An early investigative serum Raman spectroscopy study of meningioma
Kanika
Mehta
a,
Apurva
Atak
a,
Aditi
Sahu
b,
Sanjeeva
Srivastava
*a and
Murali Krishna
C
*b
aDepartment of Biosciences and Bioengineering, Indian Institute of Technology Bombay, Powai, Mumbai-400076, India. E-mail: sanjeeva@iitb.ac.in; Fax: +91-22-2572-7760; Tel: +91-22-2576-7779
bChilakapati Laboratory, ACTREC, Tata Memorial Center, Kharghar, Navi Mumbai-410210, India. E-mail: mchilakapati@actrec.gov.in; pittu1043@gmail.com; Fax: +91-22-2740-5095; Tel: +91-22-2740-5039/5544
First published on 5th March 2018
Abstract
Meningiomas represent one of the most frequently reported non-glial, primary brain and central nervous system (CNS) tumors. Meningiomas often display a spectrum of anomalous locations and morphological attributes, deterring their timely diagnosis. Majority of them are sporadic in nature and thus the present-day screening strategies, including radiological investigations, often result in misdiagnosis due to their aberrant and equivocal radiological facets. Therefore, it is pertinent to explore less invasive and patient-friendly biofluids such as serum for their screening and diagnostics. The utility of serum Raman spectroscopy in diagnosis and therapeutic monitoring of cancers has been reported in the literature. In the present study, for the first time, to the best of our knowledge, we have explored Raman spectroscopy to classify the sera of meningioma and control subjects. For this exploration, 35 samples each of meningioma and control subjects were accrued and the spectra revealed variance in the levels of DNA, proteins, lipids, amino acids and β-carotene, i.e., a relatively higher protein, DNA and lipid content in meningioma. Subsequent Principal Component Analysis (PCA) and Principal Component-Linear Discriminant Analysis (PC-LDA) followed by Leave-One-Out Cross-Validation (LOOCV) and limited independent test data, in a patient-wise approach, yielded a classification efficiency of 92% and 80% for healthy and meningioma, respectively. Additionally, in the analogous analysis between healthy and different grades of meningioma, similar results were obtained. These results indicate the potential of Raman spectroscopy in differentiating meningioma. As present methods suffer from known limitations, with the prospective validation on a larger cohort, serum Raman spectroscopy could be an adjuvant/alternative approach in the clinical management of meningioma.
Introduction
Meningiomas originate from meningeal coverings that surround the brain and the spinal cord.1 Being the most frequently reported brain tumors, they account for approximately 36.6% of all primary CNS neoplasms, with an incidence rate of 8.03 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population.2 Of all the CNS tumors, meningiomas have the highest number of new estimated cases with approximately 27
000 population.2 Of all the CNS tumors, meningiomas have the highest number of new estimated cases with approximately 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 and 27
080 and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 cases projected for the years 2016 and 2017, respectively.2 As per the guidelines of the World Health Organization (WHO), meningiomas have been classified into three grades, namely grade I (benign), grade II (atypical) and grade III (anaplastic).1 Commonly studied risk factors for meningioma include genetic predisposition and exposure to ionizing radiations.3 Additionally, since non-malignant meningiomas are 2.27 times more prevalent among females in comparison with males,2 a correlation of female sex hormones with the occurrence of meningioma has been made on numerous occasions,4,5 particularly in case of women undergoing hormone replacement therapy.6–8 Contrary to this belief, some studies have proclaimed that hormones have a limited role in the onset of meningioma, refuting the earlier theories and adding more dubiousness to the tumor etiology.9,10
110 cases projected for the years 2016 and 2017, respectively.2 As per the guidelines of the World Health Organization (WHO), meningiomas have been classified into three grades, namely grade I (benign), grade II (atypical) and grade III (anaplastic).1 Commonly studied risk factors for meningioma include genetic predisposition and exposure to ionizing radiations.3 Additionally, since non-malignant meningiomas are 2.27 times more prevalent among females in comparison with males,2 a correlation of female sex hormones with the occurrence of meningioma has been made on numerous occasions,4,5 particularly in case of women undergoing hormone replacement therapy.6–8 Contrary to this belief, some studies have proclaimed that hormones have a limited role in the onset of meningioma, refuting the earlier theories and adding more dubiousness to the tumor etiology.9,10
Meningiomas are slow-growing tumors and often become very large in size before they are diagnosed.11 The incidence of meningioma increases gradually with age and they are commonly diagnosed at the median age of 66.2 Their asymptomatic occurrence along with the associated sporadic symptoms and a wide range of clinical patterns impede their early stage diagnosis and treatment.11,12 Furthermore, misdiagnosis due to their peculiar locations and divergent morphological features along with the dearth of meningioma-specific biomarkers circumscribes the expeditious investigation of the tumor.13 In many instances, meningiomas are fortuitously discovered when a patient with head trauma or sinusitis undergoes magnetic resonance imaging (MRI)3 or as a case of incidental meningioma during autopsy.14 Hence, there is a need for a more discerning diagnostic tool that can support prompt detection of the disease. In view of the inaccessibility of the tumor site; rapid, objective and patient-friendly biofluids based methods are more suitable.
Recent advances in various spectroscopy techniques have opened up new avenues for the early screening and diagnosis of several diseases. Raman spectroscopy is one such promising technique which is extensively used in assessing a wide array of diseases since it gives insights into the small chemical changes associated with the disease and thus confers a molecular fingerprint.15 It involves inelastic scattering of a monochromatic light, wherein the incident light after interacting with the sample system undergoes a wavelength shift, which is then recorded and measured. Its non-destructive, discriminatory attributes make it a befitting diagnostic technique for both in vivo and ex vivo sample profiling.16 Its applications in early cancer detection and imaging using different biological samples have already been reported for malignancies like lung,17 breast,18–20 prostate,21 and head and neck cancer.22 Additionally, several minimally-invasive Raman spectroscopic studies, using serum, could differentiate the diseased condition from the normal state.23–25 In addition to diagnostic applications, serum Raman spectroscopy has also shown to be useful in therapeutic monitoring.26,27
In the case of meningioma, due to inaccessibility, less invasive studies involving biofluids are better suited. A recent review summarises the present status of biofluids-based spectroscopic applications.28 Previously, Koljenovic and co-workers were able to classify meningioma tissue and normal dura samples by Raman spectroscopy.29 But bio-specimens like tissue or cerebrospinal fluid (CSF) may have limited patient compliance unlike serum-based investigations that can greatly ameliorate the current diagnostic scenario and further alleviate the discomfort caused to cancer patients during screening. Serum is one of the most preferred bio-specimens as it offers numerous advantages, minimally invasive sample collection being one of them.30 Therefore in our study, Raman spectra from sera of 35 each of meningioma subjects and healthy individuals were acquired and subsequently analyzed by PCA and PC-LDA (followed by LOOCV) in a patient-wise approach. This is the first study, to the best of our knowledge, which explores the feasibility of detecting meningioma by serum Raman spectroscopy.
Materials and methods
Subject recruitment and sample collection
Serum samples were collected from 35 patients with radiologically confirmed meningioma and undergoing surgical treatment. The study was approved by the Institutional Ethics Committee of Advanced Centre for Treatment Research and Education in Cancer (ACTREC) and Tata Memorial Hospital (TMH), Mumbai, India. The samples were collected after overnight fasting, prior to any surgery-related intervention after taking a written, informed consent from the subjects. According to the WHO classification, out of these 35 meningioma samples accrued, 19 were grade I and 16 were grade II, based on their histopathology. Subject accruals are detailed in Table 1. The samples collected were aliquoted and stored at −80 °C until further use.| Subject category | Subjects recruited | Age range | Sex | Total spectra recorded |
|---|---|---|---|---|
| Healthy | 35 | 18–47 years | 23 M, 12 F | 278 |
| Meningioma | 35 | 17–81 years | 11 M, 24 F | 267 |
Raman spectroscopy
A commercial HE-785 Raman Spectrometer (LabRam, Jobin–Yvon–Horiba, France) coupled to a fiber-optic microprobe was used for spectral acquisition. The instrumentation employed in this study is described elsewhere.27 Passively thawed 30 μL serum samples, on a calcium fluoride (CaF2) window, were subjected to Raman spectroscopy. Spectral acquisition parameters are: 785 nm excitation, 30 mW power, 500–3500 cm−1 spectral range, 20 s accumulation, average of 3 accumulations, ∼8 spectra per sample. Samples were randomized during spectral acquisition to avoid any bias.Data analysis
In this study, approximately 8 spectra were recorded from each serum sample and further an average spectrum was computed. This approach, referred to as patient-wise, was undertaken to generate a representative spectrum for a given sample and eliminate any variations due to the non-homogeneous colloidal nature of serum. In the patient-wise approach, all the spectra acquired from a given sample are averaged so that each sample is represented by a single spectrum, which was then utilized in the subsequent multivariate analysis.The above-discussed spectra were subjected to following pre-processing: correction for the charge-coupled device (CCD) response, removal of the background noise, interpolation in the fingerprint region 800–1800 cm−1, first-derivatization (Savitzky-Golay, 3) to minimize slow-moving fluorescent background and area normalization to remove any intensity related variations.31,32 Then, the pre-processed spectra were analyzed by unsupervised PCA and supervised PC-LDA followed by LOOCV to delineate the classification between healthy and meningioma sera.
PCA is a routinely used statistical tool often employed in data compression and visualization. It identifies new orthogonal features, called principal components (PCs) or factors having the maximum variations. Linear Discriminant Analysis (LDA), too, works on a similar principle but additionally tries to maximize the separation of the given categories based on the optimized criterion. In other words, it tries to maximize interclass variability and minimize intraclass variability and is often used in conjunction with PCA (PC-LDA) in order to improve the classification efficiency. PC-LDA primarily focuses on the variables important for classification and reduces any noise from the data. For our analysis, statistically significant principal components with p < 0.05 were selected as inputs for subsequent LDA.33 The number of factors chosen was less than half the number of samples in the smallest group to avoid any over-fitting of the data.34,35 Also, less than 10 factors were used for analysis as it is often considered to be one of the best strategies in PC-LDA36 and the model generated by LDA was validated by LOOCV. LOOCV is a type of rotation estimation technique that is employed for determining the performance of a predictive model with a hypothetical validation set, especially in a situation where a true validation set is not available. It uses only one observation as the validation data from the original sample and all the other remaining observations are used as the training data. This step is repeated in a way that each observation from the sample set is used as the validation data and an average is calculated.37 The algorithms for the above-mentioned analyses were performed in a MATLAB (Mathworks Inc.) based in-house software.38
Spectral comparison
For spectral comparison, mean spectra were computed for each class from the pre-processed spectra (corrected for CCD and substrate and optics, interpolation in 800–1800 cm−1 region, baseline corrected by fitting a fifth order polynomial and normalized). The resulting representative spectra were used for the comparison of healthy and meningioma sera.Results and discussion
In the case of brain and CNS tumors, meningiomas represent the most frequently reported histology of 36.6% and moreover constitute 53.2% of all non-malignant brain and CNS tumors.2 Radiological examinations including MRI and computed tomography (CT) are currently the most commonly used methods for diagnosis. Since the majority of meningiomas display atypical and potentially misleading radiological features, their early diagnosis continues to remain a challenge. Additionally, they are often misdiagnosed owing to such peculiar locations and divergent morphological attributes, further worsening the prognosis. Subsequent histopathological evaluation of the biopsied tissue is the gold standard for diagnosis, which bears an additional risk of infections and seizures. Thus, these existing conventional screening modalities are cumbersome and emphasize the need for less invasive, rapid methods like serum Raman spectroscopy as surrogate or complementary screening/diagnosis strategies. Therefore, this present serum-based study investigates the efficacy of distinguishing meningioma from a control set and the results obtained are discussed below.Spectral analysis
Major spectral features were observed for β-carotene (1157, 1520 cm−1), amide linkages (1302, 1337 cm−1), CH2 deformation (1337, 1398 cm−1), DNA (1080, 1340, 1420 cm−1), tyrosine (830, 852 cm−1), tryptophan (1360 cm−1), phenylalanine (1004, 1204 cm−1) and lipids (1300 cm−1) in all the spectra (Fig. 1). The tentative assignment of spectral features is based on the existing literature.39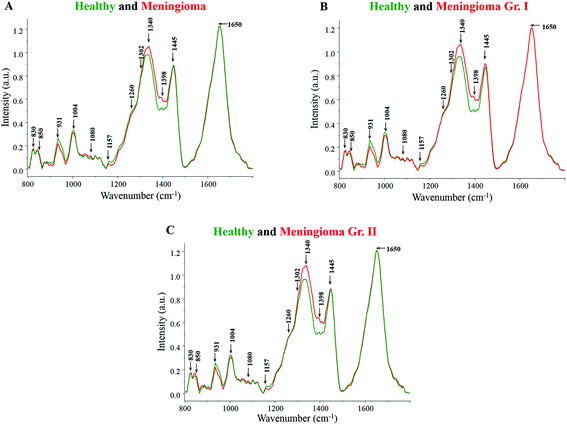 | ||
| Fig. 1 (A) Average spectra of healthy and meningioma sera, (B) average spectra of healthy and meningioma grade I serum samples, (C) average spectra of healthy and meningioma grade II serum samples. | ||
Both healthy and meningioma spectra exhibit major contributions from amino acids, proteins, DNA, lipids, and β-carotene [Fig. 1(A)]. More intense peaks of DNA backbone (1080 cm−1) and nitrogenous bases (1340, 1420 cm−1) were observed in meningioma spectrum in comparison with healthy. The presence of intense DNA peaks in meningioma may indicate high levels of cell-free DNA under tumor conditions. These findings corroborate the earlier reports of an increased expression of cell-free nucleic acids (cfNA) like DNA, RNA and mRNA in the blood circulation of cancer patients, attributed to the increased incidence of necrosis and apoptosis.40,41 Additionally, these alterations in the levels of cfNA have also been previously associated with tumor burden and their progression.40
Similarly, the peaks corresponding to amide linkages (1302, 1337 cm−1) were more intense in meningioma showing some proteomic perturbations like changes in secondary structure. Sharma et al. in their comprehensive serum-based proteomics analysis of different grades of meningioma had revealed an elevated expression of apolipoproteins, coagulation and complement factors that are involved in lipid and lipoprotein metabolism, immune and integrin signaling pathways, etc.42 In another proteomics study by Saydam and group, Minichromosome Maintenance (MCM) proteins, which play a crucial role in DNA maintenance and cellular processes such as replication, transcription, and chromatin remodelling, were found to be highly up-regulated in meningiomas. The group also performed quantitative real-time polymerase chain reaction (qRT PCR) based validation in which high expression levels of the MCM transcripts were observed in meningioma tumor samples in comparison with human arachnoidal tissues.43
Additionally, the peaks corresponding to CH2 bending were also found to be more pronounced in meningioma, which may represent high protein and lipid levels. Early reports on brain tumor have highlighted the perturbations in lipid metabolism and identified encouraging drug targets for brain tumor therapy.44 Besides, a tissue microarray study by Haase and group has revealed a heightened expression of a fatty acid synthase enzyme in meningiomas, indicating a dysregulated lipid metabolism.45 Further supporting the hypothesis, a nuclear magnetic resonance (NMR) based metabolomics study on meningioma tissue samples found an increased level of lipids like phosphocholine and phosphoethanolamine to be associated with tumor malignancy.46
Amino acids like tyrosine and tryptophan also showed relatively higher levels in meningioma in comparison with healthy samples. In the case of tryptophan, few studies on brain tumors have reported an abnormal tryptophan metabolism with an elevated tryptophan uptake and catabolism rate.47
On the other hand, peaks for phenylalanine were found to be more intense in the healthy serum samples. Likewise, more intense peaks for β-carotene were observed in the healthy spectrum in comparison with meningioma. This is in accordance with other Raman spectroscopic studies wherein too, higher levels of β-carotene were observed under normal pathological conditions in comparison with tumor samples.22,23 In the case of breast cancer, studies have suggested that a low intake of dietary carotenoids may be associated with an increased risk of cancer48 and additionally women with higher circulating levels of β-carotene may be at a lower risk of developing breast cancer.49 Similarly, in an Indian population-based study, a noticeable decline in the levels of plasma antioxidants was seen from normal to pre-cancerous condition and further to a cancerous condition.50
Similar results were obtained for grade-wise comparison in which the average healthy spectrum was first compared to the meningioma grade I spectrum and further to the meningioma grade II spectrum [Fig. 1(B and C)]. Peaks corresponding to proteins, DNA and lipids were more intense in the meningioma grade I and II spectra whereas peaks corresponding to β-carotene and phenylalanine were more prominent in the healthy spectrum.
Multivariate analysis
As mentioned, a patient-wise approach was employed wherein each patient sample was represented by a single spectrum which was computed by averaging all the spectra acquired for the given sample. The pre-processed spectra were analyzed using unsupervised (PCA) and supervised (PC-LDA) classification tools.PCA was carried out for all the three conditions, i.e., healthy vs. meningioma, healthy vs. meningioma grade I and healthy vs. meningioma grade II, wherein total percentage variance plots and scatter plots (made by plotting the various combinations of scores of factors) were computed. Since PCA is a tool used for data visualization and trends, subsequent PC-LDA was also carried out. In the case of PC-LDA, results are presented as scatter plots and a confusion matrix wherein diagonal elements are the true classifications. Akin to PCA, three different models were generated, which were evaluated LOOCV and limited test data.
Healthy vs. Meningioma
In this study, twenty-five samples each from healthy and meningioma groups were subjected to PCA and PC-LDA. PCA was carried out with 10 factors in which the first 4 factors accounted for ∼90% of the total percentage variance and the scatter plot of Factor 2 vs. 3 showed a classification between healthy and meningioma samples with minimal overlap [Fig. 2(A and B)].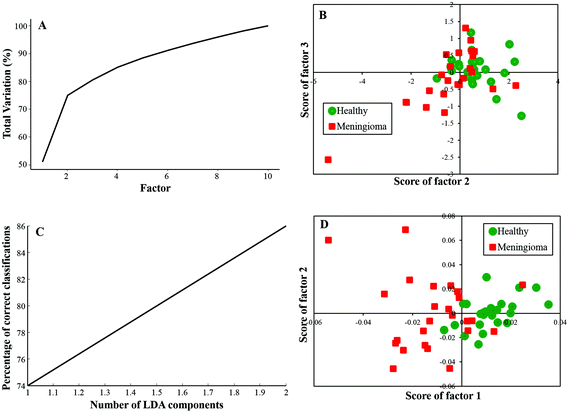 | ||
| Fig. 2 Classification of healthy and meningioma sera: (A) PCA variance plot, (B) PCA scatter plot, (C) PC-LDA scatter plot, and (D) PC-LDA scatter plot. | ||
Further, PC-LDA was performed using two factors [Fig. 2(C)] accounting for 86% of correct classification. The scatter plot of Factor 1 vs. 2 showed discrete clusters of healthy and meningioma groups (with a slight overlap), [Fig. 2(D)]. PC-LDA models were evaluated by LOOCV, which gave classification efficiency of 92% and 80% for healthy and meningioma, respectively [Table 2(a)]. The models were also evaluated using the remaining ten healthy and ten meningioma samples as independent test data. In this case, a classification efficiency of 70% was observed [Table 2(b)].
| (a) LOOCV | ||||
|---|---|---|---|---|
| Healthy | Meningioma | Total | % Efficiency | |
| Healthy | 23 | 2 | 25 | 92 |
| Meningioma | 5 | 20 | 25 | 80 |
| (b) Test prediction using an independent test data | ||||
|---|---|---|---|---|
| Model healthy | Model meningioma | Total | % Efficiency | |
| Test healthy | 7 | 3 | 10 | 70 |
| Test meningioma | 3 | 7 | 10 | 70 |
In the next step, the possibility of differentiating between healthy and different grade samples was evaluated.
Healthy vs. Meningioma Grade I
Twenty-five healthy (same samples used in Healthy vs. Meningioma model) and fifteen grade I (randomly chosen) samples were taken for analysis. First, PCA was performed with 10 factors wherein the acquired variance plot revealed that the first 4 factors account for ∼80% of the total percentage variance and the scatter plot of Factor 2 vs. 5 revealed two distinct clusters with little overlap between the two groups [Fig. 3(A and B)].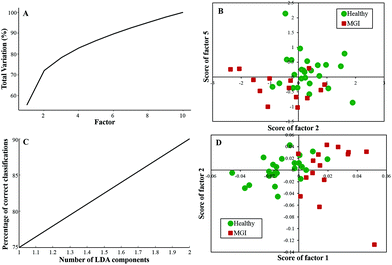 | ||
| Fig. 3 Classification of healthy and meningioma grade I sera: (A) PCA variance plot, (B) PCA scatter plot, (C) PC-LDA scatter plot, and (D) PC-LDA scatter plot. | ||
The subsequent PC-LDA was carried out using 2 factors, which accounted for 90% correct classifications [Fig. 3(C)] and the scatter plot indicated slightly overlapping clusters for the healthy and meningioma grade I group [Fig. 3(D)]. In this case, models were evaluated by LOOCV, which gave a classification efficiency of 88% and 86.66% for healthy and grade I samples, respectively [Table 3(a)]. Further, models were also evaluated by limited independent test data using the remaining ten healthy and four grade I samples. In this analysis, a classification efficiency of 70% and 75% was seen for healthy and grade I samples, respectively [Table 3(b)].
| (a) LOOCV | ||||
|---|---|---|---|---|
| Healthy | Meningioma Gr. I | Total | % Efficiency | |
| Healthy | 22 | 3 | 25 | 88 |
| Meningioma gr. I | 2 | 13 | 15 | 86.66 |
| (b) Test prediction using an independent test data | ||||
|---|---|---|---|---|
| Model healthy | Model meningioma Gr. I | Total | % Efficiency | |
| Test healthy | 7 | 3 | 10 | 70 |
| Test meningioma gr. I | 1 | 3 | 4 | 75 |
Healthy vs. Meningioma Grade II
Twenty-five healthy (same samples used in healthy vs. meningioma model) and sixteen grade II samples were employed for the analysis of the healthy vs. meningioma grade II classification. In this case, 10 factors were employed for PCA and the first 4 factors accounted for more than 80% of the total percentage variance [Fig. 4(A)]. The scatter plot of the scores of Factor 1 vs. 2 did not yield good classification [Fig. 4(B)]. In subsequent PC-LDA, three factors were used which gave ∼80% correct classification [Fig. 4(C)] and the scatter plot of Factor 1 vs. 2 is shown in Fig. 4(D) wherein two clusters with overlap can be seen. Once again models were evaluated by LOOCV and the confusion matrix obtained revealed a classification efficiency of 84% and 68.75% [Table 4(a)]. Though the specificity was good, a few of the grade II samples were misclassified. In this case, only 10 healthy samples were employed as independent test data and 80% of them were classified correctly [Table 4(b)].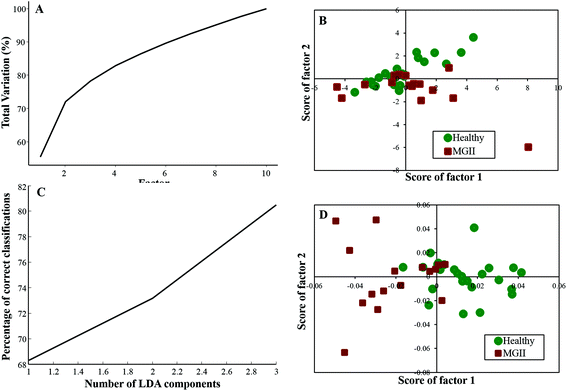 | ||
| Fig. 4 Classification of healthy and meningioma grade II sera: (A) PCA variance plot, (B) PCA scatter plot, (C) PC-LDA scatter plot, and (D) PC-LDA scatter plot. | ||
| (a) LOOCV | ||||
|---|---|---|---|---|
| Healthy | Meningioma Gr. II | Total | % Efficiency | |
| Healthy | 21 | 4 | 25 | 84 |
| Meningioma gr. II | 5 | 11 | 16 | 68.75 |
| (b) Test prediction using an independent test data | ||||
|---|---|---|---|---|
| Model healthy | Model meningioma Gr. II | Total | % Efficiency | |
| Test healthy | 8 | 2 | 10 | 80 |
Conclusions
Despite advancements in the field of cancer treatment, the disease-free survival rates remain low owing to the impediments in cancer screening. Hence, it is necessary to explore alternate discerning approaches that can circumvent the limitations of existing diagnostics or screening tools, in turn leading to a better prognosis. Ideally, such methods should be simpler, rapid, objective, less invasive and patient-compliant. Several optical spectroscopic techniques including Raman spectroscopy have been shown to classify tumor and premalignant conditions and thus are the potential tools in cancer detection.Therefore, this serum Raman spectroscopy study was taken up to explore the applicability of Raman spectroscopy in classifying meningioma. Spectral differences corresponding to DNA, amide linkages, β-carotene, phenylalanine, tryptophan, etc. were observed across the analyzed groups. The findings of patient-wise PC-LDA suggest prospects of classifying meningioma and healthy samples with classification efficiency of 80% and 92%, respectively.
To the best of our knowledge, this is the first serum-based Raman spectroscopy study that explores the distinction between healthy and meningioma samples. Early screening of meningiomas using serum Raman spectroscopy is a pragmatic and patient-friendly approach. A caveat in this work could be the limited sample size employed. Validation of this exploratory study, on a larger cohort will further evaluate the efficacy of Raman spectroscopy-based study in screening meningiomas. Upon such validation, serum Raman spectroscopy can be prospectively extended to the screening of a high-risk population and therapeutic monitoring.
Conflicts of interest
The authors declare that they have no conflicting financial interests.Acknowledgements
The Raman spectrometer employed in the study was procured from the DBT project BT/PRI11282/MED/32/83/2008 (“Development of in vivo laser Raman spectroscopy methods for diagnosis of oral precancerous and cancerous conditions”), Department of Biotechnology, Government of India. K. M. is grateful to the Department of Biotechnology grant (DRD/Rect/Project/P-12168/2015) for providing student fellowship.References
- M. J. Riemenschneider, A. Perry and G. Reifenberger, Lancet Neurol., 2006, 5, 1045–1054 CrossRef CAS PubMed.
- Q. T. Ostrom, H. Gittleman, J. Xu, C. Kromer, Y. Wolinsky, C. Kruchko and J. S. Barnholtz-Sloan, Neuro-Oncology, 2016, 18, v1–v75 CrossRef PubMed.
- J. Wiemels, M. Wrensch and E. B. Claus, J. Neuro-Oncol., 2010, 99, 307–314 CrossRef PubMed.
- A. Wigertz, S. Lonn, P. Hall, A. Auvinen, H. C. Christensen, C. Johansen, L. Klaeboe, T. Salminen, M. J. Schoemaker, A. J. Swerdlow, T. Tynes and M. Feychting, Cancer Epidemiol., Biomarkers Prev., 2008, 17, 2663–2670 CrossRef PubMed.
- E. B. Claus, P. J. Park, R. Carroll, J. Chan and P. M. Black, Cancer Res., 2008, 68, 314–322 CrossRef CAS PubMed.
- Z. Y. Qi, C. Shao, Y. L. Huang, G. Z. Hui, Y. X. Zhou and Z. Wang, PLoS One, 2013, 8, e83261 Search PubMed.
- A. Wigertz, S. Lonn, T. Mathiesen, A. Ahlbom, P. Hall and M. Feychting, Swedish Interphone Study Group, Am. J. Epidemiol., 2006, 164, 629–636 CrossRef PubMed.
- Z. X. Fan, J. Shen, Y. Y. Wu, H. Yu, Y. Zhu and R. Y. Zhan, Cancer, Causes & Control, 2013, 24, 1517–1525 CrossRef PubMed.
- G. M. Anic, M. H. Madden, L. B. Nabors, J. J. Olson, R. V. LaRocca, Z. J. Thompson, S. J. Pamnani, P. A. Forsyth, R. C. Thompson and K. M. Egan, J. Neuro-Oncol., 2014, 118, 297–304 CrossRef CAS PubMed.
- K. Korhonen, T. Salminen, J. Raitanen, A. Auvinen, J. Isola and H. Haapasalo, J. Neuro-Oncol., 2006, 80, 1–7 CrossRef CAS PubMed.
- I. R. Whittle, C. Smith, P. Navoo and D. Collie, Lancet, 2004, 363, 1535–1543 CrossRef.
- C. Mawrin and A. Perry, J. Neuro-Oncol., 2010, 99, 379–391 CrossRef CAS PubMed.
- J. T. Hallinan, A. N. Hegde and W. E. Lim, Clin. Radiol., 2013, 68, 837–844 CrossRef CAS PubMed.
- S. Nakasu, A. Hirano, T. Shimura and J. F. Llena, Surg. Neurol., 1987, 27, 319–322 CrossRef CAS PubMed.
- M. B. Fenn, P. Xanthopoulos, G. Pyrgiotakis, S. R. Grobmyer, P. M. Pardalos and L. L. Hench, Adv. Opt. Technol., 2011, 2011, 20 Search PubMed.
- L. A. Austin, S. Osseiran and C. L. Evans, Analyst, 2016, 141, 476–503 RSC.
- Z. Huang, A. McWilliams, H. Lui, D. I. McLean, S. Lam and H. Zeng, Int. J. Cancer, 2003, 107, 1047–1052 CrossRef CAS PubMed.
- A. S. Haka, K. E. Shafer-Peltier, M. Fitzmaurice, J. Crowe, R. R. Dasari and M. S. Feld, Cancer Res., 2002, 62, 5375–5380 CAS.
- B. Brozek-Pluska, J. Musial, R. Kordek, E. Bailo, T. Dieing and H. Abramczyk, Analyst, 2012, 137, 3773–3780 RSC.
- T. Bhattacharjee, A. Khan, G. Maru, A. Ingle and C. M. Krishna, Analyst, 2015, 140, 456–466 RSC.
- D. K. Medipally, A. Maguire, J. Bryant, J. Armstrong, M. Dunne, M. Finn, F. M. Lyng and A. D. Meade, Analyst, 2017, 142, 1216–1226 RSC.
- A. T. Harris, A. Lungari, C. J. Needham, S. L. Smith, M. A. Lones, S. E. Fisher, X. B. Yang, N. Cooper, J. Kirkham, D. A. Smith, D. P. Martin-Hirsch and A. S. High, Head Neck Oncol., 2009, 1, 34 CrossRef PubMed.
- J. L. Pichardo-Molina, C. Frausto-Reyes, O. Barbosa-Garcia, R. Huerta-Franco, J. L. Gonzalez-Trujillo, C. A. Ramirez-Alvarado, G. Gutierrez-Juarez and C. Medina-Gutierrez, Lasers Med. Sci., 2007, 22, 229–236 CrossRef CAS PubMed.
- A. Sahu, K. Dalal, S. Naglot, P. Aggarwal and C. Murali Krishna, PLoS One, 2013, 8, e78921 CAS.
- A. K. Sahu, S. Dhoot, A. Singh, S. S. Sawant, N. Nandakumar, S. Talathi-Desai, M. Garud, S. Pagare, S. Srivastava, S. Nair, P. Chaturvedi and C. Murali Krishna, J. Biomed. Opt., 2015, 20, 115006 CrossRef PubMed.
- T. Bhattacharjee, A. Khan, P. Kumar, A. Ingle, G. Maru and C. M. Krishna, J. Biophotonics, 2015, 8, 575–583 CrossRef CAS PubMed.
- A. Sahu, N. Nandakumar, S. Sawant and C. M. Krishna, Analyst, 2015, 140, 2294–2301 RSC.
- M. J. Baker, S. R. Hussain, L. Lovergne, V. Untereiner, C. Hughes, R. A. Lukaszewski, G. Thiefin and G. D. Sockalingum, Chem. Soc. Rev., 2016, 45, 1803–1818 RSC.
- S. Koljenovic, T. B. Schut, A. Vincent, J. M. Kros and G. J. Puppels, Anal. Chem., 2005, 77, 7958–7965 CrossRef CAS PubMed.
- D. M. Good, V. Thongboonkerd, J. Novak, J. L. Bascands, J. P. Schanstra, J. J. Coon, A. Dominiczak and H. Mischak, J. Proteome Res., 2007, 6, 4549–4555 CrossRef CAS PubMed.
- A. Sahu, S. Sawant, H. Mamgain and C. M. Krishna, Analyst, 2013, 138, 4161–4174 RSC.
- A. Nijssen, K. Maquelin, L. F. Santos, P. J. Caspers, T. C. Bakker Schut, J. C. den Hollander, M. H. Neumann and G. J. Puppels, J. Biomed. Opt., 2007, 12, 034004 CrossRef PubMed.
- S. P. Singh, A. Deshmukh, P. Chaturvedi and C. Murali Krishna, J. Biomed. Opt., 2012, 17, 105002 CAS.
- T. C. Bakker Schut, M. J. Witjes, H. J. Sterenborg, O. C. Speelman, J. L. Roodenburg, E. T. Marple, H. A. Bruining and G. J. Puppels, Anal. Chem., 2000, 72, 6010–6018 CrossRef CAS PubMed.
- T. J. Harvey, E. Gazi, A. Henderson, R. D. Snook, N. W. Clarke, M. Brown and P. Gardner, Analyst, 2009, 134, 1083–1091 RSC.
- J. G. Kelly, J. Trevisan, A. D. Scott, P. L. Carmichael, H. M. Pollock, P. L. Martin-Hirsch and F. L. Martin, J. Proteome Res., 2011, 10, 1437–1448 CrossRef CAS PubMed.
- P. Crow, B. Barrass, C. Kendall, M. Hart-Prieto, M. Wright, R. Persad and N. Stone, Br. J. Cancer, 2005, 92, 2166–2170 CrossRef CAS PubMed.
- A. D. Ghanate, S. Kothiwale, S. P. Singh, D. Bertrand and C. M. Krishna, J. Biomed. Opt., 2011, 16, 025003 CrossRef CAS PubMed.
- Z. Movasaghi, S. Rehman and I. U. Rehman, Appl. Spectrosc. Rev., 2007, 42, 493–541 CrossRef CAS.
- H. Schwarzenbach, D. S. Hoon and K. Pantel, Nat. Rev. Cancer, 2011, 11, 426–437 CrossRef CAS PubMed.
- E. Gormally, P. Hainaut, E. Caboux, L. Airoldi, H. Autrup, C. Malaveille, A. Dunning, S. Garte, G. Matullo, K. Overvad, A. Tjonneland, F. Clavel-Chapelon, P. Boffetta, H. Boeing, A. Trichopoulou, D. Palli, V. Krogh, R. Tumino, S. Panico, H. B. Bueno-de-Mesquita, P. H. Peeters, E. Lund, C. A. Gonzalez, C. Martinez, M. Dorronsoro, A. Barricarte, M. J. Tormo, J. R. Quiros, G. Berglund, G. Hallmans, N. E. Day, T. J. Key, F. Veglia, M. Peluso, T. Norat, R. Saracci, R. Kaaks, E. Riboli and P. Vineis, Int. J. Cancer, 2004, 111, 746–749 CrossRef CAS PubMed.
- S. Sharma, S. Ray, A. Moiyadi, E. Sridhar and S. Srivastava, Sci. Rep., 2014, 4, 7140 CrossRef CAS PubMed.
- O. Saydam, O. Senol, T. B. Schaaij-Visser, T. V. Pham, S. R. Piersma, A. O. Stemmer-Rachamimov, T. Wurdinger, S. M. Peerdeman and C. R. Jimenez, J. Proteome Res., 2010, 9, 485–494 CrossRef CAS PubMed.
- P. Prasanna, A. Thibault, L. Liu and D. Samid, J. Neurochem., 1996, 66, 710–716 CrossRef CAS PubMed.
- D. Haase, S. Schmidl, C. Ewald, R. Kalff, C. Huebner, R. Firsching, G. Keilhoff, M. Evert, W. Paulus, D. H. Gutmann, A. Lal and C. Mawrin, Neuro-Oncology, 2010, 12, 844–854 CrossRef CAS PubMed.
- D. Monleon, J. M. Morales, J. Gonzalez-Darder, F. Talamantes, O. Cortes, R. Gil-Benso, C. Lopez-Gines, M. Cerda-Nicolas and B. Celda, J. Proteome Res., 2008, 7, 2882–2888 CrossRef CAS PubMed.
- C. Juhasz, D. C. Chugani, O. Muzik, D. Wu, A. E. Sloan, G. Barger, C. Watson, A. K. Shah, S. Sood, E. L. Ergun, T. J. Mangner, P. K. Chakraborty, W. J. Kupsky and H. T. Chugani, J. Cereb. Blood Flow Metab., 2006, 26, 345–357 CrossRef CAS PubMed.
- P. Toniolo, A. L. Van Kappel, A. Akhmedkhanov, P. Ferrari, I. Kato, R. E. Shore and E. Riboli, Am. J. Epidemiol., 2001, 153, 1142–1147 CrossRef CAS PubMed.
- A. H. Eliassen, S. J. Hendrickson, L. A. Brinton, J. E. Buring, H. Campos, Q. Dai, J. F. Dorgan, A. A. Franke, Y. T. Gao, M. T. Goodman, G. Hallmans, K. J. Helzlsouer, J. Hoffman-Bolton, K. Hulten, H. D. Sesso, A. L. Sowell, R. M. Tamimi, P. Toniolo, L. R. Wilkens, A. Winkvist, A. Zeleniuch-Jacquotte, W. Zheng and S. E. Hankinson, J. Natl. Cancer Inst., 2012, 104, 1905–1916 CrossRef CAS PubMed.
- L. Aravindh, P. Jagathesh, S. Shanmugam, S. Sarkar, P. M. Kumar and S. Ramasubramanian, Int. J. Biol. Med. Res., 2012, 3, 1655–1657 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |
