Open Access Article
Open Access ArticleAn intramolecularly locked single molecule nanofluorophore with 13.55% quantum yield for SWIR multimodal phototheranostics†
Leilei
Si
abc,
Jun
Tang
abc,
Kaixin
Yang
abc,
Mingda
Wang
abc,
Yigang
Wang
bc,
Guomin
Xia
 *bcd and
Hongming
Wang
*bcd and
Hongming
Wang
 *ab
*ab
aCollege of Chemistry and Chemical Engineering, Nanchang University, Nanchang 330031, China. E-mail: hongmingwang@ncu.edu.cn
bJiangxi Provincial Key Laboratory of Functional Crystalline Materials Chemistry, Nanchang 330031, China. E-mail: guominxia@ncu.edu.cn
cInstitute for Advanced Study, Nanchang University, Nanchang 330031, China
dSchool of Advanced Manufacturing, Nanchang University, Nanchang 330031, China
First published on 12th March 2025
Abstract
Multimodal phototheranostics in the short-wavelength infrared range (SWIR, 900–1700 nm) holds significant promise in precision medicine, yet its progress is constrained by photosensitizers that lack effective fluorescence emission due to unwanted intermolecular aggregation and molecular vibration patterns. Herein, we present a dual electrostatic anchoring strategy to construct ultrabright co-assembled nanoparticles (NPs) of the squaraine dye SQNMe. This molecular design incorporates two peripheral quaternary ammonium cations: one interacts with the phosphate anion of the liposome mPEG2K-DSPE to achieve intermolecular isolation, while the other forms an internal salt bridge with the central oxycyclobutenolate ring, increasing intramolecular rigidity. Both molecular dynamics simulations and reorganization energy calculations are employed to illustrate the coassembly process. Spectroscopic analysis shows that SQNMe@NPs have a fluorescence brightness of approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 M−1 cm−1 and a photothermal conversion efficiency of 39.6% in aqueous media. Additionally, the high effectiveness of fluorescence and photoacoustic imaging-guided photothermal therapy for tumors in vivo was successfully demonstrated. These findings highlight the potential of the electrostatic anchoring strategy for improving multimodal tumor phototheranostics.
135 M−1 cm−1 and a photothermal conversion efficiency of 39.6% in aqueous media. Additionally, the high effectiveness of fluorescence and photoacoustic imaging-guided photothermal therapy for tumors in vivo was successfully demonstrated. These findings highlight the potential of the electrostatic anchoring strategy for improving multimodal tumor phototheranostics.
Introduction
Multimodal phototheranostics is emerging as a vital approach in precision medicine.1–5 The innovative integration of diverse modalities can overcome individual limitations and harness their combined advantages, leading to enhanced therapeutic outcomes.6–10 This approach is particularly relevant in the short-wave infrared (SWIR, 900–1700 nm), which offers superior tissue penetration, minimal scattering, and reduced autofluorescence.11–16 As an important category of photosensitizers, the low-bandgap organic small molecules often exhibit a guaranteed photothermal effect with poor fluorescence efficiency in the SWIR range.17–21 This is ascribed to the rapid nonradiative relaxation of molecules in excited states upon photoinitiation via the Sn → S0 internal conversion process, which is primarily caused by intramolecular vibrations.22,23 The issue is exacerbated in their aggregated state, where additional nonradiative relaxation pathways arise from intermolecular interactions, such as charge transfer or a reduction in the transition dipole moment in face-to-face stacked H-aggregates.24–28 Therefore, enhancing the fluorescence efficiency of these low-bandgap dye molecules is crucial for the effectiveness of multimodal phototheranostics.It is now routine to encapsulate dye molecules in liposomes to achieve biological advantages, such as high biocompatibility, prolonged blood circulation, and enhanced tumor retention, in phototheranostics.29–36 However, this often results in the encapsulated dye molecules being in an uncontrollable aggregated state at the center of the nanostructures, making the pathways for its excited state energy dissipation difficult to ascertain and thus challenging to manipulate further.37–39 Although efficient SWIR fluorescent emission from aggregated states, such as aggregation-induced emission luminogens (AIEgens)40–42 and J-aggregates,43–45 can be achieved by customizing the molecular skeletons within these nanostructures, the existing molecular archetypes are limited, and a universal design principle has yet to be established. For this, researchers have focused on introducing electrostatic interactions between phosphate anions in liposomes and dye molecules with incorporating side-chain cations. This electrostatic interaction anchors the dye molecules in a monodisperse form within the lipid layer, avoiding complex aggregation and improving fluorescence efficiency.39,46–50 Nevertheless, their fluorescence brightness, which remains below 500 M−1 cm−1, is considered unsatisfactory in multimodal phototheranostics (for details see Table S1†).
It is reasonable to conjecture that molecular vibrations are the primary factor contributing to the suboptimal efficiency of these monodispersed photosensitizers within the soft lipid layer (top in Fig. 1). In this context, our objective is to develop a strategy that addresses the challenges posed by unwanted intermolecular aggregation while minimizing the detrimental effects of intramolecular vibrations. To achieve this, we designed and synthesized a bicationic squaraine dye, SQNMe, featuring a propyl-based quaternary ammonium group at each peripheral side chain. Through co-assembly with the liposome mPEG2K-DSPE, stable nanoaggregates are formed, incorporating two types of electrostatic interactions that allow the salt bridge-rigidified SQNMe to disperse as single molecules within the lipid layer. These unified nanofluorophores exhibit ultrahigh SWIR fluorescence brightness, suitable photothermal conversion efficiency (PCE), and excellent biocompatibility and biosafety, positioning them as outstanding candidates for multimodal phototheranostic applications (bottom of Fig. 1).
Results and discussion
We initiated this investigation by conceptualizing dicationic SWIR squaraine dyes as target candidates for multimodal phototheranostics, inspired by previous literature.51,52 To begin with, a propyl-based quaternary ammonium was introduced into benzo[cd]indol-2(1H)-one, followed by methylation and subsequent condensation with squaric acid to yield the desired dye molecule, SQNMe. The compounds involved in the synthesis steps were characterized using 1H NMR, 13C NMR, and high-resolution mass spectrometry (for details see the ESI†). By examining the absorption spectra of SQNMe, we observed that the maximum absorption peak (λab) in dimethyl sulfoxide (DMSO) is at 892 nm, with a shoulder peak at 800 nm. In contrast, these values in pure water (H2O) were detected at 738 nm and 832 nm, with a slight drop in absorbance (Fig. S1†). This blue shift in the absorption spectra indicates the formation of H-aggregation modes of SQNMe in H2O, consistent with our previous literature.53Using a nanoprecipitation method, the coassembly process of oppositely charged SQNMe and the liposome mPEG2k-DSPE was then examined in an aqueous medium (for details see the ESI†). We named these nanoparticles SQNMe@NPs. As shown in Fig. S2,† an increase in the molar ratio of mPEG2k-DSPE to SQNMe results in a redshift in the absorption spectrum of the resulting SQNMe@NPs. Specifically, its absorption peak at 738 nm gradually decreases, while the peak at 890 nm increases, stabilizing at a ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This optimal absorption spectrum of SQNMe@NPs closely matches that of SQNMe in DMSO (Fig. S3†), indicating a transformation from H-aggregates to a dispersed state with the addition of mPEG2k-DSPE. Thus, it is reasonable to conjecture that SQNMe exists as a single molecule within the liposome interlayer of SQNMe@NPs due to electrostatic interactions.
1. This optimal absorption spectrum of SQNMe@NPs closely matches that of SQNMe in DMSO (Fig. S3†), indicating a transformation from H-aggregates to a dispersed state with the addition of mPEG2k-DSPE. Thus, it is reasonable to conjecture that SQNMe exists as a single molecule within the liposome interlayer of SQNMe@NPs due to electrostatic interactions.
Following the optimal ratio, SQNMe@NPs were then prepared and subjected to dialysis for subsequent experiments. From concentration dependent absorption spectra, the entrapment efficiency for SQNMe@NPs was determined to be 62.86% (Fig. S4†). As shown in Fig. 2a, the absorption peak of fresh SQNMe@NPs is at 890 nm, with a shoulder peak at 800 nm. The maximum emission peak (λem) is at 906 nm, accompanied by a shoulder peak at 1032 nm that extends up to 1100 nm. The axisymmetric relationship between the absorption and emission spectra, along with their similarity to the spectra of SQNMe in DMSO, indicates that SQNMe exists as a single molecule in the nanoparticles and undergoes no significant structural changes in its excited state upon photon excitation. Using ICG as a reference standard, the fluorescence quantum yield (ΦFL) of SQNMe@NPs was measured at 13.55%. With a molar absorption coefficient of 1.2 × 105 M−1 cm−1, the resulting fluorescence brightness was approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 M−1 cm−1 (Fig. S5†). These values are considerably higher than those of nanoparticles synthesized using the same electrostatic anchoring strategy (see Table S1† and Fig. 2b), as well as compared to other types of nanofluorophores (Table S2†). Laser irradiation of SQNMe@NPs, followed by the use of 2′,7′-dichlorofluorescin diacetate (H2DCFHDA) indicator, showed negligible generation of reactive oxygen species (Fig. S6†).
135 M−1 cm−1 (Fig. S5†). These values are considerably higher than those of nanoparticles synthesized using the same electrostatic anchoring strategy (see Table S1† and Fig. 2b), as well as compared to other types of nanofluorophores (Table S2†). Laser irradiation of SQNMe@NPs, followed by the use of 2′,7′-dichlorofluorescin diacetate (H2DCFHDA) indicator, showed negligible generation of reactive oxygen species (Fig. S6†).
Subsequently, dynamic light scattering (DLS) analysis indicated that the SQNMe@NPs had a dynamic diameter of approximately 98 nm, with a polydispersity Index (PDI) of 0.149 (Fig. 2c). These findings align with the results from transmission electron microscopy (TEM) and suggest that the particles are well-suited for in vivo enrichment through the enhanced permeability and retention (EPR) effect in tumors.54,55 Additionally, the zeta potential measurement of SQNMe@NPs revealed a value of −32.5 mV (Fig. S7†). Stability tests conducted in various media (PBS, 0.9% NaCl, RPMI-1640) on the 1st, 7th, and 15th days showed no significant changes in the absorbance spectra of SQNMe@NPs. Both the particle size and zeta potential also remained unchanged, further indicating the robust chemical stability of SQNMe@NPs (Fig. S8†).
To gain further insights, molecular dynamics simulations (MDS, for details see the ESI†) were employed to model the co-assembly process of SQNMe with the liposome mPEG2k-DSPE. The structure of the SQNMe molecule was initially optimized, revealing excellent molecular planarity, with the cationic nitrogen atoms of the ammonium salts bridged by the central oxygen atom. The distances were 3.46 Å and 3.29 Å, which aligns well with the effective range for electrostatic interactions (Fig. S9†). Theoretical calculations revealed that the highest occupied molecular orbital (HOMO) is predominantly delocalized across the conjugated backbone, while the lowest unoccupied molecular orbital (LUMO) was mainly confined to the donor region (Fig. 2d). This specific distribution of molecular orbitals, featuring a peripheral “salt bridge,” facilitate the rigidity of the whole molecule, effectively suppressing vibrational non-radiative transitions in its excited state (Fig. 2e). The calculated band gap of 1.40 eV was also in excellent agreement with experimental data (Fig. S10†).
A simplified mPEG-DSPE model was subsequently constructed in water, followed by a 60 ns molecular dynamics simulation. The results revealed that four SQNMe molecules predominantly existed as single molecule, positioned between the phosphate groups and alkyl chains of the liposome (Fig. 2f). It is worth mentioning that only one quaternary ammonium group of SQNMe functions as an “anchor” to embed itself into the hydrophilic region of the liposome via electrostatic interactions. The other one keep the electrostatic interactions with the central oxygen atom, reinforces its structural rigidity. This reasoning is well-founded, as anchoring the hydrophilic ammonium groups on opposite sides entirely with phosphate anions would inevitably result in significant structural changes, contradicting the observed spectral data. Additionally, reorganization energy analysis reveals that molecules with cationic salt bridges exhibit a lower total reorganization energy (Fig. S11†). When projected onto the corresponding internal coordinates, the analysis reveals that reorganization in the excited state is primarily driven by bond-length stretching, resulting in rearrangement. This suggests that molecular reorganization in the excited state is predominantly driven by stretching vibrations, with the cationic salt bridge stabilizing the molecular structure and enhancing its rigidity (Fig. 2g).
For comparison, we also prepared nanoparticles of the salt-bridge-free molecule SQEt, referred to as SQEt@NPs (see ESI† for details). The ΦFL of SQEt@NPs was found to be 20 times lower than that of SQNMe@NPs (Fig. S5†), further highlighting the crucial role of the electrostatic anchoring strategy.
Using an 808 nm laser (0.3 W cm−2), fluorescence images were captured with an InGaAs camera. The results showed no detectable signal for SQNMe in H2O, whereas significant signal brightness was observed in the other two solutions (Fig. 3a). These observations align with the fluorescence-quenching effect of H-aggregation in SQNMe within H2O, whereas a monodispersed, fluorescence-emitting single state is consistently formed in both DMSO and nanostructures. Additionally, they highlight the unique advantage of electrostatic interactions, which prove beneficial compared to traditional non-covalent interactions, such as dispersion forces and the antiparallel alignment of ground-state dipoles. Notably, there is a linear correlation between the concentration of SQNMe@NPs and signal brightness (Fig. 3b), with the correlation coefficient of 0.99. The permeability test in lipid emulsion solutions demonstrated that even after passing through a 1000 nm long-pass filter, the depth of penetration exceeded 3 mm (Fig. 3c). All these results indicate that SQNMe@NPs exhibits potentially superior fluorescence imaging capabilities in vivo.
Next, the temperature increase from varying concentrations of SQNMe@NPs (0–80 μM) was monitored, and the peak temperature was captured using infrared cameras. Upon laser irradiation (808 nm, 0.3 W cm−2), SQNMe@NPs reached a stable peak temperature up to 54.7 °C within 6 min, corresponding to an approximate temperature increase (ΔT) of 30 °C. These results for SQNMe@NPs demonstrated a concentration-dependent increase in temperature, and the PCE of SQNMe@NPs was calculated to be 39.6% (Fig. 3d–f and S12†), making in vivo PTT feasible. In contrast, the PCE of H-aggregated SQNMe in H2O was measured at 59.8% (Fig. S13 and S14†), which is close to the 64.25% observed for SQEt@NPs. These findings are consistent with the calculated results, indicating that the dual electrostatic anchoring strategy facilitates radiative decay (Fig. S14†). Over six cycles of laser-induced heating and cooling, SQNMe@NPs exhibited minimal changes in temperature elevation (Fig. 3g and S15†), and the nearly unchanged absorption spectra indicate that SQNMe@NPs possess excellent photostability and thermal stability (Fig. 3h). Additionally, the photoacoustic test revealed a positive correlation between signal intensity and concentration of SQNMe@NPs (Fig. 3i), establishing a foundation for in vivo photoacoustic imaging (PAI).
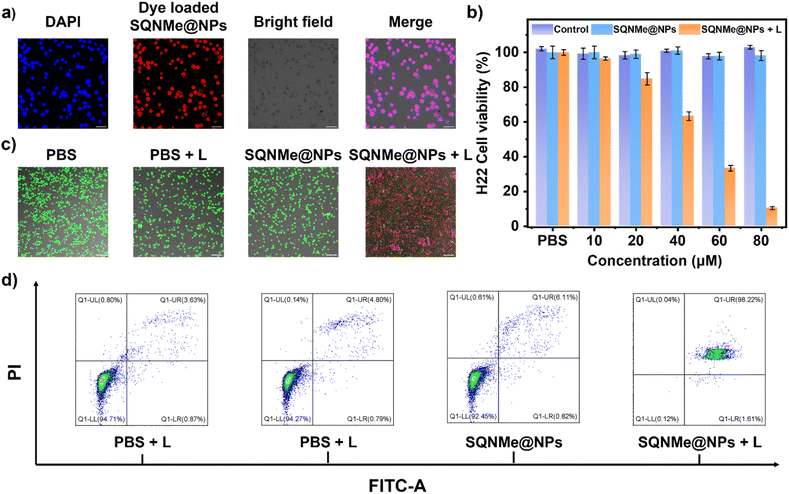
As shown in Fig. 4a, co-incubation of red-emitting dye-loaded SQNMe@NPs with H22 cells resulted in co-localization with DAPI, indicating successful cellular uptake.56 The light cytotoxicity of the SQNMe@NPs was then assessed using MTT assays. H22 cell viability in the SQNMe@NPs group was comparable to that of the control group without laser irradiation, suggesting good biocompatibility (Fig. 4b). Upon laser irradiation, H22 cell viability decreased with increasing SQNMe@NPs concentration, dropping to around 10% at 80 μM, due to the photothermal effect of SQNMe@NPs. Furthermore, live/dead staining assay observations were consistent with the results, and further confirmed by the subsequent flow cytometry (Fig. 4c and d).
The multimodal imaging performances of SQNMe@NPs were then evaluated through in vivo experiments. Intravenous administration of SQNMe@NPs enabled live vascular fluorescence imaging in the macromodel, revealing clear and detailed vascular structures (Fig. 5b). The vessel width and signal-to-background ratio (SBR) were measured and calculated by Gaussian fitting of the cross-sectional fluorescence intensity profile and calculating its full width at half-maximum (FWHM). From the micromodel, the fluorescence images of the mice's hind limbs achieved a peak SBR of 2.74, while the abdomen reached a maximum SBR of 1.92 (Fig. 5c and d). The FWHM of the hindlimb vessels measured along the solid line were 421 and 664 μm, respectively (Fig. 5c). Similarly, the widths of the abdominal vessels were 238 and 179 μm, respectively (Fig. 5d). Subsequently, SQNMe@NPs were administered intravenously to tumor-bearing mice, and fluorescence and photoacoustic imaging were performed at various time points. As shown in Fig. 5e (upper part), the signal intensity at the tumor site progressively increased over time, peaking at 12 hours before gradually declining and nearly disappearing by 48 hours. This pattern can be attributed to the accumulation of SQNMe@NPs in the tumor region via the EPR effect, along with simultaneous biological clearance within 48 hours. Additionally, the time-dependent photoacoustic signal results for the tumor were consistent with those from fluorescence imaging (Fig. 5e bottom part, Fig. S16†), indicating the potential for SWIR fluorescence imaging-guided photothermal therapy starting 12 hours post-injection (Fig. 5f and g).
The schematic of the multimodal tumor phototheranostics process over the 21 day period is depicted in Fig. 6a. Twelve hours after injection, the intratumoral temperature elevation of SQNMe@NPs was monitored using an infrared camera, as shown in Fig. 6b. Within 5 minutes of laser exposure, a significant increase of 18 °C in tumor temperature was observed in mice injected with SQNMe@NPs, while minimal change (less than 3 °C) was noted in mice injected with PBS (Fig. 6c). This temperature increase from SQNMe@NPs is sufficient to facilitate photothermal therapy (PTT) for tumor ablation.
Subsequently, we divided the mice with a tumor volume of 0.3 cm3 into four groups: PBS, PBS + L, SQNMe@NPs, and SQNMe@NPs + L. After 24 hours post-treatment, tumor tissues were extracted. Tissue sections showed irregularities in the nuclear shape of H22 cells in the SQNMe@NPs + L group, along with notable changes in cell morphology compared to the other three groups, suggesting the occurrence of cellular necrosis (Fig. S17†). As shown in Fig. 6d, complete tumor ablation was observed in the SQNMe@NPs + L group. This process is captured in Fig. S18,† indicating the strong photothermal capability of SQNMe@NPs. Additionally, the tumor volume recording curves of the mice aligned well with the experimental results (Fig. 6e). During the treatment period, the mice's body weight showed no significant changes, indicating the absence of adverse effects associated with SQNMe@NPs (Fig. 6f).
Following the administration of SQNMe@NPs, we conducted dissections of tumor-bearing mice at both 12 and 48 hours post-injection. Examination of the major organs revealed no apparent abnormalities. Results indicated that the drug primarily underwent metabolism in the liver and spleen, with a decrease of 84.12% in signal intensity associated with the liver after 48 hours (refer to Fig. 6g–i), suggesting excellent biodegradability of SQNMe@NPs. Furthermore, routine blood tests during treatment revealed no abnormalities in liver, kidney, or immune function, and there were no discernible signs of inflammation or infection (Fig. S19†). Histological analysis post-treatment showed no significant organ abnormalities or inflammation (Fig. S20†). All these findings confirm the effectiveness and biosafety of our dual electrostatic anchoring strategy in achieving SWIR multimodal phototheranostics.
Conclusion
In summary, we demonstrated an anchoring strategy to construct ultrabright nanoaggregates SQNMe@NPs, incorporating two types of electrostatic interactions that enable the salt bridge-rigidified SQNMe to disperse as single molecules within the lipid layer. This archetype is supported by molecular dynamics simulations and reorganization energy calculations. Thus, the ultrabrightness of SQNMe@NPs was calculated up to approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135 M−1 cm−1 and a PCE of 39.6% in aqueous media, permitting successful fluorescence and photoacoustic imaging-guided PTT for tumors in vivo. These findings underscore the promise of the electrostatic anchoring strategy in advancing multimodal tumor phototheranostics, paving the way for enhanced therapeutic efficacy and precision in cancer treatment.
135 M−1 cm−1 and a PCE of 39.6% in aqueous media, permitting successful fluorescence and photoacoustic imaging-guided PTT for tumors in vivo. These findings underscore the promise of the electrostatic anchoring strategy in advancing multimodal tumor phototheranostics, paving the way for enhanced therapeutic efficacy and precision in cancer treatment.
Ethical statement
All animal experiments were conducted in strict accordance with the management and technical specifications outlined by Nanchang University. The study received ethical approval from the Animal Ethics Committee of Nanchang University under the approval number NCULAE-20230610005.Data availability
Data will be made available on request.Author contributions
H. W. and G. X. conceived and designed the experiments for this project. L. S. performed the synthetic work, while L. S., Y. W., and K. Y. carried out the characterization of the compounds. L. S., Y. W., and J. T. also contributed to the photophysical experiments. Data analysis was conducted by H. W., G. X., and L. S. Additionally, H. W. and M. W performed the quantum-chemical calculations. H. W., G. X., and L. S. prepared the initial manuscript, with all other authors contributing to revisions. All authors discussed the results and provided feedback on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22063005, No. 22465023), the Natural Science Foundation of Jiangxi Province (No. 20212ACBA203012, 20224BAB214003, 20232BAB203031), the Interdisciplinary Innovation Fund of Natural Science at Nanchang University (No. 9167-27060003-ZD2101, 9167-28220007-YB2113), and the Foundation of Jiangxi Provincial Key Laboratory of Functional Crystalline Materials Chemistry (No. 2024SSY05162). The authors would like to thank Yingzhong Li for his valuable contributions to the molecular synthesis.Notes and references
- K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nat. Nanotechnol., 2009, 4, 773–780 CAS.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2015, 15, 235–242 Search PubMed.
- Z. Li, B. Z. Tang and D. Wang, Adv. Mater., 2024, 36, 2406047 CAS.
- Y. Chen, S. Wang and F. Zhang, Nat. Rev. Bioeng., 2023, 1, 60–78 CAS.
- W.-G. Qiao, T. Ma, S. Wang, L. Li, M. Liu, H. Jiang, Y. Wu, J. Zhu and Z. Li, Adv. Funct. Mater., 2021, 31, 2105452 CAS.
- C. Lu, C. Meng, Y. Li, J. Yuan, X. Ren, L. Gao, D. Su, K. Cao, M. Cui, Q. Yuan and X. Gao, Nat. Commun., 2024, 15, 5000 CAS.
- S. Song, Y. Zhao, M. Kang, F. Zhang, Q. Wu, N. Niu, H. Yang, H. Wen, S. Fu, X. Li, Z. Zhang, B. Z. Tang and D. Wang, Adv. Mater., 2024, 36, 2309748 CAS.
- B. Li, W. Wang, L. Zhao, D. Yan, X. Li, Q. Gao, J. Zheng, S. Zhou, S. Lai, Y. Feng, J. Zhang, H. Jiang, C. Long, W. Gan, X. Chen, D. Wang, B. Z. Tang and Y. Liao, ACS Nano, 2023, 17, 4601–4618 CAS.
- H. Chen, S. Yan, L. Zhang, B. Zhao, C. Zhu, G. Deng and J. Liu, Sens. Actuators, B, 2024, 405, 135346 CrossRef CAS.
- J. Liu, H. Chen, Y. Yang, Q. Wang, S. Zhang, B. Zhao, Z. Li, G. Yang and G. Deng, Mater. Horiz., 2023, 10, 3791–3796 RSC.
- E. Thimsen, B. Sadtler and M. Y. Berezin, Nanophotonics, 2017, 6, 1043–1054 CrossRef CAS.
- K.-W. Lee, Y. Gao, W.-C. Wei, J.-H. Tan, Y. Wan, Z. Feng, Y. Zhang, Y. Liu, X. Zheng, C. Cao, H. Chen, P. Wang, S. Li, K.-T. Wong and C.-S. Lee, Adv. Mater., 2023, 35, 2211632 CrossRef CAS PubMed.
- J. Guan, C. Liu, C. Ji, W. Zhang, Z. Fan, P. He, Q. Ouyang, M. Qin and M. Yin, Small, 2023, 19, 2300203 CrossRef CAS PubMed.
- Y. Chu, X.-Q. Xu and Y. Wang, J. Phys. Chem. Lett., 2022, 13, 9564–9572 CrossRef CAS PubMed.
- Z. Zhang, Y. Du, X. Shi, K. Wang, Q. Qu, Q. Liang, X. Ma, K. He, C. Chi, J. Tang, B. Liu, J. Ji, J. Wang, J. Dong, Z. Hu and J. Tian, Nat. Rev. Clin. Oncol., 2024, 21, 449–467 CrossRef PubMed.
- S. Roy, N. Bag, S. Bardhan, I. Hasan and B. Guo, Adv. Drug Delivery Rev., 2023, 197, 114821 CrossRef CAS PubMed.
- R. Ge, P. Yan, Y. Liu, Z. Li, S. Shen and Y. Yu, Adv. Funct. Mater., 2023, 33, 2301138 CrossRef CAS.
- X. Kong, J. Liang, M. Lu, K. Zhang, E. Zhao, X. Kang, G. Wang, Q. Yu, Z. Gan and X. Gu, Adv. Mater., 2024, 36, 2409041 CrossRef CAS PubMed.
- M. Shi, Z. Fu, W. Pan, K. Wang, X. Liu, N. Li and B. Z. Tang, Adv. Healthcare Mater., 2024, 13, 2303749 CrossRef CAS PubMed.
- Y. Chen, Y. Ran, Z. Rao, Q. Wang, J. Wang, Z. Liu, Q. Zhou, X. Lei, Z. Xu and J. Ming, ACS Mater. Lett., 2024, 6, 3915–3924 CrossRef CAS.
- M. Overchuk, R. A. Weersink, B. C. Wilson and G. Zheng, ACS Nano, 2023, 17, 7979–8003 CrossRef CAS PubMed.
- C.-A. Shen, M. Stolte, J.-H. Kim, A. Rausch and F. Würthner, J. Am. Chem. Soc., 2021, 143, 11946–11950 CrossRef CAS PubMed.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS.
- F. C. Spano and C. A. Silva, Annu. Rev. Phys. Chem., 2014, 65, 477–500 Search PubMed.
- Y. Wang, G. Xia, J. Wang, M. Wang, W. Guo, M. Tan, L. Si, Y. Yang, H. Wang and H. Wang, Sci. China: Chem., 2023, 67, 612–621 CrossRef.
- X. Liu, Q. Qiao, W. Tian, W. Liu, J. Chen, M. J. Lang and Z. Xu, J. Am. Chem. Soc., 2016, 138, 6960–6963 CAS.
- Z. Ye, W. Yang, C. Wang, Y. Zheng, W. Chi, X. Liu, Z. Huang, X. Li and Y. Xiao, J. Am. Chem. Soc., 2019, 141, 14491–14495 Search PubMed.
- J. Xu, M. Huang, H. Pang, Z. Weng, G. Hu, S. Zhang, Q. Yang and Q. Wu, Aggregate, 2024, 5, e546 CAS.
- Y. Li, T. Ma, H. Jiang, W. Li, D. Tian, J. Zhu and Z. Li, Angew. Chem., Int. Ed., 2022, 61, e202203093 CAS.
- Y. Yang, C. Sun, S. Wang, K. Yan, M. Zhao, B. Wu and F. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202117436 CAS.
- B. Wang, H. Zhou, L. Chen, Y. Ding, X. Zhang, H. Chen, H. Liu, P. Li, Y. Chen, C. Yin and Q. Fan, Angew. Chem., Int. Ed., 2024, 63, e202408874 CAS.
- G. Niu, X. Bi, Y. Kang, H. Zhao, R. Li, M. Ding, B. Zhou, Y. Zhai, X. Ji and Y. Chen, Adv. Mater., 2024, 36, 2407199 Search PubMed.
- D. Zhou, G. Zhang, J. Li, Z. Zhuang, P. Shen, X. Fu, L. Wang, J. Qian, A. Qin and B. Z. Tang, ACS Nano, 2024, 18, 25144–25154 Search PubMed.
- Y. Dai, F. Zhang, K. Chen, Z. Sun, Z. Wang, Y. Xue, M. Li, Q. Fan, Q. Shen and Q. Zhao, Small, 2023, 19, 2206053 CrossRef CAS PubMed.
- Z. Li, P.-Z. Liang, L. Xu, X. Zhang, K. Li, Q. Wu, X. Lou, T. Ren, L. Yuan and X. Zhang, Nat. Commun., 2023, 14, 1843 Search PubMed.
- X. Xie, Y. Dong, Y. Zhang, Z. Xie, X. Peng, Y. Huang, W. Yang, B. Li and Q. Zhang, Bioact. Mater., 2024, 43, 460–470 Search PubMed.
- M. Zhao, W. Lai, B. Li, T. Bai, C. Liu, Y. Lin, S. An, L. Guo, L. Li, J. Wang and F. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202403968 Search PubMed.
- C. Ou, L. An, Z. Zhao, F. Gao, L. Zheng, C. Xu, K. Zhang, J. Shao, L. Xie and X. Dong, Aggregate, 2022, 4, e290 Search PubMed.
- Y. Meng, J. Gao, P. Zhou, X. Qin, M. Tian, X. Wang, C. Zhou, K. Li, F. Huang and Y. Cao, Angew. Chem., Int. Ed., 2024, 63, e202318632 CAS.
- F. Ma, Q. Jia, Z. Deng, B. Wang, S. Zhang, J. Jiang, G. Xing, Z. Wang, Z. Qiu, Z. Zhao and B. Z. Tang, ACS Nano, 2024, 18, 9431–9442 Search PubMed.
- Y. Li, J. Zhuang, Y. Lu, N. Li, M. Gu, J. Xia, N. Zhao and B. Z. Tang, ACS Nano, 2021, 15, 20453–20465 CAS.
- X. Fan, Q. Xia, Y. Zhang, Y. Li, Z. Feng, J. Zhou, J. Qi, B. Z. Tang, J. Qian and H. Lin, Adv. Healthcare Mater., 2021, 10, 2101043 CAS.
- M. Fan, X. Zheng, W. Cheng, H. Wei, P. Li, C. Ji and M. Yin, Adv. Funct. Mater., 2024, 34, 2402455 CAS.
- C. Sun, B. Li, M. Zhao, S. Wang, Z. Lei, L. Lu, H. Zhang, L. Feng, C. Dou, D. Yin, H. Xu, Y. Cheng and F. Zhang, J. Am. Chem. Soc., 2019, 141, 19221–19225 Search PubMed.
- K. Wei, Y. Wu, X. Zheng, L. Ouyang, G. Ma, C. Ji and M. Yin, Angew. Chem., Int. Ed., 2024, 63, e202404395 Search PubMed.
- D. Gao, Z. Luo, Y. He, L. Yang, D. Hu, Y. Liang, H. Zheng, X. Liu and Z. Sheng, Small, 2023, 19, 2206544 Search PubMed.
- C. Zhou, Z. Li, Z. Zhu, G. W. N. Chia, A. Mikhailovsky, R. J. Vázquez, S. J. W. Chan, K. Li, B. Liu and G. C. Bazan, Adv. Mater., 2022, 34, 2201989 CrossRef CAS PubMed.
- K. Liu, D. Hu, L. He, Z. Wang, P. Cheng, P. Sun, Y. Chen and D. Li, J. Nanobiotechnol., 2024, 22, 451 CrossRef CAS PubMed.
- P. Chen, F. Qu, S. Chen, J. Li, Q. Shen, P. Sun and Q. Fan, Adv. Funct. Mater., 2022, 32, 2208463 CrossRef CAS.
- C. Ayala-Orozco, D. Galvez-Aranda, A. Corona, J. M. Seminario, R. Rangel, J. N. Myers and J. M. Tour, Nat. Chem., 2023, 16, 456–465 CrossRef PubMed.
- Y. Yadav, E. Owens, S. Nomura, T. Fukuda, Y. Baek, S. Kashiwagi, H. S. Choi and M. Henary, J. Med. Chem., 2020, 63, 9436–9445 CrossRef CAS PubMed.
- T. Fukuda, S. Yokomizo, S. Casa, H. Monaco, S. Manganiello, H. Wang, X. Lv, A. D. Ulumben, C. Yang, M. Kang, K. Inoue, M. Fukushi, T. Sumi, C. Wang, H. Kang, K. Bao, M. Henary, S. Kashiwagi and H. S. Choi, Angew. Chem., Int. Ed., 2022, 61, e202117330 CrossRef CAS PubMed.
- Y. Wang, G. Xia, M. Tan, M. Wang, Y. Li and H. Wang, Adv. Funct. Mater., 2022, 32, 2113098 CrossRef CAS.
- J. Park, Y. Choi, H. Chang, W. Um, J. H. Ryu and I. C. Kwon, Theranostics, 2019, 9, 8073–8090 CrossRef CAS PubMed.
- H. Kang, S. Rho, W. R. Stiles, S. Hu, Y. Baek, D. W. Hwang, S. Kashiwagi, M. S. Kim and H. S. Choi, Adv. Healthcare Mater., 2019, 9, 1901223 CrossRef PubMed.
- Y. Wang, Y. Li, Q. Yan, X. Liu, G. Xia, Q. Shao, K. Liang, L. Hong, B. Chi and H. Wang, Dyes Pigm., 2020, 173, 107977 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00089k |
| This journal is © The Royal Society of Chemistry 2025 |