Equivalent cation-tuning to realize a new Ce(IV) fluoride with excellent comprehensive nonlinear optical performances†
Bei-Bei
Zhang‡
,
Wen-Ye
Gao‡
,
Wei
Xu
,
Liang
Ma
,
Wenlong
Liu
,
Ru-Ling
Tang
 * and
Sheng-Ping
Guo
* and
Sheng-Ping
Guo

School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, Jiangsu 225002, P. R. China. E-mail: rltang@yzu.edu.cn
First published on 30th September 2024
Abstract
Exploring nonlinear optical (NLO) halide materials has become a hot research field owing to their diverse structures and excellent optical properties. In this paper, a new inorganic tetravalent cerium fluoride NH4Ce3F13 was obtained. It crystallizes in the polar space group of Pmc21 and exhibits a three-dimensional {[Ce3F13]−}∞ framework, which is composed of {[CeF8]4−}∞ chains and {[CeF6]2−}∞ layers formed by CeF9 polyhedra, with the NH4+ cation balancing the charge. NH4Ce3F13 exhibits a strong second-harmonic generation (SHG) response among inorganic metal fluorides of about 1.2 times greater than that of KH2PO4 (KDP) and a high laser-induced damage threshold (LIDT), which is 39 times more than that of AgGaS2. Besides, NH4Ce3F13 possesses the largest band gap among Ce(IV)-containing NLO materials. This work provides a good case for exploring high-performance NLO fluoride materials.
Introduction
Metal halides play an important role in the field of photoelectronic functional materials. They are widely used in photovoltaics, light-emitting diodes (LEDs), nonlinear optical (NLO) materials, and laser technology on account of their diverse structural features and excellent photoelectric properties.1–4 In the past several years, several NLO metal halides, including K2SbF2Cl3, Pb7F12Cl2, BaClBF4, Ag2HgI4, HgBr2, CsHgBr3, Cs2HgI2Cl2, Rb2CdBr2I2, Rb2CdBrI3, and Rb4Sn3Cl2Br8, have been reported. Some of them exhibit strong SHG effects and high laser-induced damage thresholds (LIDTs), such as Pb7F12Cl2, HgBr2, and Rb2CdBrI3.5–14 In addition, some metal halides with stereochemically active lone-pair electrons (LPEs) exhibit excellent optical anisotropy. For example, A3SnCl5, ASn2Cl5 (A = NH4 and Rb), A2Sn2F5Cl, and ASnFCl2 (A = Rb and Cs) exhibit outstanding birefringence and a wide transparent region.15,16In recent years, NLO metal fluoride crystals have attracted more attention. The combination of the strongest electronegative F atom and metal cations can form compounds with excellent optical performances. Many NLO fluorides have been investigated, such as SbF3, NaSb3F10, BaZnF4, KBi4F13, SrAlF5, KNa2ZrF7, K3Ba2Zr6F31, K2BaM2F12, and Li2CaMF8 (M = Zr and Hf).17–25 Evidently, Zr- and Hf-based fluorides show large optical band gaps and high LIDTs, such as KNa2ZrF7. Attractively, K3Ba2Zr6F31, K2BaM2F12 and Li2CaMF8 (M = Zr and Hf) possess a wide transparency window below 200 nm. However, although fluorides have advantages in generating compounds with large band gaps, most acentric fluoride crystals show weaker SHG intensity than commercial NLO crystal KH2PO4 (KDP). Apart from group IVB metal fluorides, our group studied cerium(IV) fluorides owing to their similar ionic radii and valence states. Based on these studies, highly electropositive cations (Zr4+, Ti4+, Hf4+, Ce4+, etc.) could lead to diverse asymmetric structural units and thus produce many interesting crystal structures and promising NLO properties. To date, except for lone pair electronic metal fluorides, only Na2CeF6 exhibits an SHG intensity stronger than KDP (2.1 × KDP).26 Clearly, tetravalent cerium fluorides have great potential for NLO performance. To date, among Ce(IV)-containing fluorides, only Na2CeF6 has been reported to show SHG effects. Hence, exploring new fluorides containing Ce(IV) is a meaningful task not just for exploring new NLO materials, but also for enriching the structural diversity of rare earth fluorides. Designing new acentric fluorides is the prerequisite for obtaining new NLO Ce(IV) fluoride materials. In current popular design strategies, the synthesis of new NLO materials using isovalent ion substitution based on non-centrosymmetric (NCS) or centrosymmetric (CS) parent compounds is an effective approach. Generally, different ionic radii may cause structural changes and variations in SHG activity. In recent years, several new NLO compounds have been discovered through equivalent ion regulation strategies. For instance, the NCS compounds CsNaTaF7 were obtained from CS compound CsKTaF7 by substituting the alkali–metal cation. CS-to-NCS structural transformation can be achieved from Cs2M3(P2O7)2 (M = Zn and Mg) to Rb2Zn3(P2O7)2, as well as from K2YB3O6F2 to A2YB3O6F2 (A = Cs, Rb), achieving CS to NCS structural transformation. The substitution of Li+ to K+ results in optimizing the optical properties of α-AZnPO4 (A = Li, K). The physical properties vary due to the different cations based on the AB4O6F (A = NH4, Na, K, Rb, and Cs) family. The NLO carbonates ABCO3F (A = K, Rb, Cs; B = Ca, Sr, Ba) were designed by adjusting the metal cations. A series of NLO rare-earth borates, K7MRE2B15O30 (M = Zn, Cd, Pb; RE = Sc, Y, Gd, Lu), were also obtained by the substitution of isovalent cations.27–38 Obviously, isovalent cation regulation is a preferred method for developing new NLO materials.
According to the above thoughts, a new ammonium cerium(IV) fluoride crystal, NH4Ce3F13, was obtained by a hydrothermal reaction. It adopts the acentric space group of Pmc21 and shows a phase-matching SHG effect. Synthesis, crystal structure, and the relationship between structure and performance based on theoretical calculations are presented in this work.
Experimental section
Synthesis
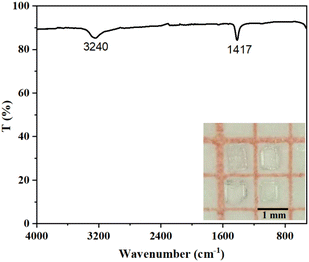
All commercial reagents of CeO2 (Tansoole, 99.99%), NH4F (Sinopharm, AR), ZnF2 (Sinopharm, AR), and HF solution (40% in water, by weight) were used as received. Caution! Hydrofluoric acid is toxic and volatile. Proper precautions and extreme caution must be exercised during the experiment. The single crystals of NH4Ce3F13 were obtained by applying the hydrothermal method. A mixture of NH4F (0.9 mmol), CeO2 (1.8 mmol), and ZnF2 (0.3 mmol) was put into the 23 mL Teflon liners with adding 1.5 mL of HF and 3 mL of deionized water solution. Then, the sealed Teflon liners in the oven were heated to 230 °C for 1 day and cooled to room temperature for 2 days. Finally, colourless single crystals (inset of Fig. 3) of NH4Ce3F13 were obtained with yields of 28–35% based on CeO2.Single crystal structure determination
The crystal data of NH4Ce3F13 were collected by applying a Bruker D8 QUEST X-ray diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The structures were solved using direct methods refined by full-matrix least-squares techniques on F2 with anisotropic displacement parameters for all atoms. The PLATON program was used to check the correctness of the structure, and no errors were found.39,40 The crystallographic data and refinement parameters of NH4Ce3F13 are shown in Table 1. Bond lengths, bond angles, atomic coordinates and equivalent isotropic displacement parameters are summarized in Tables S1–S4,† respectively. CCDC number 2371443† was assigned to the deposition of NH4Ce3F13.| Empirical formula | NH4Ce3F13 |
|---|---|
| a R 1 = ||Fo| − |Fc||/|Fo|. b wR2 = [w(Fo2 − Fc2)2]/[w(Fo2)2]1/2. | |
| Mr (g mol−1) | 685.40 |
| Cryst syst. | Orthorhombic |
| T (K) | 296(2) |
| Space group | Pmc21 |
| a (Å) | 7.9167(8) |
| b (Å) | 7.2697(7) |
| c (Å) | 8.3413(7) |
| V (Å3) | 480.06(8) |
| Z | 2 |
| D c (g cm−3) | 4.742 |
| μ(mm−1) | 14.161 |
| F(000) | 604.0 |
| Crystal size/mm3 | 0.12 × 0.11 × 0.1 |
| Radiation | MoKα (λ = 0.71073) |
| 2θ range for data collection/° | 5.146 to 54.952 |
| Index ranges | −6 ≤ h ≤ 10, −9 ≤ k ≤ 9, −10 ≤ l ≤ 9 |
| Reflns collected | 2327 |
| Independent reflections | 1046 [Rint = 0.0287, Rsigma = 0.0393] |
| Data/restraints/parameters | 1046/10/99 |
| Goodness-of-fit on F2 | 1.047 |
| Final R indexes [I ≥ 2σ(I)]a,b | R 1 = 0.0178, wR2 = 0.0380 |
| Final R indexes [all data]a,b | R 1 = 0.0182, wR2 = 0.0382 |
| Largest diff. peak/hole/e Å−3 | 0.76/−1.35 |
| Flack parameter | 0.030(19) |
Energy-dispersive X-ray spectroscopy (EDS)
EDS analysis of selected several crystals was performed using a Bruker quantum dispersive X-ray spectroscope, which confirms the presence of N, Ce and F in the crystals. The EDS elemental analysis images for the single crystals and the molar ratios of N/Ce/F are shown in Fig. S2 and Table S5,† respectively.Powder X-ray diffraction (PXRD) analysis
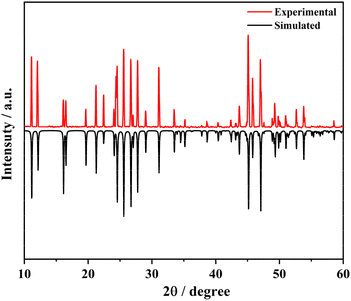
The PXRD characterization for the NH4Ce3F13 sample was collected using a Smart Lab powder X-ray diffractometer for Cu Kα radiation (λ = 1.5406 Å), with the 2θ range of 10–70° and a scan speed of 0.1 s per step. The simulated patterns were generated on the Mercury v3.8 program using the single-crystal structure data of NH4Ce3F13. The experimental and simulated PXRD patterns match well, suggesting that the obtained sample is pure (Fig. 1).Thermogravimetric analysis
Thermogravimetric characterization was performed on Netzsch STA 449 F3 in flowing N2 gas. The powder sample was placed in an alumina crucible with a heating procedure ranging from 20 to 1000 °C at a rate of 15 °C per minute.Optical properties
The IR spectrum was measured using a Magna 750 FI-IR spectrometer with a wavelength of 4000–400 cm−1 with pure KBr as the background. The pure BaSO4 sample was used as the standard reference. The UV–vis–NIR diffuse reflectance spectrum of NH4Ce3F13 was collected using a Carry 5000 spectrometer in the range of 200–800 nm. The reflection spectrum was calculated using the Kubelka–Munk function to calculate the band gap.Second-harmonic generation and laser-induced damage threshold (LIDT) measurements
The SHG response of the NH4Ce3F13 powder sample was measured by applying the Kurtz and Perry method using a Q-switched Nd:YAG laser under 1064 nm. The frequency-doubling effect depended largely on the granular size, so the sample was sieved into six consecutive sizes (25–45, 45–75, 75–105, 105–150, 150–200 and 200–250 μm), with KDP samples of the same size used as the reference. The single-pulse measurement method was used to measure the LIDTs of NH4Ce3F13, with AgGaS2 (AGS) selected for comparison under the same conditions. The samples were radiated with a 1064 nm laser with a pulse width τp of 10 ns in a 1 Hz repetition. The laser emission energy was adjusted by a Nova II sensor display with a PE50-DIT-C energy sensor until the damaged spot was observed.Theoretical calculation
The theoretical calculations of electronic structure, optical properties and the density of states (DOS) were carried out on the CASTEP mode based on the density functional theory (DFT) method. The generalized gradient approximation (GGA) and the Perdew–Burke–Ernzerhof (PBE) function were applied for the exchange–correlation energy.41–43 The orbital electrons of each atom as valence electrons were H 1s1, N 2s22p3, F 2s22p5 and Ce 4f15d16s2. The cut-off kinetic energy of 410 eV was adopted, and the Brillouin zone numerical integration was implemented by employing 4 × 3 × 3 for Monkhorst–Pack k-point sampling to determine the numbers of plane waves, with the Fermi level setting at zero as the energy reference.Results and discussion
Crystal structure
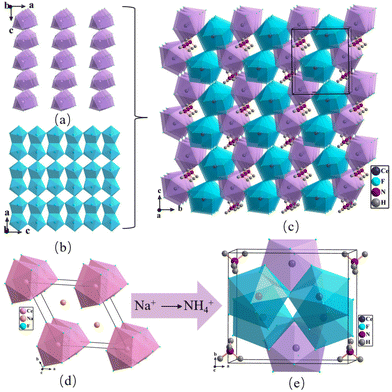
NH4Ce3F13 crystallizes in the orthorhombic space group Pmc21, which includes two kinds of Ce, one unique N, and nine types of F atoms in the asymmetric unit. The crystal structure of NH4Ce3F13 consists of NH4+ cations and the 3D {[Ce3F13]−}∞ anion framework. Both Ce(1) and Ce(2) atoms are connected with nine F atoms to construct the CeF9 polyhedra (Fig. S1†). The Ce(1)F9 polyhedra are linked together via corner-sharing F(2) atoms along the c axis to form {[Ce(1)F8]4−}∞ chains (Fig. 2a), whereas the Ce(2)F9 polyhedra are bonded together via edge-sharing to make up {[Ce(2)F6]2−}∞ layers in the ac plane (Fig. 2b). Furthermore, the {[Ce(1)F8]4−}∞ chains are corner- and edge-shared with {[Ce(2)F6]2−}∞ layers to construct the 3D {[Ce3F13]−}∞ framework, with NH4+ distributed in the cavity to balance the charge (Fig. 2c). The Ce–F and N–H bond distances are in the ranges of 2.197(6)–2.455(4) Å and 0.847(7)–0.851(1) Å, respectively.The reported inorganic monovalent cation Ce(IV)-based fluorides are summarized in Table S6† for comparison with NH4Ce3F13. In Na2CeF6 (P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2m) and LiCeF5 (I41/a), Ce atoms also form CeF9 polyhedra.26,44 Cs3CeF7 (Fm
2m) and LiCeF5 (I41/a), Ce atoms also form CeF9 polyhedra.26,44 Cs3CeF7 (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and Rb3CeF7 (Fm
m) and Rb3CeF7 (Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) feature 3D frameworks composed of CeF6 polyhedra. CsCeF5 (P21/c), Na3CeF7 (I4/mmm), Li4CeF8 (Pnma), (NH4)7Ce6F31 (R
m) feature 3D frameworks composed of CeF6 polyhedra. CsCeF5 (P21/c), Na3CeF7 (I4/mmm), Li4CeF8 (Pnma), (NH4)7Ce6F31 (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ), (NH4)2CeF6 (Pbcn) and (NH4)4CeF8 (C2/c) comprised CeF8 polyhedra to display 3D frameworks.45–50 In the structure of LiCeF5, the distorted CeF9 polyhedra form {[CeF6]2−}∞ chains by edge-sharing along the c axis. Then, these chains are linked together via corner sharing to build a 3D {[CeF5]−}∞ framework. To date, only Na2CeF6 has been reported as an NLO material. Although the two compounds are composed of CeF9 polyhedra, it is noteworthy that the arrangements of CeF9 polyhedra have transformed. Na2CeF6 only contains one Ce atom, which forms {[CeF6]2−}∞ chains, with the 3D {[Na6F18]12−}∞ framework to build the whole structure (Fig. S3†). However, there are two kinds of Ce atoms in NH4Ce3F13, showing two different structural parts, {[CeF8]4−}∞ chains and {[CeF6]2−}∞ layers, and then further to build a 3D {[Ce3F13]−}∞ framework. Besides, the Na–F bond distances are in the range of 2.2748(11)–2.6453(6) Å, which are close to the bond distances of Ce–F in the range of 2.182(2)–2.370(2) Å, so it can be observed that the structure of Na2CeF6 is composed of MF9 (M = Na, Ce) polyhedra. When the Na+ cation is substituted by an NH4+ cation with a larger ionic radius, NH4+ cations are further away from CeF9 polyhedra, resulting in the CeF9 polyhedra adopting different configurations to fit different cations, from 1D chains in Na2CeF6 to a 3D framework in NH4Ce3F13, which further leads to structural transformation. On the other hand, fluorides with the same number of fluorine atoms in the formulae are summarized in Table S7,† including KBi4F13 (I
), (NH4)2CeF6 (Pbcn) and (NH4)4CeF8 (C2/c) comprised CeF8 polyhedra to display 3D frameworks.45–50 In the structure of LiCeF5, the distorted CeF9 polyhedra form {[CeF6]2−}∞ chains by edge-sharing along the c axis. Then, these chains are linked together via corner sharing to build a 3D {[CeF5]−}∞ framework. To date, only Na2CeF6 has been reported as an NLO material. Although the two compounds are composed of CeF9 polyhedra, it is noteworthy that the arrangements of CeF9 polyhedra have transformed. Na2CeF6 only contains one Ce atom, which forms {[CeF6]2−}∞ chains, with the 3D {[Na6F18]12−}∞ framework to build the whole structure (Fig. S3†). However, there are two kinds of Ce atoms in NH4Ce3F13, showing two different structural parts, {[CeF8]4−}∞ chains and {[CeF6]2−}∞ layers, and then further to build a 3D {[Ce3F13]−}∞ framework. Besides, the Na–F bond distances are in the range of 2.2748(11)–2.6453(6) Å, which are close to the bond distances of Ce–F in the range of 2.182(2)–2.370(2) Å, so it can be observed that the structure of Na2CeF6 is composed of MF9 (M = Na, Ce) polyhedra. When the Na+ cation is substituted by an NH4+ cation with a larger ionic radius, NH4+ cations are further away from CeF9 polyhedra, resulting in the CeF9 polyhedra adopting different configurations to fit different cations, from 1D chains in Na2CeF6 to a 3D framework in NH4Ce3F13, which further leads to structural transformation. On the other hand, fluorides with the same number of fluorine atoms in the formulae are summarized in Table S7,† including KBi4F13 (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ), NH4Sb4F13 (I
), NH4Sb4F13 (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ), KSb4F13 (I
), KSb4F13 (I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ), RbU3F13 (Pmc21), NH4UF13 (Pmc21), RbTh3F13 (Pmc21) and [(C5H6N2)2H](Sb4F13) (P1).20,51–56 RbU3F13, NH4UF13 and RbTh3F13 are isotypes with the title compound, but these compounds are radioactive and have only been reported on their structures without studying the SHG effects. The structures of the three compounds also feature a combination of layers and chains consisting of edge- and corner-shared MF9 (M = U and Th) polyhedra.
), RbU3F13 (Pmc21), NH4UF13 (Pmc21), RbTh3F13 (Pmc21) and [(C5H6N2)2H](Sb4F13) (P1).20,51–56 RbU3F13, NH4UF13 and RbTh3F13 are isotypes with the title compound, but these compounds are radioactive and have only been reported on their structures without studying the SHG effects. The structures of the three compounds also feature a combination of layers and chains consisting of edge- and corner-shared MF9 (M = U and Th) polyhedra.
Optical measurements
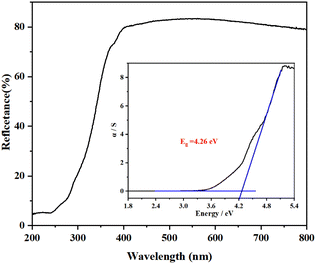
The IR spectrum shows two distinct absorption peaks (Fig. 3). The peaks at 3240 and 1417 cm−1 are related to the N–H vibrations of NH4+, which is close to the reported compounds containing NH4+.57,58 As the UV–vis–NIR diffuse reflectance spectrum shown in Fig. 4, the optical band gap of NH4Ce3F13 is determined to be 4.26 eV, which is the largest among Ce(IV)-containing NLO materials, including Na2CeF6 (3.89 eV),26 CeF2(SO4) (2.71 eV), Ce(IO3)2(SO4) (2.42 eV), CeF2(IO3)2 (2.17 eV), Ce(IO3)2F2·H2O (2.60 eV), Ce3F4(SO4)4 (2.5 eV), Ba2Ce(IO3)8(H2O) (2.44 eV), and Rb2Ce(IO3)5F (2.35 eV).59–65Thermal properties
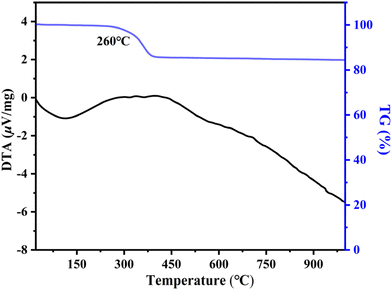
The TG–DTA curves of NH4Ce3F13 are presented in Fig. 5. From the curves, we can observe that NH4Ce3F13 can be stable at temperatures below about 260 °C.SHG activity and LIDT
Due to the acentric structure of NH4Ce3F13, its SHG measurement was performed using the Kurtz–Perry method under a 1064 nm laser. The result shows that NH4Ce3F13 exhibits an SHG efficiency of about 1.2 times that of KDP and can achieve phase-matching (Fig. 6), which is larger than most of the NLO fluoride crystals, including BaZnF4 (0.16 × KDP), KBi4F13 (0.5 × KDP), SrAlF5 (0.65 × KDP), KNa2ZrF7 (0.4 × KDP), K3Ba2Zr6F31 (0.5 × KDP),19–23 CsNaTaF7 (0.2 × KDP),27 and Na2SbF5 (0.17 × KDP).66 Since NH4Ce3F13 exhibits a large band gap and good SHG effect, the LIDTs of NH4Ce3F13 and AgGaS2 were measured under the same conditions. The LIDT measurement results show that NH4Ce3F13 possesses a large LIDT value of 169.4 MW cm−2, which is 39 times that of AGS (4.3 MW cm−2). The LIDT value of NH4Ce3F13 is much enhanced compared to other NLO halides (Table S8†), such as Na2CeF6 (20 × AGS), Pb7F12Cl2 (15.4 × AGS), and KBi4F13 (24 × AGS). Although the SHG signal is not stronger than Na2CeF6 (2.1 × KDP), NH4Ce3F13 shows a larger band gap and higher LIDT than Na2CeF6. Hence, NH4Ce3F13 exhibits balanced NLO performance.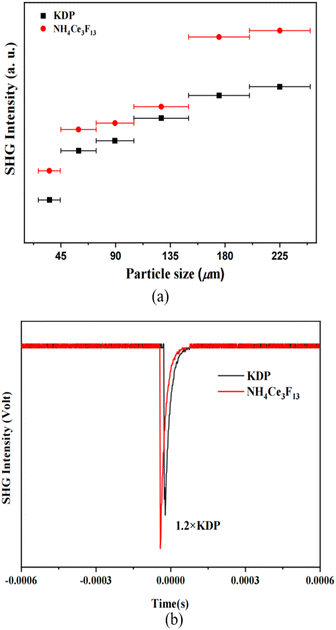 | ||
| Fig. 6 (a) Size-dependence SHG responses of NH4Ce3F13 and KDP under 1064 nm radiation, and (b) SHG responses of 200–250 μm particles of NH4Ce3F13 and KDP. | ||
Theoretical studies
Based on the first principles calculations, we further study the relationship between structures and optical performances.67 The calculated results indicate that the theoretical band gap of NH4Ce3F13 is 2.47 eV (Fig. 7a). Due to the derivative discontinuity of the exchange–correlation function of GGA-PBE, the calculated band gap is smaller than the measured band gap. From the partial density of state (PDOS) graphs of NH4Ce3F13 shown in Fig. 7b, the top of the valence band is mainly contributed by the F-2p orbitals. The bottom of the conduction band is mainly composed of Ce-4f and partial F-2p orbitals. Therefore, it can be observed that the charge transfer between the valence and conduction bands is mainly determined by the Ce and F atoms.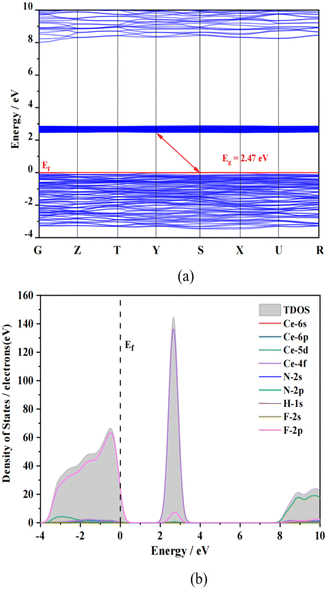 | ||
| Fig. 7 Calculated (a) band gap and (b) density of states (DOS) of NH4Ce3F13. The Fermi level is set at 0 eV. | ||
We deeply explore the NLO properties of NH4Ce3F13 by calculation. As crystallized in the mm2 point group and considered Kleinman symmetry, NH4Ce3F13 has three independent SHG tensors (χ31, χ32, and χ33), which are calculated to be 0.50, 0.53, and 0.57 pm V−1 under a 1064 nm laser, respectively (Fig. 8a). The largest value is 0.7 times that of the KDP (d36 = 0.39 pm V−1), which is relatively smaller than the experimental value. The underestimation is a deviation between the experiment and calculation, and similar cases have been reported in other works.68,69 As the calculated refractive index dispersion curves of NH4Ce3F13 shown in Fig. 8b, the calculated birefringence is 0.03@1064 nm, which is suitable for the phase-matching requirement. The phase-matching wavelength condition was also calculated (Fig. 8c), indicating that the shortest SHG phase-matching wavelength of NH4Ce3F13 is 685 nm, which confirms the experimental results.
 | ||
| Fig. 8 Energy-dependent SHG tensors (a), calculated refractive index dispersion curves (b), and phase-matching conditions of NH4Ce3F13 (c). | ||
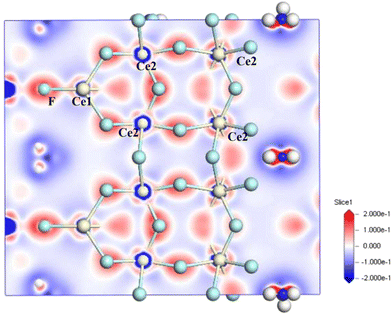
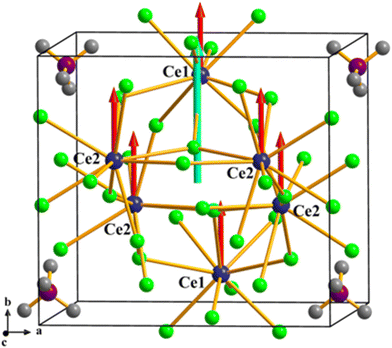
To further study the contribution of the groups in NH4Ce3F13 to optical properties, the calculation of the electronic density difference is carried out. As presented in Fig. 9, the electron density of the F− anion connected by the Ce4+ cation is higher. Therefore, the electron density difference map confirms that the CeF9 polyhedra in NH4Ce3F13 are the main contributors to NLO performance. Besides, the degree of distortion of the total CeF9 polyhedra is also calculated, as shown in Table S9.† The calculation results reveal that CeF9 polyhedra have a relatively large degree of distortion, which is beneficial for NLO properties. The dipole moments of two types of CeF9 units and the total structure in one unit cell of NH4Ce3F13 were calculated to analyse the contribution to the NLO effects. The results are shown in Fig. 10 and Table S10,† indicating that the dipole moments of CeF9 units are arranged in almost the same orientation ranging from 0.55 to 1.10 D and the total dipole moment of CeF9 units is 2.40 D, which results in SHG performance for NH4Ce3F13. Obviously, the optical properties of NH4Ce3F13 are determined by CeF9 units.
Conclusions
In conclusion, NH4Ce3F13, a Ce(IV)-based fluoride NLO crystal, was synthesized using a hydrothermal reaction. It displays a three-dimensional framework comprising corner- and edge-shared CeF9 polyhedra. NH4Ce3F13 exhibits a strong SHG response and high LIDT. Besides, NH4Ce3F13 possesses the largest band gap among all the reported Ce(IV)-based NLO materials. This study provides a good route to explore Ce(IV) halides with NLO effects. We will continue to develop more new promising NLO rare earth halide compounds.Data availability
The data supporting the research findings of this work are available from the corresponding author on reasonable request.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (22101248), the Lvyangjinfeng Talent Program of Yangzhou (YZLYJFJH2021YXBS083), and the Qinglan Project of Yangzhou University.References
- P. F. Gong, F. Liang, L. Kang, X. G. Chen, J. G. Qin, Y. C. Wu and Z. S. Lin, Recent advances and future perspectives on infrared nonlinear optical metal halides, Coord. Chem. Rev., 2019, 380, 83–102 CrossRef CAS.
- Y. Pan, S. P. Guo, B. W. Liu, H. G. Xue and G. C. Guo, Second-order nonlinear optical crystals with mixed anions, Coord. Chem. Rev., 2018, 374, 464–496 CrossRef CAS.
- H. Cho, S. H. Jeong, M. H. Park, Y. H. Kim, C. Wolf, C. L. Lee, J. H. Heo and A. Sadhanala, Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes, Science, 2015, 350, 1222–1225 CrossRef CAS PubMed.
- F. G. You, F. Liang, Q. Huang, Z. G. Hu, Y. C. Wu and Z. S. Lin, Pb2GaF2(SeO3)2Cl: Band Engineering Strategy by Aliovalent Substitution for Enlarging Bandgap while Keeping Strong Second Harmonic Generation Response, J. Am. Chem. Soc., 2019, 141, 748–752 CrossRef CAS PubMed.
- Y. Huang, X. G. Meng, P. F. Gong, Z. S. Lin, X. G. Chen and J. G. Qin, A Study on K2SbF2Cl3 as A New Mid-IR Nonlinear Optical Material: New Synthesis and Excellent Properties, J. Mater. Chem. C, 2015, 3, 9588–9593 RSC.
- Q. Wu, X. Liu, F. Liang, S. R. Xu, H. B. Pi, X. Han, Y. Liu, Z. S. Lin and Y. J. Li, Pb7F12Cl2: A Promising Infrared Nonlinear Optical Material with High Laser Damage Threshold, Dalton Trans., 2019, 48, 13529–13535 RSC.
- M. Zhang, S. L. Pan, Z. H. Yang, Y. Wang, X. Su, Y. Yang, Z. J. Huang, S. J. Han and K. R. Poeppelmeier, BaClBF4: a new noncentrosymmetric pseudo-Aurivillius type material with transparency range from deep UV to middle IR and a high laser damage threshold, J. Mater. Chem. C, 2013, 1, 4740–4745 RSC.
- C. Yang, X. Liu, C. Teng, X. Cheng, F. Liang and Q. Wu, Hierarchical molecular design of high-performance infrared nonlinear Ag2HgI4 material by defect engineering strategy, Mater. Today Phys., 2021, 19, 100432 CrossRef CAS.
- Q. Wu, C. Yang, J. Ma, X. Liu and Y. J. Li, Halogen-Ion-Induced Structural Phase Transition Giving a Polymorph of HgBr2 with Balanced Nonlinear Optical Properties, Inorg. Chem., 2021, 60, 19297–19303 CrossRef CAS PubMed.
- S. W. Lv, Q. Wu, X. G. Meng, L. Kang, C. Zhong, Z. S. Lin, Z. G. Hu, X. G. Chen and J. G. Qin, A promising new nonlinear optical crystal with high laser damage threshold for application in the IR region: synthesis, crystal structure and properties of noncentrosymmetric CsHgBr3, J. Mater. Chem. C, 2014, 2, 6796–6801 RSC.
- G. Zhang, Y. J. Li, K. Jiang, H. Y. Zeng, T. Liu, X. G. Chen, J. G. Qin, Z. S. Lin, P. Z. Fu, Y. C. Wu and C. T. Chen, A New Mixed Halide, Cs2HgI2Cl2: Molecular Engineering for a New Nonlinear Optical Material in the Infrared Region, J. Am. Chem. Soc., 2012, 134, 14818–14822 CrossRef CAS PubMed.
- Q. Wu, X. G. Meng, C. Zhong, X. G. Chen and J. G. Qin, Rb2CdBr2I2: A New IR Nonlinear Optical Material with a Large Laser Damage Threshold, J. Am. Chem. Soc., 2014, 136, 5683–5686 CrossRef CAS PubMed.
- Q. Wu, X. Liu, Y. S. Du, C. L. Teng and F. Liang, Abnormal bandgap enlargement resulted in a promising mid-infrared nonlinear optical material Rb2CdBrI3 with an ultrahigh laser damage threshold, J. Mater. Chem. C, 2020, 8, 9005–9011 RSC.
- P. F. Gong, S. Y. Luo, Y. Yang, F. Liang, S. Z. Zhang, S. G. Zhao, J. H. Luo and Z. S. Lin, Nonlinear Optical Crystal Rb4Sn3Cl2Br8: Synthesis, Structure, and Characterization, Cryst. Growth Des., 2017, 18, 380–385 CrossRef.
- J. Y. Guo, J. B. Huang, A. Tudi, X. L. Hou, S. J. Han, Z. H. Yang and S. L. Pan, Birefringence Regulation by Clarifying the Relationship Between Stereochemically Active Lone Pairs and Optical Anisotropy in Tin-based Ternary Halides, Angew. Chem., Int. Ed., 2023, 62, e202304238 CrossRef CAS PubMed.
- J. B. Wang, M. M. Zhu, Y. Q. Chu, J. D. Tian, L. L. Liu, B. B. Zhang and P. S. Halasyamani, Rational Design of the Alkali Metal Sn-Based Mixed Halides with Large Birefringence and Wide Transparent Range, Small, 2024, 20, 2308884 CrossRef CAS PubMed.
- G. Zhang, T. Liu, T. X. Zhu, J. G. Qin, Y. C. Wu and C. T. Chen, SbF3: A new second-order nonlinear optical material, Opt. Mater., 2008, 31, 110–113 CrossRef CAS.
- G. Zhang, J. G. Qin, T. Liu, Y. J. Li, Y. C. Wu and C. T. Chen, NaSb3F10: A new second-order nonlinear optical crystal to be used in the IR region with very high laser damage threshold, Appl. Phys. Lett., 2009, 95, 261104 CrossRef.
- J. G. Bergman, G. R. Crane and H. Guggenheim, Linear and nonlinear optical properties of ferroelectric BaMgF4 and BaZnF4, J. Appl. Phys., 1975, 46, 4645 CrossRef CAS.
- Q. Wu, H. M. Liu, F. C. Jinang, X. G. Meng, X. G. Chen, L. Yang, Z. G. Hu and J. G. Qin, KBi4F13: An Infrared Nonlinear Optical Material with High Laser Damage Threshold, Inorg. Chem., 2015, 31, 1875–1880 CAS.
- H. Huang, Z. S. Lin, L. Bai, R. He and C. T. Chen, Mechanism of the linear and nonlinear optical effects of SrAlF5 and BaMgF4 crystals, Solid State Commun., 2010, 150, 2318–2321 CrossRef CAS.
- X. Lian, W. D. Yao, W. L. Liu, R. L. Tang and S. P. Guo, KNa2ZrF7: A Mixed-Metal Fluoride Exhibits Phase-Matchable Second-Harmonic-Generation Effect and High Laser-Induced Damage Threshold, Inorg. Chem., 2021, 60, 19–23 CrossRef CAS PubMed.
- M. Yan, R. L. Tang, W. D. Yao, W. L. Liu and S. P. Guo, Exploring a new short-wavelength nonlinear optical fluoride material featuring unprecedented polar cis-[Zr6F34]10− clusters, Chem. Sci., 2024, 15, 2883–2888 RSC.
- M. Yan, R. L. Tang, W. D. Yao, W. L. Liu and S. P. Guo, From CaBaM2F12 to K2BaM2F12 (M = Zr, Hf): Heterovalent Cation-Substitution-Induced Symmetry Break and Nonlinear-Optical Activity, Inorg. Chem., 2024, 63, 10949–10953 CrossRef CAS PubMed.
- M. Yan, R. L. Tang, W. Xu, W. L. Liu and S. P. Guo, Centrosymmetric CaBaMF8 and Noncentrosymmetric Li2CaMF8 (M = Zr, Hf): Dimension Variation and Nonlinear Optical Activity Resulting from an Isovalent Cation Substitution-Oriented Design, Inorg. Chem., 2024, 63, 5260–5268 CrossRef CAS PubMed.
- R. L. Tang, W. Xu, X. Lian, Y. Q. Wei, Y. L. Lv, W. L. Liu and S. P. Guo, Na2CeF6: A Highly Laser Damage-Tolerant Double Perovskite Type Ce(IV) Fluoride Exhibiting Strong Second-Harmonic-Generation Effect, Small, 2024, 20, 2308348 CrossRef CAS PubMed.
- R. L. Tang, X. Lian, X. H. Li, L. Huai, W. L. Liu and S. P. Guo, From CsKTaF7 to CsNaTaF7: Alkali Metal Cations Regulation to Generate SHG Activity, Chem. – Eur. J., 2022, 28, e202201588 CrossRef CAS PubMed.
- Z. F. Song, H. W. Yu, H. P. Wu, Z. G. Hu, J. Y. Wang and Y. C. Wu, Syntheses, structures and characterization of non-centrosymmetric Rb2Zn3(P2O7)2 and centrosymmetric Cs2M3(P2O7)2 (M = Zn and Mg), Inorg. Chem. Front., 2020, 7, 3482–3490 RSC.
- Y. Zheng, Z. J. Wei, H. P. Wu, Z. G. Hu, J. Y. Wang, Y. C. Wu and H. W. Yu, Breaking through the “200 nm deep-ultraviolet wall” of phase matching region by cation structural modulation, Mater. Today Phys., 2024, 46, 101529 CrossRef.
- H. P. Wu, Z. J. Wei, Z. G. Hu, J. Y. Wang, Y. C. Wu and H. W. Yu, Assembly of π-Conjugated [B3O6] Units by Mer-Isomer [YO3F3] Octahedra to Design a UV Nonlinear Optical Material, Cs2YB3O6F2, Angew. Chem., Int. Ed., 2024, 63, e202406318 CrossRef CAS PubMed.
- X. M. He, L. Qi, W. Y. Zhang, R. X. Zhang, X. Y. Dong, J. H. Ma, M. Abudoureheman, Q. Jing and Z. H. Chen, Controlling the Nonlinear Optical Behavior and Structural Transformation with A-Site Cation in α-AZnPO4 (A = Li, K), Small, 2023, 19, 2206991 CrossRef CAS PubMed.
- Z. Z. Zhang, Y. Wang, B. B. Zhang, Z. H. Yang and S. L. Pan, Polar Fluorooxoborate, NaB4O6F: A Promising Material for Ionic Conduction and Nonlinear Optics, Angew. Chem., Int. Ed., 2018, 57, 6577–6581 CrossRef CAS PubMed.
- G. Q. Shi, Y. Wang, F. F. Zhang, B. B. Zhang, Z. H. Yang, X. L. Hou, S. L. Pan and K. R. Poeppelmeier, Finding the Next Deep-Ultraviolet Nonlinear Optical Material: NH4B4O6F, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef CAS PubMed.
- F. Liang, L. Kang, P. F. Gong, Z. S. Lin and Y. C. Wu, Rational Design of Deep-Ultraviolet Nonlinear Optical Materials in Fluorooxoborates: Toward Optimal Planar Configuration, Chem. Mater., 2017, 29, 7098–7102 CrossRef CAS.
- X. F. Wang, Y. Wang, B. B. Zhang, F. F. Zhang, Z. H. Yang and S. L. Pan, CsB4O6F: A Congruent-Melting Deep-Ultraviolet Nonlinear Optical Material by Combining Superior Functional Units, Angew. Chem., Int. Ed., 2017, 56, 14119–14123 CrossRef CAS PubMed.
- Y. Wang, B. B. Zhang, Z. H. Yang and S. L. Pan, Cation-Tuned Synthesis of Fluorooxoborates: Towards Optimal Deep-Ultraviolet Nonlinear Optical Materials, Angew. Chem., Int. Ed., 2018, 57, 2150–2154 CrossRef CAS PubMed.
- G. h. Zou, N. Ye, L. Huang and X. S. Lin, Alkaline-Alkaline Earth Fluoride Carbonate Crystals ABCO3F (A = K, Rb, Cs; B = Ca, Sr, Ba) as Nonlinear Optical Materials, J. Am. Chem. Soc., 2011, 133, 20001–20007 CrossRef CAS PubMed.
- Z. Q. Xie, M. Mutailipu, G. J. He, G. P. Han, Y. Wang, Z. H. Yang, M. Zhang and S. L. Pan, A Series of Rare-Earth Borates K7MRE2B15O30 (M = Zn, Cd, Pb; RE = Sc, Y, Gd, Lu) with Large Second Harmonic Generation Responses, Chem. Mater., 2018, 30, 2414–2423 CrossRef CAS.
- V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: A complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef.
- J. Tauc, R. Grigorovici and A. Vancu, Optical Properties and Electronic Structure of Amorphous Germanium, Phys. Status Solidi B, 1966, 15, 627–637 CrossRef CAS.
- S. K. Kurtz and T. T. Perry, A Powder Technique for the Evaluation of Nonlinear Optical Materials, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Z. Payne, First Principles Methods Using CASTEP, Z. Kristallogr. - Cryst. Mater., 2005, 220, 567–570 CrossRef CAS.
- D. D. Macdonald and M. Urquidi-Macdonald, Application of Kramers-Kronig Transforms in the Analysis of Electrochemical Systems: I. Polarization Resistance, J. Electrochem. Soc., 1985, 132, 2316–2319 CrossRef CAS.
- A. Grzechnik, C. C. Underwood, J. W. Kolis and K. Friese, Crystal structures and stability of LiCeF5 and LiThF5 at high pressures: A comparative study of the coordination around the Ce4+ and Th4+ ions, J. Fluor. Chem., 2013, 156, 124–129 CrossRef CAS.
- R. Hoppe and K. M. Roedder, Fluorokomplexe des vierwertigen Cers, Z. Anorg. Allg. Chem., 1961, 313, 154–160 CrossRef CAS.
- A. Grzechnik, C. C. Underwood and J. W. Kolis, Twinned caesium cerium(IV) pentafluoride, Acta Crystallogr., Sect. B: Struct. Sci., 2014, 70, 112–113 Search PubMed.
- C. J. Windorff, A. T. Chemey, J. M. Sperling, B. E. Klamm and T. E. Albrecht-Schmitt, Examination of Molten Salt Reactor Relevant Elements Using Hydrothermal Synthesis, Inorg. Chem., 2020, 59, 4176–4180 CrossRef CAS PubMed.
- C. C. Underwood, C. D. Mcmillen and J. W. Kolis, Hydrothermal Synthesis and Crystal Chemistry of Novel Fluorides with A7B6F31 (A = Na, K, NH4, Tl; B = Ce, Th) Compositions, J. Chem. Crystallogr., 2014, 44, 493–500 CrossRef CAS.
- R. R. Ryan, A. C. Larson and F. H. Kruse, Crystal Structure of Ammonium Hexafluorocerate(IV), (NH4)2CeF6, Inorg. Chem., 1969, 8, 33–36 CrossRef CAS.
- S. J. Patwe, B. N. Wani, U. R. K. Rao and K. S. Venkateswarlu, Synthesis and thermal study of tris(ammonium) hexafluoro metallates(III) of some rare earths, Can. J. Chem., 1989, 67, 1815–1818 CrossRef CAS.
- L. A. Zemnukhova, A. A. Udovenko, N. V. Makarenko, S. I. Kuznetsov and T. A. Babushkina, Crystal structure and Sb NQR Parameters of ammonium tridecafluorotetraantimonate(III) NH4Sb4F13, J. Struct. Chem., 2017, 58, 694–699 CrossRef CAS.
- A. A. Udovenko, L. A. Zemnukhova, Y. E. Gorbunova, Y. N. Mikhailov and R. L. Davidovich, Crystal Structure of Thallium Tridecafluorotetraantimonate(III) TlSb4F13, Russ. J. Coord. Chem., 2003, 29, 310–311 CrossRef CAS.
- J. Yeon, M. D. Smith, J. Tapp, A. Möller and H. C. zur Loye, Mild Hydrothermal Crystal Growth, Structure, and Magnetic Properties of Ternary U(IV) Containing Fluorides: LiUF5, KU2F9, K7U6F31, RbUF5, RbU2F9, and RbU3F13, Inorg. Chem., 2014, 53, 6289–6298 CrossRef CAS PubMed.
- H. Abazli, A. Cousson, A. Tabuteau and M. Pages, Fluorure d'Ammonium et d'Uranium(IV): NH4U3F13, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2765–2766 CrossRef.
- C. C. Underwood, M. Mann, C. D. McMillen and J. W. Kolis, Hydrothermal Descriptive Chemistry and Single Crystal Structure Determination of Cesium and Rubidium Thorium Fluorides, Inorg. Chem., 2011, 50, 11825–11831 CrossRef CAS PubMed.
- J. H. Wu, C. L. Hu, Y. F. Li, J. G. Mao and F. Kong, [(C5H6N2)2H](Sb4F13): a polyfluoroantimonite with a strong second harmonic generation effect, Chem. Sci., 2024, 15, 8071–8079 RSC.
- Q. Wang, J. X. Ren, D. Wang, L. L. Cao, X. H. Dong, L. Huang, D. J. Gao and G. H. Zou, Low temperature molten salt synthesis of noncentrosymmetric (NH4)3SbF3(NO3)3 and centrosymmetric (NH4)3SbF4(NO3)2, Inorg. Chem. Front., 2023, 10, 2107–2114 RSC.
- Q. Wu, C. Yang, X. Liu, J. Ma, F. Liang and Y. S. Du, Dimensionality reduction made high-performance mid-infrared nonlinear halide crystal, Mater. Today Phys., 2021, 21, 100569 CrossRef CAS.
- C. Wu, T. H. Wu, X. X. Jiang, Z. J. Wang, H. Y. Sha, L. Lin, Z. S. Lin, Z. P. Huang, X. F. Long, M. G. Humphrey and C. Zhang, Large Second-Harmonic Response and Giant Birefringence of CeF2(SO4) Induced by Highly Polarizable Polyhedra, J. Am. Chem. Soc., 2021, 143, 4138–4142 CrossRef CAS PubMed.
- T. H. Wu, X. X. Jiang, Y. R. Zhang, Z. J. Wang, H. Y. Sha, C. Wu, Z. S. Lin, Z. P. Huang, X. F. Long, M. G. Humphrey and C. Zhang, From CeF2(SO4)·H2O to Ce(IO3)2(SO4): Defluorinated Homovalent Substitution for Strong Second-Harmonic-Generation Effect and Sufficient Birefringence, Chem. Mater., 2021, 33, 9317–9325 CrossRef CAS.
- T. H. Wu, X. X. Jiang, C. Wu, H. Y. Sha, Z. J. Wang, Z. S. Lin, Z. P. Huang, X. F. Long, M. G. Humphrey and C. Zhang, From Ce(IO3)4 to CeF2(IO3)2: fluorinated homovalent substitution simultaneously enhances SHG response and bandgap for mid-infrared nonlinear optics, J. Mater. Chem. C, 2021, 9, 8987–8993 RSC.
- T. Abudouwufu, M. Zhang, S. C. Cheng, Z. H. Yang and S. L. Pan, Ce(IO3)2F2·H2O: The First Rare-Earth-Metal Iodate Fluoride with Large Second Harmonic Generation Response, Chem. – Eur. J., 2019, 25, 1221–1226 CrossRef CAS PubMed.
- T. H. Wu, X. X. Jiang, C. Wu, Z. S. Lin, Z. P. Huang, M. G. Humphrey and C. Zhang, Ce3F4(SO4)4: cationic framework assembly for designing polar nonlinear optical material through fluorination degree modulation, Inorg. Chem. Front., 2023, 10, 5270–5277 RSC.
- X. Y. Zhang, X. H. Zhang, B. P. Yang and J. G. Mao, A new polar alkaline earth–rare earth iodate: Ba2Ce(IO3)8(H2O), Dalton Trans., 2023, 52, 4423–4428 RSC.
- O. P. Grigorieva, T. B. Shatalova, A. N. Kuznetsov, P. S. Berdonosov, S. Yu. Stefanovich, K. A. Lyssenko and V. A. Dolgikh, New iodate fluoride Rb2Ce(IO3)5F with nonlinear optical properties, Dalton Trans., 2024, 53, 7367–7375 RSC.
- J. G. Bergman, D. S. Chemla, R. Fourcade and G. Mascherpa, Linear and nonlinear optical properties of Na2SbF5, J. Solid State Chem., 1978, 23, 187–190 CrossRef CAS.
- J. P. Perdew and M. Levy, Physical Content of the Exact Kohn-Sham Orbital Energies: Band Gaps and Derivative Discontinuities, Phys. Rev. Lett., 1983, 51, 1884–1887 CrossRef CAS.
- Y. Q. Wei, W. Xu, L. Huai, Y. L. Lv, W. L. Liu, S. P. Guo and R. L. Tang, From ZnF2 to ZnF2(H2O)4: Partial Substitution Achieves Structural Transformation and Nonlinear Optical Activity while Keeping Short Ultraviolet Cutoff Edge, Inorg. Chem., 2024, 63, 1714–1719 CrossRef CAS PubMed.
- M. Yan, C. L. Hu, R. L. Tang, W. D. Yao, W. L. Liu and S. P. Guo, KBa3M2F14Cl (M = Zr, Hf): novel short-wavelength mixed metal halides with the largest second-harmonic generation responses contributed by mixed functional moieties, Chem. Sci., 2024, 15, 8500–8505 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional tables and figures. CCDC 2371443. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02088j |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2024 |