS PubMed.
- S. M. Taghdisi, N. M. Danesh, P. Lavaee, A. Sarreshtehdar Emrani, M. Ramezani and K. Abnous, Anal. Lett., 2014, 48, 672–681 CrossRef.
- Y. Wang and J. Li, Anal. Chim. Acta, 2009, 650, 49–53 CrossRef CAS PubMed.
- C. T. Marco Frasconi, F. Botrè and F. Mazzei, Anal. Chem., 2010, 82, 7335–7342 CrossRef PubMed.
- Y. Li, D. J. Young and X. J. Loh, Mater. Chem. Front., 2019, 3, 1489–1502 RSC.
- X. Luo, M. Xu, C. Freeman, T. James and J. J. Davis, Anal. Chem., 2013, 85, 4129–4134 CrossRef CAS PubMed.
- E. R. Feeney and P. W. Mallon, Best Pract. Res., Clin. Endocrinol. Metab., 2011, 25, 443–458 CrossRef CAS PubMed.
- Y. Xiao, H. Sun and J. Du, J. Am. Chem. Soc., 2017, 139, 7640–7647 CrossRef CAS PubMed.
- A. GhavamiNejad, B. Lu, A. Giacca and X. Y. Wu, Nanoscale, 2019, 11, 10167–10171 RSC.
- A. GhavamiNejad, J. Li, B. Lu, L. Zhou, L. Lam, A. Giacca and X. Y. Wu, Adv. Mater., 2019, 31, 1901051 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2020 |


