Late transition metal recovery from a silver nitrate electrolyte using a phosphine-oxide bearing scavenger†
Abstract
Silver is one of the eight precious metals, along with gold and the six platinum-group metals (PGM). Most silver is obtained as a by-product of mining and refining of other metals. Fine silver is produced in electrolytic processes, using silver anodes (electrolytic refining) or inert ones (electrowinning) and an acidified silver nitrate electrolyte. Because the standards for refined silver are very high, it is necessary to control the behaviour of the impurities. Depending on the refining process parameters, it is not uncommon to find precious metals, such as palladium, platinum or even gold, dissolved in the electrolyte. In particular, palladium is very challenging because of its standard oxidation–reduction potential similar to the one of silver 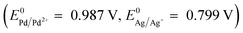 . The recovery of palladium and platinum from the silver refining processes is important for two reasons: to produce high quality silver and to recover the valuable precious metals. In this work, Pd and to a lesser extent Pt recovery from a silver nitrate effluent was investigated using a phosphine oxide – bearing coordinating resin (MPX-310). Laboratory scale studies, in batch and continuous modes, have shown the ability of MPX-310 to capture Pd and Pt from a matrix highly concentrated in Ag (up to 131 g l−1) and Cu (up to 70 g l−1) from industrial effluents. Moreover, adiabatic calorimetry studies were performed in order to determine the reactivity of the resin in the silver nitrate effluent and the optimal conditions where this resin can be safely used in an industrial process. Pilot scale experiments were done and the results obtained in the laboratory were confirmed.
. The recovery of palladium and platinum from the silver refining processes is important for two reasons: to produce high quality silver and to recover the valuable precious metals. In this work, Pd and to a lesser extent Pt recovery from a silver nitrate effluent was investigated using a phosphine oxide – bearing coordinating resin (MPX-310). Laboratory scale studies, in batch and continuous modes, have shown the ability of MPX-310 to capture Pd and Pt from a matrix highly concentrated in Ag (up to 131 g l−1) and Cu (up to 70 g l−1) from industrial effluents. Moreover, adiabatic calorimetry studies were performed in order to determine the reactivity of the resin in the silver nitrate effluent and the optimal conditions where this resin can be safely used in an industrial process. Pilot scale experiments were done and the results obtained in the laboratory were confirmed.

- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...