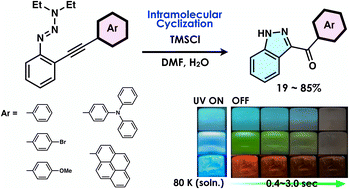
To advance the development of luminescent materials based on indazoles, a class of nitrogen-containing aromatic compounds, it is crucial to establish a reliable synthetic method for their derivatives. Five 1H-indazole derivatives with a ketoaryl group at the 3-position were synthesized by a cyclisation reaction using phenyltriazene derivatives. In solution state, only 3-ketoindazole derivatives bearing 4-diphenylaminophenyl or pyrenyl groups showed fluorescence at room temperature, whereas all 3-ketoindazole derivatives showed blue, green, or red phosphorescence, depending on the substituents, at 80 K. In addition, double luminescence has been observed at 80 K for 3-ketoindazole derivatives bearing 4-diphenylaminophenyl or pyrenyl groups. Furthermore, the 3-ketoindazole derivative did not exhibit room-temperature phosphorescence in either solution or solid state; however, when it was dispersed in a phenylbenzoate matrix at a concentration of 0.1 wt%, room-temperature phosphorescence was successfully produced in a variety of colours. These optical properties were elucidated through theoretical calculations.