Unsymmetrical squaraines with new linkage manner for high-performance solution-processed small-molecule organic photovoltaic cells†
Lin Yang‡
a,
Daobin Yang‡ab,
Yao Chena,
Qian Luoa,
Mangang Zhanga,
Yan Huang*a,
Zhiyun Lu*a,
Hisahiro Sasabe*b and
Junji Kidob
aKey Laboratory of Green Chemistry and Technology (Ministry of Education), College of Chemistry, Sichuan University, Chengdu 610064, P. R. China. E-mail: huangyan@scu.edu.cn; luzhiyun@scu.edu.cn
bDepartment of Organic Device Engineering, Yamagata University, 4-3-16 Jonan, Yonezawa, Yamagata 992-8510, Japan. E-mail: h-sasabe@yz.yamagata-u.ac.jp
First published on 15th December 2015
Abstract
Squaraines are promising donor materials because of their strong and broad absorption band in the visible and near infrared regions which is suitable for application in organic photovoltaic (OPV) cells. Two unsymmetrical squaraines (USQs), namely BIBISQ and TIBISQ, with two electron-donating aryls directly linked to the electron-withdrawing squaric acid core (Y-manner) can act as high performance donor materials for solution-processed bulk-heterojunction (BHJ) OPV cells. Both USQs show ideal low bandgaps (1.47 eV for BIBISQ and 1.39 eV for TIBISQ) with an intense and broad absorption band in the range of 500–900 nm, and relatively low HOMO levels of ∼−5.10 eV. The BHJ-OPV device based on both of them simultaneously showed excellent short current density (Jsc) (over 13 mA cm−2), open circuit voltage (Voc) (0.84 V), fill factor (FF) (0.49) and power conversion efficiency (PCE) of over 5% under a blend ratio of USQs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1
PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. These results indicate that the two USQs are quite promising candidates for small molecular (SM) OPV cells and the Y-manner should be a quite successful linking method for USQs.
3. These results indicate that the two USQs are quite promising candidates for small molecular (SM) OPV cells and the Y-manner should be a quite successful linking method for USQs.
1. Introduction
Owing to its nanometer-scaled phase separation in the donor–acceptor (D–A) blending system with more efficient D/A interfacial contact, the bulk-heterojunction (BHJ) organic photovoltaic (OPV) device has been considered to be a promising technique for renewable energy source applications.1,2 In the last decade, small molecular (SM) photovoltaic donor materials have attracted considerable attention for preparing BHJ-OPVs due to their unique advantages over their polymer counterparts, such as well-defined molecular structure and molecular weight, facile synthesis and high purity without batch-to-batch variations.3 Therefore, many kinds of small molecular photovoltaic donor materials have been developed, including oligothiophenes, dyes, fused acenes, triphenylamine-based molecules and so on.4–8 Up to now, power conversion efficiencies (PCEs) of SMOPV cells as high as >10% have been achieved with a high donor content (D/A = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8) when an oligothiophene derivative (DRCN5T) is employed as donor material.9
0.8) when an oligothiophene derivative (DRCN5T) is employed as donor material.9
Recently, squaraines (SQs) have emerged as promising donor materials in OPVs due to their strong and broad absorption band in the visible and near infrared regions.10–16 Thus far, the record PCE of squaraine-based BHJ-OPV cells is 5.50% with a rather low donor content (D/A = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6)17 and the PCE of tandem devices based on squaraines is up to 8.3%.18
6)17 and the PCE of tandem devices based on squaraines is up to 8.3%.18
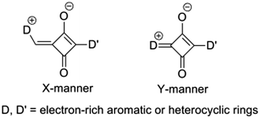
In recent years, our group has designed a series of unsymmetrical squaraines (USQs) in which the D and D′ segments are connected to the A subunit directly and via a methylidene bridge, respectively (X-manner in Scheme 1), and the PCE of these USQ-based BHJ-OPV devices is improved from 1.54% to 4.29%; however, the optimized D/A ratios fall into the region of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–1
8–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 with a low donor content.19–22 According to the literature, the photophysical and chemical properties of squaraines are highly correlated with their linkage manner,23,24 which is closely related to their OPV performance. Therefore, very recently, our group has firstly designed some USQs with their two electron-donating aryls linked directly to the electron-withdrawing squaric acid core in Y-manner (Scheme 1).25 The OPV device based on them rendered a short current density (Jsc) of up to 12.03 mA cm−2 and a PCE of 4.35% with a D/A ratio of 1
5 with a low donor content.19–22 According to the literature, the photophysical and chemical properties of squaraines are highly correlated with their linkage manner,23,24 which is closely related to their OPV performance. Therefore, very recently, our group has firstly designed some USQs with their two electron-donating aryls linked directly to the electron-withdrawing squaric acid core in Y-manner (Scheme 1).25 The OPV device based on them rendered a short current density (Jsc) of up to 12.03 mA cm−2 and a PCE of 4.35% with a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. These preliminary results indicated that D and D′ linked to A in Y-manner should be a quite promising linking manner for USQ donor materials. However, their PCE is unsatisfactory due to their relatively low open circuit voltage (Voc, 0.78 V) and fill factor (FF, 0.45). It is noteworthy that one of their donor segments is a pyrrole derivative, whose electron-donating ability is much better than phenyl groups, which leads to a relatively high HOMO energy level and then low Voc.
5. These preliminary results indicated that D and D′ linked to A in Y-manner should be a quite promising linking manner for USQ donor materials. However, their PCE is unsatisfactory due to their relatively low open circuit voltage (Voc, 0.78 V) and fill factor (FF, 0.45). It is noteworthy that one of their donor segments is a pyrrole derivative, whose electron-donating ability is much better than phenyl groups, which leads to a relatively high HOMO energy level and then low Voc.
Therefore, from these previous results, two novel USQs with Y-manner linking have been designed and synthesized. Phenyl groups, such as N,N-dibutylaniline16,26 or 4-(p-tolyl)-1,2,3,3a,4,8b-hexahydrocyclopenta[b]indole, which are promising groups in OPV donor materials, were used as the D subunit;20,27 and 5-(8,9,10,10a-tetrahydrobenzo[e]cyclopenta[b]indol-7(7aH)-yl)benzene-1,3-diol was chosen as the D′ subunit.25 The molecular structures of two novel USQs (BIBISQ and TIBISQ) are shown in Scheme 2. Both show ideal low bandgaps of 1.47 eV for BIBISQ and 1.39 eV for TIBISQ with an intense and broad absorption band in the range 500–900 nm,28 and relatively low HOMO levels of ∼−5.10 eV. The BHJ-SMOPV cells based on both of them simultaneously showed enhanced Voc (0.84 V) and FF (0.49). Meanwhile, an excellent Jsc of over 13 mA cm−2 was achieved due to the relatively high content of USQs (USQs/PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Ultimately, the PCEs of BHJ-SMOPV cells based on USQs were over 5%. In particular, the BHJ-SMOPV cell based on TIBISQ showed a high Jsc of up to 13.50 mA cm−2 and an excellent PCE of 5.49%, which could compare to the highest Jsc29 and PCE17,30,31 among all of the reported squaraine-based BHJ-OPV cells. These results indicate that Y-manner is indeed a quite promising linking method for the construction of high performance USQ-based OPV cells.
3). Ultimately, the PCEs of BHJ-SMOPV cells based on USQs were over 5%. In particular, the BHJ-SMOPV cell based on TIBISQ showed a high Jsc of up to 13.50 mA cm−2 and an excellent PCE of 5.49%, which could compare to the highest Jsc29 and PCE17,30,31 among all of the reported squaraine-based BHJ-OPV cells. These results indicate that Y-manner is indeed a quite promising linking method for the construction of high performance USQ-based OPV cells.
2. Materials and methods
2.1. Instruments and characterization
1H and 13C NMR spectra were recorded on a Bruker Avance AV II-400 MHz instrument with tetramethylsilane as internal standard. High resolution mass spectra were measured on a Shimadzu LCMS-IT-TOF. The purity of the two USQs was measured by Hitachi EZChrom Elite high performance liquid chromatography (diode array detector (DAD) and refractive index detector (RID)). Absorption spectra of the two USQs in 5 × 10−6 mol L−1 chloroform solution and thin film states were measured with a PerkinElmer Lambda 950 UV-Vis scanning spectrophotometer.2.2. Electrochemistry measurement
Cyclic voltammetry was performed in 0.10 mol L−1 tetrabutylammonium perchlorate/anhydrous dichloromethane (2.5 × 10−4 mol L−1) with a LK 2010 electrochemical workstation, using a three-electrode cell with a Pt disk working electrode, a Pt wire counter electrode and a Ag/AgNO3 (0.1 mol L−1 in acetonitrile) reference electrode. Solutions were pre-degassed by argon bubbling for 30 min before each experiment. At the end of each measurement, the ferrocenium/ferrocene redox couple was added as an internal potential reference.2.3. Preparation of organic photovoltaic cells
Photovoltaic devices were fabricated on indium-tin oxide (ITO)-coated glass substrate (sheet resistance = 15 Ω sq−1) with a layered structure of ITO/MoO3 (8 nm)/USQs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (60 nm)/BCP (6 nm)/Al (100 nm). The patterned ITO-coated glass substrates were cleaned through sequential sonication in detergent, deionized water, acetone, and isopropanol for 20 min each. The cleaned substrates were dried in an oven at 65 °C for 12 h before use. The substrate was treated with UV-ozone for 30 min, then immediately transferred into a high vacuum chamber for deposition of 8 nm MoO3 at a pressure of less than 1 × 10−4 Pa with a rate of 0.2 Å s−1. Subsequently, the photoactive layer (thickness: 60 ± 5 nm) was fabricated by spin-casting a blend of the USQs and PC71BM in chloroform solution with a total concentration of 20 mg mL−1 (3500 rpm, 45 s) under a N2-filling glove box at 25 °C. Finally, the substrates were transferred back to the high-vacuum chamber, where BCP (6 nm) and Al (100 nm) were deposited as the top electrode at pressures of less than 2 × 10−4 Pa with a rate of 0.20 Å s−1 and 2.0–3.0 Å s−1, respectively. The active area of the OPV cells was 9 mm2. To obtain the average data related to device performance, several batches of devices (4 cells per batch) were fabricated and tested for each set of conditions. Current density–voltage (J–V) and external quantum efficiency (EQE) characterization of organic photovoltaic cells were performed on a CEP-2000 integrated system manufactured by Bunkoukeiki Co. The integration of EQE data over a AM 1.5G solar spectrum yielded calculated Jsc values with an experimental variation of less than 5% relative to Jsc measured under 100 mW cm−2 simulated AM 1.5G light illumination. Hole-only devices were fabricated with structures of ITO/MoO3 (8 nm)/USQs
PC71BM (60 nm)/BCP (6 nm)/Al (100 nm). The patterned ITO-coated glass substrates were cleaned through sequential sonication in detergent, deionized water, acetone, and isopropanol for 20 min each. The cleaned substrates were dried in an oven at 65 °C for 12 h before use. The substrate was treated with UV-ozone for 30 min, then immediately transferred into a high vacuum chamber for deposition of 8 nm MoO3 at a pressure of less than 1 × 10−4 Pa with a rate of 0.2 Å s−1. Subsequently, the photoactive layer (thickness: 60 ± 5 nm) was fabricated by spin-casting a blend of the USQs and PC71BM in chloroform solution with a total concentration of 20 mg mL−1 (3500 rpm, 45 s) under a N2-filling glove box at 25 °C. Finally, the substrates were transferred back to the high-vacuum chamber, where BCP (6 nm) and Al (100 nm) were deposited as the top electrode at pressures of less than 2 × 10−4 Pa with a rate of 0.20 Å s−1 and 2.0–3.0 Å s−1, respectively. The active area of the OPV cells was 9 mm2. To obtain the average data related to device performance, several batches of devices (4 cells per batch) were fabricated and tested for each set of conditions. Current density–voltage (J–V) and external quantum efficiency (EQE) characterization of organic photovoltaic cells were performed on a CEP-2000 integrated system manufactured by Bunkoukeiki Co. The integration of EQE data over a AM 1.5G solar spectrum yielded calculated Jsc values with an experimental variation of less than 5% relative to Jsc measured under 100 mW cm−2 simulated AM 1.5G light illumination. Hole-only devices were fabricated with structures of ITO/MoO3 (8 nm)/USQs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (60 nm)/MoO3 (8 nm)/Al (100 nm).
PC71BM (60 nm)/MoO3 (8 nm)/Al (100 nm).
Samples for atomic force microscopy (AFM) measurements were prepared by spin-casting from USQs/PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in chloroform solution on glass substrates.
3 in chloroform solution on glass substrates.
2.4. Synthesis
The synthetic routes of intermediates and target molecule USQs are outlined in Scheme 2. Compounds 1–9 were prepared according to the procedures described in the literature.25,27,32,33 n-Butanol and toluene were freshly distilled with sodium before use. All of the other chemicals, reagents, and solvents were used as received from the suppliers.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v N,N-dimethylformamide (DMF)/N-methylpyrrolidinone (NMP) was heated at 120 °C for 12 h. The reaction mixture was then cooled to room temperature and filtered to remove the insoluble material. The precipitate was washed with ethyl acetate, and the combined filtrate was evaporated to dryness. Water was then added, and the aqueous phase was extracted with ethyl acetate. After silica gel column chromatography (hexane), a pale yellow oil (9.18 g, 85%) was obtained.
1 v/v N,N-dimethylformamide (DMF)/N-methylpyrrolidinone (NMP) was heated at 120 °C for 12 h. The reaction mixture was then cooled to room temperature and filtered to remove the insoluble material. The precipitate was washed with ethyl acetate, and the combined filtrate was evaporated to dryness. Water was then added, and the aqueous phase was extracted with ethyl acetate. After silica gel column chromatography (hexane), a pale yellow oil (9.18 g, 85%) was obtained.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 5 (0.71 g, 30%) as a yellow solid. 1HNMR (400 MHz, CDCl3, ppm) δ 8.15 (d, J = 9.2 Hz, 2H, ArH), 6.75 (d, J = 9.2 Hz, 2H, ArH), 3.44 (t, J = 7.6 Hz, 4H, CH2), 1.69–1.61 (m, 4H, CH2), 1.46–1.37 (m, 4H, CH2), 1.03 (t, J = 7.2 Hz, 6H, CH3).
1) to give 5 (0.71 g, 30%) as a yellow solid. 1HNMR (400 MHz, CDCl3, ppm) δ 8.15 (d, J = 9.2 Hz, 2H, ArH), 6.75 (d, J = 9.2 Hz, 2H, ArH), 3.44 (t, J = 7.6 Hz, 4H, CH2), 1.69–1.61 (m, 4H, CH2), 1.46–1.37 (m, 4H, CH2), 1.03 (t, J = 7.2 Hz, 6H, CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6 (0.22 g, 25%) as an orange solid. 1HNMR (400 MHz, CDCl3, ppm) δ 8.01 (d, J = 8.4 Hz, 1H, ArH), 7.96 (s, 1H, ArH), 7.26–7.16 (m, 4H, ArH), 6.74 (d, J = 8.8 Hz, 1H, ArH), 4.99 (t, J = 8.4 Hz, 1H, CH), 3.89 (t, J = 8.4 Hz, 1H, CH), 2.38 (s, 3H, CH3), 2.14–2.01 (m, 1H, CH2), 1.92–1.85 (m, 2H, CH2), 1.76–1.66 (m, 2H, CH2), 1.56–1.44 (m, 1H, CH2).
1) to give 6 (0.22 g, 25%) as an orange solid. 1HNMR (400 MHz, CDCl3, ppm) δ 8.01 (d, J = 8.4 Hz, 1H, ArH), 7.96 (s, 1H, ArH), 7.26–7.16 (m, 4H, ArH), 6.74 (d, J = 8.8 Hz, 1H, ArH), 4.99 (t, J = 8.4 Hz, 1H, CH), 3.89 (t, J = 8.4 Hz, 1H, CH), 2.38 (s, 3H, CH3), 2.14–2.01 (m, 1H, CH2), 1.92–1.85 (m, 2H, CH2), 1.76–1.66 (m, 2H, CH2), 1.56–1.44 (m, 1H, CH2).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). Mp 219–221 °C. 1HNMR (400 MHz, CDCl3, ppm) δ 12.84 (s, 2H, OH), 8.15 (d, J = 8.8 Hz, 2H, ArH), 7.87 (d, J = 8.0 Hz, 1H, ArH), 7.83 (d, J = 8.4 Hz, 1H, ArH), 7.79 (d, J = 9.2 Hz, 1H, ArH), 7.71 (d, J = 8.8 Hz, 1H, ArH), 7.53 (t, J = 7.2 Hz, 1H, ArH), 7.44 (t, J = 7.2 Hz, 1H, ArH), 7.09 (s, 2H, ArH), 6.40 (s, 2H, ArH), 4.98–4.93 (m, 1H, CH), 4.39–4.34 (m, 1H, CH), 3.44 (t, J = 7.6 Hz, 4H, CH2), 2.39–2.25 (m, 2H, CH2), 2.12–1.99 (m, 2H, CH2), 1.79–1.67 (m, 4H, CH2), 1.58–1.49 (m, 2H, CH2), 1.44–1.35 (m, 4H, CH2), 0.99 (t, J = 7.2 Hz, 6H, CH3). 13CNMR (100 MHz, CDCl3, ppm) δ 182.8, 178.1, 166.5, 164.6, 155.2, 152.6, 140.1, 132.5, 130.9, 130.7, 130.1, 128.8, 128.6, 126.9, 124.5, 123.3, 117.8, 115.2, 112.3, 108.0, 97.0, 69.7, 51.2, 44.7, 35.1, 33.3, 29.6, 24.8, 20.2, 13.9. HRMS (ESI)+ m/z: [M + H]+ calcd for C39H41N2O4, 601.3061; found, 601.3062. Anal. calcd for C39H40N2O4: C, 77.97; H, 6.71; N, 4.66. Found: C, 78.11; H, 6.94; N, 5.12.
9). Mp 219–221 °C. 1HNMR (400 MHz, CDCl3, ppm) δ 12.84 (s, 2H, OH), 8.15 (d, J = 8.8 Hz, 2H, ArH), 7.87 (d, J = 8.0 Hz, 1H, ArH), 7.83 (d, J = 8.4 Hz, 1H, ArH), 7.79 (d, J = 9.2 Hz, 1H, ArH), 7.71 (d, J = 8.8 Hz, 1H, ArH), 7.53 (t, J = 7.2 Hz, 1H, ArH), 7.44 (t, J = 7.2 Hz, 1H, ArH), 7.09 (s, 2H, ArH), 6.40 (s, 2H, ArH), 4.98–4.93 (m, 1H, CH), 4.39–4.34 (m, 1H, CH), 3.44 (t, J = 7.6 Hz, 4H, CH2), 2.39–2.25 (m, 2H, CH2), 2.12–1.99 (m, 2H, CH2), 1.79–1.67 (m, 4H, CH2), 1.58–1.49 (m, 2H, CH2), 1.44–1.35 (m, 4H, CH2), 0.99 (t, J = 7.2 Hz, 6H, CH3). 13CNMR (100 MHz, CDCl3, ppm) δ 182.8, 178.1, 166.5, 164.6, 155.2, 152.6, 140.1, 132.5, 130.9, 130.7, 130.1, 128.8, 128.6, 126.9, 124.5, 123.3, 117.8, 115.2, 112.3, 108.0, 97.0, 69.7, 51.2, 44.7, 35.1, 33.3, 29.6, 24.8, 20.2, 13.9. HRMS (ESI)+ m/z: [M + H]+ calcd for C39H41N2O4, 601.3061; found, 601.3062. Anal. calcd for C39H40N2O4: C, 77.97; H, 6.71; N, 4.66. Found: C, 78.11; H, 6.94; N, 5.12.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). Mp 248–249 °C. 1HNMR (400 MHz, CDCl3, ppm) δ 12.69 (s, 2H, OH), 8.02 (d, J = 8.4 Hz, 1H, ArH), 7.97 (s, 1H, ArH), 7.84 (d, J = 8.4 Hz, 1H, ArH), 7.79 (d, J = 8.4 Hz, 1H, ArH), 7.75 (d, J = 8.8 Hz, 1H, ArH), 7.69 (d, J = 8.8 Hz, 1H, ArH), 7.52 (t, J = 7.2 Hz, 1H, ArH), 7.40 (t, J = 7.2 Hz, 1H, ArH), 7.26 (d, J = 8.0 Hz, 2H, ArH), 7.20 (d, J = 8.4 Hz, 2H, ArH), 6.75 (d, J = 8.0 Hz, 1H, ArH), 6.38 (s, 2H, ArH), 4.98 (s, 1H, CH), 4.91 (s, 1H, CH), 4.32 (s, 1H, CH), 3.85 (s, 1H, CH), 2.39 (s, 3H, CH3), 2.36–2.19 (m, 2H, CH2), 2.12–1.84 (m, 5H, CH2), 1.77–1.66 (m, 3H, CH2), 1.57–1.45 (m, 2H, CH2). 13CNMR (100 MHz, CDCl3, ppm) δ 182.9, 177.6, 165.8, 164.5, 155.1, 154.5, 140.1, 136.9, 136.8, 135.7, 133.1, 130.9, 130.8, 130.3, 130.1, 128.8, 128.6, 126.9, 126.2, 124.5, 123.3, 123.2, 120.6, 115.2, 108.1, 108.0, 97.0, 70.9, 69.7, 44.7, 44.4, 35.5, 35.1, 33.3, 32.8, 24.8, 24.0, 21.1. HRMS (ESI)+ m/z: [M + H]+ calcd for C43H37N2O4, 645.2748; found, 645.2748. Anal. calcd for C43H36N2O4: C, 80.10; H, 5.63; N, 4.34. Found: C, 80.52; H, 5.74; N, 4.82.
9). Mp 248–249 °C. 1HNMR (400 MHz, CDCl3, ppm) δ 12.69 (s, 2H, OH), 8.02 (d, J = 8.4 Hz, 1H, ArH), 7.97 (s, 1H, ArH), 7.84 (d, J = 8.4 Hz, 1H, ArH), 7.79 (d, J = 8.4 Hz, 1H, ArH), 7.75 (d, J = 8.8 Hz, 1H, ArH), 7.69 (d, J = 8.8 Hz, 1H, ArH), 7.52 (t, J = 7.2 Hz, 1H, ArH), 7.40 (t, J = 7.2 Hz, 1H, ArH), 7.26 (d, J = 8.0 Hz, 2H, ArH), 7.20 (d, J = 8.4 Hz, 2H, ArH), 6.75 (d, J = 8.0 Hz, 1H, ArH), 6.38 (s, 2H, ArH), 4.98 (s, 1H, CH), 4.91 (s, 1H, CH), 4.32 (s, 1H, CH), 3.85 (s, 1H, CH), 2.39 (s, 3H, CH3), 2.36–2.19 (m, 2H, CH2), 2.12–1.84 (m, 5H, CH2), 1.77–1.66 (m, 3H, CH2), 1.57–1.45 (m, 2H, CH2). 13CNMR (100 MHz, CDCl3, ppm) δ 182.9, 177.6, 165.8, 164.5, 155.1, 154.5, 140.1, 136.9, 136.8, 135.7, 133.1, 130.9, 130.8, 130.3, 130.1, 128.8, 128.6, 126.9, 126.2, 124.5, 123.3, 123.2, 120.6, 115.2, 108.1, 108.0, 97.0, 70.9, 69.7, 44.7, 44.4, 35.5, 35.1, 33.3, 32.8, 24.8, 24.0, 21.1. HRMS (ESI)+ m/z: [M + H]+ calcd for C43H37N2O4, 645.2748; found, 645.2748. Anal. calcd for C43H36N2O4: C, 80.10; H, 5.63; N, 4.34. Found: C, 80.52; H, 5.74; N, 4.82.3. Results and discussion
3.1. Synthesis and characterization
The synthetic routes of intermediate and target molecule USQs are outlined in Scheme 2. Compounds 1–9 were prepared according to the procedures described in the literature.25,27,32,33 The two objective molecules BIBISQ and TIBISQ were prepared by condensation of 9 with 7 and 8, respectively. The molecular structures of the USQs were characterized by 1H NMR, 13C NMR, HR-ESIMS and element analysis. The purity of both USQs was confirmed to be >99.0% by HPLC analysis.3.2. Optical properties
The UV-Vis absorption spectra of BIBISQ and TIBISQ in dilute chloroform solution and thin solid films (spin-casted from chloroform solution) are shown in Fig. 1, and the relative data are summarized in Table 1. In solution, analogous to most squaraines,19–22,25 both compounds showed intense absorption (molar extinction coefficient > 105 M−1 cm−1) in Vis-NIR regions. λmax values of BIBISQ and TIBISQ were 691 nm and 713 nm, respectively, with full width at half maxima (FWHM) of 53 nm or 61 nm. In the thin film state, both compounds displayed drastically red-shifted and broadened absorption bands (λmax 748 nm for BIBISQ, 760 nm for TIBISQ; FWHM 184 nm for BIBISQ, 217 nm for TIBISQ). This is attributed to their intense intermolecular interactions in the condensed state. The intense and wide absorption band of the two compounds in the range 500–900 nm should be beneficial to the harvesting of sunlight hence the enhancement of Jsc in these OPV devices. Through the absorption spectra of their solid film samples, the optical bandgap of BIBISQ and TIBISQ was estimated to be 1.47 eV and 1.39 eV, respectively, which are ideal values for photovoltaic donor materials.28| Compound | λabsa (nm) (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) ε) |
λabsb (nm) | FWHM (nm) | Eoptg (eV) | Eox (V) | HOMOc (eV) | LUMOd (eV) | |
|---|---|---|---|---|---|---|---|---|
| Solution | Film | |||||||
| a Measured in dilute chloroform solution (5.00 × 10−6 M).b Measured in thin film state.c HOMO= (−4.80 − Eox) eV.d LUMO = Eoptg + HOMO. | ||||||||
| BIBISQ | 691 (5.32) | 748 | 53 | 184 | 1.47 | 0.32 | −5.12 | −3.65 |
| TIBISQ | 713 (5.50) | 760 | 61 | 217 | 1.39 | 0.28 | −5.08 | −3.69 |
3.3. Electrochemical properties
Cyclic voltammetry experiments were carried out on BIBISQ and TIBISQ in dichloromethane solution with tetrabutylammonium perchlorate as the supporting electrolyte, and the results are shown in Table 1 and Fig. 2. The HOMO levels of BIBISQ and TIBISQ were estimated to be −5.12 eV and −5.08 eV, respectively, by comparing the oxidation wave peak of the compounds with the Fc/Fc+ redox couple whose energy level is 4.80 eV below vacuum.15 And the corresponding LUMO energy level of BIBISQ and TIBISQ was calculated to be −3.65 eV and −3.69 eV, respectively through their HOMO level and optical bandgap data.34 From these results, we can roughly guess the Voc of BHJ-OPV cells based on these two USQs and PC71BM may be in the range 0.7–0.9 V.35 | ||
| Fig. 2 Cyclic voltammograms of the two USQs (a) and the energy levels of the components for OPV devices (b). | ||
3.4. Photovoltaic characteristics
To evaluate their photovoltaic performance, BHJ-OPV cells with BIBISQ or TIBISQ as electron donor material and [6,6]-phenyl-C71butyric acid methyl ester (PC71BM) as electron acceptor material were fabricated with different D/A blending ratios. The device structure was ITO/MoO3 (8 nm)/USQs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (60 nm)/BCP (6 nm)/Al (100 nm). The corresponding data and curves are shown in Table 2, Fig. 3 and 4. The results indicated that a similar trend in photovoltaic performance of OPV devices based on both BIBISQ and TIBISQ could be observed when their D/A ratio was varied from 1
PC71BM (60 nm)/BCP (6 nm)/Al (100 nm). The corresponding data and curves are shown in Table 2, Fig. 3 and 4. The results indicated that a similar trend in photovoltaic performance of OPV devices based on both BIBISQ and TIBISQ could be observed when their D/A ratio was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. Taking TIBISQ as an example, when the blend ratio was changed from 1
8. Taking TIBISQ as an example, when the blend ratio was changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, the Voc and FF values of the corresponding BHJ-OPV devices were almost unaltered, while Jsc showed a distinct variation, which matched with the corresponding EQE spectra of the devices. As shown in Fig. 3b, three major bands could be identified in the EQE spectra of TIBISQ devices, with their peaks approximately located at 380 nm, 480 nm and 760 nm, respectively. The first two high-energy bands should stem from the absorption of PC71BM, and the third low-energy band at ∼760 nm should arise from the absorption of TIBISQ. With increasing composition of TIBISQ, the intensity of the third band peaked at ∼760 nm increased gradually, which is consistent with the corresponding absorption spectrum of D/A blend film with a similar D/A mixing ratio (shown in Fig. 3c). Accordingly, with increasing D/A ratio from 1
8, the Voc and FF values of the corresponding BHJ-OPV devices were almost unaltered, while Jsc showed a distinct variation, which matched with the corresponding EQE spectra of the devices. As shown in Fig. 3b, three major bands could be identified in the EQE spectra of TIBISQ devices, with their peaks approximately located at 380 nm, 480 nm and 760 nm, respectively. The first two high-energy bands should stem from the absorption of PC71BM, and the third low-energy band at ∼760 nm should arise from the absorption of TIBISQ. With increasing composition of TIBISQ, the intensity of the third band peaked at ∼760 nm increased gradually, which is consistent with the corresponding absorption spectrum of D/A blend film with a similar D/A mixing ratio (shown in Fig. 3c). Accordingly, with increasing D/A ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to 1
8 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, Jsc of the TIBISQ device increased gradually from 10.92 to 13.02 mA cm−2. However, in the case of the TIBISQ device with a D/A ratio of 1
3, Jsc of the TIBISQ device increased gradually from 10.92 to 13.02 mA cm−2. However, in the case of the TIBISQ device with a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the intensity of the third band in its EQE spectrum decreased drastically rather than increasing, despite the fact that the 1
1, the intensity of the third band in its EQE spectrum decreased drastically rather than increasing, despite the fact that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend film displayed the strongest absorption in this band, as shown in Fig. 3c. This may be ascribed to the drastically different morphology of the blend films between 1
1 blend film displayed the strongest absorption in this band, as shown in Fig. 3c. This may be ascribed to the drastically different morphology of the blend films between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and other D/A ratios (Fig. 5). Similarly, for BIBISQ devices, the optimum D/A ratio was also found to be 1
1 and other D/A ratios (Fig. 5). Similarly, for BIBISQ devices, the optimum D/A ratio was also found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Therefore, taking advantage of the more effective absorption in the NIR region due to the increased content of USQ in the 1
3. Therefore, taking advantage of the more effective absorption in the NIR region due to the increased content of USQ in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 optoelectronic film, the resulting devices both showed impressive Jsc values of ∼13 mA cm−2, which is much higher than that of the reported USQ-based BHJ-SMOPV devices.15,25 Additionally, according to the experimental and calculated results (Fig. 6), the hole mobility of the as-prepared 1
3 optoelectronic film, the resulting devices both showed impressive Jsc values of ∼13 mA cm−2, which is much higher than that of the reported USQ-based BHJ-SMOPV devices.15,25 Additionally, according to the experimental and calculated results (Fig. 6), the hole mobility of the as-prepared 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 D/A blending films was relatively high (3.25 × 10−5 and 6.21 × 10−5 cm2 V−1 s−1 for BIBISQ- and TIBISQ-based film in sequence), which would also contribute to the high Jsc and FF,36 hence the high PCE of 4.97–5.08% of the devices.
3 D/A blending films was relatively high (3.25 × 10−5 and 6.21 × 10−5 cm2 V−1 s−1 for BIBISQ- and TIBISQ-based film in sequence), which would also contribute to the high Jsc and FF,36 hence the high PCE of 4.97–5.08% of the devices.
| Active layer (w/w) | Voc (V) | Jsc (mA cm−2) | FF | PCEb (%) |
|---|---|---|---|---|
| a Thermally annealed at 80 °C for 10 min.b The average PCE values are shown in parentheses. | ||||
TIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.83 | 9.95 | 0.45 | 3.72 (3.65) |
TIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.83 | 13.02 | 0.47 | 5.08 (5.03) |
TIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0.84 | 11.15 | 0.50 | 4.68 (4.57) |
TIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
0.84 | 10.92 | 0.48 | 4.40 (4.32) |
TIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a 3a |
0.83 | 13.50 | 0.49 | 5.49 (5.39) |
BIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.83 | 8.23 | 0.43 | 2.94 (2.81) |
BIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.84 | 12.87 | 0.46 | 4.97 (4.92) |
BIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0.85 | 10.97 | 0.49 | 4.57 (4.46) |
BIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
0.86 | 10.41 | 0.47 | 4.21 (4.13) |
BIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a 3a |
0.83 | 13.13 | 0.49 | 5.34 (5.24) |
 | ||
| Fig. 3 The J–V characteristics (a); and EQE characteristics (b) of the TIBISQ devices; and absorption spectra (c) of the photoactive layers with different D/A ratios. | ||
 | ||
| Fig. 4 The J–V characteristics (a); and EQE characteristics (b) of the BIBISQ devices; and absorption spectra (c) of the photoactive layers with different D/A ratios. | ||
 | ||
Fig. 5 Tapping-mode AFM height images (5 × 5 µm) of TIBISQ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM blend films with different blend ratios ((a) 1 PC71BM blend films with different blend ratios ((a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (b) 1 1; (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). 3). | ||
 | ||
Fig. 6 Current density–voltage characteristics of hole-only single-carrier devices using USQs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM = 1 PC71BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 blend films as active layer. 3 blend films as active layer. | ||
Upon thermal annealing (80 °C, 10 min), the device performance of both BIBISQ and TIBISQ was enhanced, with PCE values of 5.34% and 5.49%, respectively. The enhanced PCEs are attributed not only to the improved Jsc (for BIBISQ, from 12.87 to 13.13 mA cm−2; for TIBISQ, from 13.02 to 13.50 mA cm−2), which is consistent with their EQE measurement results (shown in Fig. 3b and 4b), but also to the enhanced FF (for BIBISQ, from 0.46 to 0.49; for TIBISQ, from 0.47 to 0.49). Moreover, the impressive Jsc (13.50 mA cm−2) and excellent PCE (5.49%) of TIBISQ-based BHJ-OPV are among the highest Jsc29 and PCE17,30,31 values of all of the reported squaraine-based BHJ-OPV cells. These results indicate that the Y-mannered molecular backbone is indeed quite promising for the construction of successful OPV USQs with increased donor content in the photoactive layer.
It is worth mentioning that the optimized D/A composite ratio in these devices based on two USQs is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Generally, a larger content of donor material in the blend film is propitious to the harvesting of sunlight, hence the enhancement of PCE of the device,37 therefore, the typical D/A ratio in highly efficient OPV devices lies in the range of 1
3. Generally, a larger content of donor material in the blend film is propitious to the harvesting of sunlight, hence the enhancement of PCE of the device,37 therefore, the typical D/A ratio in highly efficient OPV devices lies in the range of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9 However, although many D/A composite ratios have been employed in BHJ-OPV devices based on squaraines, the record PCE of 5.50% for BHJ-OPV devices using symmetrical squaraines (SSQs, D–A–D-type) was achieved with a rather low D/A ratio (1
2.9 However, although many D/A composite ratios have been employed in BHJ-OPV devices based on squaraines, the record PCE of 5.50% for BHJ-OPV devices using symmetrical squaraines (SSQs, D–A–D-type) was achieved with a rather low D/A ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), according to Wei's report.17 Similarly, for our reported USQ donor materials, the optimized D/A ratios fell in the region 1
6), according to Wei's report.17 Similarly, for our reported USQ donor materials, the optimized D/A ratios fell in the region 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–1
8–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.19–22 The low content of donor material in the photoactive layer may hamper the photovoltaic performance of these squaraine materials. Therefore, the present investigation shows that a much enhanced content of USQ donor materials in the active layers (the optimized D/A ratios of BHJ-OPV cells based on BIBISQ and TIBISQ are both 1
5.19–22 The low content of donor material in the photoactive layer may hamper the photovoltaic performance of these squaraine materials. Therefore, the present investigation shows that a much enhanced content of USQ donor materials in the active layers (the optimized D/A ratios of BHJ-OPV cells based on BIBISQ and TIBISQ are both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) is beneficial to absorb more solar light in the NIR region, which contributes to the impressive Jsc over 13 mA cm−2 and PCE over 5% of BHJ-OPV devices, which would open a more promising route to improve the OPV performance of squaraine materials by molecular tailoring.
3) is beneficial to absorb more solar light in the NIR region, which contributes to the impressive Jsc over 13 mA cm−2 and PCE over 5% of BHJ-OPV devices, which would open a more promising route to improve the OPV performance of squaraine materials by molecular tailoring.
4. Conclusion
Two novel USQs bearing Y-manner molecular platforms have been developed. Both showed a relatively low bandgap of <1.5 eV, an intense and wide absorption band in the range 500–900 nm, and a relatively low HOMO energy level of ∼−5.10 eV as well. BHJ-OPV devices based on these platforms showed a relatively high Voc of 0.84 V, a rather impressive Jsc of over 13 mA cm−2 and high FF of 0.49, consequently, a high PCE of over 5.0%, even at a relatively high donor content (the optimized D/A ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Consequently, Y-manner is indeed a quite promising linking method for the construction of high performance OPV USQs in terms of increased donor content.
3). Consequently, Y-manner is indeed a quite promising linking method for the construction of high performance OPV USQs in terms of increased donor content.
Acknowledgements
We acknowledge the financial support for this work by the National Natural Science Foundation of China (project No. 51573108, 21190031, 21372168 and 21432005), Fujian Key Laboratory of Polymer Materials (Fujian Normal University) (FJKL-POLY 201502) and China Scholarship Council. We are grateful to the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan University for providing NMR and HR-MS data for the intermediate and target compounds.Notes and references
- A. J. Heeger, Adv. Mater., 2014, 26, 10–27 CrossRef CAS PubMed.
- Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Chem. Rev., 2014, 114, 7006–7043 CrossRef CAS PubMed.
- J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821–3838 CrossRef CAS PubMed.
- Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245–4272 RSC.
- A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- P. Gautam, R. Misra, S. A. Siddiqui and G. D. Sharma, ACS Appl. Mater. Interfaces, 2015, 7, 10283–10292 CAS.
- P. Gautam, R. Misra, S. A. Siddiqui and G. D. Sharma, Org. Electron., 2015, 19, 76–82 CrossRef CAS.
- T. Jadhav, R. Misra, S. Biswas and G. D. Sharma, Phys. Chem. Chem. Phys., 2015, 17, 26580–26588 RSC.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang, H. Feng, Y. Zuo, M. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. Chen, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS PubMed.
- G. Chen, H. Sasabe, W. Lu, X. Wang, J. Kido, Z. Hong and Y. Yang, J. Mater. Chem. C., 2013, 1, 6547–6552 RSC.
- Y. Fu, D. A. da Silva Filho, G. Sini, A. M. Asiri, S. G. Aziz, C. Risko and J. Brédas, Adv. Funct. Mater., 2014, 24, 3790–3798 CrossRef CAS.
- G. Chen, H. Sasabe, T. Igarashi, Z. Hong and J. Kido, J. Mater. Chem. A, 2015, 3, 14517–14534 CAS.
- H. Sasabe, T. Igarashi, Y. Sasaki, G. Chen, Z. Hong and J. Kido, RSC Adv., 2014, 4, 42804–42807 RSC.
- J. Huang, T. Goh, X. Li, M. Y. Sfeir, E. A. Bielinski, S. Tomasulo, M. L. Lee, N. Hazari and A. D. Taylor, Nat. Photonics, 2013, 7, 479–485 CrossRef CAS.
- S. So, H. Choi, H. M. Ko, C. Kim, S. Paek, N. Cho, K. Song, J. K. Lee and J. Ko, Sol. Energy Mater. Sol. Cells, 2012, 98, 224–232 CrossRef CAS.
- S. Wang, L. Hall, V. V. Diev, R. Haiges, G. Wei, X. Xiao, P. I. Djurovich, S. R. Forrest and M. E. Thompson, Chem. Mater., 2011, 23, 4789–4798 CrossRef CAS.
- G. Wei, S. Wang, K. Sun, M. E. Thompson and S. R. Forrest, Adv. Energy Mater., 2011, 1, 184–187 CrossRef CAS.
- J. D. Zimmerman, B. E. Lassiter, X. Xiao, K. Sun, A. Dolocan, R. Gearba, D. A. V. Bout, K. J. Stevenson, P. Wickramasinghe, M. E. Thompson and S. R. Forrest, ACS Nano, 2013, 7, 9268–9275 CrossRef CAS PubMed.
- D. Yang, Y. Zhu, Y. Jiao, L. Yang, Q. Yang, Q. Luo, X. Pu, Y. Huang, S. Zhao and Z. Lu, RSC Adv., 2015, 5, 20724–20733 RSC.
- L. Yang, Q. Yang, D. Yang, Q. Luo, Y. Zhu, Y. Huang, S. Zhao and Z. Lu, J. Mater. Chem. A, 2014, 2, 18313–18321 CAS.
- D. Yang, Q. Yang, L. Yang, Q. Luo, Y. Chen, Y. Zhu, Y. Huang, Z. Lu and S. Zhao, Chem. Commun., 2014, 50, 9346–9348 RSC.
- D. Yang, Q. Yang, L. Yang, Q. Luo, Y. Huang, Z. Lu and S. Zhao, Chem. Commun., 2013, 49, 10465–10467 RSC.
- I. A. Karpenko, A. S. Klymchenko, S. Gioria, R. Kreder, I. Shulov, P. Villa, Y. Mély, M. Hiberta and D. Bonnet, Chem. Commun., 2015, 51, 2960–2963 RSC.
- C. Gude and W. Rettig, J. Phys. Chem. A, 2000, 104, 8050–8057 CrossRef CAS.
- Y. Chen, Y. Zhu, D. Yang, Q. Luo, L. Yang, Y. Huang, S. Zhao and Z. Lu, Chem. Commun., 2015, 51, 6133–6136 RSC.
- G. Chen, H. Sasabe, Y. Sasaki, H. Katagiri, X. Wang, T. Sano, Z. Hong, Y. Yang and J. Kido, Chem. Mater., 2014, 26, 1356–1364 CrossRef CAS.
- N. F. Montcada, L. Cabau, C. V. Kumar, W. Cambarau and E. Palomares, Org. Electron., 2015, 20, 15–23 CrossRef CAS.
- X. Liu, Y. Sun, B. B. Hsu, A. Lorbach, L. Qi, A. J. Heeger and G. C. Bazan, J. Am. Chem. Soc., 2014, 136, 5697–5708 CrossRef CAS PubMed.
- S. Spencer, H. Hu, Q. Li, H. Ahn, M. Qaddoura, S. Yao, A. Ioannidis, K. Belfield and C. J. Collison, Prog. Photovoltaics, 2014, 22, 488–493 CAS.
- D. Yang, Y. Jiao, L. Yang, Y. Chen, S. Mizoi, Y. Huang, X. Pu, Z. Lu, H. Sasabe and J. Kido, J. Mater. Chem. A, 2015, 3, 17704–17712 CAS.
- D. Yang, L. Yang, Y. Huang, Y. Jiao, T. Igarashi, Y. Chen, Z. Lu, X. Pu, H. Sasabe and J. Kido, ACS Appl. Mater. Interfaces, 2015, 7, 13675–13684 CAS.
- W. Wang, A. Fu, J. You, G. Gao, J. Lan and L. Chen, Tetrahedron, 2010, 66, 3695–3701 CrossRef CAS.
- D. Yang, Z. Guan, L. Yang, Y. Huang, Q. Wei, Z. Lu and J. Yu, Sol. Energy Mater. Sol. Cells, 2012, 105, 220–228 CrossRef CAS.
- N. Lim, N. Cho, S. Paek, C. Kim, J. K. Lee and J. Ko, Chem. Mater., 2014, 26, 2283–2288 CrossRef CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789–794 CrossRef CAS.
- D. Bagnis, L. Beverina, H. Huang, F. Silvestri, Y. Yao, H. Yan, G. A. Pagani, T. J. Marks and A. Facchetti, J. Am. Chem. Soc., 2010, 132, 4074–4075 CrossRef CAS PubMed.
- X. Guo, M. Zhang, J. Tan, S. Zhang, L. Huo, W. Hu, Y. Li and J. Hou, Adv. Mater., 2012, 24, 6536–6541 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The 1H NMR, 13C NMR and HRMS spectra of BIBISQ and TIBISQ. See DOI: 10.1039/c5ra24186c |
| ‡ The first two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |