Ibrayim Saidalimu, Takuya Yoshioka, Yumeng Liang, Etsuko Tokunaga and Norio Shibata
Chem. Commun., 2018,54, 8761-8764
DOI:
10.1039/C8CC05409F,
Communication
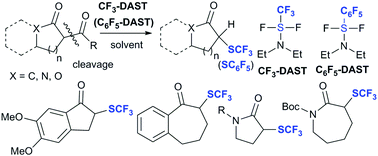
A trifluoromethyl analogue of diethylaminosulfur trifluoride (CF3-DAST)-induced deacylative trifluoromethylthiolation of cyclic 1,3-diketones, lactams, and lactones that provides cyclic α-trifluoromethylthioketones, lactams, and lactones is reported. To the best of our knowledge, this method represents the first example of the trifluoromethylthiolation of lactams. A corresponding deacylative pentafluorophenylthiolation using a pentafluorophenyl analogue of diethylaminosulfur trifluoride (C6F5-DAST) was also attempted.