An advanced plasmonic bimetallic nanostar composite for ultra-sensitive SERS detection of crystal violet†
Sintayehu Leshe
Kitaw
 ad,
Yohannis Wondosen
Ahmed
a,
Andy
Candra
a,
Tsung-Yun
Wu
a,
Beyadgalem Endawoke
Anley
a,
Ying-Yu
Chen
a,
Yu-Ting
Cheng
a,
Kuan-Ju
Chen
a,
Chayaporn
Thammaniphit
a,
Chen Chu
Hsu
a,
Yi Ting
Wu
a,
Mahvash Hira
Khan
a and
Hsieh-Chih
Tsai
ad,
Yohannis Wondosen
Ahmed
a,
Andy
Candra
a,
Tsung-Yun
Wu
a,
Beyadgalem Endawoke
Anley
a,
Ying-Yu
Chen
a,
Yu-Ting
Cheng
a,
Kuan-Ju
Chen
a,
Chayaporn
Thammaniphit
a,
Chen Chu
Hsu
a,
Yi Ting
Wu
a,
Mahvash Hira
Khan
a and
Hsieh-Chih
Tsai
 *abc
*abc
aGraduate Institute of Applied Science and Technology, National Taiwan University of Science and Technology, Taipei, 10607, Taiwan, Republic of China. E-mail: h.c.tsai@mail.ntust.edu.tw
bAdvanced Membrane Materials Center, National Taiwan University of Science and Technology, Taipei, 10607, Taiwan, Republic of China
cR&D Center for Membrane Technology, Chung Yuan Christian University, Chungli, Taoyuan, 320, Taiwan, Republic of China
dDepartment of Chemistry, College of Natural and Computational Sciences, Debremarkos University, Ethiopia
First published on 21st November 2024
Abstract
The controlled synthesis of Ag/Au nanocomposite particles has remained a significant challenge in nanomaterial research. This study presents the synthesis, characterization, and surface-enhanced Raman scattering (SERS) performance of silver (Ag) and gold (Au) nanostar composites. The structural and plasmonic properties of these nanocomposites were optimized by varying the molar ratios of silver nanostars (AgNSs) and gold nanostars (AuNSs). By synthesizing composite nanostars with differing AgNS/AuNS ratios, we systematically compared their optical and spectroscopic behaviors. The results demonstrated that Ag/Au nanostar composites function as highly effective SERS substrates for the detection of rhodamine 6G (R6G), with solutions tested at concentrations from 10−15 to 10−6 M. Compared to individual AgNS or AuNS substrates, the Ag/Au nanocomposites exhibited significantly enhanced SERS signals, with superior consistency and sensitivity. Notably, the nanostar composite with a 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 Ag/Au ratio showed the highest SERS performance, achieving an enhancement factor of 8.9 × 106 and a detection limit of 10−15 M for R6G. Additionally, this composite demonstrated excellent long-term stability, maintaining performance until ten weeks of storage. To our knowledge, this represents the highest sensitivity reported for R6G detection using label-free SERS. The study further provides a detailed analysis of the composition-dependent SERS activity, underscoring the potential of Ag/Au nanocomposites as advanced SERS substrates for applications in chemical and biological sensing, as well as environmental monitoring.
25 Ag/Au ratio showed the highest SERS performance, achieving an enhancement factor of 8.9 × 106 and a detection limit of 10−15 M for R6G. Additionally, this composite demonstrated excellent long-term stability, maintaining performance until ten weeks of storage. To our knowledge, this represents the highest sensitivity reported for R6G detection using label-free SERS. The study further provides a detailed analysis of the composition-dependent SERS activity, underscoring the potential of Ag/Au nanocomposites as advanced SERS substrates for applications in chemical and biological sensing, as well as environmental monitoring.
1. Introduction
While all materials possessing conducting electrons have the potential to generate plasmons, only a select few remain stable as nanostructures and exhibit plasmon resonances of substantial intensity when irradiated with light.1,2 The majority of metals such as sodium, potassium, or aluminum have a tendency to oxidize or degrade under normal conditions. Conversely, almost all other elements are characterized by limited plasmonic behavior, quantifiable by the plasmonic quality factor at specific optical frequencies.3 This drawback has confined much of the plasmonic research to nanostructures composed of gold,4 silver,5 and to a lesser extent copper,6 thereby dominating the wide array of magnetic, chemical, optical, and other properties. This challenge is frequently cited due to the demand for more cost-effective alternative nanomaterials possessing plasmonic, catalytic and magnetic properties. The simultaneous integration of these attributes within a single nanoparticle makes such nanosystems highly promising for numerous cutting-edge research pursuits. In certain instances, nanocomposites give rise to novel magnetic, optical and electrical phenomena that remain unexplored, influenced by the plasmon characteristics. It enables modifications of undesirable characteristics associated with plasmonic materials, such as price, environmental oxidation, and stability.7Plasmonic bimetallic nanocomposite fabrication has emerged as a strategic approach to reintroduce diverse properties of bulk metallic elements into nanostructures while maintaining a notable plasmonic response.8,9 It is widely recognized that combining two or more metallic nanomaterials yields new materials characterized by properties that surpass those of their monometallic counterparts. Effectively, this methodology paves the way for multifunctional plasmonic entities with considerable appeal for various applications, offering the opportunity to tailor the nanoconfinement of the metals to enhance and optimize specific plasmonic effects.10
This architype extends beyond a nanomaterial's “bulk” properties and encompasses surface properties, particularly the surface characteristics of nanocomposites, undergoing significant modification when compared with single-element nanoparticles. We must also consider the percentage or molar composition of each metal achievable to grasp the potential afforded by nanocompositing a plasmonic metal with other plasmonic or non-plasmonic materials. Moreover, factors such as size distribution, particle size, morphology, and chemical environment must be considered in advance.11,12
1.1. Current trends and future challenges in nanocomposite synthesis
Over the past few decades, advancements in fabrication methods for nanomaterials have eased the exploration of new plasmonic composites. The traditional wet-chemistry methods have offered superior control over nanomaterial composition and morphology but are restricted to the nature of metal precursors soluble in liquid and gaseous phases at thermodynamic equilibrium. Consequently, one of the current and future challenges in nanoscience spins around creating metastable phase composites, necessitating more demanding fabrication conditions that deviate from equilibrium. These conditions can alleviate issues related to the nature of the solvent and the varying reactivity of the component metals within a nanocomposite.12,13 Hence, physicochemical approaches have become prevalent in the top-down and bottom-up methods of plasmonic nanocomposite preparation, as depicted in recent literature.14 The reaction conditions, the precursor type, and the dispersion mode of the final nanocomposites are generally used to distinguish between different synthetic approaches for nanocomposites. Even though several fabrication procedures for plasmonic metal nanocomposites are available in the literature such as amalgamation seeded growth, ultrasonication, supercritical reactive metallization, hydrothermal reduction, carbothermal shocking, photoreduction, and ion implantation,15–19 the most common method for creating plasmonic metal nanoparticles is chemical reduction of the corresponding metal salts dissolved in water, as in the preparation of colloidal gold nanostar particles, where citrate is used as a reducing and stabilizing agent.20 An alternate synthesis approach was also given by Martin et al.21 using regulated relative compositions of NaOH, NaBH4, HauCl4, and HCl to stabilize physisorbed boron-based anions and control nanoparticle sizes ranging from 3.1 to 5.2 nm. By employing sodium citrate as the reducing agent, this method was also used to create monodisperse nanoalloys. When a stabilizing agent wasn't used in combination with a stronger reducing agent, such as sodium borohydride, chemical reduction, and seed formation became rapid and concurrent, leading to the formation of inhomogeneous nanoparticles via Ostwald ripening and coalescence.22 Depending on the synthesis conditions, the final morphology of bimetallic plasmon nanoparticles varies. The creation of uniform bimetallic plasmonic nanoalloys characterized by a distinct shape, restricted size distribution, adjustable composition, and nanostructure necessitates the meticulous choice of reaction conditions that provide uniformity of the metal lattice formation nucleation and growth phases.231.2. Applications of silver–gold nanocomposite particles in SERS
The application of colloidal Au and Ag nanoparticles in surface-enhanced Raman scattering has been extensively documented. However, the search for surface active SERS substrates has persisted for many years and is still a subject of considerable research. To create tunable, sensitive, and repeatable SERS substrates based on immobilizing plasmonic nanoparticles on solid surfaces through the use of lithographic or template techniques, a variety of strategies have been used.24,25 Consequently, the Au/Ag system has attracted special attention since it allows metallic nanosubstrates with varying degrees of SERS performances to be prepared by adjusting the size/shape-dependent optical absorption of nanoparticles. Using various percentage compositions of Au/Ag in the range of 0/100 to 12/88, 52/48, 81/19, and 100/0, Park et al.26 fabricated an Au/Ag nanocomposite substrate on cellulose materials for SERS biosensing.In contrast to monometallic Ag or Au nanoparticles, the Au/Ag nanocomposite particles were chosen to better complement the 532 nm laser excitation,27 highlighting the significance of composition for best SERS performance. In this context, the majority of documented works, however, concentrated on Au/Ag spherical nanoparticle morphologies, core–shell structures, nanofilm depositions, and surface modifiers whose fabrication necessitates controlling thickness and ensuring shell homogeneity28,29 which limited the availability of conventional and homogeneous Au/Ag nanocomposites.30
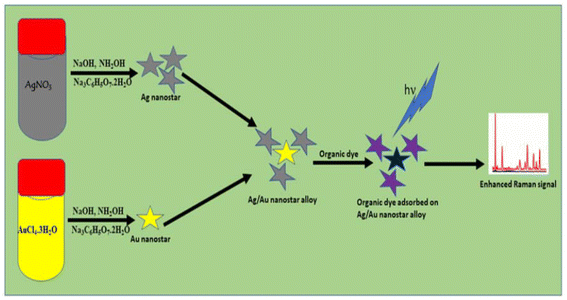
This research is aimed at developing a composite material consisting of noble metal nanostar particles for a highly sensitive SERS sensor that can accurately detect crystal violet molecules. In this research, an effective method to fabricate surfactant free Ag and Au homogeneous bimetallic nano-stars in an aqueous solution was presented, which had not been done before. Unlike other investigations, we avoided the use of surface modifying polymers, heating, lasing, electrolyzing, annealing, dewetting, sputtering, and pyrolysis saving time and energy. Importantly, we have discovered that using plasmonic metal nanocomposite substrates yields higher SERS analytical enhancement factors for R6G. Furthermore, we demonstrate the practical application of this method for quantitative and qualitative analysis of crystal violet in real-world scenarios.
2. Materials and methods
2.1. Materials
Chloroauric acid with a purity of 99.99%, silver nitrate with a purity of 99.92%, trisodium citrate with a purity of 99%, hydroxylamine, rhodamine 6G (C28H31ClN2O3), sodium hydroxide, and crystal violet (C25H30N3Cl) were obtained from Merck (Germany). All chemicals used were of analytical grade and were used without any further processing. De-ionized water was used for all solution preparations and cleaning of glassware.2.2. Preparation of nanostar colloids
Ag, Au, and Ag/Au bimetallic nanostar colloids were prepared using hydroxylamine (NH2OH) and trisodium citrate dihydrate (Na3C6H5O7·2H2O) sequential reduction, following a detailed process outlined by our team.31 To summarize, 9 mL of 1 mM AgNO3 and 9 mL of 1 mM HAuCl4·3H2O were separately added dropwise with vigorous swirling into two beakers, each containing a mixture of 1 mL of NaOH and NH2OH, to synthesize Au and Ag nanostars. After 15 minutes of magnetic stirring, 0.1 mL of 0.34 mM Na3C6H5O7·2H2O was added to both beakers.2.3. Characterization of the nanostar particles
A Jasco V-770 UV-VIS instrument with a resolution of 2 nm was used to record SPR absorption spectra of the as-synthesized nanostar colloids in the wavelength range of 300–800 nm. To measure the average particle size distributions, zeta potential, and polydispersity indexes of Ag, Au, and Ag/Au composites, we used a dynamic light scattering apparatus (HORIBA Scientific, Kyoto, Japan). A JEOL JSM-6500F scanning electron microscope (SEM) coupled with an EDS detector, operating at an accelerating voltage of 9 kV, was employed to obtain comprehensive information on the morphology of the nanostructures and elemental composition through energy-dispersive X-ray spectroscopy. The morphology and size of the nanoparticles were further measured using a transmission electron microscope (TEM, Philips CM 300) at 300 kV accelerating voltage.With a high degree of spatial resolution and accuracy, energy dispersive X-ray spectroscopy32 hyphenated within a scanning electron microscope (SEM) offered elemental analysis that produced elemental maps with atomic resolution. The method involves incidentally measuring the energy of X-rays that are released from a tiny sample using a highly concentrated electron beam in order to identify the atomic species of the nanocomposite inside the beam path. The samples for SEM and EDS investigation were prepared by drip-coating Ag, Au, and Ag/Au nanocomposite colloid roughened silica wafers. The samples were allowed to dry in air at room temperature prior to the analysis.
2.4. SERS spectroscopic measurements
A JASCO NRS-5100 Raman instrument was employed to obtain the SERS spectra within the 550–1800 cm−1 spectral region. The resonant Raman effect of R6G and CV was effectively supported by the 532 nm excitation line.3. Results and discussion
3.1. Plasmonic resonance characteristics
Because of their great miscibility and strong surface plasmon resonance (SPR) effects in the visible light spectrum, AgNS and AuNS were selected as plasmonic components for the fabrication of the composition-adjustable Ag/Au nanostar composite. NH2OH and HOC(COONa)(CH2COONa)2·2H2O were used to reduce the corresponding metal salts in water to create nanostar colloids of Au, Ag, and their nanostar composite equivalent (Ag/Au). Fig. 1 shows the synthesis processes for AgNS, AuNS, and the composition-adjustable Ag/Au nanostar composite.Ag and Au nanoparticles have readily measurable UV-VIS spectral and localized surface plasmonic characteristics indicative of their nanostructure, composition, surface chemistry, and aggregation state. These characteristics can be used to identify nanoparticle growth processes and dynamics. Understanding how processes like colloid formation, aggregation, oxidation, and surface adsorption affect these optical signals is crucial since they are widely used in plasmonic nanoparticle characterization in environmental detection and chemical sensing. To describe the growth of nanoparticle composites in colloidal solutions, an effective method is to analyze the UV-vis spectra, paying particular attention to the absorbance curve, LSPR peak positions and intensity.
Characteristic UV-VIS absorptions of Ag, Au and Ag/Au caused by surface plasmon vibration excitations are presented in Fig. 2A. Differential double plasmon absorption bands (∼368 & 600 nm) originating from separate Ag and Au nanostar colloids were observed. In this experiment, we varied the molar ratio of AgNS and AuNS colloids to control the size distribution, morphology, and plasmon resonance. As shown in Fig. 2A, tests were conducted on Ag/Au nanostar composites with AgNS/AuNS molar ratios of 100/0, 87.5/12.5, 75/25, 60/40, 50/50, 25/75, 12.5/87.5, and 0/100% in order to optimize uniformity of size distribution and surface plasmon resonances. The 75/25 AgNS/AuNS composite exhibited a distinct single band SPR absorption at 435 nm, which is typical of nanocomposite particles. The optical absorption of the bimetallic AgNS/AuNS colloids ranged between the absorption maxima (λmax) values of the monometallic colloids, approximately 368 nm for pure AgNSs and 600 nm for pure AuNSs, which aligns well with the findings of a previous publication.33 This composition was used to investigate the SERS activities of the nanostar composite and to characterize the Ag/Au nanostar composites.
Fig. 2B shows the linear calibration curve of the absorbance intensity of the 368 nm peak intensity versus the Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au percentage in the AgNS
Au percentage in the AgNS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AuNS composites. It is observed that the absorbance increased as the amount of AgNSs in the nanocomposite increased and almost completely vanished for the pure AuNSs sample. This increase can be explained by the prevention of aggregation between gold and silver nanoparticles.34 In contrast, the pure AgNSs sample did not show an adsorption peak at 600 nm. This peak is only present in nanostar composite samples and becomes stronger as the concentration of AuNSs in the colloidal solution increases. The 600 nm peak is generally weaker than the 368 nm peak, indicating that the optical characteristic of silver is more pronounced than that of gold. Other concurrent changes include the sharpening of the SPR peak, the shift of the plasmon peak towards shorter wavelengths, and the color change of the colloidal solution. For the purpose of comparison and clarity, the color variations and the corresponding UV-VIS spectra of AgNSs, AuNSs and AgNS/AuNS (75
AuNS composites. It is observed that the absorbance increased as the amount of AgNSs in the nanocomposite increased and almost completely vanished for the pure AuNSs sample. This increase can be explained by the prevention of aggregation between gold and silver nanoparticles.34 In contrast, the pure AgNSs sample did not show an adsorption peak at 600 nm. This peak is only present in nanostar composite samples and becomes stronger as the concentration of AuNSs in the colloidal solution increases. The 600 nm peak is generally weaker than the 368 nm peak, indicating that the optical characteristic of silver is more pronounced than that of gold. Other concurrent changes include the sharpening of the SPR peak, the shift of the plasmon peak towards shorter wavelengths, and the color change of the colloidal solution. For the purpose of comparison and clarity, the color variations and the corresponding UV-VIS spectra of AgNSs, AuNSs and AgNS/AuNS (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) composites are shown in Fig. 2C.
25) composites are shown in Fig. 2C.
There is currently a lot of interest in SERS for the development of nanoscale substrates and systems for environmental detection. The fundamental justification for this is that nanoparticles possess structural and functional characteristics that are unavailable from bulk materials or single molecules. As one of the primary factors influencing their uniformity and stability in colloidal systems, particle size is the most fundamental information available about the wide range of nanoparticle types. Fig. 2D demonstrates that the hydrodynamic sizes for the AgNS, AuNS and AgNS/AuNS particles are in a comparatively closer range that allow proper mixing.
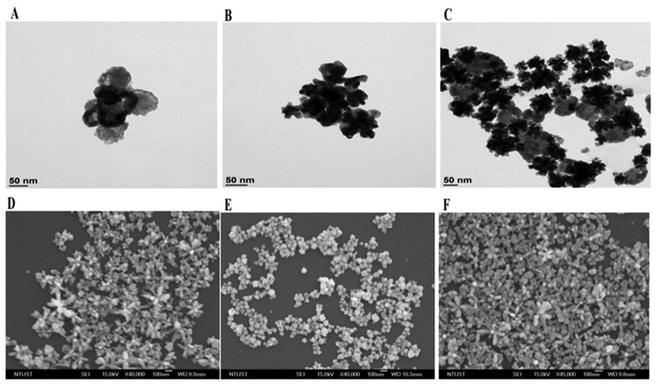
3.2. TEM, SEM, and DLS measurements
Fig. 3A–F display typical TEM and SEM images of AgNS, AuNS, and Ag/Au NS. Exclusively monodisperse nanostar particles with diameters ranging from 20 to 40 nm were observed. The average zeta potential (ζ) and hydrodynamic size (z-average) were determined through Dynamic Light Scattering (DLS) experiments, and the results are presented in Fig. 2D and Table 1. Morphologies of resulting Ag/Au nanostar composites were thoroughly examined using TEM and SEM images shown in Fig. 3C and F. The images displayed numerous evenly distributed Ag/Au nanoscale particles (NSs) with a narrow size distribution ranging from 40 to 60 nm and a consistent shape.| Sample | SPR, nm | Z-average, nm | Zeta potential, mV |
|---|---|---|---|
| Ag | 368 | 61.9 | −54.7 |
| Au | 600 | 81.7 | −38.8 |
| Ag/Au | 435 | 74.3 | −44.1 |
TEM, SEM, hydrodynamic size and polydispersity index results all support the uniformity of particle size distributions in the nanostar colloid. Citrate ions adsorbed at nanostar surfaces led to the formation of anionic surfaces. These species resulted in highly negative zeta potential values (Table 1) and provided colloidal stability through interparticle electrostatic repulsion.34 The zeta (ζ) potential, which represents surface charge, has a significant impact on how nanoparticles interact with their surroundings. It can be utilized to forecast the stability of nanoparticle dispersions over an extended period of time as well as to investigate the properties of their surfaces and associated adsorption effects.
SEM scan (Fig. 4A) and SEM elemental mapping (Fig. 4C) were used to indicate the elemental distribution of the Ag/Au nanocomposite in two dimensions. As shown in Fig. 4C, the surface segregation of both elements is clearly identified demonstrating the utility of the SEM mapping technique in helping to explain the two-dimensional distribution of the component elements in the multi-nanoparticle system. The high-intensity signals around 2, 8, 10, and 11 keV in the EDS spectrum (Fig. 4B) confirm the presence of Au nanoparticles.
 | ||
| Fig. 4 SEM image (A), EDS spectrum of Au and Ag (B) and the separated SEM elemental mappings of Ag and Au (C). | ||
3.3. Reproducibility and stability of the nanocomposite SERS substrate
The homogeneity of the hotspots may have contributed to the excellent spot-to-spot repeatability. Despite the coffee ring effect,35 which is the result of the probe molecules being introduced into a substrate such that the nanoparticles in the colloid were preferentially collected at the edge at higher concentrations than at the middle, the minuscule droplets may prove to be highly efficient and practical in producing even dispersions of the Ag/Au NSs/R6G blend onto the support.Based on the data observed, it can be concluded that the Ag/Au (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) nanostar composite showed a very low RSD value and a very sensitive and repeatable SERS response. Furthermore, a good chemosensor should have outstanding chemical stability in addition to its sensitivity.
25) nanostar composite showed a very low RSD value and a very sensitive and repeatable SERS response. Furthermore, a good chemosensor should have outstanding chemical stability in addition to its sensitivity.
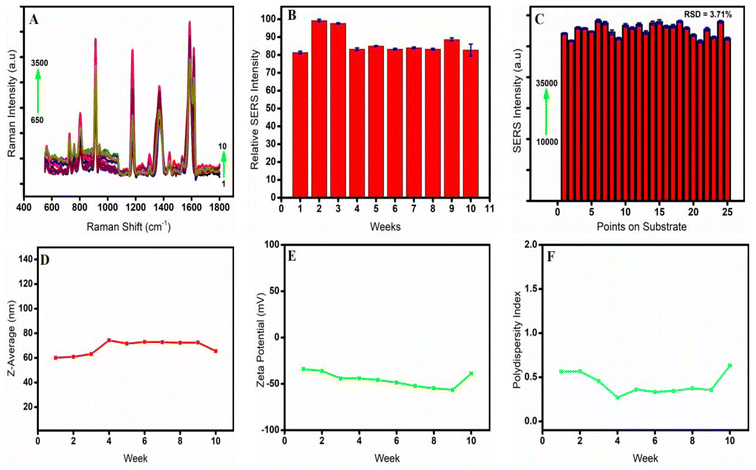
The SERS spectra showed that these bimetallic plasmonic colloids were stable, with no appreciable variations in the peak intensity and position. Using the Ag/Au nanostar composite that had been stored for ten weeks, SERS experiments were conducted to record the SERS spectra of CV for investigation of the chemical stability of the Ag/Au nanostar substrate (Fig. 5A). When assessing the viability of SERS substrates, one important factor to consider is the uniformity of the SERS signals.36 As demonstrated in Fig. 5C, 25 SERS spectra were recorded at random spots on the substrate using 10−6 M R6G to further demonstrate the repeatability of the Ag/Au (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) nanostar SERS substrate. There are no discernible changes in the substrate's repeatability, as seen in the SERS intensity histogram in Fig. 5C. Reproducible and accurate results were revealed by RSD 3.71%, which was computed from the SERS signal intensity at the notable peak of 614 cm−1 from various places. The hydrodynamic size, zeta potential, and polydispersity index did not significantly vary in the DLS tests too (Fig. 5D–F). From weeks 4 to 9, the Ag/Au nanostar composite had exceptional SERS stability, as evidenced by consistently uniform values of SERS intensity (Fig. 5A), SERS relative intensity (Fig. 5B), z-average (Fig. 5D), zeta potential (Fig. 5E), and polydispersity index (Fig. 5F).
25) nanostar SERS substrate. There are no discernible changes in the substrate's repeatability, as seen in the SERS intensity histogram in Fig. 5C. Reproducible and accurate results were revealed by RSD 3.71%, which was computed from the SERS signal intensity at the notable peak of 614 cm−1 from various places. The hydrodynamic size, zeta potential, and polydispersity index did not significantly vary in the DLS tests too (Fig. 5D–F). From weeks 4 to 9, the Ag/Au nanostar composite had exceptional SERS stability, as evidenced by consistently uniform values of SERS intensity (Fig. 5A), SERS relative intensity (Fig. 5B), z-average (Fig. 5D), zeta potential (Fig. 5E), and polydispersity index (Fig. 5F).
From week 1 to weeks 2 and 3, there was an almost 20% rise in the SERS spectra. This could be explained by the development of mature nanostar composite seeds. A stable Ag/Au nanostar composite at a fixed composition was shown by the spectra, which then decreased to 85% and remained consistent until week 10.
3.4. SERS performance of the nanostar composite
To assess the enhancement capacity of different samples, we selected R6G as the probe molecule in the SERS experiment. Very dilute solutions of R6G (10−15–10−3 M) were prepared by serial dilution of standard R6G in distilled water to be employed for the SERS experiment using a green 532 nm laser excitation source. The synthesized nanosubstrates were completely air dried before performing SERS spectra measurements, as depicted in Fig. 6A and B, which illustrate the Raman spectrum of different R6G concentrations displaying distinct characteristic signals within the range of 550 to 1800 cm−1.The SERS spectra showed a prominent peak at 614 cm−1, which was attributed to the in-plane bending vibration mode of the xanthene ring. The remaining peaks at Raman shifts of approximately 773, 1185, 1310, 1363, 1509, 1575, and 1649 cm−1 can be explained as follows: the bending mode of SP3 C–H (out-of-plane, xanthene ring); bending of SP2 C–H (in-plane, aromatic); the stretching vibration mode of C–C (in-plane) in the xanthene ring; the symmetric stretching vibration mode of C–C (aromatic); the stretching vibration mode of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (aromatic); and the stretching vibrational mode of the C
C (aromatic); and the stretching vibrational mode of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the xanthene ring structure, respectively.37
O group in the xanthene ring structure, respectively.37
When R6G was coated on Ag/Au NSs, the SERS molecular characteristic signals of target analytes were significantly stronger compared to those of Ag or Au NSs. Fig. 6B shows SERS spectra collected from 10−6 M R6G solution adsorbed on Ag, Au, and Ag/Au nanostar composite substrates. It is clear that in the absence of noise and background interferences, the SERS spectra displayed distinct and highly resolved Raman spectral peaks.
Nanocomposites of Ag and Au can prevent the individual defects of gold and silver nanostructures, and a great deal of study has been done to rationally design a bimetallic Ag–Au nanostructure in various forms. Compared to silver nanostructures, gold nanoparticles exhibit substantially lower LSPR properties despite having higher stability and monodispersity.38 Unfortunately, the weak oxidation resistance and easy aggregation of Ag with significant plasmonic characteristics limit its practical value. Thus, combining the exceptional plasmonic characteristics of silver with the high stability of gold in a single nanostructure is critical. Ag–Au nanostructures’ strong optical response and chemical stability as well as the location and bandwidth of SPR peaks were found to be significantly dependent on the precise ratios of the metals in the nanocomposite structure.39
As shown in the TEM and SEM images (Fig. 3C and F, respectively), the Ag/Au nanostar colloid resulted in a larger number of short armed plasmonic hotspots, which are the crucial reason for improving the SERS signals. This could explain why Ag/Au NS with a composition ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 exhibited the strongest SERS signals. Apart from electromagnetic enhancement, the SERS activity is further boosted by chemical enhancement resulting from charge transfer between probe molecules and the substrate. In the case of the plasmonic bimetallic nanostar composite and probe R6G molecules, the charge-transfer process is the source of the chemical (CM) enhancement.40
25 exhibited the strongest SERS signals. Apart from electromagnetic enhancement, the SERS activity is further boosted by chemical enhancement resulting from charge transfer between probe molecules and the substrate. In the case of the plasmonic bimetallic nanostar composite and probe R6G molecules, the charge-transfer process is the source of the chemical (CM) enhancement.40
A 532 nm green laser stimulation can effectively excite Ag or Au nanostars, resulting in an enhanced charge transfer effect that increases the intensity of surface-enhanced Raman scattering.41 To determine the analytical SERS enhancement factor (AEF) of Ag/Au nanostar composites, the signals obtained from the substrate with and without Ag nanostars, Au nanostars, or Ag/Au nanostar composites were compared. Calculation of the analytical SERS enhancement factor is based on the following equation:
 | (1) |
The SERS intensity of R6G on the metallic nanostructure substrate is denoted as ISERS. The R6G concentration on substrates exposed to the laser spot-focused area is denoted as CSERS. The R6G concentration that yields the Raman signal (IRaman) acquired under the same conditions is represented by CRaman = 0.1 M.
Seven spectral features that were used to determine the AEF values of different concentrations of R6G are presented in Table S1 (ESI†). These features are located at 614, 773, 1182, 1310, 1363, 1509, 1573, and 1649 cm−1. Among these features, the peak 614 cm−1 is the most typical, as shown by the spectra. Its EF is higher than those of the other peaks, and its intensity is highly sensitive to changes in the R6G concentration. Therefore, it was selected as the ideal peak for the calculation of EF values and the quantitative analysis of R6G.
The computed SERS enhancement factors for the Ag nanostar substrate and the Au nanostar substrate were 4.1 × 106 and 3.96 × 106, respectively, with very slight variations. However, the best Raman activity was observed in the Ag/Au nanostar composite-based substrate (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) with an analytical enhancement factor of 8.9 × 106, surpassing both AgNS and AuNS substrates. This is attributed to the formation of numerous short-armed stable nanostructure composites creating the smallest distance between the nanostar plasmons and analytes caused by the aggregation effect between Ag and Au nanostar composites. As a result, the nanostar composites create high-density homogeneous and heterogeneous hotspots, which greatly enhance the SERS effect. The fact that the 75/25 Ag/Au ratio provided a better SERS substrate could be attributed to the reduction of potential energy. As a matter of fact, 3 moles of Ag+ get reduced when 1 mole of Au3+ is reduced per unit of time. This is the point where a stable uniform Ag/Au nanostar composite is created. Fig. 6A displays the SERS spectra of R6G on the Ag/Au (75
25) with an analytical enhancement factor of 8.9 × 106, surpassing both AgNS and AuNS substrates. This is attributed to the formation of numerous short-armed stable nanostructure composites creating the smallest distance between the nanostar plasmons and analytes caused by the aggregation effect between Ag and Au nanostar composites. As a result, the nanostar composites create high-density homogeneous and heterogeneous hotspots, which greatly enhance the SERS effect. The fact that the 75/25 Ag/Au ratio provided a better SERS substrate could be attributed to the reduction of potential energy. As a matter of fact, 3 moles of Ag+ get reduced when 1 mole of Au3+ is reduced per unit of time. This is the point where a stable uniform Ag/Au nanostar composite is created. Fig. 6A displays the SERS spectra of R6G on the Ag/Au (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) nanostar composite across concentrations between 10−15 and 10−3 M. At lower concentrations, the SERS signal is relatively weak. However, as more molecules are adsorbed on the substrate with increasing R6G concentration, a higher Raman signal is observed. Notably, the signal intensity at 10−3 M R6G solution is practically strong (∼18
25) nanostar composite across concentrations between 10−15 and 10−3 M. At lower concentrations, the SERS signal is relatively weak. However, as more molecules are adsorbed on the substrate with increasing R6G concentration, a higher Raman signal is observed. Notably, the signal intensity at 10−3 M R6G solution is practically strong (∼18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 a.u.) at 614 cm−1.
000 a.u.) at 614 cm−1.
Even at a very low R6G concentration (10−15 M), the SERS signal intensity remains relatively robust (∼7700 a.u.), highlighting the substrate's superior amplification capacity. The Ag/Au nanostar composite substrate exhibits a low limit of detection42 at the femtomolar level (10−15 M) as demonstrated by the characteristic signals of R6G molecules, which remain visible even at such low concentrations. This LOD value is significantly lower than the previously reported value (5 × 10−9 M) for detection of rhodamine B using core–shell Au@Ag nanoparticles as the SERS substrate.43 This showed that Ag/Ag composite NSs have a great deal of potential for use as a standard SERS substrate to detect organic dye toxins in wastewater.
3.5. Detection of CV using the optimized Ag/Au SERS substrate
The Ag/Au nanostar composite achieved the practical requirements for the sensitive detection of CV in the aqueous environment and functions as a dependable chemo-sensor in an oxidative environment due to its exceptional oxidation resistance and chemical stability. The Ag/Au nanostar substrate's exceptional sensitivity allowed it to be utilized as a chemo-sensor to detect CV at ultra-trace levels. CV is a triphenylmethane organic dye used mainly as a biological staining agent in veterinary and drug applications. It is also useful in the textile industry and acts as a deep violet colorant in the manufacture of paints and printing ink. It is also employed as an antibiotic to retard the development of fungal molds in chicken feed and as a mutagenic as well as bacteriostatic agent in medical treatments. Despite this wide range of applications, CV is considered as a stubborn organic dye molecule that has harmful effects and lingers in the environment for a long time. In certain fish species, it functions as a clastogenic, strong carcinogen, and mitotic toxin, intensifying the growth of tumors.27It is therefore essential to create a simple ultra-trace detection technique for this dye residue in an aqueous environment. The SERS spectra of CV using the Ag/Au nanostar are displayed in Fig. 6C, where the Ag/Au nanocomposite substrate allowed for the recording of well-resolved CV spectra with a significantly higher signal-to-noise ratio. The characteristic signals of CV molecules are located at 810, 913, 1180, 1363, 1585, and 1621 cm−1. The 1621 cm−1 SERS signal split-up at lower concentrations most probably due to the simultaneous occurrence of symmetric and asymmetric SP3 C–C stretching modes where the symmetric stretch vanished at higher concentrations. The accompanying modes of vibrations for each peak and their bond representations44,45 are available in Table 2.
| Peak position, cm−1 | Peak assignment | Vibration mode |
|---|---|---|
| 807 | Aromatic ring | Deformation |
| 913 | C–H, aromatic | Bending, out-of-plane |
| 1178 | C–H, aromatic | Bending, in-plane |
| 1374 | SP3 C–H | Bending |
| 1585 | Aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
Stretching |
| 1620 | SP3 C–C | Stretching |
The intensity of the notable 1588 cm−1 spectral characteristic was monitored against the analyte concentration to develop a linear calibration. Mathematically, the resulting fitted curve is expressed as follows:
| I = 128.3C − 192 | (2) |
In this case, ‘I’ stands for the 1588 cm−1 peak's SERS intensity and ‘C’ for the CV concentration, which ranges from 2.5 to 20 μM. The calibration showed good accuracy as discerned by its coefficient of determination (R2), 0.987, showing a strong linear correlation. Remarkably, the characteristic spectral peaks of CV molecules were discernible even at extremely low concentrations of 10−12 M (Fig. 6D).
3.6. Comparison of the method with other SERS techniques for detection of CV
A significant number of research articles were investigated to compare the feasibility and workability of our procedures and findings. In our procedures, we followed simple nanosubstrate synthesis steps and omitted the use of surface modifiers, aggregating agents, electrolysis and heating. As can be seen in Table 3, the sensitivity (demonstrated by the analytical enhancement factor and limit of detection) of the optimized procedure for the detection of CV is superior to other SERS techniques.| No | SERS substrate | Surfactant | AEF | LOD | Ref. |
|---|---|---|---|---|---|
| 1 | Au colloid | None | — | 10−6 M | 46 |
| 2 | Ag nanostar | PVP | — | 10−9 M | 42 |
| 3 | AgNP colloids | None | 1.3 × 108 | 10−15M | 47 |
| 4 | e-AgNP | MoS2 | 2.9 × 106 | 10−9 M | 48 |
| 5 | AgNP network | None | 6.2 × 105 | 10−12 M. | 49 |
| 6 | AgNPs/SiO2 | PVA | 10−8 M | 50 | |
| 7 | HAp/Ag nanocomposite | None | — | 10−5 M | 51 |
| 8 | AgNP | PVA hydrogel | — | 10−12 M | 52 |
| 9 | Gold nanoparticle superlattices | N-Acetyl glutathione | 3.6 × 105 | 10−8 M | 53 |
| 10 | Gold nanorods on tapered optical fiber | None | — | 10−10 M | 54 |
| 11 | Ag/Au nanostar composite | None | 8.9 × 106 | 10−12 M | This study |
4. Conclusion
Overall, we demonstrated that Ag/Au nanostar composites with tunable compositions and sizes can be developed using a straightforward NH2OH/Na3C6H7O5·2H2O reduction procedure. This method proved to be effective and efficient, allowing for the adjustment of the Ag/Au percentage ratio in the nanostar composite. As the AgNS/AuNS ratio decreased, the intensity of the Ag/Au nanostar composite at λmax red-shifted, correlating with changes in the particle size. The drip-coated Ag/Au composite nanostar colloid remained stable and uniform, making it well-suited for use as a SERS substrate possessing superior signal reproducibility and improved SERS performance. The composite nanostars were drip-coated on a silicon wafer in a uniformly aggregated nanostructure to maximize signal enhancements. The 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 Ag/Au nanostar percentage composition demonstrated excellent SERS performances. This optimized nanosubstrate is highly sensitive to detect R6G and CV molecules. SERS measurements of R6G probe solution indicated that the Ag/Au nanocomposite improved SERS performance, with an LOD of 10−15 M R6G and an EF exceeding 1013. We achieved excellent SERS reproducibility with the RSD below 3.71% at many spots on the substrate. Because of these attractive features, 75
25 Ag/Au nanostar percentage composition demonstrated excellent SERS performances. This optimized nanosubstrate is highly sensitive to detect R6G and CV molecules. SERS measurements of R6G probe solution indicated that the Ag/Au nanocomposite improved SERS performance, with an LOD of 10−15 M R6G and an EF exceeding 1013. We achieved excellent SERS reproducibility with the RSD below 3.71% at many spots on the substrate. Because of these attractive features, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 Ag/Au composite nanostars are a promising alternative for SERS applications in environmental and food quality control, chemical sensing, and the fast detection of CV in wastewater.
25 Ag/Au composite nanostars are a promising alternative for SERS applications in environmental and food quality control, chemical sensing, and the fast detection of CV in wastewater.
Author contributions
Sintayehu Leshe Kitaw: conceptualization, investigation, visualization, and manuscript writing – original draft. Yohannis Wondosen Ahmed: investigation and data curation. Andy Candra: data curation. Yu-Ting Cheng: resources. Ying-Yu Chen: resources. Beyadgalem Endawoke Anley: methodology. Tsung-Yun Wu: methodology. Kuan-Ju Chen: methodology. Chayaporn Thammaniphit: methodology. Chen Chu Hsu: resources. Yi Ting Wu: resources. Mahvash Hira Khan: methodology. Hsieh-Chih Tsai: conceptualization, supervision, project administration, funding acquisition, and manuscript writing – review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Ministry of Science and Technology, Taiwan (MOST 109-2221-E-011-146-MY3, 108-2221-E-011-110-MY3 and 108-2923-E-011-005-MY3) for financial support.References
- Y. Gutiérrez, et al., Nanoplasmonic photothermal heating and near-field enhancements: a comparative survey of 19 metals, J. Phys. Chem. C, 2020, 124(13), 7386–7395 CrossRef.
- D. Ray, et al., A Low–Temperature Annealing Method for Alloy Nanostructures and Metasurfaces: Unlocking a Novel Degree of Freedom, Adv. Mater., 2022, 34(17), 2108225 CrossRef CAS.
- B. Doiron, et al., Quantifying figures of merit for localized surface plasmon resonance applications: a materials survey, ACS Photonics, 2019, 6(2), 240–259 CrossRef CAS.
- A. Candra, et al., A green method for fabrication of a biocompatible gold-decorated-bacterial cellulose nanocomposite in spent coffee grounds kombucha: A sustainable approach for augmented wound healing, J. Drug Delivery Sci. Technol., 2024, 105477 CrossRef CAS.
- T. Liu, et al., Green Synthesis of Silver Nanoparticles with Size Distribution Depending on Reducing Species in Glycerol at Ambient pH and Temperatures, ACS Omega, 2020, 5(26), 16246–16254 CrossRef CAS.
- M. B. Gawande, et al., Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis, Chem. Rev., 2016, 116(6), 3722–3811 CrossRef CAS.
- V. Coviello, D. Forrer and V. Amendola, Recent developments in plasmonic alloy nanoparticles: synthesis, modelling, properties and applications, ChemPhysChem, 2022, 23(21), e202200136 CrossRef CAS.
- V. Amendola, Laser–Assisted Synthesis of Non–Equilibrium Nanoalloys, Wiley Online Library, 2021, pp. 622–624 Search PubMed.
- T. Gong, et al., Emergent opportunities with metallic alloys: from material design to optical devices, Adv. Opt. Mater., 2020, 8(23), 2001082 Search PubMed.
- T. T. H. Pham, N. D. Dien and X. H. Vu, Facile synthesis of silver/gold alloy nanoparticles for ultra-sensitive rhodamine B detection, RSC Adv., 2021, 11(35), 21475–21488 RSC.
- X. Wang, et al., Composition-adjustable Ag–Au substitutional alloy microcages enabling tunable plasmon resonance for ultrasensitive SERS, Chem. Sci., 2018, 9(16), 4009–4015 RSC.
- K. Loza, M. Heggen and M. Epple, Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals, Adv. Funct. Mater., 2020, 30(21), 1909260 CrossRef CAS.
- J. Li and S. Sun, Intermetallic nanoparticles: synthetic control and their enhanced electrocatalysis, Acc. Chem. Res., 2019, 52(7), 2015–2025 CrossRef CAS PubMed.
- B. M. Munoz-Flores, et al., Recent advances in the synthesis and main applications of metallic nanoalloys, Ind. Eng. Chem. Res., 2011, 50(13), 7705–7721 CrossRef CAS.
- Y. Wang, et al., Ultrastable plasmonic Cu-based core–shell nanoparticles, Chem. Mater., 2020, 33(2), 695–705 CrossRef.
- J. Clarysse, et al., Size-and composition-controlled intermetallic nanocrystals via amalgamation seeded growth, Sci. Adv., 2021, 7(31), eabg1934 CrossRef CAS.
- P. F. Siril and M. Türk, Synthesis of metal nanostructures using supercritical carbon dioxide: A green and upscalable process, Small, 2020, 16(49), 2001972 Search PubMed.
- S. Deng, et al., Green synthesis of proanthocyanidins-functionalized Au/Ag bimetallic nanoparticles, Green Chem. Lett. Rev., 2021, 14(1), 45–50 Search PubMed.
- M. Köroğlu, et al., One step production of silver-copper (AgCu) nanoparticles, Metals, 2021, 11(9), 1466 Search PubMed.
- M. Fan, G. F. Andrade and A. G. Brolo, A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry, Anal. Chim. Acta, 2011, 693(1–2), 7–25 Search PubMed.
- M. N. Martin, et al., Charged gold nanoparticles in non-polar solvents: 10 min synthesis and 2D self-assembly, Langmuir, 2010, 26(10), 7410–7417 Search PubMed.
- N. T. Thanh, N. Maclean and S. Mahiddine, Mechanisms of nucleation and growth of nanoparticles in solution, Chem. Rev., 2014, 114(15), 7610–7630 CAS.
- I. Ojea-Jiménez, N. G. Bastús and V. Puntes, Influence of the sequence of the reagents addition in the citrate-mediated synthesis of gold nanoparticles, J. Phys. Chem. C, 2011, 115(32), 15752–15757 Search PubMed.
- S. J. Barcelo, et al., Nanoimprint lithography of plasmonic platforms for SERS applications, Appl. Phys. A, 2015, 121, 443–449 CAS.
- I. Sow, et al., Revisiting surface-enhanced Raman scattering on realistic lithographic gold nanostripes, J. Phys. Chem. C, 2013, 117(48), 25650–25658 Search PubMed.
- M. Park, C. S. Hwang and K.-H. Jeong, Nanoplasmonic alloy of Au/Ag nanocomposites on paper substrate for biosensing applications, ACS Appl. Mater. Interfaces, 2018, 10(1), 290–295 Search PubMed.
- S. Mani and R. N. Bharagava, Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety, Rev. Environ. Contam. Toxicol., 2016, 237, 71–104 CAS.
- J. Wang, et al., A controlled Ag–Au bimetallic nanoshelled microsphere array and its improved surface-enhanced Raman scattering effect, RSC Adv., 2014, 4(17), 8758–8763 CAS.
- S. H. Park, et al., Galvanic synthesis of three-dimensional and hollow metallic nanostructures, Nanoscale Res. Lett., 2014, 9, 1–7 Search PubMed.
- N. L. Netzer, et al., Gold–silver bimetallic porous nanowires for surface-enhanced Raman scattering, ChemComm, 2011, 47(34), 9606–9608 CAS.
- S. L. Kitaw, et al., Fabrication of Ag nanostar and PEI-based SERS substrate for sensitive and rapid detection of SO2: Application for detection of sulfite residues in beer, Spectrochim. Acta, Part A, 2023, 302, 123113 Search PubMed.
- S. Rostami, et al., High-throughput label-free detection of Ochratoxin A in wine using supported liquid membrane extraction and Ag-capped silicon nanopillar SERS substrates, Food Control, 2020, 113, 107183 CrossRef CAS.
- S. Ristig, et al., Nanostructure of wet-chemically prepared, polymer-stabilized silver–gold nanoalloys (6 nm) over the entire composition range, J. Mater. Chem. B, 2015, 3(23), 4654–4662 RSC.
- G. G. Guisbiers, et al., Electrum, the gold–silver alloy, from the bulk scale to the nanoscale: synthesis, properties, and segregation rules, ACS Nano, 2016, 10(1), 188–198 CrossRef CAS.
- K. Morinaga, N. Oikawa and R. Kurita, Emergence of different crystal morphologies using the coffee ring effect, Sci. Rep., 2018, 8(1), 12503 CrossRef PubMed.
- S. L. Kitaw, et al., Fabrication of polydopamine functionalized AgNF SERS substrate: Applications for sensitive detection of nivalenol in food samples and cell biocompatibility, Colloids Surf., A, 2024, 688, 133398 CrossRef CAS.
- Q. Shao, et al., Fast one-step silicon–hydrogen bond assembly of silver nanoparticles as excellent surface-enhanced Raman scattering substrates, RSC Adv., 2012, 2(5), 1762–1764 RSC.
- A. Candra, et al., Eco-benign synthesis of nano-gold chitosan-bacterial cellulose in spent ground coffee kombucha consortium: Characterization, microbiome community, and biological performance, Int. J. Biol. Macromol., 2023, 253, 126869 CrossRef CAS PubMed.
- G. Awiaz, J. Lin and A. Wu, Recent advances of Au@ Ag core–shell SERS–based biosensors, in Exploration, 2023, Wiley Online Library Search PubMed.
- Z. Ding, et al., TiO2 compact layer induced charge transfer enhancement in a three-dimensional TiO2–Ag array SERS substrate for quantitative and multiplex analysis, RSC Adv., 2023, 13(12), 8270–8280 RSC.
- C. J. Fredricksen, et al., Plasmonic enhancement of thin-film solar cells using gold-black coatings, in Next Generation (Nano) Photonic and Cell Technologies for Solar Energy Conversion II, SPIE, 2011 Search PubMed.
- J. L. Zamora-Navarro, et al., SERS Detection of Methylene Blue and Crystal Violet Using Silver Nanostars, Mater. Process., 2022, 9(1), 27 Search PubMed.
- H. Wang, et al., Optimized core–shell Au@ Ag nanoparticles for label-free Raman determination of trace Rhodamine B with cancer risk in food product, Food Chem., 2015, 188, 137–142 CrossRef CAS.
- B. Morovvati and R. Malekfar, Surface Enhanced Raman Scattering of Crystal Violet with Low Concentrations Using Self-Assembled Silver and Gold-Silver Core-Shell Nanoparticles, Int. J. Opt. Photonics, 2019, 13(2), 89–96 CrossRef.
- X. Meng, et al., Significant surface-enhanced Raman scattering effect of Ag-loaded iron hydroxide enabled by coordination effect between Ag and hydroxyl group, Inorg. Chem. Front., 2023, 10(12), 3648–3655 RSC.
- K. Zhang, et al., A facile surface-enhanced Raman scattering (SERS) detection of rhodamine 6G and crystal violet using Au nanoparticle substrates, Appl. Surf. Sci., 2015, 347, 569–573 CrossRef CAS.
- R. M. Liu, et al., The ultratrace detection of crystal violet using surface enhanced Raman scattering on colloidal Ag nanoparticles prepared by electrolysis, Chin. Chem. Lett., 2009, 20(6), 711–715 CrossRef CAS.
- M. K. Pham, et al., Ultrasensitive detection of crystal violet using a molybdenum sulfide–silver nanostructure-based sensing platform: roles of the adsorbing semiconductor in SERS signal enhancement, Anal. Methods, 2023, 15(39), 5239–5249 RSC.
- E.-Z. Tan, P.-G. Yin and L. Guo, Fabrication of a network structure SERS substrate and the application in ultra-sensitive crystal violet detection, Optoelectron. Lett., 2013, 9(5), 381–384 CrossRef.
- Y. Xu, Y. Wang and Z. Jia, PVA-Coated AgNPs/SiO2 for Detection of Gentian Violet with High Sensitivity, Plasmonics, 2022, 17(6), 2451–2457 Search PubMed.
- Y. Lin, et al., Quantitative detection of crystal violet using a surface-enhanced Raman scattering based on a flower-like HAp/Ag nanocomposite, Anal. Methods, 2021, 13(36), 4143–4149 Search PubMed.
- C. Wu, et al., Fabrication of hydrogels with nanoparticles as surface-enhanced Raman scattered (SERS) substrates and their application in Raman imaging, Mater. Res. Express, 2021, 8(1), 015008 Search PubMed.
- E. Shibu, K. Kimura and T. Pradeep, Gold nanoparticle superlattices: Novel surface enhanced Raman scattering active substrates, Chem. Mater., 2009, 21(16), 3773–3781 CrossRef CAS.
- N. Kaur, V. Varsha and G. Das, Surface-enhanced Raman spectroscopy (SERS) for the detection of Rhodamine 6G and Crystal Violet, in Bio-Optics: Design and Application, Optica Publishing Group, 2023 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03299c |
| This journal is © The Royal Society of Chemistry 2025 |