 Open Access Article
Open Access ArticleDirect vicinal sulfonyloximation of alkenes: an efficient and straightforward approach towards the synthesis of α-sulfonyl ketoximes
Zinatossadat Hossaini *a,
Shahrzad Abdolmohammadib,
Shakir Mahmood Saeedc,
Wadhah Hasan Alkhazalid,
Waam mohammed Tahere,
Mariem Alwanf,
Mahmood Jasem Jawadg,
Hiba Mushtaqh and
Esmail Vessally
*a,
Shahrzad Abdolmohammadib,
Shakir Mahmood Saeedc,
Wadhah Hasan Alkhazalid,
Waam mohammed Tahere,
Mariem Alwanf,
Mahmood Jasem Jawadg,
Hiba Mushtaqh and
Esmail Vessally ij
ij
aDepartment of Chemistry, QaS.C., Islamic Azad University, Qaemshahr, Iran. E-mail: zshossaini@yahoo.com
bDepartment of Chemistry, ST.C., Islamic Azad University, Tehran, Iran
cCollege of Pharmacy, Alnoor University, Nineveh, Iraq
dAhl Al Bayt University, Kerbala, Iraq
eCollege of Nursing, National University of Science and Technology, Dhi Qar, Iraq
fPharmacy College, Al-Farahidi University, Iraq
gDepartment of Pharmacy, Al-Zahrawi University College, Karbala, Iraq
hGilgamesh Ahliya University, Baghdad, Iraq
iDepartment of Chemistry, Payame Noor University, P. O. Box 19395-3697, Tehran, Iran
jComposite Materials Scientific Research Center of Azerbaijan State University of Economics (UNEC), 194 M. Mukhtarov Str., Baku, Azerbaijan
First published on 22nd May 2025
Abstract
Direct vicinal sulfonylative difunctionalization of simple alkenes represents a powerful strategy for the rapid assembly of β-functionalized sulfones from simple starting materials. In this context, the direct sulfonyloximation of alkene substrates has recently received much attention from the chemical community owing to important applications of α-sulfonyl ketoxime products in organic synthesis. This review provides an overview of recent research on the titled reactions, with an emphasis on the reaction patterns and mechanisms. Literature has been surveyed until the end of 2024.
1. Introduction
Sulfones (R–SO2–R) are an important family of organosulfur compounds with extensive applications across a number of disciplines, including organic synthesis,1 materials science,2 pharmaceutical chemistry,3 and agricultural chemistry.4 Notably, over the past several decades, sulfones have emerged as the key functional groups in more than eight FDA-approved drugs, such as apremilast (an anti-rheumatic drug), vismodegib (an anti-cancer drug), and chlormezanone (an anxiolytic drug), examples of which are shown in Scheme 1.5 Because of their interest in many areas, the development of efficient and straightforward synthetic methods to access various types of sulfone compounds from cheap and easily accessible starting materials has a high influence on the chemistry community.In a similar way, ketoximes (ketone–oximes) represent a fundamental structural motif prevalent in organic transformations6 and ubiquitous in medicinally relevant molecules7 agrochemicals,8 and natural products.9 The most important biological application of ketoximes is their ability to treat organophosphate (OP) poisoning by the reactivation of acetylcholinesterase.10 Diacetylmonoxime (DAM) is a ketoxime discovered by Grob et al., that was initially used in the treatment of OP poisoning in 1956.11 Drawing inspiration form DAM, several OP antidotes have been developed over the past decades. However, to our knowledge, none have yet been approved by the FDA. Very recently, Mathew and co-workers published an interesting review paper entitled “oxime derivatives: a valid pharmacophore in medicinal chemistry” that highlights some of the important discoveries on the ketoxime-derived biological active compounds.12 Some selected examples of biologically active ketoximes are given in Scheme 2.
 | ||
| Scheme 2 Selected examples of bioactive compounds containing a ketoxime moiety in their overall structure. | ||
α-Sulfonyl ketoximes (Scheme 3) are one of the most specific classes of ketoximes that are not only found in various bioactive molecules but also exhibit diverse reaction patterns due to their multiple functional groups and have recently attracted widespread attention as useful and versatile building blocks for the synthesis of many significant pharmaceutically relevant molecules.13–19
Conventional methods for the preparation of α-sulfonyl ketoximes mainly relay on tedious and costly multiple steps syntheses that suffer from limited substrate scope, poor overall yields, prolonged reaction times and/or require harsh conditions.20 In order to bypass these limitations, the direct sulfonyloximation of cheap and easily accessible alkenes has recently been developed as an efficient and straightforward strategy for the synthesis of titled compounds from simple starting materials within a single click. Since significant progress has been made in this rapidly growing research area over the past few years and no review article has yet been published on this chemistry, it seems an appropriate time to summarize those discoveries and developments in a comprehensive review. In connection with our recent works on vicinal difunctionalization reactions,21 herein, we try to summarize available literature on the synthesis of α-sulfonyl ketoximes through the direct vicinal sulfonyloximation of alkenes (Fig. 1), with an aim to encourage researchers to make further progress in this attractive research field.
2. Two-component reactions
The direct 1,2-sulfonyloximation of alkenes using bifunctional reagents has been scarcely investigated.22 To the best of our knowledge, there is only one reported example of such a reaction in the literature so far. In this study, the research group of He disclosed that the treatment of various styrene derivatives 1 with commercially available N-hydroxy-arenesulfonamides 2 in the presence of stoichiometric amounts of tetrabutylammonium periodate (nBu4NIO4) as an oxidant in DCM afforded the corresponding α-sulfonyl ketoximes 3 in good to excellent yields within 3 h (Scheme 4a). The results demonstrated that nBu4NIO4 was essential for this transformation, as no reaction occurred in its absence. Notably, replacing nBu4NIO4 with some other oxidants (e.g., PhIO, PhI(OAc)2, IBX, HITB, DMP, HITB) resulted in significantly reduced yields or even no desired product at all. Regarding the substrate scope, the reaction proved largely insensitive to the electronic nature of both partners. As a result, substrates bearing either electron-donating or electron-withdrawing groups on both components were well tolerated under the optimized conditions. In contrast, steric effects had a pronounced influence; while 2-chlorostyrene afforded a 78% yield, the 2,6-dichlorinated analogue was quite inert under the identical conditions. It should be mentioned that next to aromatic alkenes, acrylates and conjugated dienes were also compatible substrates with this scenario. Unfortunately, the potential use of aliphatic alkenes as starting materials was not explored in this study. Based on a series of control experiments, the authors suggested a potential mechanistic pathway for this sulfonyloximation reaction, as outlined in Scheme 4b. Initially, the oxidation of hydroxylamine 2 with nBu4NIO4 a low temperature leads to the formation of nitroso intermediate A, which after homolytic cleavage of the S–N bond delivers sulfonyl free radical B and NO free radical (NO˙). Subsequently, the regioselective addition of sulfonyl radical B to styrene 1 forms the carbon-centered radical intermediate C. Then the newly generated radical captures NO˙ to yield intermediate D. Finally, this intermediate D undergoes a quick intramolecular hydrogen transfer to produce the observed α-sulfonyl ketoxime products 3.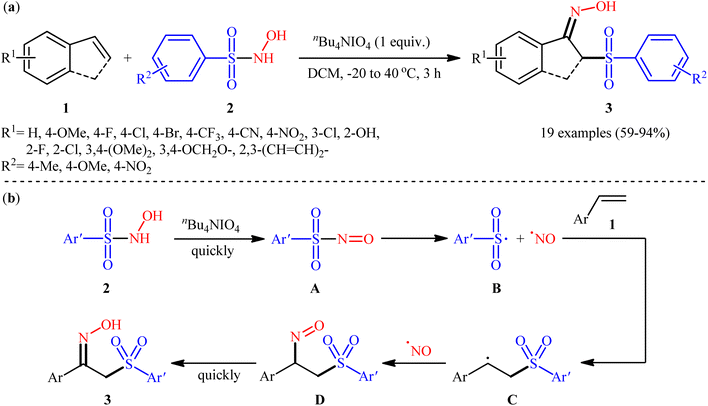 | ||
| Scheme 4 (a) He's synthesis of α-sulfonyl ketoximes 3; (b) mechanism proposed to explain the formation of α-sulfonyl ketoximes 3. | ||
3. Three-component reactions
In the current section, we will look at the available literature on the direct sulfonyloximation of alkenes via three-component methodologies. For clarity, the section is organized into five major subsections according to the type of sulfonating agents.3.1. Sulfinic acids as sulfonating agents
In 2017, Han and Yu along with their co-workers unraveled an elegant protocol for the synthesis of α-sulfonyl ketoximes 6 through the three-component reaction between alkenes 4, sulfinic acids 5, and tert-butyl nitrite (tBuONO) under catalyst-free conditions (Scheme 5a).23 According to the authors, in this reaction tBuONO played double roles; both as the oxime source and the radical initiator. The transformation was highly general and functional-group tolerant, compatible with a wide range of alkene substrates, including aromatic (electron-rich and electron-poor), aliphatic (cyclic and acyclic), and acrylate derivatives. Additionally, the scope of sulfinic acids that participated in the reaction was broad, encompassing aromatic, aliphatic, and naphthenic sulfinic acids. Interestingly, when the alkene substrate contains an alkynyl group within its structure, the reaction exhibits a high level of selectivity, allowing the alkenyl group to be functionalized without affecting the carbon–carbon triple bond. In this study, the authors identified several limitations in their methodology, some of which are listed below: (i) when cyclohexene was used as the substrate, the destabilizing steric effects and dipole–dipole interactions caused the expected product (1-methyl-4-((2-nitrosocyclohexyl)sulfonyl)benzene) to further react with two additional sulfonyl radicals, resulting in the formation of the unexpected 4-methyl-N-(2-tosylcyclohexyl)-N-(tosyloxy)benzenesulfonamide as the sole product in 30% yield; (ii) performing the reaction with 4-bromobut-1-ene as the substrate resulted in the formation of 3-(tosylmethyl)-4,5-dihydroisoxazole in moderate yield, which was clearly formed through a sulfoximation/intramolecular-SN2 cascade sequence; and (iii) subjection of 3,4-dimethylene-1-(phenylsulfonyl)pyrrolidine to the reaction under identical conditions resulted in no detection of the desired product, only a complex mixture was obtained. The proposed mechanism for this sulfonyloximation, as outlined in Scheme 5b, begins with the formation of sulfinyl anion A through deprotonation of sulfinic acid 5 by pyridine. This anion is then oxidized via single electron transfer (SET) by tBuONO to generate the sulfonyl free radical B. Subsequently, radical B adds to the double bond of alkene 4 to form the C-centered radical C that, after reaction with NO free radical converts to the nitroso compound D. Finally, intermediate D undergoes tautomerization to deliver the target product 6.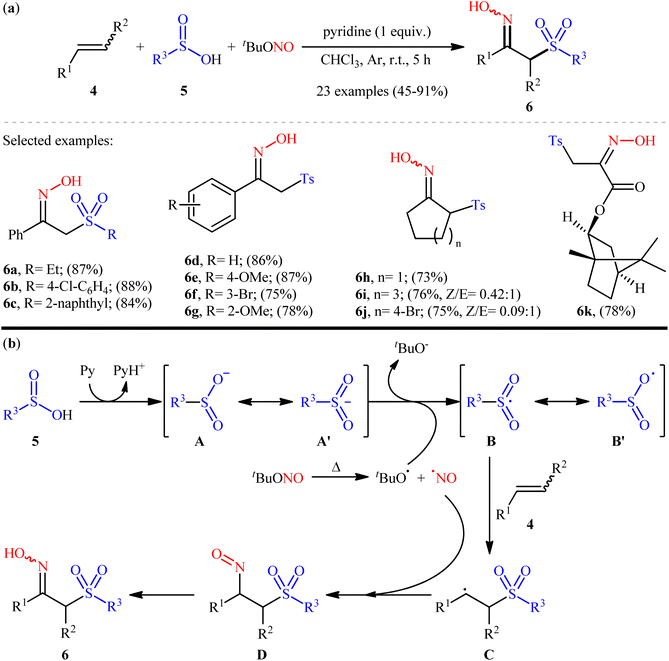 | ||
| Scheme 5 (a) Selected examples of tBuONO-mediated vicinal sulfoximation of alkenes 4 with sulfinic acids 5; (b) plausible mechanism for the formation of α-sulfonyl ketoximes 6. | ||
3.2. Sodium sulfinates as sulfonating agents
In 2016, Yang et al. investigated the possibility of synthesizing α-sulfonyl ketoximes through the three-component reaction of alkenes with sodium sulfinates and tBuONO.24 To determine the optimum conditions, they carefully screened various copper catalysts (e.g., CuBr, CuI, CuOTf, CuBr2, Cu(OAc)2) and different solvents (toluene, MeCN, DCM, DCE, DMF, DMSO) in the sulfonyloximation of 1-methyl-4-vinylbenzene with sodium benzenesulfinate and tBuONO, as a model reaction. The optimal system was recognized using 10 mol% of CuBr in toluene at 60 °C. It should be noted that the above-mentioned temperature was crucial for the success of this reaction, as either an increase or decrease in temperature reduced the reaction efficiency. Under the standard reaction, a range of aromatic alkenes 7 (terminal and internal) were reacted well with various sodium aryl sulfinates 8 to provide the expected α-sulfonyl ketoximes 9 in modest to high yields (Scheme 6). Unfortunately, aliphatic alkenes failed to undergo the desired transformation under the optimized reaction conditions. Additionally, the potential applicability of alkyl sulfinates in the reaction was not investigated in this study. The mechanism proposed by the authors to explain this reaction is illustrated in Scheme 7. At first, in the presence of water, tBuONO readily decomposes into HNO2 and tBuOH, and the resulting HNO2 is rapidly converted into NO2, NO, and H2O. In parallel, in the presence of CuBr, sodium aryl sulfinates 8 generates the oxygen-centered radical A, which is in resonance with the sulfonyl radical B. Afterwards, the addition of newly generated radical B to styrene 7 yields intermediate C, which subsequently reacts with NO to form intermediate D. Finally, intermediate D undergoes tautomerization to produce the desired oxime 9.In a significant contribution in this field, Zhang and co-workers developed an interesting direct sulfonyloximation of styrene derivatives 10 with sodium sulfinates 11 using sodium nitrite (NaNO2) as a nitrosating agent under metal-free conditions.25 The reactions were performed in the presence of fluoroboric acid (HBF4) as a Brønsted acid in the most environmentally benign solvent, water, and provided the desired α-sulfonyl ketoximes 12 in poor to excellent yields (Scheme 8). Evaluation of the substrate scope revealed that the reaction was tolerant to both aromatic and aliphatic sodium sulfinates. However, the scope of alkenes was limited to the use of (hetero)aromatic alkenes. As an extension of the substrate scope of the methodology, it was demonstrated that a variety of functionalized pyridine alkenes could be successfully employed in the reaction. Thus, in this study, twenty-two 2-(alkyl/aryl-sulfonyl)-1-(pyridin-2-/3-/4-yl)ethanone oximes were also synthesized with yield ranging from 37% to 95%, using the corresponding 2-/3-/4-vinylpyridines under the standard reaction conditions. The following mechanistic pathway was proposed by the authors for this sulfonyloximation reaction (Scheme 9): initially, the combination of NaNO2 with HBF4 generates the NO positive ion, which after reaction with alkene 10 produces cationic intermediate A. Afterwards, this cationic reacts with sodium sulfinate 11 to form intermediate B, which undergoes a tautomerization process to afford the observed product 12.
3.3. Sulfonyl hydrazides as sulfonating agents
In 2017, Wang and co-workers informed for the first time the usefulness of sulfonyl hydrazides as sulfonating agents for the direct sulfonyloximation of olefinic double bonds,26 when various terminal and internal alkenes 13 in the treatment with (hetero)aryl sulfonyl hydrazides 14 and tBuONO under basic conditions in binary solvent NMP/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), underwent regioselective sulfonyloximation to afford corresponding α-sulfonyl ketoximes 15 in fair to good yields (Scheme 10a). The procedure proved to be general and a diverse range of internal and terminal (aromatic and aliphatic) alkenes successfully participated in the reaction. Moreover, both aromatic and heteroaromatic sulfonyl hydrazides demonstrated good reactivity under the standard conditions. However, phenylmethanesulfonyl hydrazide did not work well in the reaction and therefore no other aliphatic sulfonyl hydrazides were investigated in the protocol. Noteworthy, the authors demonstrated the scalability of the reaction since 1-phenyl-2-tosylethanone oxime could be obtained in 2.25 g scale in a high yield of 78%. According to the authors, the reaction proceeds through a mechanism analogous to that proposed by Han and Yu for sulfonyloximations using sulfinic acids and tBuONO.
1), underwent regioselective sulfonyloximation to afford corresponding α-sulfonyl ketoximes 15 in fair to good yields (Scheme 10a). The procedure proved to be general and a diverse range of internal and terminal (aromatic and aliphatic) alkenes successfully participated in the reaction. Moreover, both aromatic and heteroaromatic sulfonyl hydrazides demonstrated good reactivity under the standard conditions. However, phenylmethanesulfonyl hydrazide did not work well in the reaction and therefore no other aliphatic sulfonyl hydrazides were investigated in the protocol. Noteworthy, the authors demonstrated the scalability of the reaction since 1-phenyl-2-tosylethanone oxime could be obtained in 2.25 g scale in a high yield of 78%. According to the authors, the reaction proceeds through a mechanism analogous to that proposed by Han and Yu for sulfonyloximations using sulfinic acids and tBuONO.
Two years later, this reach group extended the scope of their methodology for regioselective sulfonyloximation of alkyne substrates.27 The reactions were conducted under an inert atmosphere in a EtOH/H2O solvent mixture, tolerated various terminal (hetero)aromatic alkynes 16 and a range of functionalized (hetero)aryl sulfonyl hydrazides 17, and provided the desired α-sulfonyl ketoximes 18 in yields ranging from 25% to 80% (Scheme 10b). In addition, a tolerance for phenylmethanesulfonyl hydrazide was also demonstrated. However, the methodology was unfruitful with both internal and aliphatic alkynes. It is important to note that an inert atmosphere is essential for the success of this reaction, as performing the process under air results in the formation of sulfonyl ketone by-products, which significantly reduce the yield of the desired α-sulfonyl ketoximes.
3.4. Sulfonyl chlorides as sulfonating agents
One of the earliest reports of the direct sulfonyloximation of alkenes using sulfonyl chlorides as sulfonating agents was published by Li and colleagues in 2024,28 who showed that the treatment of styrene 19 with 4-toluenesulfonyl chloride 20 and tBuONO in the presence of an excess amount of tetramethylethylenediamine (TMEDA) under visible light irradiation at room temperature resulted in the formation of corresponding α-sulfonyl ketoximes 21 in a 66% yield (Scheme 11). Notably, no product was obtained when the respective sulfonyl fluoride was used in place of sulfonyl chloride. Although only a single example was presented, this work can serve as an inspiration for future research on the topic. Mechanistically, the reaction is believed to proceed through the formation of an electron-donor–acceptor (EDA) complex between sulfonyl chloride and TMEDA (Scheme 12).Concurrently, this research group presented an interesting visible-light-mediated direct sulfonyloximation of styrene 19 with a series of aryl sulfonyl chlorides 22 and tBuONO through a hydrogen atom transfer (HAT) and halogen-atom transfer (XAT) relay strategy.29 The optimized condition for this difunctionalization reaction is the use of 2 equiv. of inexpensive borane trimethylamine complex (Me3N–BH3) as the XAT reagent and 395 nm LEDs as the light source. Under these conditions, the desired α-sulfonyl ketoximes 23 were obtained in moderate yields, ranging from 38% to 58% (Scheme 13). The results demonstrated that electron-rich aryl sulfonyl chlorides afforded better yields compared to their electron-deficient counterparts. Unfortunately, the scope and limitations of alkene substrates were not explored in this study. Worth noting is that this synthetic strategy was also extended to one-pot sulfamoyl-oximation of various terminal and internal alkenes, allowing the synthesis of a broad range of structurally diverse oxime-containing alkyl sulfonamides (40 examples, yields ranging from 23% to 73%). Evidence from radical trapping experiments suggests that this transformation proceeds through a radical-mediated pathway as depicted in Scheme 14.
3.5. Miscellaneous
Tosylmethyl isocyanide (TosMIC) is a versatile and multipurpose reagent that is widely employed not only as a C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C synthon in the synthesis of various heterocycles via cyclization reactions, but also as an effective sulfonating and sulfomethylating agent.30 In 2019, Shen and co-workers disclosed the usefulness of this reagent as the source of tosyl group in the direct vicinal sulfonyloximation of olefinic double bonds,31 when a series of styrene derivatives 24 underwent regioselective tosyloximation with TosMIC 25 and tBuONO in the presence of Co(salen)2/nC4F9I/Na2CO3 combination as a catalytic system in THF to form corresponding α-sulfonyl ketoximes 26 in 32–73% yields (Scheme 15). In this study, the authors identified some limitations in their methodology when they attempted to apply sterically congested 2-chlorostyrene and heteroaromatic 3-vinylpyridine as the substrates. Unfortunately, in both cases, no desired products were obtained. Furthermore, the process appears to be unsuitable for gram-scale synthesis, as a significant decrease in yield was observed (from 73% in the 0.6 mmol scale, to 51% in the 20 mmol scale). The proposed mechanism for this transformation is outlined in Scheme 16.
C synthon in the synthesis of various heterocycles via cyclization reactions, but also as an effective sulfonating and sulfomethylating agent.30 In 2019, Shen and co-workers disclosed the usefulness of this reagent as the source of tosyl group in the direct vicinal sulfonyloximation of olefinic double bonds,31 when a series of styrene derivatives 24 underwent regioselective tosyloximation with TosMIC 25 and tBuONO in the presence of Co(salen)2/nC4F9I/Na2CO3 combination as a catalytic system in THF to form corresponding α-sulfonyl ketoximes 26 in 32–73% yields (Scheme 15). In this study, the authors identified some limitations in their methodology when they attempted to apply sterically congested 2-chlorostyrene and heteroaromatic 3-vinylpyridine as the substrates. Unfortunately, in both cases, no desired products were obtained. Furthermore, the process appears to be unsuitable for gram-scale synthesis, as a significant decrease in yield was observed (from 73% in the 0.6 mmol scale, to 51% in the 20 mmol scale). The proposed mechanism for this transformation is outlined in Scheme 16.
Very recently, in a beautiful approach, Li's research group disclosed a photoinduced three-component reaction involving alkenes 27, N-nitrosamines 28, and 1,4-diazabicyclo[2.2.2]octane bis-(sulfur dioxide) (DABSO) for the synthesis of α-oximino sulfonamides 29 using 30 W 395 nm LEDs as the light source and DCM as the solvent.32 The reactions were carried out in absence of any transition metal catalysts or additives, tolerated the presence of a wide array of important functional groups such as fluoro, chloro, bromo, cyano, nitro, amino, hydroxy, ketone, ester, ether, and amide functionalities, and provided the desired α-oximino sulfonamides 29 in synthetically useful yields (Scheme 17). Notably, the authors further demonstrated the synthetic utility of their methodology through the late-stage functionalization of various alkenes and N-nitrosamines derived from natural products and medicinal agents (e.g., diacetone-D-glucose, leelamine, L-menthol, febuxostat, atomoxetine). It is worth noting that, in this transformation, N-nitrosamines acted as bifunctional reagents by simultaneously generating aminyl and NO radicals via photoinduced N–N bond hemolysis. Additionally, DABSO functioned not only as the sulfonyl source but also as an effective scavenger of aminyl radicals, thereby facilitating the bifunctional use of N-nitrosamines under neutral conditions. With respect to the reaction mechanism, the authors proposed the following pathways, as illustrated in Scheme 18: (i) homolytic cleavage of the N–N bond of N-nitrosamines 28 upon light irradiation to form the aminyl radical A and NO radical; (ii) trapping of aminyl radical A by SO2 to produce key sulfamoyl radical B;33 (iii) addition of the sulfamoyl radical B to the double bond of alkene 27 to afford nucleophilic alkyl C-centered radical C; (iv) radical/radical cross-coupling between radical C and NO˙ to give nitroso compound D; and (v) tautomerization of intermediate D to deliver the observed α-oximino sulfonamides 29. Another independent sulfamoyloximation method was concurrently published by Sang et al., employing almost identical conditions demonstrated by Li group.34
4. Four-component reactions
In 2021, Zhao and co-workers explored the synthesis of α-sulfonyl ketoximes 32 through a four-component reaction involving alkenes 30, amines 31, tBuONO, and DABSO via the insertion of sulfur dioxide.35 Thus, a detailed analysis of the optimized reactions revealed that the best conditions involved performing the process in the presence of a catalytic amount of salicylic acid in MeCN under an inert atmosphere, resulting the desired α-sulfonyl ketoximes 32 in poor to good yield, ranging from 26% to 68% (Scheme 19). Various styrene and aniline derivatives were compatible with this scenario. However, the protocol was unsuccessful with alkyl-substituted alkenes, as well as with aliphatic and heteroaromatic amines. It is worthy of note that an inert atmosphere has essential impact on the success of this transformation. Performing the process under an oxygen atmosphere resulted in the predominant formation of α-sulfonyl ketones via the selective oxo-sulfonylation pathway.36 Although α-sulfonyl ketones can be easily converted to the corresponding α-sulfonyl ketoximes by condensation with hydroxylammonium chloride,37 the overall yield of the final product using this sequential procedure may be significantly lower than that of the direct sulfonyloximation, and the substrate scope may also be more limited. A plausible mechanism that explains the formation of α-sulfonyl ketoximes 32 is depicted in Scheme 20 and involves the following steps: (i) initial formation of the diazonium salt A through the reaction of aniline 31 with tBuONO; (ii) reaction of A with salicylic acid to generate the diazo intermediate B; (iii) decomposition of intermediate B to form the hydrogen-bond stabilized salicyloyl radical C and aryl radical along with the release of N2; (iv) reduction of radical C by intermediate F (or DABCO) to produce the salicylate anion D; (v) abstraction of a proton from reaction mixture by salicylate anion D to regenerate salicylic acid; (vi) reaction of aryl radical with DABSO to afford aryl sulfonyl radical E and intermediate F; (vii) addition of radical F to styrene 30 to produce the alkyl radical G; (viii) reaction of radical G with in situ generated nitrosyl radical to give nitroso compound H; and (ix) tautomerization of H to yield the observed product 32.5. Conclusion
During the past decade, significant progress has been made in the area of direct vicinal sulfonylative functionalization of alkene substrates, to achieve complex sulfone molecules from simple starting materials within a single click. In this review, recent advances in the direct sulfonyloximation of simple alkenes is discussed. This synthetic strategy provides an atom and step economical approach for preparing synthetically useful α-sulfonyl ketoxime derivatives, whose traditional synthetic methods mainly relied on tedious and costly multi-step syntheses. As illustrated, over the past decade, various two-, three-, and four-component reactions have been developed for the direct sulfonyloximation of olefinic bonds, which predominantly benefit from the use of inexpensive and commercially available starting materials. Despite recent advances, this field of research is still in its infancy and we believe that the highly versatile and extremely effective methodologies for the synthesis of these compounds through the titled reactions will be attainable in the near future.Data availability
All data supporting this study are included in the article.Conflicts of interest
There are no conflicts to declare.References
- (a) B. M. Trost and C. A. Kalnmals, Sulfones as chemical chameleons: versatile synthetic equivalents of small-molecule synthons, Chem.–Eur. J., 2019, 25, 11193–11213 Search PubMed; (b) A. M. Kmkm, M. M. Tawfiq, H. Mushtaq, E. A. Mahmood, D. F. Hasan and H. Bahair, Chem. Rev. Lett., 2024, 7, 413–424 CAS; (c) A. K. Obaid Aldulaimi, E. A. Mahmood and E. Vessally, Sulfaguanidines: A new class of carbonic anhydrase inhibitors, Med. Med. Chem., 2024, 1, 2–9 Search PubMed; (d) T. A. Qassem, H. Mushtaq, H. Bahair, E. A. Mahmood, D. F. Hasan and A. H. Idan, Chem. Rev. Lett., 2024, 7, 500–512 Search PubMed.
- M. Feng, B. Tang, S. H. Liang and X. Jiang, Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS PubMed.
- (a) T. Zhang, M. H. Litt and C. E. Rogers, Sulfone-containing polymers as high barrier materials, J. Polym. Sci., Part B: Polym. Phys., 1994, 32, 1671–1676 CrossRef CAS; (b) K. Xu and C. A. Angell, Sulfone-based electrolytes for lithium-ion batteries, J. Electrochem. Soc., 2002, 149, A920 CrossRef CAS; (c) T. Hua, J. Miao, H. Xia, Z. Huang, X. Cao, N. Li and C. Yang, Sulfone-incorporated multi-resonance TADF emitter for high-performance narrowband blue OLEDs with EQE of 32%, Adv. Funct. Mater., 2022, 32, 2201032 CrossRef CAS.
- P. Devendar and G. F. Yang, Sulfur-containing agrochemicals, Top. Curr. Chem., 2017, 375, 82 CrossRef PubMed.
- K. A. Scott and J. T. Njardarson, Analysis of US FDA-approved drugs containing sulfur atoms, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed.
- K. A. Rykaczewski, E. R. Wearing, D. E. Blackmun and C. S. Schindler, Reactivity of oximes for diverse methodologies and synthetic applications, Nat. Synth., 2022, 1, 24–36 CrossRef CAS.
- I. A. Schepetkin, M. B. Plotnikov, A. I. Khlebnikov, T. M. Plotnikova and M. T. Quinn, Oximes: Novel therapeutics with anticancer and anti-inflammatory potential, Biomolecules, 2021, 11, 777 CrossRef CAS PubMed.
- C. Lamberth, Oxime chemistry in crop protection, Pest Manag. Sci., 2024, 80, 4163–4174 CrossRef CAS PubMed.
- H. P. Chen, Z. Z. Zhao, Z. H. Li, Z. J. Dong, K. Wei, X. Bai, L. Zhang, C. N. Wen, T. Feng and J. K. Liu, Novel natural oximes and oxime esters with a vibralactone backbone from the basidiomycete Boreostereum vibrans, ChemistryOpen, 2016, 5, 142–149 CrossRef CAS PubMed.
- J. Dhuguru, E. Zviagin and R. Skouta, FDA-approved oximes and their significance in medicinal chemistry, Pharmaceuticals, 2022, 15, 66 CrossRef CAS PubMed.
- D. Grob and R. J. Johns, Use of oximes in the treatment of intoxication by anticholinesterase compounds in normal subjects, Am. J. Med., 1958, 24, 497–511 CrossRef CAS.
- N. Chandran, K. Bose, A. C. Thekkantavida, R. R. Thomas, K. Anirudhan, S. Bindra, S. Sura, H. A. Hasan, S. Kumar, T. M. Rangarajan, A. G. Al-Sehemi and B. Mathew, Oxime derivatives: a valid pharmacophore in medicinal chemistry, ChemistrySelect, 2024, 9, e202401726 CrossRef CAS.
- X. M. Zhou, Y. Y. Hu, B. Fang and C. H. Zhou, Benzenesulfonyl thiazoloimines as unique multitargeting antibacterial agents towards Enterococcus faecalis, Eur. J. Med. Chem., 2023, 248, 115088 CrossRef CAS PubMed.
- R. Chen, J. Qi, Z. Mao and S. Cui, Rh (iii)-catalyzed C–H activation/cyclization of oximes with alkenes for regioselective synthesis of isoquinolines, Org. Biomol. Chem., 2016, 14, 6201–6204 RSC.
- J. M. Ontoria, S. Altamura, A. Di Marco, F. Ferrigno, R. Laufer, E. Muraglia, M. C. Palumbi, M. Rowley, R. Scarpelli, C. Schultz-Fademrecht and S. Serafini, Identification of novel, selective, and stable inhibitors of class II histone deacetylases. Validation studies of the inhibition of the enzymatic activity of HDAC4 by small molecules as a novel approach for cancer therapy, J. Med. Chem., 2009, 52, 6782–6789 CrossRef CAS PubMed.
- E. Muraglia, S. Altamura, D. Branca, O. Cecchetti, F. Ferrigno, M. V. Orsale, M. C. Palumbi, M. Rowley, R. Scarpelli, C. Steinkühler and P. Jones, 2-Trifluoroacetylthiophene oxadiazoles as potent and selective class II human histone deacetylase inhibitors, Bioorg. Med. Chem. Lett., 2008, 18, 6083–6087 CrossRef CAS PubMed.
- V. Padmavathi, B. C. O. Reddy, P. Thriveni and A. V. N. Mohan, Synthesis of 2-oxazolines and 2-thiazolines using lanthanide amino alkoxide, Synth. Commun., 2007, 37, 3127–3142 CrossRef CAS.
- V. Padmavathi, P. Thriveni, B. C. O. Reddy and K. Mahesh, Phenacylsulfonylacetic esters—source for thiadiazoles, triazoles and oxadiazoles, J. Heterocycl. Chem., 2007, 44, 93–98 CrossRef CAS.
- J. Xiang, M. Ipek, V. Suri, M. Tam, Y. Xing, N. Huang, Y. Zhang, J. Tobin, T. S. Mansour and J. McKew, β-Keto sulfones as inhibitors of 11β-hydroxysteroid dehydrogenase type I and the mechanism of action, Bioorg. Med. Chem., 2007, 15, 4396–4405 CrossRef CAS.
- (a) H. A. Abdel-Aziz, H. A. Ghabbour, M. A. Bhat and H. K. Fun, Microwave-assisted synthesis and characterization of certain oximes, hydrazones, and olefins derived from β-keto sulfones, J. Chem., 2014, 532467 Search PubMed; (b) G. Dilauro, L. Cicco, F. M. Perna, P. Vitale and V. Capriati, Solvent-catalyzed umpolung carbon sulfur bond-forming reactions by nucleophilic addition of thiolate and sulfinate ions to in situ–derived nitrosoalkenes in deep eutectic solvents, C. R. Chim., 2017, 20, 617–623 CrossRef CAS; (c) V. S. Nikonova, A. R. Kaliev, T. N. Borodina, V. I. Smirnov, I. B. Rozentsveig and N. A. Korchevin, Synthesis, structure, and chemical transformations of 2-chloroprop-2-en-1-yl sulfones, Russ. J. Org. Chem., 2019, 55, 1912–1917 CrossRef CAS; (d) W. Zhang and X. Yang, Difluoroaminosulfonylation of styrenes with N,N-difluorobenzenesulfonamide, J. Fluorine Chem., 2021, 248, 109823 Search PubMed.
- Selected reviews: (a) B. Azizi, M. R. P. Heravi, Z. Hossaini, A. Ebadi and E. Vessally, Intermolecular difunctionalization of alkenes: synthesis of β-hydroxy sulfides, RSC Adv., 2021, 11, 13138–13151 Search PubMed; (b) L. Yan-Mei, F. Jin-Feng, H. Long-Qiang, L. Wei-Na and E. Vessally, Recent advances in intermolecular 1, 2-difunctionalization of alkenes involving trifluoromethylthiolation, RSC Adv., 2021, 11, 24474–24486 RSC; (c) Y. Zhang and E. Vessally, Direct halosulfonylation of alkynes: an overview, RSC Adv., 2021, 11, 33447–33460 RSC; (d) Z. Hossaini, E. A. Mahmood, M. R. P. Heravi, A. G. Ebadi and E. Vessally, Hydroxysulfonylation of alkenes: an update, RSC Adv., 2021, 11, 21651–21665 Search PubMed; (e) A. Bakhtiary, M. R. P. Heravi, A. Hassanpour, I. Amini and E. Vessally, Recent trends in the direct oxyphosphorylation of C–C multiple bonds, RSC Adv., 2021, 11, 470–483 Search PubMed; (f) Y. Cao, S. Soleimani-Amiri, R. Ahmadi, A. Issakhov, A. G. Ebadi and E. Vessally, Alkoxysulfenylation of alkenes: development and recent advances, RSC Adv., 2021, 11, 32513–32525 Search PubMed.
- N. Liu, P. Yin, Y. Chen, Y. Deng and L. He, Preparation of α-sulfonylethanone oximes from oxidized hydroxylamine, Eur. J. Org Chem., 2012, 2711–2714 CrossRef CAS.
- F. Chen, N. N. Zhou, J. L. Zhan, B. Han and W. Yu, tert-Butyl nitrite-mediated vicinal sulfoximation of alkenes with sulfinic acids: a highly efficient approach toward α-sulfonyl ketoximes, Org. Chem. Front., 2017, 4, 135–139 RSC.
- J. Yang, Y. Y. Liu, R. J. Song, Z. H. Peng and J. H. Li, Copper-mediated 1, 2-difunctionalization of styrenes with sodium arylsulfinates and tert-butyl nitrite: facile access to α-sulfonylethanone oximes, Adv. Synth. Catal., 2016, 358, 2286–2292 CrossRef CAS.
- Z. Zhang, N. Zhu and T. Liu, Facile synthesis of α-sulfonyl ketoximes from alkenes using sodium sulfinate and NaNO2 in water, Org. Chem. Front., 2024, 11, 5054–5060 Search PubMed.
- B. Wang, L. Tang, L. Liu, Y. Li, Y. Yang and Z. Wang, Base-mediated tandem sulfonylation and oximation of alkenes in water, Green Chem., 2017, 19, 5794–5799 Search PubMed.
- B. Wang, Z. Yan, L. Liu, J. Wang, Z. Zha and Z. Wang, TBN-mediated regio-and stereoselective sulfonylation & oximation (oximosulfonylation) of alkynes with sulfonyl hydrazines in EtOH/H2O, Green Chem., 2019, 21, 205–212 Search PubMed.
- W. Li, Z. Li, D. Zhong, N. Wang and H. Li, Photoinduced perfluoroalkyloximation of alkenes with simple perfluoroalkyl halides, Chin. J. Chem., 2024, 42, 2217–2222 CrossRef CAS.
- W. Li, Z. Huang, D. Zhong and H. Li, Photocatalyst-free activation of sulfamoyl chlorides for regioselective sulfamoyl-oximation of alkenes via hydrogen atom transfer (HAT) and halogen-atom transfer (XAT) relay strategy, Org. Lett., 2024, 26, 2062–2067 Search PubMed.
- (a) A. D. Mathiyazhagan and G. Anilkumar, Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC), Org. Biomol. Chem., 2019, 17, 6735–6747 RSC; (b) K. Kumar, TosMIC: a powerful synthon for cyclization and sulfonylation, ChemistrySelect, 2020, 5, 10298–10328 CrossRef CAS.
- X. Q. Chu, D. Ge, T. P. Loh and Z. L. Shen, Oxidant-directed chemoselective sulfonylation and sulfonyloximation of alkenes via cleaving the C–S bond in TosMIC, Org. Chem. Front., 2019, 6, 835–840 Search PubMed.
- W. Li, C. Diao, Y. Lu and H. Li, Photoinduced vicinal sulfamoyloximation of alkenes: harnessing bifunctional nitrosamines via a rapid radical trapping strategy, Org. Lett., 2024, 26, 6253–6258 CrossRef CAS PubMed.
- Selected recent studies on the utilization of SO2 for radical trapping: (a) H. Zhang, X. Sun, C. Ma, C. Li, Y. Ni, Y. Yu, Y. Q. Xu, S. F. Ni and Z. Y. Cao, Copper-mediated radical fluorine-atom transfer to sulfonyl radical: a dramatic 4-methoxypyridine 1-oxide ligand effect, ACS Catal., 2024, 14, 3115–3127 Search PubMed; (b) L. Lin, G. Pei, Z. Y. Cao and S. Liao, Recent advances in developing radical methods for the synthesis of aliphatic sulfonyl fluorides, Eur. J. Org Chem., 2024, 27, e202400279 CrossRef CAS.
- J. W. Sang, H. Chen, Y. Zhang, J. Wang and W. D. Zhang, Photo-mediated radical relay oximinosulfonamidation of alkenes with N-nitrosamines triggered by DABSO, Green Chem., 2024, 26, 7849–7856 Search PubMed.
- S. Jakkampudi, N. Sakkani and J. G. Zhao, Synthesis of α-sulfonyl ketoximes through a salicylic acid-catalyzed four-component reaction involving radical sulfonylation followed by arylsulfonylation and oximation, Tetrahedron Lett., 2021, 76, 153229 Search PubMed.
- N. Sakkani, S. Jakkampudi, N. Sadiq and J. C. G. Zhao, Synthesis of α-sulfonyl ketones through a salicylic acid-catalyzed multicomponent reaction involving arylsulfonation and oxidation, ChemistrySelect, 2021, 6, 13577–13581 Search PubMed.
- R. Chen, Y. Tang, X. He, K. K. Wang, L. Ding and L. Liu, Catalyst-controlled direct oxysulfonylation of alkenes by using sulfonylazides as the sulfonyl radical, Org. Lett., 2023, 25, 5454–5458 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |


















