A lysosome-targeting dual-functional fluorescent probe for imaging intracellular viscosity and beta-amyloid†
Hui-ya
Tan
 a,
Yu-tai
Qiu
a,
Han
Sun
a,
Jin-wu
Yan
a,
Yu-tai
Qiu
a,
Han
Sun
a,
Jin-wu
Yan
 *abc and
Lei
Zhang
*abc
*abc and
Lei
Zhang
*abc
aSchool of Biology and Biological Engineering, South China University of Technology, Guangzhou 510006, P. R. China
bGuangdong Provincial Engineering and Technology Research Center of Biopharmaceuticals, South China University of Technology, Guangzhou 510006, P. R. China
cJoint International Research Laboratory of Synthetic Biology and Medicine, South China University of Technology, Guangzhou 510006, P. R. China. E-mail: yjw@scut.edu.cn; lzhangce@scut.edu.cn
First published on 6th February 2019
Abstract
A lysosome-targeting dual-functional fluorescent probe was rationally designed and developed for imaging intracellular lysosomal viscosity and beta-amyloid. More importantly, the real-time tracking of the dynamic movement of lysosomes, as vesicle structures, has been achieved using Lyso-MC.
Alzheimer's disease (AD), an age-related condition, leads to progressive cognitive decline, a communication barrier, language disorder and loss of the ability to self-care.1–3 Besides this, AD affects millions of people worldwide with 5 million new cases every year.4 However, symptomatic treatment only mildly improves the symptoms of AD, but the disease itself is still incurable due to its unclear pathophysiological mechanism.5,6 A toxic amyloid-β peptide (Aβ) is generated from the APP (amyloid precursor protein) after the sequential action of β- and γ-secretase and accumulates constantly to form senile plaques, the hallmark of AD, which are considered to be basic pathological targets for primary prevention, early diagnosis and treatment of the disease.5,6 It has been pointed out that intracellular Aβ is involved in the early stages of AD and spreads from neuron to neuron, directly causing neurotoxicity and triggering AD.7–9 Several studies have found that APP and Aβ are generated as a result of autophagy and the endocytic pathway.10–13 Besides this, AD can be characterized by lysosomal dysfunction and a massive accumulation of lysosomal-related vesicles in the degenerating neurons.11,14,15 Moreover, an increasing number of studies have pointed out that the dysfunction of the autophagy pathway is conducive to Aβ accumulation in pathological conditions.11 Also, much evidence advocates that the lysosomal system, an acidic vesicular compartment containing various hydrolases, is related to Aβ production and neurotoxicity.16,17 In addition, restoring autophagy or improving the lysosomal degradation of intracellular Aβ may be a potential therapeutic approach to treating AD.10,13 Therefore, monitoring cellular lysosomal movement or physiological changes and intracellular Aβ could provide a better understanding of AD pathology and its early diagnosis.
It has been reported that lysosomal viscosity is an important benchmark for lysosome condition and reflects the status and function of this organelle.16,18,19 Therefore, tracing the lysosome or monitoring its viscosity changes is of great benefit for AD diagnosis and pathological studies. Recently, many Aβ fluorescent probes20–22 and many lysosome-targetable fluorescent probes used to image viscosity19,23,24 have been reported. However, as is known, the imaging of both viscosity and Aβ aggregates at the cellular level using a lysosome-targetable dual-functional fluorescent probe has not been reported.
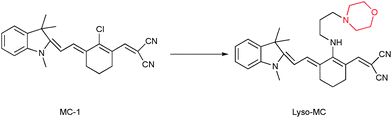
Herein, a fluorescent probe (Lyso-MC) for the sensitive imaging of intracellular lysosomes and Aβ aggregates was reasonably designed and developed. Neutral merocyanine dye (MC-1)25 was selected as the fluorophore to design this probe, which was previously reported by our group as a good scaffold that has been used to image Aβ plaques in vivo and image viscosity at the cellular level by Lin's group.26 However, the low quantum yield and photo-stability of MC-1 restricts its further application. In this study, a 3-morpholinopropylamine moiety was rationally introduced as an electron-donor to enhance the π electron conjugation so as to improve the fluorescence quantum yield, and function as a lysosomal targeting group.19,23,24 As is known, Lyso-MC was the first lysosome-targeting dual-functional probe developed to monitor viscosity and Aβ aggregates at the cellular level.
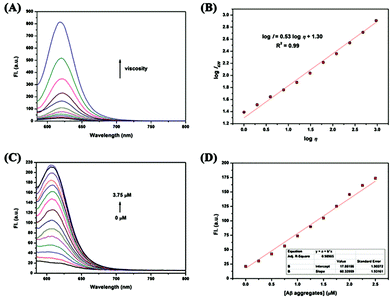
The synthesis of Lyso-MC is outlined in Scheme S1 (ESI†) and its structure (Scheme 1) was confirmed by 1H and 13C NMR spectroscopy and high-resolution mass spectrometry (HR-MS). The fluorescence response spectra of Lyso-MC were first investigated in a water–glycerol system with increasing viscosity. As shown in Fig. 1A, Lyso-MC exhibits its weakest fluorescence emission at 615 nm in water. Meanwhile, the emission intensity showed a dramatic fluorescence enhancement (33-fold, Table S1, ESI†) and the emission wavelength red-shifted to 620 nm due to an increase in the viscosity caused by an increase in the ratio of glycerol to water in the water–glycerol system. In addition, as shown in Fig. 1B, a good linear relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I620 and log
I620 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η (R2 = 0.99, x = 0.53) was quantitatively observed according to the Förster–Hoffmann equation. Moreover, as shown in Fig. S2 (ESI†), the polarity environment only had an influence on the emission wavelength; Lyso-MC exhibited longer absorption and emission wavelengths upon an increase in the polarity of the surrounding environment. Compared with the strong fluorescence of Lyso-MC in glycerol, its fluorescence in other solvents with different polarities was negligible (Fig. S3, ESI†), therefore, its fluorescence intensity was insensitive to polarity changes. In the pH titration experiment, Lyso-MC was not disturbed by the lysosomal pH range (pH = 4.5–5.0) and was only affected by viscosity (Fig. S4, ESI†). Besides this, Lyso-MC was not affected by any interference from various amino acids and ions (Fig. S5, ESI†), exhibiting good selectivity towards viscosity. These results indicated that the Lyso-MC is a stable fluorescent probe for detecting viscosity in lysosomes.
η (R2 = 0.99, x = 0.53) was quantitatively observed according to the Förster–Hoffmann equation. Moreover, as shown in Fig. S2 (ESI†), the polarity environment only had an influence on the emission wavelength; Lyso-MC exhibited longer absorption and emission wavelengths upon an increase in the polarity of the surrounding environment. Compared with the strong fluorescence of Lyso-MC in glycerol, its fluorescence in other solvents with different polarities was negligible (Fig. S3, ESI†), therefore, its fluorescence intensity was insensitive to polarity changes. In the pH titration experiment, Lyso-MC was not disturbed by the lysosomal pH range (pH = 4.5–5.0) and was only affected by viscosity (Fig. S4, ESI†). Besides this, Lyso-MC was not affected by any interference from various amino acids and ions (Fig. S5, ESI†), exhibiting good selectivity towards viscosity. These results indicated that the Lyso-MC is a stable fluorescent probe for detecting viscosity in lysosomes.
We further investigated the fluorescence response of Lyso-MC towards the synthetic Aβ1–42 aggregates, Aβ monomer, BSA and HSA. As expected, the probe exhibited a remarkable (10-fold) fluorescence intensity in the presence of Aβ1–42 aggregates at a low final concentration of 0.25 μM. As displayed in Fig. S5 (ESI†), lower fluorescence responses were observed upon interaction with BSA, HSA and Aβ monomer, indicating low nonspecific binding and its good selectivity towards Aβ1–42 aggregates. Next, the interaction between Lyso-MC and Aβ1–42 aggregates was further studied by carrying out fluorescence titrations. As shown in Fig. 1C, upon titration with Aβ1–42 aggregates, the emission wavelength blue-shifted to 610 nm and the emission intensity was significantly enhanced. Besides this, it showed a good linear relationship (R2 = 0.9899) with Aβ1–42 aggregates in the concentration range of 0 to 2.5 μM (Fig. 1D). Moreover, the detection limit of Lyso-MC was determined to be as low as 10.2 nM (Table S2, ESI†). After the saturation binding assay between Lyso-MC and Aβ1–42 aggregates, the binding constant was calculated to be 31.74 nM, indicating that the Lyso-MC probe exhibited moderate affinity towards the Aβ aggregates (Table S2, ESI†). In addition, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value was measured to be 2.63, predicting that Lyso-MC has the potential for good blood–brain barrier (BBB) penetration. Besides this, red and blue shifts were observed for Lyso-MC in viscous and hydrophobic environments, respectively, therefore it could be used as a dual-functional fluorescent probe to distinguish viscosity changes and Aβ aggregates on the basis of the different emission wavelengths in the different environments.
P value was measured to be 2.63, predicting that Lyso-MC has the potential for good blood–brain barrier (BBB) penetration. Besides this, red and blue shifts were observed for Lyso-MC in viscous and hydrophobic environments, respectively, therefore it could be used as a dual-functional fluorescent probe to distinguish viscosity changes and Aβ aggregates on the basis of the different emission wavelengths in the different environments.
Due to its good response to viscosity, we further investigated the application of Lyso-MC in living cells. Before fluorescence imaging in living cells, the cytotoxicity of Lyso-MC was first assessed in SH-SY5Y and HeLa cells. A standard MTT assay in SH-SY5Y and HeLa cells showed that over 80% of the cells remained alive when 12.5 μM of Lyso-MC was internalized for 12 h (Fig. S7, ESI†), indicating that the probe exhibited weak cytotoxicity. Then, the fluorescence imaging of Lyso-MC in living SH-SY5Y and HeLa cells was performed by confocal laser scanning microscopy (CLSM). The cells exhibited negligible fluorescence in the absence of Lyso-MC, while the cells incubated with Lyso-MC exhibited strong red fluorescence and the fluorescence intensity was found to be dose and time dependent, indicating that Lyso-MC permeated the membrane and stained the living cells (Fig. S8 and S9, ESI†).
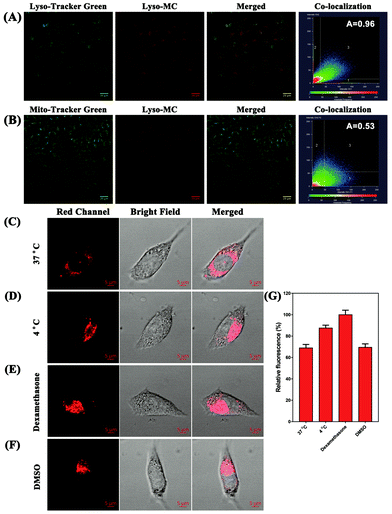
Encouraged by the good membrane-permeability of the probe, we further investigated the co-localization of Lyso-MC in lysosomes of SH-SY5Y cells with commercially available LysoTracker Green DND-26 and MitoTracker Green FM. As shown in Fig. 2, Lyso-MC overlapped very well with LysoTracker Green, showing an overlap coefficient of as high as 0.96 between Lyso-MC and LysoTracker Green, while the coefficient between Lyso-MC and MitoTracker green was only 0.53. The results indicated that Lyso-MC is a lysosome-targetable probe. Meanwhile, we investigated the photo-stability of Lyso-MC inside the cells, which is of great importance for imaging the viscosity of lysosomes. Then, commercially-available LysoTracker Green was used as a comparison. The SH-SY5Y cells were exposed to the 561 nm channel after incubating with Lyso-MC, while the LysoTracker Green group was exposed to the 488 nm channel. After 10 min of continuous illumination, the signal losses for Lyso-MC and LysoTracker Green were 72% and 88%, respectively (Fig. S10, ESI†). Therefore, the probe exhibited moderate photo-stability both in solution (Fig. S6, ESI†) and in living cells.
It is known that a lower temperature contributes to an increase in the intracellular viscosity and that dexamethasone when used as a clinic drug has been proven to be effective in increasing lysosomal viscosity.27,28 With the aim of investigating the ability of Lyso-MC to monitor the variation in the intracellular lysosomal viscosity, living SH-SY5Y cells were first incubated with the probe (5 μM) at different temperatures. As shown in Fig. 2, the cells incubated at 4 °C exhibited a stronger red fluorescence in the red channel when compared with the cells at 37 °C due to the greater viscosity exhibited at 4 °C. As a result, Lyso-MC was only able to detect the lysosomal viscosity in living cells. Besides this, it was clearly observed that the fluorescence intensity increased after treatment with dexamethasone (5 μM), indicating that the lysosomal viscosity was greater than that at 37 °C. In addition, there were no obvious fluorescence changes when incubated with DMSO (10 μL) compared with being incubated with the probe at 37 °C, which further demonstrated that the probe could be used to monitor the variation in the lysosomal viscosity without any interference from polarity fluctuation in living cells.
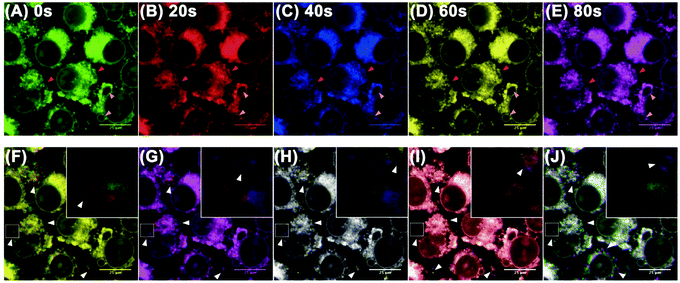
Due to the good lysosome-targeting performance of the probe, we investigated the ability of the probe to trace lysosomes in real time and observed the subcellular structure of the lysosomes using CLSM in ultra-high-resolution mode. Images of the SH-SY5Y cells incubated with Lyso-MC were taken every 4 seconds by CLSM. As shown in Fig. 3, it can be seen that the dynamic lysosomes in living cells have vesicular structures and five different colors were used to clearly show the movements of the lysosomes at various time points (0, 20, 40, 60 and 80 s). The merged images at various time points and dynamic video indicate that the movements of the lysosomes were rather active and that Lyso-MC could be used to trace intracellular lysosomes in real-time.
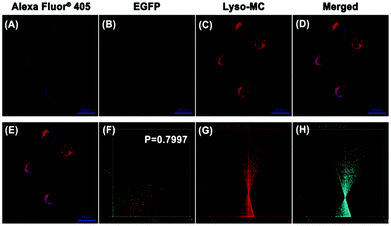
Considering the specific binding of the probe to Aβ1–42 aggregates in solution and the important pathological action of intracellular Aβ aggregates, PC12 cells transfected for the overexpression of Aβ (PC12-EGFP-Aβ cells) and normal PC12 cells taken as controls were used to investigate whether Lyso-MC could detect Aβ aggregates at the cellular level. As shown in Fig. S11B (ESI†), granular aggregates were detected with Lyso-MC in the cytoplasm, consistent with an immunofluorescence group (Fig. S11A, ESI†). Meanwhile, there was negligible fluorescence observed in the control PC12 cells (Fig. S12, ESI†). Besides this, there was also no fluorescence observed in the PC12-EGFP-Aβ cells with no staining with Lyso-MC or immunofluorescence (Fig. S13, ESI†). We also conducted a co-localization experiment for Aβ aggregates in the transfected PC12-EGFP-Aβ cells through co-staining with Lyso-MC after immunofluorescence assay with the secondary antibody Alexa Fluor®405. As shown in Fig. 4, the Lyso-MC and Alexa Fluor®405 merged well with a good Pearson's coefficient of 0.7997. The ICA (intensity correlation analysis, which was used to evaluate the intensity distribution of the two co-existing stains) plots for the Lyso-MC and Alexa Fluor®405 created an unsymmetrical hourglass-shaped scatterplot, which tended toward positive values (Fig. 4G and H). No matter whether ex situ monitored or co-localized for Aβ aggregates, the Lyso-MC probe was confirmed to detect Aβ aggregates at the cellular level.
In conclusion, a red-emitting dual-functional fluorescent probe with low cytotoxicity based on a neutral merocyanine dye (MC-1) was rationally designed and developed for imaging intracellular lysosomal viscosity and Aβ aggregates. The spectral results showed that Lyso-MC exhibited an excellent linear relationship between the logarithm of fluorescence intensity and the logarithm of viscosity (R2 = 0.99, x = 0.53). Meanwhile, the probe also showed a significant turn-on fluorescence response when bound to Aβ aggregates and a high sensitivity towards Aβ aggregates (detection limit: 10.2 nM). Besides this, Lyso-MC was applied to monitor lysosomal viscosity changes and detect intracellular Aβ aggregates. More importantly, the real-time tracking of the dynamic movement of lysosomes, as vesicle structures, was achieved using Lyso-MC. Taking advantage of its remarkable properties, this versatile probe could be a promising imaging tool for use in Alzheimer's disease pathology-related fields.
This work was financially supported by the Natural Science Foundation of Guangdong Province, China (2016A030310463 and 2016A010121004), the National Natural Science Foundation of China (21502056) and the Medical Scientific Research Foundation of Guangdong Province, China (A2018080).
Conflicts of interest
There are no conflicts to declare.Notes and references
- X. Zhang, Y. Tian, C. Zhang, X. Tian, A. W. Ross, R. D. Moir, H. Sun, R. E. Tanzi, A. Moore and C. Ran, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 9734–9739 CrossRef CAS PubMed.
- C. Ballard, S. Gauthier, A. Corbett, C. Brayne, D. Aarsland and E. Jones, Lancet, 2011, 377, 1019–1031 CrossRef.
- M. Staderini, M. A. Martin, M. L. Bolognesi and J. C. Menendez, Chem. Soc. Rev., 2015, 44, 1807–1819 RSC.
- H. Tong, K. Lou and W. Wang, Acta Pharm. Sin. B, 2015, 5, 25–33 CrossRef PubMed.
- F. Mangialasche, A. Solomon, B. Winblad, P. Mecocci and M. Kivipelto, Lancet Neurol., 2010, 9, 702–716 CrossRef CAS PubMed.
- S. Gauthier, L. Wu, P. Rosa-Neto and J. Jia, Transl. Neurodegener., 2012, 1, 1–13 CrossRef PubMed.
- J. Naslund, V. Haroutunian, R. Mohs, K. L. Davis, P. Davies, P. Greengard and J. D. Buxbaum, J. Am. Med. Assoc., 2000, 283, 1571–1577 CrossRef CAS PubMed.
- M. Koistinaho, M. Ort, J. M. Cimadevilla, R. Vondrous, B. Cordell, J. Koistinaho, J. Bures and L. S. Higgins, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 14675–14680 CrossRef CAS PubMed.
- S. Nath, L. Agholme, F. R. Kurudenkandy, B. Granseth, J. Marcusson and M. Hallbeck, J. Neurosci., 2012, 32, 8767–8777 CrossRef CAS PubMed.
- R. A. Nixon and A. M. Cataldo, J. Alzheimer's Dis., 2006, 9, 277–289 CAS.
- R. A. Nixon, J. Cell Sci., 2007, 120, 4081–4091 CrossRef CAS PubMed.
- R. A. Nixon, D.-S. Yang and J.-H. Lee, Autophagy, 2008, 4, 590–599 CrossRef CAS PubMed.
- F. Pickford, E. Masliah, M. Britschgi, K. Lucin, R. Narasimhan, P. A. Jaeger, S. Small, B. Spencer, E. Rockenstein, B. Levine and T. Wyss-Coray, J. Clin. Invest., 2008, 118, 2190–2199 CAS.
- K. Inoue, J. Rispoli, H. Kaphzan, E. Klann, E. I. Chen, J. Kim, M. Komatsu and A. Abeliovich, Mol. Neurodegener., 2012, 7, 48 CrossRef CAS PubMed.
- J.-H. Lee, W. H. Yu, A. Kumar, S. Lee, P. S. Mohan, C. M. Peterhoff, D. M. Wolfe, M. Martinez-Vicente, A. C. Massey, G. Sovak, Y. Uchiyama, D. Westaway, A. M. Cuervo and R. A. Nixon, Cell, 2010, 141, 1146–1158 CrossRef CAS PubMed.
- R. A. Nixon, A. M. Cataldo and P. M. Mathews, Neurochem. Res., 2000, 25, 1161–1172 CrossRef CAS PubMed.
- J. Han and K. Burgess, Chem. Rev., 2010, 110, 2709–2728 CrossRef CAS PubMed.
- T. Liu, X. Liu, D. R. Spring, X. Qian, J. Cui and Z. Xu, Sci. Rep., 2014, 4, 5418 CrossRef CAS PubMed.
- L.-L. Li, K. Li, M.-Y. Li, L. Shi, Y.-H. Liu, H. Zhang, S.-L. Pan, N. Wang, Q. Zhou and X.-Q. Yu, Anal. Chem., 2018, 90, 5873–5878 CrossRef CAS PubMed.
- J.-y. Zhu, L.-f. Zhou, Y.-k. Li, S.-b. Chen, J.-w. Yan and L. Zhang, Anal. Chim. Acta, 2017, 961, 112–118 CrossRef CAS PubMed.
- K. Rajasekhar, N. Narayanaswamy, N. A. Murugan, K. Viccaro, H.-G. Lee, K. Shah and T. Govindaraju, Biosens. Bioelectron., 2017, 98, 54–61 CrossRef CAS PubMed.
- Y. Li, K. Wang, K. Zhou, W. Guo, B. Dai, Y. Liang, J. Dai and M. Cui, Chem. Commun., 2018, 54, 8717–8720 RSC.
- B. Guo, J. Jing, L. Nie, F. Xin, C. Gao, W. Yang and X. Zhang, J. Mater. Chem. B, 2018, 6, 580–585 RSC.
- L. Hou, P. Ning, Y. Feng, Y. Ding, L. Bai, L. Li, H. Yu and X. Meng, Anal. Chem., 2018, 90, 7122–7126 CrossRef CAS PubMed.
- J.-w. Yan, J.-y. Zhu, K.-x. Zhou, J.-s. Wang, H.-y. Tan, Z.-y. Xu, S.-b. Chen, Y.-t. Lu, M.-c. Cui and L. Zhang, Chem. Commun., 2017, 53, 9910–9913 RSC.
- R. Guo, J. Yin, Y. Ma, G. Li, Q. Wang and W. Lin, Sens. Actuators, B, 2018, 271, 321–328 CrossRef CAS.
- S. Humphries, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 14693–14698 CrossRef CAS PubMed.
- L. Wang, Y. Xiao, W. Tian and L. Deng, J. Am. Chem. Soc., 2013, 135, 2903–2906 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of the probe, experimental procedures, and supplemental spectra and graphs. See DOI: 10.1039/c9cc00113a |
| This journal is © The Royal Society of Chemistry 2019 |