A DFT investigation of the mechanism of photoinduced radical borylation of alkyl bromides: elucidation of base-mediated photoinduced SET and SN2 processes†
Nana
Ma
 *,
Qiongjin
Wang
,
Die
Zhao
,
Bowen
Duan
,
Shujun
Li
*,
Qiongjin
Wang
,
Die
Zhao
,
Bowen
Duan
,
Shujun
Li
 * and
Guisheng
Zhang
* and
Guisheng
Zhang
 *
*
School of Chemistry and Chemical Engineering, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education; NMPA Key Laboratory for Research and Evaluation of Innovative Drug, Henan Normal University, Xinxiang, Henan 453007, China. E-mail: mann076@htu.edu.cn; lisj@htu.edu.cn; zgs@htu.edu.cn
First published on 29th March 2025
Abstract
The transition-metal-free borylation of alkyl halides has received widespread attention due to environmental and economic considerations. In particular, the visible-light-induced radical borylation of alkyl halides catalyzed by 4-phenylpyridine has been achieved, even for unactivated alkyl bromides. In these reactions, the visible-light-induced radical borylation of alkyl bromides has demonstrated the formation of nucleophilic products and proposed a mechanism where the activation of unreactive alkyl bromides occurs through an SN2/photoinduced radical formation pathway. However, a systematic investigation of the mechanism of this type of reaction is lacking. Several crucial aspects necessitate further elucidation, including the formation of a super electron donor (SED), the competition between photoinduced single-electron transfer (SET) and SN2 processes, and the precise nature of MeO−. In this study, we employed density functional theory (DFT) calculations to address these issues. The calculated results indicate that in the 4-PhPy/B2cat2/MeONa system, the generation of alkyl radicals mainly results from the two successive photoinduced SET processes alongside SN2/photoinduced SET. Notably, in addition to the N-boryl pyridyl anion (INT-A) and the ate complex (INT-B), the resulting anionic radical (2C) and radical 1E as SEDs also play crucial roles in generating R˙ in the pathways subsequent to the SET of INT-A and INT-B, respectively. MeO− plays a critical role in both the nucleophilic substitution and SET processes through the Oδ−⋯Bδ+ Lewis acid–base interaction, facilitated by decreased electron delocalization from sp2 to sp3 of the B center, making it easier to donate electrons.
1. Introduction
Organoboron compounds have extensive applications in organic synthesis, medicinal chemistry, and life sciences.1–3 Due to their diverse applications, the synthesis of various organoboron compounds has attracted increasing attention.4–10 In organoboron compounds, alkylboronates serve as versatile intermediates in organic synthesis owing to the capacity of the C–B bond to undergo a wide array of transformations, leading to the formation of C–H, C–C, C–N, C–O, and C–X bonds.11–14 Consequently, the development of efficient synthetic approaches for alkylboronates holds significant appeal for organic chemists. Traditionally, alkylboronates have been synthesized from alkyl halides through the use of transition-metal catalysis involving elements such as Pd, Cu, Ni, Zn, Fe, and Mn.15–21 However, in light of environmental and economic considerations, there is a growing demand for transition-metal-free methods for the preparation of alkylboronates. Radical-type borylation reactions present an avenue for generating alkylboronates by utilizing redox-active esters, pyridinium salts, and xanthates/oxalates as alkyl sources, thus obviating the need for a transition-metal catalyst.22–25In recent years, significant progress has been made in achieving transition-metal-free borylation of alkyl halides, particularly alkyl iodides.26,27 However, due to the comparatively greater difficulty in transforming alkyl bromides into the corresponding alkyl radicals,28 developing a new activation method for the borylation of unactivated alkyl bromides has posed a substantial challenge. Addressing this, Jiao et al. reported that the visible-light-induced radical borylation of alkyl bromides catalyzed by 4-phenylpyridine (4-PhPy) features a broad substrate scope.29
Prior to this groundbreaking work, Jiao et al. delved into the reaction between pyridine, bis(pinacolato)diboron (B2pin2), and MeONa, revealing the formation of an N-boryl pyridyl anion (Int-A) and an ate complex (Int-B) which are capable of acting as super electron donors (SEDs), activating aryl iodides via single-electron transfer (SET).30,31 Building upon these findings, Jiao et al. proposed the existence of similar SEDs (Int-A and Int-B) in the 4-PhPy/B2cat2/MeONa system, where they serve to activate alkyl bromides through photoinduced SET. Notably, they discovered that these SED complexes are not only electron donors but also nucleophiles, introducing a mechanism where the activation of unreactive alkyl bromides occurs through an SN2/photoinduced radical formation pathway.
We are deeply intrigued by the mechanistic details governing the radical borylation of alkyl bromides catalyzed by the pyridine/diboron/base system. While the proposed mechanism (Scheme 1) has undergone experimental scrutiny,29 several crucial aspects necessitate further elucidation. First, we aim to comprehend the formation mechanism of possible super electron donors (SEDs) and their specific roles in the reaction process. Second, we seek a comprehensive understanding of the photoinduced SET and SN2 processes. Third, it is essential to uncover the precise nature of MeONa's involvement in the experiments. Furthermore, we are committed to conducting a thorough investigation of the B–B bond cleavage of B2cat2 under the experimental conditions. To investigate these aspects, we plan to employ density functional theory (DFT) calculations, with the anticipation that the theoretical results will offer a more profound comprehension of the borylation of alkyl bromides.
 | ||
| Scheme 1 The proposed mechanism by Jiao et al.29 | ||
2. Computational methods and details
All DFT calculations were conducted using the Gaussian 09 program.32 The molecular structures of the studied systems were optimized using the B3LYP33–35 functional with a 6-31G(d)36 basis set. Vibrational frequencies were computed at the same level to confirm whether each optimized structure corresponded to an energy minimum or a transition state (TS) and to assess its thermal corrections at 298 K. Additionally, an intrinsic reaction coordinate (IRC)37 calculation was performed to uniquely identify the transition states connecting the reactants to the products. Solvent effects were incorporated through single-point calculations on the gas-phase stationary points using a self-consistent reaction field (SCRF) with the SMD38 model in MeCN solvent. Furthermore, solvation single-point calculations were conducted at the M06L/6-311++G(d,p) level to attain more precise energetic information. Vertical excitation values were calculated by the time-dependent (TD) DFT method at the B3LYP/6-31+G(d) level to simulate the wavelength of experimental irradiation, and the determination of the computational level was discussed in the ESI.† The excited states of possible SEDs were obtained by geometry optimization of the triplet state of SEDs at B3LYP/6-31+G(d).39,40The discussion of the reaction involved the use of Gibbs free energy changes (ΔG°) and corrected Gibbs activation energies (ΔG°‡) in MeCN solution. Activation barriers for single-electron transfer (SET) steps involve in this work have been calculated using Marcus–Hush theory41–43 (details provided in the ESI†). In addition, natural population analysis (NPA) charges were computed based on the gas-phase optimized geometries using the NBO program.44,45 This analysis was integral for identifying potential reaction active sites as well as for assessing frontier molecular orbitals. The visualization of molecular orbital composition was achieved using the Multiwfn46 and VMD47 programs.
3. Results and discussion
3.1. The formation mechanism of INT-A and INT-B
 | ||
| Fig. 2 The energy profile of activation and cleavage of the B–B bond in B2cat2 (red values represent bond lengths (Å), green values represent NPA charges, and blue values represent spin densities). | ||
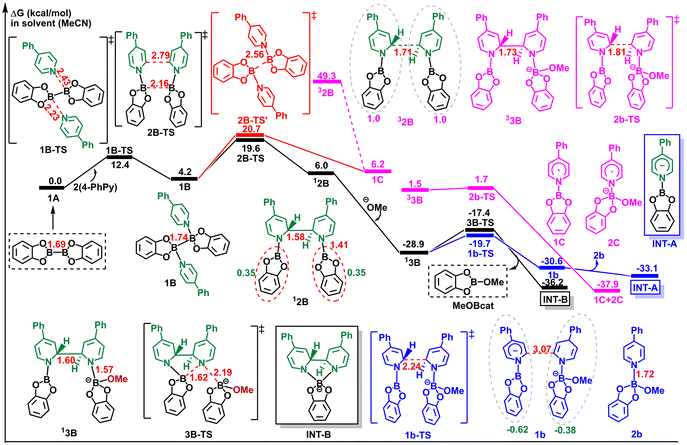
Another possible pathway (b) involves the initial reaction of MeO− with 1Avia an Oδ−⋯Bδ+ interaction, followed by a reaction with 4-PhPy through a dative Nδ−⋯Bδ+ interaction to activate the B–B bond. As shown in Fig. 2, the resulting 2a then reacts with 4-PhPy to form intermediate 3A with a ΔG°‡ value of 8.9 kcal mol−1. From a kinetic standpoint, the ΔG°‡ value of intermediate 3A obtained via pathway (a) is 3.3 kcal mol−1 lower than that obtained via pathway (b). However, the generated intermediate 2a in pathway (b) exhibits an exothermic ΔG° value of 31.7 kcal mol−1, indicating that the interaction of Oδ− in MeO− with Bδ+ in B2cat2 is thermodynamically favorable. Moreover, intermediate 2a is more stable than 3A, indicating that 3A readily reverts to 2a. Consequently, we concluded that the activation of the B–B bond in B2cat2 occurs through the initial interaction of B2cat2 with 4-PhPy, followed by the interaction with MeO−.
Following the activation of the B–B bond, we further investigated the cleavage of the B–B bond in intermediate 3A. Although INT-A can only be generated by heterolysis from 3A, we also explored the homolytic pathway for a more comprehensive understanding of the electron transfer process (see Fig. 1). The calculated results in Fig. 2 reveal that the heterolytic cleavage of the B–B bond in 3A proceeds via the transition state 3A-TS, resulting in the formation of intermediate 4A, with a ΔG°‡ value of 13.6 kcal mol−1. The NPA charge analysis of intermediate 4A indicates that the N-boryl pyridine moiety carries a charge of −0.94, while the MeOBcat moiety carries a charge of −0.06, thereby confirming the formation of the N-boryl pyridyl anion (INT-A).
We meticulously examined the spin state of electrons during the homolytic cleavage process. The singlet state 13a of intermediate 3A required overcoming an activation energy of 13.1 kcal mol−1 (3a-ts), resulting in the same intermediate 4A as observed in the heterolytic cleavage process. Analysis of the transition state 3a-ts revealed different spin densities for the two B atoms (0.10 and 0.15), compared to the heterolysis process. To accurately capture the electron transfer process, an IRC calculation was conducted to analyze the change in spin density during B–B homolysis (see Fig. S1†). The results indicated that prior to the transition state, the homolysis process occurred, with the two electrons of opposite spins pairing up for a stable state, ultimately leading to intermediate 4A as observed in heterolysis. In the case of homolysis from the triplet state 33a, it was found to be 20.1 kcal mol−1 less stable than 13a. The homolysis of 33a required a ΔG°‡ value of 10.7 kcal mol−1 through the transition state 3a-ts1, resulting in intermediate 4a. Further analysis of the electron transfer process using spin density, from 33a to 4a, revealed spin densities of 0.25 for B1 and 0.35 for B2 in 33a, evolving into 0.04 and 0.77, respectively, in the transition state 3a-ts1. Ultimately, the analysis of 4a showed a spin density of 1.94 for the N-boryl pyridine moiety, 0.80 for B2, 0.97 for the 4-PhPy moiety, and 0.06 for the MeOBcat moiety, indicating the presence of an unpaired electron on B and the other electron being delocalized in the pyridine ring. This suggests the formation of a diradical anion (5a) and MeOBcat, rather than the expected MeOBcat˙ and 4-PhPyBcat˙. However, the relatively high activation energy barrier of 35.8 kcal mol−1 and the thermodynamic instability both indicate the difficulty of obtaining 5a compared to the formation of INT-A.
Hence, regardless of whether the spin state is 13a or 33a, the homolytic cleavage of the B–B bond in the presence of 4-PhPy and MeO− is hindered. This is due to the superior electron dispersion capability of the π-delocalized 4-PhPy, while the resulting MeOBcat exhibits difficulty in accepting additional electrons. Collectively, these results underscore that the formation of INT-A depends on the initial interaction of B2cat2 with the base MeO− and subsequent interaction with 4-PhPy, culminating in the heterolysis of the B–B bond in intermediate 3A, with a ΔG°‡ value of 18.1 kcal mol−1. Furthermore, we explored the homolysis of the B–B bond with two identical 4-PhPy or MeO−. The calculated energy barriers, as depicted in Fig. S2,† surpass those observed for the heterolysis of the B–B bond with 4-PhPy and MeO−.
Based on the calculated energy profile in Fig. 4, in pathway (a), B2cat2 interacts with two 4-PhPy through Nδ−⋯Bδ+ interactions, resulting in the formation of intermediate 1B. This step exhibits a ΔG°‡ value of 12.4 kcal mol−1via transition state 1B-TS. Subsequently, intermediate 1B undergoes transition state 2B-TS, facilitating the concerted cleavage of the B–B bond and the formation of the C–C bond. This process requires an activation energy of 19.6 kcal mol−1, producing the pyridine-substituted intermediate 2B (12B). The calculated Natural Population Analysis (NPA) charge of 12B indicates an identical charge of 0.35 on the two Bcat moieties, signifying an even split of B2cat2. Intermediate 12B then interacts with MeO− through Bδ+⋯Oδ− interactions, yielding intermediate 13B and MeOBcat. This step is exothermic, with a ΔG° value of 34.9 kcal mol−1. Notably, the interaction between MeO− and the B center results in the elongation of the N–B bond (from 1.41 Å in 12B to 1.57 Å in 13B), facilitating the cleavage of the N–B bond and providing the potential for a new N–B bond. Subsequently, INT-B is formed via transition state 3B-TS with a ΔG°‡ value of 11.5 kcal mol−1. Alternatively, 13B may undergo the cleavage of the C–C bond between the two pyridine rings to generate complex 1b, with a ΔG°‡ value of 11.5 kcal mol−1 and a ΔG° value of 2.5 kcal mol−1. The NPA charge for the two moieties of 1b (−0.62 and −0.38) suggests that the heterolysis of the C–C bond can yield INT-A and intermediate 2b.
Furthermore, we also explored the homolytic cleavage of the C–C bond, and the calculated results indicate that the energy of the 33B state is 30.4 kcal mol−1 higher than that of 13B, although the homolysis of the 33B state has a ΔG°‡ value of 0.2 kcal mol−1, resulting in the formation of the N-arylpyridine free radical intermediate 1C and the anionic radical 2C. Consequently, we can conclude that the rate-determining step for the formation of INT-B, starting from B2cat2 and two 4-PhPy, is the concerted process of C–C bond formation and B–B bond cleavage, with a ΔG°‡ value of 19.6 kcal mol−1. Importantly, in this pathway, the formation of INT-A (ΔG°‡ = 9.2 kcal mol−1) is more favorable than that of INT-B (ΔG°‡ = 11.5 kcal mol−1). Additionally, the generation of intermediate 1C was also considered through the C–C cleavage of 32B, but 32B exhibits significantly higher energy with respect to 12B.
In pathway (b), INT-B can be formed through the interaction of INT-A with 4-PhPy. As depicted in Fig. 5, INT-A can react with 4-PhPy through a B ← N bonding process (AB-TS1) with a ΔG°‡ value of 7.5 kcal mol−1, affording intermediate INT-AB. Subsequently, INT-AB undergoes C–C bond formation between the two pyridine rings, resulting in the formation of INT-B. This step has a ΔG°‡ value of 18.5 kcal mol−1, which is close to the rate-determining step of pathway (a) (ΔG°‡ = 19.6 kcal mol−1). This suggests that the generation of INT-B may occur via these two pathways, and the transformation between INT-A and INT-B is feasible, aligning with experimental propositions.
Interestingly, the intermediate INT-AB demonstrates similar thermodynamic stability to that of INT-A and INT-B, as illustrated in Fig. 5. Consequently, we intended to assess the electron-donating capability of INT-AB to explore its potential role in this reaction. The oxidation potentials (vs. SCE) of these three species were calculated,48 (the computational details are provided in ESI†), revealing the values Eox for INT-A, INT-AB, and INT-B as −1.61 V, −1.51 V, and −1.53 V, respectively. This suggests that INT-AB possesses electron-donating capabilities close to those of INT-A and INT-B. Furthermore, the Eox values of other intermediates 1C and 2C, although their formations are not favorable according to Fig. 4, were also computed. These calculations indicate an Eox value of −1.40 V for 2C, suggesting that it possesses a certain level of electron-donating capability. Consequently, in subsequent investigations, we will consider pathways involving INT-AB or 2C.
3.2. The reaction of INT-A with RBr
Indeed, INT-B and 2C demonstrate exceptional single electron donor (SED) properties, making them well-suited for generating aryl radicals from aryl iodides or bromides through single-electron reduction.21 However, the high ΔG°‡ value of 30.6 kcal mol−1 indicates that the formation of 2C is highly unfavorable. Consequently, we conducted calculations to assess the abilities of INT-B, INT-A, and INT-AB to generate alkyl radicals through SET without light, comparing these processes with the outcomes of photoinduced SET. Moreover, Jiao et al. observed that a synthesized analogue of INT-B reacts with alkyl bromide under both illuminated and non-illuminated conditions, resulting in the observation of nucleophilic substitution products exclusively.29 This suggests that nucleophilic substitution might precede photoinduced SET. Despite these findings, given the continuity and intricacy of this reaction, we aim to comprehensively explore all potential avenues through theoretical calculations.Therefore, we delved into the sequence of nucleophilic substitution and (photoinduced) SET processes when considering the roles of INT-A, INT-AB, and INT-B as either electron donors or nucleophiles, respectively. Given the substantial influence of the base MeO− in such reactions, it is crucial to elucidate the involvement of base. Our initial exploration involved calculating the process wherein INT-A undergoes nucleophilic substitution with alkyl bromide followed by SET. Factoring in the addition of MeO−, we examined potential reaction pathways (a), (b), and (c), represented in Fig. 6.
 | ||
| Fig. 6 The possible reaction pathway involving the nucleophilic substitution of INT-A with alkyl bromide followed by SET in the presence of MeO−. | ||
In pathway (b), MeO− initially interacts with INT-A with an energy barrier of 14.5 kcal mol−1, forming intermediate 2D. Following this, 2D undergoes nucleophilic substitution with RBr, affording intermediate 6A with a ΔG°‡ value of 7.9 kcal mol−1. This sequence of events indicates that MeO− more readily engages with INT-A than with RBr, leading to the formation of a dianionic intermediate 2D, which is more favorable for the subsequent nucleophilic substitution with RBr.
Subsequently, intermediate 6A undergoes a second SET process with another RBr to generate alkyl radical R˙ and radical species 7A. The free energy barrier for the electron transfer process without illumination is calculated to be 35.1 kcal mol−1, which indicates that this process is challenging. Under illumination, 6A can be excited to its singlet excited state (67.9 kcal mol−1), which subsequently undergoes intersystem crossing (ISC) to form the thermodynamically more stable triplet excited state (6A*). This optimized triplet state then participates in the SET process with RBr, generating radical species 7A and the alkyl radical R˙ with an activation barrier of 9.2 kcal mol−1. These findings demonstrate that photoirradiation significantly facilitates the production of the alkyl radical R˙via this pathway. Furthermore, we explored an alternative radical generation mechanism involving the C–C bond homolysis of 6A*. This process yields R˙ and 2C through transition state 6A*-TS with a comparable activation barrier of 9.0 kcal mol−1. The nearly identical activation barriers suggest that these two R˙ formation pathways are energetically competitive under the reaction conditions.
Furthermore, pathway (c) involves a direct SET process following nucleophilic substitution without the involvement of MeO−. The findings reveal that the SET process between 5A and RBr exhibits a high ΔG°‡ value of 51.8 kcal mol−1. Although the SET process between the excited state 5A* and RBr exhibits a relatively lower activation barrier (ΔG°‡ = 21.9 kcal mol−1), the required excitation energy of 103.6 kcal mol−1 far exceeds the standard experimental conditions (400 nm). Therefore, it can be concluded that MeO− plays a critical role in both the nucleophilic substitution and SET processes through the Oδ−⋯Bδ+ Lewis acid–base interaction. This outcome represents an elucidation of the role of MeO− for the first time in this context.
As shown in the potential energy profile depicted in Fig. 8, in pathway (a′), INT-A engages in a SET process with RBr, resulting in the formation of the alkyl radical R˙ and radical 1C with a ΔG°‡ value of 18.1 kcal mol−1. This process is exergonic by 34.4 kcal mol−1. Compared with the SN2 reaction between INT-A and RBr (ΔG°‡ = 15.2 kcal mol−1) in Fig. 7, this SET process is less favorable. In the presence of MeO−, the energy barrier for the SET process has a lower ΔG°‡ value of 9.4 kcal mol−1, occurring in the reaction of intermediate 2D and RBr. This lower barrier suggests that the SET process is also disadvantageous when compared to the nucleophilic substitution of 2D with RBr (ΔG°‡ = 7.9 kcal mol−1) in Fig. 7. These results imply that in the absence of illumination, the interaction between INT-A and RBr is more likely to result in nucleophilic substitution. This observation aligns with the fact that borylation reactions can yield products of nucleophilic substitution both with and without illumination, thus illustrating the dual nature of these reactions under different conditions.
Under photoirradiation, the ΔG°‡ value for the SET process between the excited state INT-A* and the alkyl bromide is 5.6 kcal mol−1, which is lower than the energy barrier of the nucleophilic substitution (14.5 kcal mol−1). This result suggests that both SET and SN2 processes can occur under illumination when the alkyl group is phenylpropane (R = PhCH2CH2CH3–). Additionally, the dianionic species 2D demonstrates enhanced SET reactivity toward RBr under photoirradiation, exhibiting a significantly reduced activation barrier (ΔG°‡ = 3.9 kcal mol−1) compared to the non-illuminated conditions (ΔG°‡ = 9.4 kcal mol−1). These findings indicate that illumination can significantly impact the reaction pathways, making both SET and SN2 processes viable under these conditions.
In the case of reaction pathways (a′) and (b′), which involve base-mediated SET, the generation of the alkyl radical R˙ also produces an intermediate 2C. Recognizing the formation of 2C in this context, with a lower ΔG°‡ value of 10.2 kcal mol−1 and strong electron-donating capability, we further investigated the SET and photoinduced SET processes involving 2C. As depicted in Fig. 10, the SET process exhibits a ΔG°‡ value of 10.2 kcal mol−1, generating R˙, and under illumination, this process has a ΔG°‡ value of 2.3 kcal mol−1. These results suggest that 2C is also a crucial species in generating R˙ in this reaction—an aspect that has been overlooked in the proposed mechanism by experimental scientists. Furthermore, we explored the potential for intermediate 2C to undergo a nucleophilic reaction with a second RBr. The high activation energy barrier of 23.1 kcal mol−1 indicates that nucleophilic substitution is not feasible in this context. Additionally, as we considered the breaking of the N–B bond in 2C, which results in the formation of the radical anion 4C and the byproduct MeOBcat, the associated step exhibits a ΔG°‡ value of 20.6 kcal mol−1. It is plausible that the 4C radical anion may undergo recombination with the generated Bcat˙ in subsequent reactions, effectively regenerating INT-A. These findings emphasize the potential significance of 2C within the reaction, indicating its role as a significant species in single electron transfer, while also shedding light on its unique reactivity and its contribution to the overall reaction mechanism.
Given that intermediate 1C has an electrochemical potential (Eox) value of −0.64 V, indicating a reduced tendency to donate electrons, we investigated whether this intermediate could undergo an SN2 reaction with RBr. As pathway (c′) in Fig. 6 depicts, intermediate 1C first participates in a nucleophilic reaction with a second RBr molecule, which generates the radical 3C with a high activation energy barrier of 33.8 kcal mol−1. As a result, the SN2 reaction between 1C and RBr is highly improbable.
Based on the potential energy surfaces depicted in Fig. 7 and 8 and considering the potential reaction pathways, two routes are possible for the generation of R˙. (i) Initial SN2 nucleophilic substitution followed by photo-induced SET, leading to R˙ and intermediate 7A. (ii) A sequence involving photo-induced SET, in which 2C also serves as a significant SED, as illustrated in Fig. 9. These calculated free energy barriers indicate that the successive photoinduced SET pathway is in parallel with the SN2/SET reaction pathway. Importantly, the involvement of MeO− is essential, as it interacts with INT-A through an Oδ−⋯Bδ+ interaction before the reaction proceeds.
To account for experimentally relevant methoxide conditions, we performed comparative energy calculations for INT-A → 7A under free MeO− anions and the MeONa system. The complete free energy profiles are shown in Fig. S3,† with Fig. S4† highlighting key activation barriers for comparison. Analysis of Fig. 9 and Fig. S5† reveals that Na+ coordination systematically elevates activation barriers by 1.1–6.0 kcal mol−1 relative to the cation-free system. Crucially, this ionic pairing preserves the overall reaction mechanism. Based on this result, subsequent mechanistic investigations employed the free MeO− model to simplify electronic structure analysis while maintaining chemical accuracy.
The N–B bond in the resulting intermediate 7A measures 1.61 Å, facilitating heterolysis to yield the byproduct MeOBcat and the radical 8A, with a lowered ΔG°‡ value of 6.9 kcal mol−1, as depicted in Fig. 10. Additionally, given that the C–C bond stretches to 1.60 Å, the radical 8A can decompose into the radical R˙ and the catalyst 4-PhPy, with a ΔG°‡ value of 8.0 kcal mol−1. Subsequently, R˙ generated during the process can be captured by B2cat2, forming the radical intermediate 9A through the C–B bond formation. This step has a ΔG°‡ value of 9.2 kcal mol−1. Notably, 9A has the potential to interact with the second resulting R˙ radical through a concerted C–B bond formation and B–B cleavage, generating the product P with a ΔG°‡ value of 9.1 kcal mol−1. As an alternative pathway, 9A might undergo cleavage into Bcat˙ and reactant diboronate ester 1Avia B–B homolysis. However, this process needs a relatively high activation energy of 21.2 kcal mol−1. These findings elucidate the experimental requirement of a near 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for 1A to RBr.
1 ratio for 1A to RBr.
Both experimental findings and theoretical calculations have underscored the crucial role of MeO− in the reaction. Regardless of its sequential involvement, the addition of MeO− leads to increased stability and electron richness. As a result, the presence of MeO− promotes electron transfer and facilitates the generation of R˙, irrespective of illumination. These insights not only enhance our comprehension of the reaction mechanism but also establish a theoretical basis for optimizing and controlling related reactions. To gain insight into the influence of MeO−, we compared the electronic structures between INT-A and 2D, and between 1C and 2C. Analysis of the composition of the highest occupied molecular orbital (HOMO) in Fig. 11 reveals a significant decrease in boron distribution, dropping from 7.40% to 1.19% (INT-A → 2D) and from 5.06% to 0.55% (1C → 2C). This reduction is attributed to the transformation of B hybridization (sp2 → sp3), signifying a decrease in electron delocalization and making it easier to donate electrons.
 | ||
| Fig. 11 Composition (isosurface value = 0.02) and energy levels of the HOMO of INT-A, 2D, 1C, 2C, and 6A. | ||
Consequently, following the interaction of the base with boron, the phenylpyridine π system can accept the delocalized electron from the boron atom, thereby reducing electron density concentration and stability. Moreover, due to the similar HOMO compositions of these SEDs, the HOMO energy level (EH) can be used to gauge their electron-donating ability. Notably, the EH values of 2D and 2C, formed by interacting with MeO−, are higher than the corresponding systems INT-A and 1C without MeO−. These results indicate that the electron-donating capacity of SED is enhanced when MeO− participates in the reaction through B–O binding. Similarly, with a lower EH value of −1.42 eV, 6A has weak donating ability to RBr and hardly generate the alkyl radical R˙ easily. However, the EH value of the excited state 6A* is 1.19 eV, resulting in enhanced electron-donating capacity.
 | ||
| Fig. 13 The energy profile of the SN2 reaction followed by SET and the direct SET process starting from INT-B. | ||
Alternatively, the direct electron transfer from INT-AB to alkyl bromide needs to overcome an activation energy barrier of 16.1 kcal mol−1 to afford R˙ and radical 1E. Then, the nucleophilic substitution of the resulting radical 1E with a second alkyl bromide generates intermediate 2E with an activation energy barrier of 28.1 kcal mol−1. The interaction of 2E with MeO− generates 3E through an exothermic process, with a ΔG° value of 12.2 kcal mol−1. Subsequent B–N bond cleavage in 3E leads to the formation of 7A and the regeneration of 4-PhPy with an activation energy barrier of 4.7 kcal mol−1. Also, the direct SET from INT-B to alkyl bromide can result in the alkyl radical R˙ and radical 1E with a ΔG°‡ value of 20.9 kcal mol−1. Under illumination, this SET barrier reduces to 16.8 kcal mol−1.
For radical 1E, it can interact with MeO− to form the single electron donor 2C with a ΔG°‡ value of 20.5 kcal mol−1. Meanwhile, the SET of 1E has a lower ΔG°‡ value of 11.2 kcal mol−1 (7.1 kcal mol−1 under photoirradiation), forming the alkyl radical R˙ and intermediate 4E. The subsequent interaction of 4E with MeO− can afford 5C (ΔG°‡ = 12.0 kcal mol−1), in which the cleavage of the N–B bond is facile, producing the byproduct MeOBcat and regenerating 4-PhPy (as shown in Fig. 8). In addition, the nucleophilic substitution of intermediate 1E with RBr was calculated and the ΔG°‡ value is 28.1 kcal mol−1. The results suggest that the nucleophilic substitution of intermediate 1E is less favorable, but the SET process is more likely to occur due to the sp3 hybridization of the B center.
Based on the pathways involving INT-B, it is evident from the data presented in Fig. 14 that the successive photoinduced SET pathway is also a viable mechanism alongside the SN2/SET reaction pathway for generating the alkyl radical R˙. Additionally, when combined with the reaction pathway from INT-A to R˙ (Fig. 9), it is clear that intermediates 2C and 1E also play a role in electron donation in two successive SET processes, and the observed products formed via SN2 in experiments result from the involvement of both INT-A and INT-B.
3.3. The mechanism of tertiary alkyl bromides
The experimental results indicating that certain tertiary alkyl bromides disfavor nucleophilic substitution but exhibit good reactivity imply the presence of a direct photoinduced SET pathway for these specific alkyl bromide substrates. To further investigate this, we calculated the case of tertiary alkyl (R = adamantine) for comparison. As shown in Fig. S5,† the SN2 reaction of a tertiary alkyl substrate with intermediate 2D exhibits a higher ΔG°‡ value of 16.7 kcal mol−1 and the SET between the tertiary alkyl and 2D has a ΔG°‡ value of 13.6 kcal mol−1 (5.6 kcal mol−1 under illumination). Likewise, as provided in Fig. S6,† the SN2 reaction of a tertiary alkyl substrate with intermediate INT-AB has a higher ΔG°‡ value of 31.2 kcal mol−1 than the SET between the tertiary alkyl and INT-AB with a ΔG°‡ value of 21.6 kcal mol−1 (17.9 kcal mol−1 under illumination). This discrepancy explains why SN2 products cannot be observed when the substrate is a tertiary alkyl bromide, which further supports the hypothesis that the real pathway to generate R˙ involves two successive photoinduced SET processes between Int-A/Int-B and the alkyl bromide. These findings provide valuable insight into the reactivity of tertiary alkyl substrates and the mechanisms underlying the observed product formation.3.4. The comparison of B2cat2 with B2pin2
Jiao et al. demonstrated the feasibility of alkyl bromide borylation using B2pin2 as an alternative to B2cat2.29 The experiments confirmed the generation of alkyl radicals from alkyl bromides, but revealed inefficient subsequent borylation due to the markedly slower radical trapping kinetics of B2pin2 compared to B2cat2. To elucidate this difference, we computationally evaluated the radical borylation pathway using B2pin2 (Fig. S7†). The calculations reveal a significantly higher activation barrier for alkyl radical trapping by B2pin2 (ΔG°‡ = 16.8 kcal mol−1) relative to B2cat2 (ΔG°‡ = 9.2 kcal mol−1). Furthermore, the resulting radical intermediate (12a) exhibits severely diminished reactivity toward another alkyl radical capture to form dual borylation products. This suppressed reactivity arises from the pronounced steric hindrance caused by the pinacolato ligands, contrasting with the compact catecholato groups in B2cat2 that facilitate efficient radical coupling.4. Conclusions
Based on the DFT calculations for the mechanism of visible-light-induced radical borylation of alkyl bromides catalyzed by 4-phenylpyridine, we can conclude that the reaction includes the following processes: (i) the initial interaction of B2cat2 with the base MeO− and subsequent interaction with 4-PhPy afford the N-boryl pyridyl anion (INT-A). (ii) The ate complex (INT-B) can be formed through the interaction of INT-A with 4-PhPy or the synergistic effect of two 4-PhPy molecules followed by the interaction with MeO−. (iii) The successive photoinduced SET generating alkyl radicals is also a plausible mechanism alongside the SN2/SET reaction pathway. Notably, MeO− plays a critical role in both the nucleophilic substitution and SET processes through the Oδ−⋯Bδ+ Lewis acid–base interaction, facilitated by decreased electron delocalization from sp2 to sp3 of the B center, making it easier to donate electrons. Additionally, the resulting intermediates 2C and 1E act as SEDs, facilitating the generation of R˙ in the pathways subsequent to the SET of INT-A and INT-B, respectively.Author contributions
Nana Ma: conceptualization, formal analysis, investigation, data curation, supervision, and writing – original draft; Qiongjin Wang: data curation, formal analysis, conducting the calculations, and investigation; Die Zhao: data curation and investigation; Bowen Duan: data curation and investigation; Shujun Li: investigation and funding acquisition; and Guisheng Zhang: writing – review & editing and funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 22171073) and the Natural Science Foundation of Henan Province (grant no. 252300421190), and the 111 Project (D17007). We also thank the financial support from the Henan Key Laboratory of Organic Functional Molecules and Drug Innovation. The calculations were supported by the High-Performance Computing (HPC) Centre of Henan Normal University.References
- N. Miyaura and A. Suzuki, Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
.
- S. Manna, K. K. Das, S. Nandy, D. Aich, S. Paul and S. Panda, A new avenue for the preparation of organoboron compounds via nickel catalysis, Coord. Chem. Rev., 2021, 448, 214165 Search PubMed
.
- R. Bisht, C. Haldar, M. M. M. Hassan, M. E. Hoque, J. Chaturvedi and B. Chattopadhyay, Metal-catalysed C–H bond activation and borylation, Chem. Soc. Rev., 2022, 51, 5042–5100 RSC
.
- C. Haldar, M. E. Hoque, J. Chaturvedi, M. M. M. Hassana and B. Chattopadhyay, Ir-catalyzed proximal and distal C–H borylation of arenes, Chem. Commun., 2021, 57, 13059–13074 RSC
.
- M. E. Hoque, M. M. M. Hassana and B. Chattopadhyay, Remarkably Efficient Iridium Catalysts for Directed C(sp2)–H and C(sp3)–H Borylation of Diverse Classes of Substrates, J. Am. Chem. Soc., 2021, 143, 5022–5037 CrossRef CAS PubMed
.
- T. Ishiyama, M. Murata and N. Miyaura, Palladium(0)-Catalyzed Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters, J. Org. Chem., 1995, 60, 7508–7510 CrossRef CAS
.
- C. Shu, A. Noble and V. K. Aggarwal, Metal-free photoinduced C(sp3)–H borylation of alkanes, Nature, 2020, 586, 714–719 CrossRef CAS PubMed
.
- M.-A. Légaré, M.-A. Courtemanche, É. Rochette and F.-G. Fontaine, Metal-free catalytic C-H bond activation and borylation of heteroarenes, Science, 2015, 349, 513–516 CrossRef PubMed
.
- M. M. M. Hassan, B. Mondal, S. Singh, C. Haldar, J. Chaturvedi, R. Bisht, R. B. Sunoj and B. Chattopadhyay, Ir-Catalyzed Ligand-Free Directed C–H Borylation of Arenes and Pharmaceuticals: Detailed Mechanistic Understanding, J. Org. Chem., 2022, 87, 4360–4375 CrossRef PubMed
.
- S. Guria, M. M. M. Hassan, J. Ma, S. Dey, Y. Liang and B. Chattopadhyay, A tautomerized ligand enabled meta selective C–H borylation of phenol, Nat. Commun., 2023, 14, 6906 CrossRef CAS PubMed
.
- A. Suzuki, Organoborates in new synthetic reactions, Acc. Chem. Res., 1982, 15, 178–184 Search PubMed
.
- N. Miyaura and A. Suzuki, Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
.
- Y. L. Phang, J. Jin, F. Zhang and Y. Wang, Radical hydroboration for the synthesis of organoboron compounds, Chem. Commun., 2024, 60, 4275–4289 RSC
.
- Y. Tian, X. Guo, H. Braunschweig, U. Radius and B. Marder, Photoinduced Borylation for the Synthesis of Organoboron Compounds, Chem. Rev., 2021, 121, 3561–3597 Search PubMed
.
- J. Li, C. Chen, Y. Dong, J. Lv, J. Peng, Y. Jiang and D. Yang, Ligand-promoted reductive coupling between aryl iodides and cyclic sulfonium salts by nickel catalysis, Chin. Chem. Lett., 2024, 35, 109732 CrossRef CAS
.
- M. Zhang, Z. Ye and W. Zhao, Cobalt-Catalyzed Asymmetric Remote Borylation of Alkyl Halides, Angew. Chem., Int. Ed., 2023, 62, e202306248 Search PubMed
.
- X. Li, L. Kong, S. Yin, H. Zhou, A. Lin, H. Yao and S. Gao, Palladium-Catalyzed Atroposelective Suzuki–Miyaura Coupling to Construct Axially Chiral Tetra-Substituted α-Boryl Styrenes, Adv. Sci., 2024, 11, 2309706 Search PubMed
.
- L. Britton, M. Skrodzki, G. S. Nichol, A. P. Dominey, P. Pawluć, J. H. Docherty and S. P. Thomas, Manganese-Catalyzed C(sp2)–H Borylation of Furan and Thiophene Derivatives, ACS Catal., 2021, 11, 6857–6864 CrossRef CAS
.
- R. Sang, W. Han, H. Zhang, C. M. Saunders, A. Noble and V. K. Aggarwal, Copper-Mediated Dehydrogenative C(sp3)–H Borylation of Alkanes, J. Am. Chem. Soc., 2023, 145, 15207–15217 CrossRef CAS PubMed
.
- R. J. Procter, M. Uzelac, J. Cid, P. J. Rushworth and M. J. Ingleson, Low-Coordinate NHC–Zinc Hydride Complexes Catalyze Alkyne C–H Borylation and Hydroboration Using Pinacolborane, ACS Catal., 2019, 9, 5760–5771 CrossRef CAS
.
- T. Yoshida, L. Ilies and E. Nakamura, Iron-Catalyzed Borylation of Aryl Chlorides in the Presence of Potassium t-Butoxide, ACS Catal., 2017, 7, 3199–3203 CrossRef CAS
.
- Q. Wei, Y. Lee, W. Liang, X. Chen, B. Mu, X. Cui, W. Wu, S. Bai and Z. Liu, Photocatalytic direct borylation of carboxylic acids, Nat. Commun., 2022, 13, 7112 CrossRef CAS PubMed
.
- P. Kumar Someswara Ashwathappa, T. Higashi, V. Desrosiers, A. A. Omaña and F. Fontaine, Metal-Free Directed Site-Selective Csp3-H Borylation of Saturated Cyclic Amines, Angew. Chem., Int. Ed., 2023, 62, e202309295 CrossRef CAS PubMed
.
- J. Wu, L. He, A. Noble and V. K. Aggarwal, Photoinduced Deaminative Borylation of Alkylamines, J. Am. Chem. Soc., 2018, 140, 10700–10704 CrossRef CAS
.
- W. Guan, Y. Chang and S. Lin, Electrochemically Driven Deoxygenative Borylation of Alcohols and Carbonyl Compounds, J. Am. Chem. Soc., 2023, 145, 16966–16972 CrossRef CAS PubMed
.
- Y. Cheng, C. Mück-Lichtenfeld and A. Studer, Transition Metal-Free 1,2-Carboboration of Unactivated Alkenes, J. Am. Chem. Soc., 2018, 140, 6221–6225 CrossRef CAS PubMed
.
- Y. Cheng, C. Mück-Lichtenfeld and A. Studer, Metal-Free Radical Borylation of Alkyl and Aryl Iodides, Angew. Chem., Int. Ed., 2018, 57, 16832–16836 CrossRef CAS PubMed
.
- D. J. Carlsson and K. U. Ingold, Kinetics and rate constants for the reduction of alkyl halides by organotin hydrides, J. Am. Chem. Soc., 1968, 90, 7047–7055 CrossRef CAS
.
- L. Zhang, Z. Wu and L. Jiao, Photoinduced Radical Borylation of Alkyl Bromides Catalyzed by 4-Phenylpyridine, Angew. Chem., Int. Ed., 2020, 59, 2095–2099 CrossRef CAS PubMed
.
- L. Zhang and L. Jiao, Visible-Light-Induced Organocatalytic Borylation of Aryl Chlorides, J. Am. Chem. Soc., 2019, 141, 9124–9128 CrossRef CAS PubMed
.
- L. Zhang and L. Jiao, Super electron donors derived from diboron, Chem. Sci., 2018, 9, 2711–2722 Search PubMed
.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013 Search PubMed
.
- A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS
.
- C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 Search PubMed
.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS
.
- M. Dolg, U. Wedig, H. Stoll and H. Preuss, Energy-adjusted ab initio pseudopotentials for the first row transition elements, J. Chem. Phys., 1987, 86, 866–872 CrossRef CAS
.
- K. Fukui, The path of chemical reactions-the IRC approach, Acc. Chem. Res., 1981, 14, 363–368 CrossRef CAS
.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions, J. Phys. Chem., 2009, 113, 6378–6396 CrossRef CAS PubMed
.
- Z. Gu, L. Zhang, H. Li, S. Cao, Y. Yin, X. Zhao, X. Ban and Z. Jiang, Deracemization through Sequential Photoredox-Neutral and Chiral Brønsted Acid Catalysis, Angew. Chem., Int. Ed., 2022, 61, e202211241 CrossRef CAS
.
- H. Ren, G.-F. Li, B. Zhu, X.-D. Lv, L.-S. Yao, X.-L. Wang, Z.-M. Su and W. Guan, How Does Iridium(III) Photocatalyst Regulate Nickel(II) Catalyst in Metallaphotoredox-Catalyzed C–S Cross-Coupling? Theoretical and Experimental Insights, ACS Catal., 2019, 9, 3858–3865 CrossRef CAS
.
- R. A. Marcus, On the Theory of Oxidation–Reduction Reactions Involving Electron Transfer. I, J. Chem. Phys., 1956, 24, 966–978 Search PubMed
.
- R. A. Marcus, On the Theory of Oxidation–Reduction Reactions Involving Electron Transfer. III. Applications to Data on the Rates of Organic Redox Reactions, J. Chem. Phys., 1957, 26, 872–877 Search PubMed
.
- V. Lemaur, M. Steel, D. Beljonne, J. L. Brédas and J. Cornil, Photoinduced Charge Generation and Recombination Dynamics in Model Donor/Acceptor Pairs for Organic Solar Cell Applications: A Full Quantum-Chemical Treatment, J. Am. Chem. Soc., 2005, 127, 6077–6086 CrossRef CAS PubMed
.
- J. E. Carpenter and F. Weinhold, Analysis of the geometry of the hydroxymethyl radical by the “different hybrids for different spins” natural bond orbital procedure, J. Mol. Struct.:THEOCHEM, 1988, 169, 41–62 Search PubMed
.
- A. E. Reed and F. Weinhold, Natural bond orbital analysis of near–Hartree–Fock water dimer, J. Chem. Phys., 1983, 78, 4066–4073 CrossRef CAS
.
- T. Lu and F. Chen, Multiwfn: A multifunctional wavefunction analyser, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed
.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graphics Modell., 1996, 14, 33–38 CrossRef CAS PubMed
.
- Y. Fu, L. Liu, H. Yu, Y. Wang and Q. Guo, Quantum-Chemical Predictions of Absolute Standard Redox Potentials of Diverse Organic Molecules and Free Radicals in Acetonitrile, J. Am. Chem. Soc., 2005, 127, 7227–7234 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Change in spin density during B–B hemolysis; the presence of two MeO− and 4-PhPy promotes the cleavage of B–B bonds in B2cat2; the SET process between 2D/INT-AB and alkyl bromide or nucleophilic substitution; the photoinduced SET process following the SN2 reaction of INT-A with a countercation; the comparison of B2cat2/B2pin2 trapping R˙; the calculations of excitation; the computational details of oxidation potentials; the computational details of the activation barrier of the SET step; estimation of the activation barriers for SET steps; calculated energies and energy corrections; Cartesian coordinates of all calculated compounds. See DOI: https://doi.org/10.1039/d5qo00048c |
| This journal is © the Partner Organisations 2025 |