Open Access Article
Open Access ArticleExperimental and computational studies on the effects of C(2) methylation on the properties and gas separation performance of polyimide-ionene membranes†
Grayson P.
Dennis
 a,
Kathryn E.
O'Harra
a,
Kathryn E.
O'Harra
 a,
Xiaoyang
Liu
a,
Xiaoyang
Liu
 a,
Enrique M.
Jackson
b,
C.
Heath Turner
a,
Enrique M.
Jackson
b,
C.
Heath Turner
 a and
Jason E.
Bara
a and
Jason E.
Bara
 *a
*a
aUniversity of Alabama, Department of Chemical & Biological Engineering, Tuscaloosa, AL 35487-0203, USA. E-mail: Jbara@eng.ua.edu
bNASA Marshall Space Flight Center, Huntsville, AL 35812, USA
First published on 6th September 2023
Abstract
As methylation of the imidazolium C(2) position is known to affect the intermolecular properties of ionic liquids (ILs), polyimide (PI)-ionenes were designed to determine what effects the presence of a C(2)–Me group might have on the properties and membrane performance characteristics relative to analogous PI-ionenes with a C(2)–H group. A commonly used IL (1-butyl-3-methylimidazolium bistriflimide, [C4mim][Tf2N]) was added to the synthesized polymers which formed homogeneous PI-ionene + IL composites that were amenable to the formation of flexible films suitable for membrane testing. The gas permeation behaviors of the resultant membranes were measured for H2, CO2, N2, and CH4. The 6FDA-containing PI-ionene exhibited greater gas permeabilities than the PMDA-containing material, while both materials exhibited comparable selectivity for CO2 relative to other gases. The permeability–selectivity relationships were visualized on Robeson plots, and these PI-ionene membranes were comparable to other ionene and poly(IL) materials. Molecular dynamics (MD) simulations and quantum mechanics (QM) calculations were performed on the C(2)–H and C(2)–Me monomeric units to determine their conformations and other structural properties. The results obtained are useful in the further development of ionene polymers for gas separation membranes.
1. Introduction
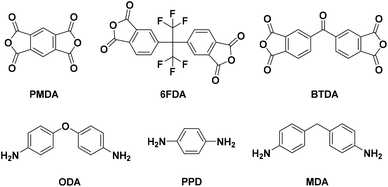
Although multiple types of processes exist to separate CO2 from various industrial gas streams, membranes offer inherent advantages of pressure-driven, solution–diffusion (S–D) transport of solutes through dense polymer materials. In the S–D mechanism, separation of gases through polymer membranes is influenced by the available free volume through which diffusion can occur for a given gas (and its relative size) and the thermodynamics of gas-polymer interactions which govern solubility – essentially the product of “how much” (i.e., S) and “how fast” (i.e., D). The S and D properties of polymer gas separation membranes can be controlled/tailored via molecular design.Among the many different polymer types that have been studied as gas separation membranes, polyimides (PIs) exhibit CO2 selectivity in addition to robust thermal and mechanical properties. PIs are condensation polymers formed through the reaction of a dianhydride and diamine (i.e., ring closure with loss of water molecule). Common dianhydrides used in polyimide synthesis include pyromellitic dianhydride (PMDA), 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA), and 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (BTDA) (Fig. 1). Examples of common diamines used in polyimide synthesis such as 4,4′-oxydianiline (ODA), p-phenylenediamine (PPD), and 4,4′-methylenedianiline (MDA) are also shown in Fig. 1.
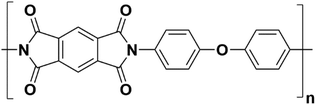
Combinations of these dianhydrides and diamines are used to form polymers with excellent chemical, thermal, and mechanical properties – traits which are required for viable membrane formation. Kapton® (Fig. 2) is a well-known commercial PI formed via condensation polymerization between PMDA and ODA.
O'Brien et al. measured the CO2 permeability (PCO2) of a commercial sample of Kapton H® to be 0.22 barrer, yet a polymer with an identical backbone synthesized in their work from PMDA + ODA was measured as PCO2 = 2.71 barrer. This ∼12× increase was attributed to the processing method(s) which influenced the aggregation and chain orientations within the film.1
One of the main conclusions of a review by Hirayama and co-workers on the structure–property relationship of PIs was that the addition of methyl groups to the PI backbone increases PCO2.2 The presence of pendant groups on polymer backbones can have significant effects on the performances of gas separation membranes. Stern, et al. showed that the selection of diamine, which varied only with the presence of a methyl group, greatly affected the PI microstructure and gas permeation behaviors.3 When a methyl group was added onto the CH2CH2 bridge of 4,4′-(ethane-1,2-diylbis(oxy))dianiline (Fig. 3) and reacted with 6FDA to form PIs, the CO2/CH4 permselectivities increased by over 50% while PCO2 decreased by 30%.3
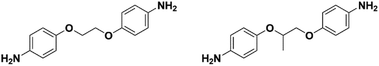 | ||
| Fig. 3 Structures of 4,4′-(ethane-1,2-diylbis(oxy))dianiline (left) and 4,4′-(propane-1,2-diylbis(oxy))dianiline (right). | ||
Hirayama and co-workers did an extensive test of over 43 PI homopolymers formed from various combinations of dianhydrides and diamines.2 Comparing two PI materials formed using 6FDA, 3,3′-dimethyl-4,4′-diaminodiphenylmethane (Fig. 4a), and 3,3′,5,5′-tetramethyl-4,4′-diaminodiphenylmethane (Fig. 4b), the results show that the addition of methyl groups drastically changed the sterics and thus the permeabilities of the resultant materials. The addition of two more methyl groups onto a phenylene diamine caused the resulting 6FDA-containing PI to increase in PCO2 by 500% while only decreasing the CO2/CH4 permselectivity by 33%.2 Depending on the position and bulk of R group(s), the final properties of materials with similar backbones can be markedly different.
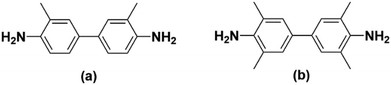 | ||
| Fig. 4 Methylated biphenyl diamine monomers used by Hirayama et al. where (a) is 3,3′-dimethyl-4,4′-diaminodiphenylmethane and (b) is 3,3′,5,5′-tetramethyl-4,4′-diaminodiphenylmethane. | ||
Ionenes are a broad class of polymers which contain ionic groups (e.g., ammonium, imidazolium, phosphonium, etc.) directly within the polymer backbone.4–8 Historically, the vast majority of ionenes in the literature have contained ammonium cations tethered by simple alkyl linkages.9,10 However, in recent years, imidazolium ionenes are becoming of greater interest due to tremendous growth in the study of ionic liquids (ILs), particularly in membrane-based separation of CO2 from various gas streams.11,12 The numerous synthetic strategies available for imidazolium ionenes present opportunities to create highly tailored charged polymers that also contain structural elements found in the aforementioned PIs. Due to their “hybrid” characteristics, the design of imidazolium PI-ionenes and composites with ILs present unique possibilities in the design of polymer gas separation membranes.
One example of a PI-ionene was presented by Li,13 where 6FDA-containing random or block copolymers containing imidazolium moieties and MDA (see Fig. 1) demonstrated good CO2 selectivities. Although the PCO2 decreased with increasing ionic content, the CO2/CH4 selectivity increased; however, it should be noted that the number average molecular weight (MN) of both random and block copolymers were considerably lower as the fraction of the ionic components increased, indicating potential synthetic challenges when starting from imidazolium-containing diamines.13–15
Rather than form PI-ionenes directly through reaction of an imidazolium-containing diamine with a dianhydride, our group has found that higher molecular weights are achieved by first forming a bis(imidazole) diimide that is then polymerized with an α,ω-dihalide (or similar compound) via the Menshutkin reaction (forming the imidazolium group upon polymerization), followed by anion-exchange from a halide to a molecular anion. Mittenthal et al., demonstrated this concept forming a PMDA-containing PI-ionene (Fig. 5) which showed excellent film forming qualities.16
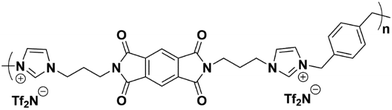 | ||
| Fig. 5 PMDA-containing PI-ionene reported by Mittenthal et al.16 | ||
Although the neat PI-ionene in Fig. 5 had a PCO2 of ∼1 barrer, soaking the film in 1-butyl-3-methylimidazolium bistriflimide ([C4mim][Tf2N]) IL (which was absorbed at ∼1 eq. by the polymer) increased PCO2 by more than 2000%.16 Multiple works from our group have since expanded on the concept of PI-ionenes built from PMDA or 6FDA, demonstrating that these synthetic methods can be applied to provide great control over the backbone structure, while achieving molecular weights >100 kDa that are favorable for formation of mechanically stable, thin films. Furthermore, each of these PI-ionenes also exhibits favorable interactions with ILs to form composites with ILs with enhanced PCO2 relative to the neat PI-ionenes.17,18
Although our group has examined multiple structural variations within PI-ionenes and related polymers, the effects of methyl groups present on the imidazolium ring has not yet been explored. Within the study of IL properties, there has been a strong focus on understanding the effects of methylation of the imidazolium C(2) position of the imidazolium ring.19–24 The C(2)–H is considered to be “acidic” and able to experience H-bonding with anions, and this behavior is attributed as a differentiating factor between low melting imidazolium salts and other organic salts (e.g., quaternary ammonium). “Blocking” this H-bonding via introduction of a methyl (or other) group at the C(2) position is usually correlated with increases in IL viscosity and melting point compared to analogous ILs with a C(2)–H, with a benefit of increased stability to alkaline media.25 However, the effects of this methylation on CO2 solubility are not as obvious. These changes indicate that the interactions in imidazolium ILs with C(2)–Me groups are distinctly different than those with C(2)–H groups. Although the exact nature of these interactions are still under debate, Fumino et al. confirms the methylation of the imidazolium C(2) position causes the H-bonding effect to transition toward coulombic interactions between the ions.26
Carlisle, Bara, and co-workers have shown that relatively simple imidazolium ionenes can be formed in two steps via the reaction of 2-methylimidazole and 1,10-dibromodecane (Fig. 6).27
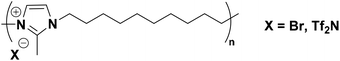 | ||
| Fig. 6 “Main-chain imidazolium ionene” containing 2-methylimidazolium cations tethered by n-decyl chains. | ||
To the best of our knowledge, the alkyl-linked imidazolium ionene depicted in Fig. 6 is the only instance of an imidazolium ionene with an imidazolium C(2)–Me group in the literature.27 Carlisle's imidazolium ionenes were amenable to casting into thin films via melt processing, whether as the Br− or Tf2N− forms. However, gas permeabilities varied greatly with anion type, with PCO2 = 0.13 barrer and 5.3 barrer, for the Br− and Tf2N− forms, respectively.27 Carlisle was unable to compare the ionene in Fig. 6 to a structurally analogous polymer without methylation (i.e., C(2)–H) due to limitations of the synthetic method.
He et al. synthesized imidazolium oligomers of exact molecular weight using 2-methylimidazole.28 These oligomers offered excellent synthetic control, allowing for differing anions to be coordinated with the C(2)–Me imidazolium cations. Even though these were used for peptide synthesis, this approach is notable as it is likely the first report of a unidirectional ionene synthesis.28
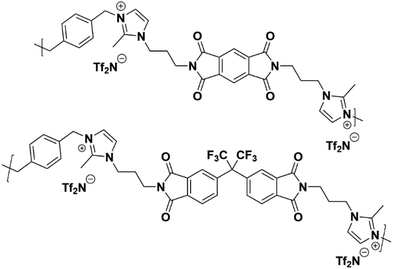
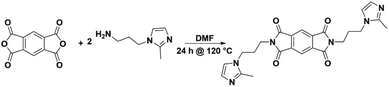
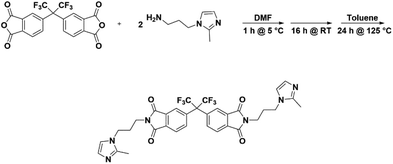
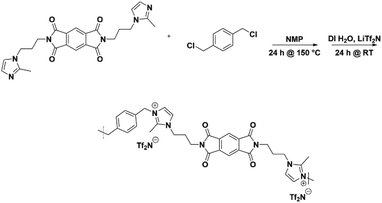
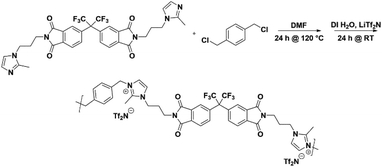
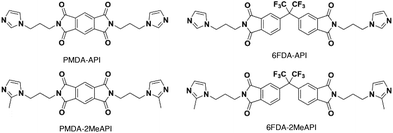
Thus, the effects of a C(2)–Me functional group on the properties of imidazolium ionenes and associated gas separation membranes remain unexplored. Herein, we explore these behaviors through two new PI-ionenes: [PMDA-2MeAPI(pXy)][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] (Fig. 7), which were respectively synthesized by first reacting PMDA or 6FDA with 1-(3-aminopropyl)-2-methylimidazole (2-MeAPI), followed by polymerization via Menshutkin reaction with para-dichloroxylene and subsequent anion metathesis to the [Tf2N]− form. To aid in the formation of thin films suitable for gas separation membranes, IL was directly added to the casting solution, and the non-volatile IL is held within the polymer film after solvent evaporation. The resulting materials were characterized and tested as gas separation membranes to begin developing an understanding as to how the presence of a methyl group at the C(2) position of the imidazolium cation affects the structure–property relationships in PI-ionenes. Furthermore, quantum mechanics (QM) and molecular dynamics (MD) simulations were performed on the PMDA-2MeAPI and 6FDA-2MeAPI monomers to gain further insight into the effects on the film's characteristics and gas separation ability.
2. Experimental section
2.1 Materials
PMDA (>99%) and 6FDA (>99%) were purchased from Akron Polymer Systems. para-dichloroxylene (pDCXy, >98%) was purchased from TCI. LiTf2N was purchased from 3M. N-Methylpyrrolidone (NMP, ACS grade), tetrahydrofuran (THF, ACS grade), diethyl ether (Et2O, ACS grade), methanol (MeOH), ammonium hydroxide (NH4OH, 28–30% in water, ACS Grade), and N,N-dimethylformamide (DMF, anhydrous), were purchased from VWR. RANEY® Nickel®2800 slurry in H2O and triethylamine (Et3N) were purchased from Oakwood Chemical. 2-Methylimidazole was purchased from Alfa Aesar. Celite® 545 was purchased from Millipore-Sigma. Acrylonitrile (≥ 99%) was purchased from Sigma-Aldrich. All materials were used as obtained, without further purification. Deionized H2O (DI H2O, 12 MW) was obtained from the Department of Chemistry at The University of Alabama.2.2 Synthesis of 3-(2-methyl-1H-imidazol-1-yl)propan-1-amine (“2MeAPI”)
The synthesis of 2MeAPI has been detailed in our previous work29 based on methods originally reported by Schwan for 1-(3-aminopropyl)imidazole.30 The corresponding nitrile (i.e., R-CN) precursor was prepared from 2-methylimidazole, acrylonitrile, and Et3N in refluxing toluene, and was subsequently reduced to 2MeAPI (i.e., R-NH2) with H2 in the presence of RANEY® nickel in MeOH/NH4OH (aq).2.3 Synthesis of 2MeAPI-diimide monomers
1H-NMR (500 MHz, d6-DMSO) δ 8.21 (s, 2H), 7.12 (s, 2H), 6.74 (s, 2H), 3.98 (t, J = 7.24 Hz, 4H), 3.66 (t, J = 6.10 Hz, 4H), 2.29 (s, 6H), 2.06 (p, J = 6.51, 6.71 Hz, 4H). 13C-NMR (500 MHz, d6-DMSO) δ 166.85, 144.11, 137.48, 126.65, 119.88, 117.51, 43.37, 36.06, 29.12, 13.04.
1H-NMR (500 MHz, d6-DMSO) δ 8.08 (d, J = 8.06 Hz, 2H), 7.88 (d, J = 7.99 Hz, 2H), 7.66 (s, 2H), 7.11(s, 2H), 6.73 (s, 2H), 3.97 (t, J = 7.11 Hz, 4H), 3.63 (t, J = 6.62 Hz, 4H), 2.29 (s, 6H), 2.03 (p, J = 6.76, 6.93 Hz, 4H). 13C-NMR (500 MHz, d6-DMSO) δ167.55, 167.42, 144.18, 137.44, 135.99, 133.63, 133.22, 125.62, 124.34, 123.61, 120.15, 64.61, 43.55, 35.75, 29.09, 12.75.
2.4 Synthesis of PI-ionenes
To remove residual low molecular weight content, THF (100 mL) was added to [PMDA-2MeAPI(pXy)][Tf2N] and the two-phase mixture stirred at RT for 4 h. The lower polymer layer separates as a viscous brown gel, and the upper THF layer containing solvent and oligomeric compounds was decanted. Finally, the polymer was reprecipitated in Et2O and filtered. The ionene product was dried to yield a light tan glassy solid (9.42 g, 77%).
1H NMR (500 MHz, DMSO) δ 8.24 (s, 2H), 7.74 (d, J = 15.8 Hz, 4H), 7.38 (s, 4H), 5.44 (s, 4H), 4.24 (s, 4H), 3.72 (s, 4H), 2.64 (s, 6H), 2.16 (s, 4H).
Low molecular weight content was removed using THF in an identical manner to that for the PMDA-containing PI-ionene. [6FDA-2MeAPI(pXy)][Tf2N] was dried to yield a tan powder (7.57 g, 74%).
1H NMR (500 MHz, DMSO) δ 8.09 (d, J = 8.1 Hz, 2H), 7.84 (s, 2H), 7.70 (s, 6H), 7.36 (s, 4H), 5.41 (s, 4H), 4.20 (s, 4H), 3.67 (s, 4H), 2.63 (s, 6H), 2.10 (d, J = 8.0 Hz, 4H).
2.5 Membrane preparation
The non-methylated PI-ionene counterparts were able to form films by solvent casting methods, without the use of IL. The membrane forming ability of the non-methylated counterparts demonstrated the essential nature of the C(2) position of the imidazolium cation. The addition of [C4mim][Tf2N] to these membranes allowed for thin, flexible membrane formation. Membranes were prepared by dissolving ∼1 g of polymer in ∼10 mL of acetone in a 20 mL scintillation vial. One molar equivalent of [C4mim][Tf2N] per polymer repeat unit was then added to each solution. Rain-X®, a hydrophobic coating, was buffed onto a 6′′ × 6′′ glass plate to aid in the eventual release of the polymer films from the glass surface. The solution was cast onto these glass plates and left overnight at ambient conditions. The resulting film was then placed into a vacuum oven at 80 °C for 24 h. These films peeled easily from the plates yet were slightly tacky, so the resulting films was placed onto highly permeable Supor® PES supports prior to insertion in the membrane systems. Although the membrane was supple, it remained as a thin (i.e., 20–30 μm) layer selective later atop the Supor® substrate, which can be seen in the SEM images in the Fig. S12 and S13.†2.6 Material characterization
1H and 13C NMR were used to characterize the monomers PMDA-2MeAPI and 6FDA-2MeAPI, and 1H NMR for the polymers [PMDA-2MeAPI(pXy)][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] using a Bruker Avance (360 MHz or 500 MHz for 1H, 500 MHz for 13C). 1H- and 13C-NMR spectra are presented in Fig. S1–S6.†FT-IR spectroscopy was performed using a PerkinElmer Spectrum Two spectrometer with the UATR accessory. (Fig. S7†). The spectra were normalized at the carbonyl peak (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations at ∼1720 cm−1).
O stretching vibrations at ∼1720 cm−1).
Matrix assisted laser deionization/ionization-time of flight (MALDI-TOF) mass spectroscopy was performed using a Bruker Ultraflex on both polymers to determine the mass of the most prominent peak or mode of the distribution (Mp), and thus alongside NMR end-group analysis provide excellent estimates of MN. The graphs can be seen in Fig. S8.†
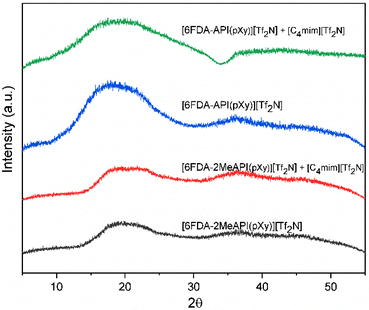
Wide-angle X-ray diffraction (WAXD) was carried out using a Bruker D8 Discover with GADDS using a cobalt source (Kα = 1.79 Å) with a point collimator. The detector was a Vantec 500 area detector. The domain spacing (d-spacing) was determined for each material along with their [C4mim][Tf2N] composite counterparts. The d-spacing was calculated using Bragg's law. The 2θ range for WAXD was 5 to 55°.
Differential scanning calorimetry (DSC) was performed using a TA instruments Q20. Each sample was cycled three times from 25 °C to 250 °C at a rate of 10 °C min−1 under N2 atmosphere. Thermal gravimetric analysis (TGA) was carried out on a Seiko TG-DTA 7300. The samples were run starting at room temperature running up to 700 °C at a heating rate of 10 °C min−1 under inert environment. All thermal graphs can be seen in Fig. S9–S11.†
Thicknesses of the materials were determined using a Mitutoyo micrometer. Membranes were measured at five different locations. Since the PI-IL composite membranes were tacky, to determine the thickness, the difference was taken between the film and PES support were measured, and then the known thickness of the Supor® was subtracted. An average of their differences of the membrane-PES and PES was used for the thickness variable within the permeation calculations. The thickness of the film was also observed using SEM to ensure the accuracy of the micrometer.
2.7 Gas permeability measurements
Gas permeation behavior of the ionene composite membranes were analyzed using pure gases (H2, CO2, N2, and CH4) to determine their permeability (P, in barrer), with the assumption that the mechanism of transport is solution–diffusion (S–D). P is taken to be the product of solubility (S) and diffusivity (D). The high vacuum time-lag systems are based on a constant-volume/variable-pressure method and used in this research, as described in previous works from our group,16 and throughout the polymer gas separation membrane literature. A 47 mm diameter composite membrane is placed into a Millipore membrane holder with an O-ring. After the feed and permeate are held at dynamic vacuum for 24 h, pure gas is applied to ∼3 atm (∼44 psia) on the feed while the downstream pressure is at vacuum (∼0.01 psia). According to the S–D model, the gas permeates through the dense film while the pressure of the downstream is recorded. The pure gas permeations are determined by calculating the linear steady-state increase of the permeate pressure against time (dp/dt). The time lag (θ) is used in part to determine the diffusivity of gas through the membrane. The ideal selectivities (αi/j) are determined as the quotient of the measured P (in barrer) of the individual gases of interest.3. Results and discussion
To contextualize the experimental characterization and gas permeation results, computational studies focused on the impacts of C(2)-methylation were conducted to better understand the properties and performance trends for these materials.3.1 Simulation outcomes
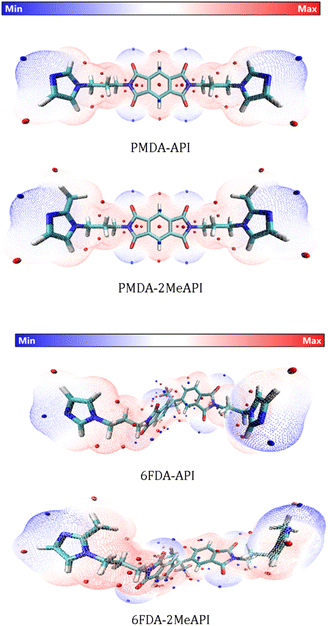 | ||
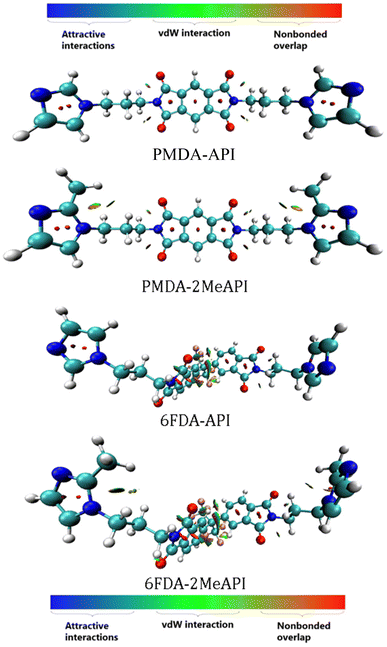
| Fig. 9 ESP-mapped molecular vdW surfaces of the neutral monomers, corresponding to the ρ = 0.001 e/Bohr3 isosurface. The color scale bar is shown at the top, while the corresponding ESP values (units of kcal mol−1) are −50–50 kcal mol−1. The local minima and maxima points on the ESP surface are represented as blue and red spheres, respectively. These species are represented as licorice models (cyan: C, white: H, red: O, blue: N, and pink: F). | ||
In order to further investigate the structure–property relationships of these molecules, especially how the C(2)–Me group changes the electronic properties around the imidazolium cation, the electrostatic potential (ESP) and the reduced density gradient (RDG)39 of these molecules were analyzed by Multiwfn and visualized by VMD.40 The general interaction property functions (GIPF)41 of these species were also generated to analyze the molecular electrostatic properties. The GIPFs are derived from the molecular surface electrostatic potentials by mapping the electrostatic potential surface according to the van der Waals surfaces, as defined by Bader42 (with the electron density isosurface corresponding to 0.001 e/Bohr3).
The ESP surface is mapped to the molecular vdW surface of these four species, as shown in Fig. 9, and the corresponding ESP distributions and GIPF descriptors are shown in Fig. S15 and Table S2,† respectively. From the ESP distributions of these molecules, the methylation for both PMDA-API and 6FDA-API make the ESP curves less positive. For the neutral species, the sites possessing more positive values (i.e., the red spheres distributed on the red surface) have a stronger tendency to attract electrons and thus, are most likely the interaction sites with either the anions or the O atoms of CO2. However, from the ESP surfaces, the methylation on the C(2) positions of both PMDA-API and 6FDA-API slightly weakens the ESP values around the C(2) atoms, which potentially decreases the electrostatic intermolecular interactions between these corresponding cationic species in the polymer chains with the anions.
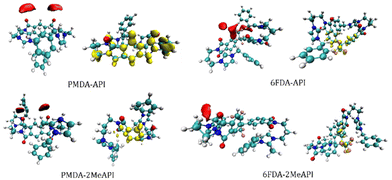
Furthermore, the RDG analyses based on our DFT calculations are shown in Fig. 10. The RDG images, which help visualize the intramolecular non-covalent interactions, show weaker interactions between the methyl groups with the –CH2 on the backbones of both PMDA-2MeAPI and 6FDA-2MeAPI, while these weaker interactions are not presented in either PMDA-API or 6FDA-API molecules. The RDG results indicate that the methylation on the C(2) positions slightly increase the intramolecular interactions between the imidazolium rings with the polymer chains in these molecules.
In order to perform the MD simulations, the optimized geometries of the ionic molecules and anions were inserted into the simulation boxes at close distances (∼5 Å), followed by a few pre-optimization steps to adjust the covalent-bonding network. Then, NVT ensemble simulations (constant volume and temperature) were performed at 300 K using a total of 250 ps for equilibrium, followed by another 250 ps for production (time step of 1 fs). The simulations were accelerated by constraining the X–H bonds in the system. A total of 104 trajectories were used to generate the spatial distribution function (SDF) and connection matrix (CMat) using the Travis package.45,46
For reference, the initial and final snapshots of these systems are shown in Fig. S16.† The initial molecular geometries optimized using DFT calculations are almost linear structures, while after the MD simulations, the polymer chains tend to fold together. In order to gain a deeper insight into the intermolecular and intramolecular interactions within these polymer chains, the SDFs are shown in Fig. 11, and the CMats of all non-carbon atoms in the cationic molecules with the anions and the CMats for the intramolecular interactions within the positively-charged species are shown in Fig. S17 and S18,† respectively. In Fig. 11, the anionic species are located around the O atom sites of the backbones and near the H atoms of the imidazolium rings in both PMDA-API and PMDA-2MeAPI. While for both 6FDA-API and 6FDA-2MeAPI, the anionic species are located around the O and F atom sites of the backbones and near the H atoms of the imidazolium ring (of 6FDA-API) and near the O atoms and the ring-bound H atoms (of 6FDA-2MeAPI).
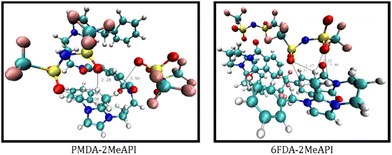
The CMats for these monomers show the potential binding sites between the cationic species with the anions. Here, we focus on the changes of intermolecular H-bonding between the counterions and the intramolecular H-bonding within cationic polymer chains before and after C(2) methylation. We focus on the interactions between the H atoms in PMDA-API (H49 and H50) and 6FDA-API (H68 and H69) and the H atoms in the –CH3 groups in PMDA-2MeAPI (H52–54 and H56–58) and 6FDA-2MeAPI (H71–73 and H75–77) with the H-bond acceptors. The slightly stronger H-bonding via the O atom sites, and the weaker H-bonding via the F atom sites of the anions highlight the increased H-bonding interaction after methylation of both PMDA-API and 6FDA-API. Examples of potential H-bonding networks between the –CH3 groups in PMDA-2MeAPI and 6FDA-2MeAPI with the O atom sites in the [Tf2N]− anions during MD simulations are shown in Fig. 12. Due to thermal fluctuations, the binding sites and bond distances change during the MD simulations. Although the true liquid state is not captured, the main types of anion–cation interactions should still be consistent. Thus, based on the CMat analyses from the MD simulations, the methylation of C(2) positions on the imidazolium ring potentially increases the chance of H-bonding between the cationic species with the anions by providing more binding sites via –CH3. Furthermore, the SDFs show that the methylation of the C(2) positions slightly decreases the intramolecular interactions via electrostatic interaction, while increasing the hydrogen bonding interactions by providing more H sites in –CH3 (confirmed by the CMat intramolecular H-bonding analyses in Fig. S18†).
In conclusion, the intermolecular SDF results show that the methylation at the C(2) position of the imidazolium rings decreases the intermolecular interactions (mainly via electrostatic interactions) between the positively-charged polymer chain with the [Tf2N]− anions, while slightly increasing the intramolecular H-bonding interaction within positively-charged polymer chains. This is corroborated with the inter- and intra-molecular CMat analyses, along with the ESP and RDG analyses based on DFT calculations.
3.2 Material characterization
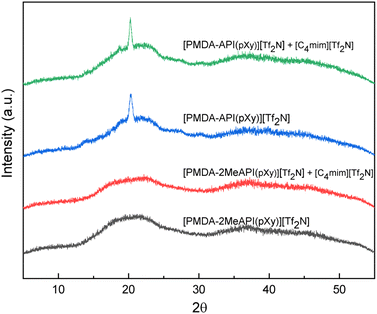
Glass transition temperatures (Tg) were determined from the DSC thermograms. The Tg values were less than their counterparts from a previous work.47 The changes in Tg can be attributed to the reduction of H-bonding within the supramolecular polymer structure of both polymers. While both ionenes contain diimides, it should be noted that the PMDA segment is essentially planar while 6FDA is non-planar (due to the CF3 group repulsions) and known to introduce a kink along polyimide chains. Additionally, larger delocalized anions like [Tf2N−] are known to decrease the Tg in corresponding cationic polymers, due to weakened electrostatic interactions with the associated cation. Further, [Tf2N−] corresponds to a significant portion of the total polymer mass (41–50 wt%), and this anion bulk has been shown to notably influence the mobility of the corresponding cationic chains. Comparing [PMDA-API(pXy)][Tf2N] and [PMDA-2MeAPI(pXy)][Tf2N], the reduction in Tg was from 106.8 °C to 87.0 °C, which according to the computational results may be attributed to the further decreased electrostatic interactions between the anion and cationic backbone from methylation of the C(2) position. Due to the presence of a semi-crystalline peak in the XRD profiles (Fig. 13), [PMDA-API(pXy)][Tf2N] neat or with IL, is slightly more regularly ordered and may entangle and/or align more efficiently, due to the stronger interactions which affect chain mobility, when compared to [PMDA-2MeAPI(pXy)][Tf2N]. These stacking interactions and the planarity of the PMDA-based diimide segment appears to be disrupted by the C(2)–Me presence and corresponding suppression in cation–anion associations.However, the Tg values of [6FDA-API(pXy)][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] were observed to increase from 93.5 °C to 107.4 °C, respectively. Due to the bent nature of 6FDA the contortion and conformation which dictate chain entanglement may be more significantly affected by the increased intramolecular H-bonding and decreased electrostatic interactions. The interchain interactions may be amplified for the C(2)–Me derivative, relative to the more irregular stacking interactions, due to the inherent kinks and increased distance between the anion and cationic backbone.
WAXD was used to approximate the domain spacings of the polymer films, as well as to elucidate the effects of the IL on the chain–chain spacing, coordinating effects, and molecular interactions. Due to their random nature, amorphous polymers produced a broad halo, corresponding to a range of chain–chain spacings of a polymer. [PMDA-2MeAPI(pXy)][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] produced profiles with 2θ = 19.5–22.5° and 2θ = 18.3–20.5°, respectively whereas their counterparts, [PMDA-API(pXy)][Tf2N] and [6FDA-API(pXy)][Tf2N], show halos from 2θ = 20–24° and 2θ = 17.5–22.0° (Fig. 13 and 14). The d-spacing ranges are reported in Table 1 along with the estimated mean or most intense “peak” value noted. With the presence of a C(2)–Me group on the imidazolium cation, the 2θ distribution slightly shifted, corresponding to increased d-spacings. This is in agreement with the simulated decrease in electrostatic interactions observed for the C(2)-methylated derivatives. In [PMDA-2MeAPI(pXy)][Tf2N], the semicrystalline peak is diminished, indicating the C(2)–H played a vital role in the regularity and strength of intermolecular forces present between anion–cation pairs, and by extension other stacking interactions may have been disrupted. Regardless, these d-spacing ranges correlated well to the range of interchain spacings reported for other polyimides (∼ 5.5 Å).48
| Neat | Composite | Neat | Composite | |
|---|---|---|---|---|
| [PMDA-2MeAPI(pXy)][Tf 2 N] | [6FDA-2MeAPI(pXy)][Tf 2 N] | |||
| Halo (Å) | 4.77–5.72 | 4.56–5.72 | 5.03–5.81 | 4.51–5.81 |
| Peak (Å) | 4.77 | 4.56 | 5.15 | 4.75 |
| [PMDA-API(pXy)][Tf 2 N] | [6FDA-API(pXy)][Tf 2 N] | |||
| Halo (Å) | 4.54–5.36 | 4.45–5.48 | 5.10–6.16 | 4.93–6.23 |
| Peak (Å) | 5.05 | 5.07 | 5.65 | 5.15 |
The addition of IL was shown to broaden the main halo for all derivatives, and thus decreases the regularity and uniformity of interchain spacings amongst ionene chains. Addition of [C4mim][Tf2N] decreased the d-spacing range as well. This is attributed to the voids filled by IL, as well as the increase in electrostatic and H-bonding participants within these composite systems, yet the decrease is not substantial. The n-butyl group of the [C4mim][Tf2N] may also hinder interactions between charged segments or impede the packing, causing only a slight reduction of 2θ range within both [PMDA-2MeAPI(pXy)][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] + IL composites.
FT-IR measurements were performed on all materials to validate the structural composition/functional groups along verifying the presence of IL within the polymer matrix. The spectra are provided in Fig. S7.† FT-IR spectra were normalized to the carbonyl stretching (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) peak at 1720 cm−1. The formation of the imide ring and imidazolium can be found in the C–N–C out-of-plane bending and stretching at 721 cm−1 and 1100 cm−1, respectively. The sulfonyl (S
O) peak at 1720 cm−1. The formation of the imide ring and imidazolium can be found in the C–N–C out-of-plane bending and stretching at 721 cm−1 and 1100 cm−1, respectively. The sulfonyl (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching within a sulfonimide at peaks 1370–1335 cm−1 and the S–N–S stretching at 760 cm−1 indicate complete exchange of Cl− to [Tf2N]− in the final PI-ionenes. The increase of the S
O) stretching within a sulfonimide at peaks 1370–1335 cm−1 and the S–N–S stretching at 760 cm−1 indicate complete exchange of Cl− to [Tf2N]− in the final PI-ionenes. The increase of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S–N–S stretching were denoted in the composite membranes to demonstrate the incorporation [C4mim][Tf2N] into the polymer matrix.
O and S–N–S stretching were denoted in the composite membranes to demonstrate the incorporation [C4mim][Tf2N] into the polymer matrix.
3.3 Gas separation performances of PI-ionene + IL composite membranes
The permeabilities, diffusivities, and solubilities are summarized in Table 2. Both the [PMDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N] showed moderate gas separation ability for all gases (N2, CH4, CO2, and H2) where both C(2)–Me composites decreased when compared to their direct counterpart with C(2)–H groups. Comparing the [PMDA-API(pXy)][Tf2N] + [C4mim][Tf2N] and [PMDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N], the addition of the methyl group caused significant decreases in all gas diffusivities by factors over 10×, while slight increases in solubility were observed. As seen in research related to ILs with C(2)–Me imidazolium cations, the CO2 solubility did not change with the addition of a methyl group on the acidic C(2) position. The gas solubility of similar ILs was determined to be directly related to the anion affinity for CO2 within the IL.48| [PMDA-2MeAPI(pXy)][Tf2N] + IL | [6FDA-2MeAPI(pXy)][Tf2N] + IL | |
|---|---|---|
| a Three replicate experiments were performed, and thus uncertainties are presented as a single standard deviation. b Permeability in barrer (10−10 (cm3 (STP) cm) (cm2 s cmHg)−1). – P data for [6FDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N] from O'Harra et al.49 | ||
| N2 | 0.07 ± 0.01 | 0.16 ± 0.01 |
| CH4 | 0.08 ± 0.01 | 0.16 ± 0.02 |
| H2 | 1.34 ± 0.02 | 3.89 ± 0.05 |
| CO2 | 2.75 ± 0.03 | 5.75 ± 0.03 |
| [PMDA-API(pXy)][Tf 2 N] + IL | [6FDA-API(pXy)][Tf 2 N] + IL | |
| N2 | 0.52 ± 0.02 | 0.20 ± 0.03 |
| CH4 | 0.79 ± 0.02 | 0.18 ± 0.02 |
| H2 | 5.18 ± 0.04 | 3.59 ± 0.25 |
| CO2 | 20.4 ± 0.1 | 6.58 ± 0.15 |
The structural influence on permeabilities of both [PMDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N] can be compared, since these differ with respect to the linearity and planarity of the dianhydride precursor. Koros and co-workers compared PMDA and 6FDA in two instances, and the increase in the permeability for the more contorted 6FDA analogues are consistent.50,51 In both cases, the addition of a methyl group at the imidazolium C(2) was shown to decrease PCO2, although comparable selectivities when compared to the C(2)–H derivatives were maintained. In this instance, C(2)-methylation further supports that 6FDA-based ionic polyimides may be superior to PMDA derivatives, when looking to improve gas permeabilities by increasing FFV. Yet, with respect to the ionic polymer architecture and regularity of chain ordering, the C(2) position can be seen as an integral influence on these polymer composites. When comparing the permeabilities of the IL composites with and without a methyl group, the non-methylated counterparts have higher permeabilities with respect to all gases tested than either [PMDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N] or [6FDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N]. As an indicator of permeability, the d-spacing relationships can be a correlative factor since these are related to chain–chain spacings within a polymer matrix. This phenomenon is also demonstrated here, as the derivatives with decreased d-spacing distributions exhibited diminished permeabilities. It should also be noted that the method of IL impregnation may influence this, as the C(2)–H PMDA derivative was allowed to soak in [C4mim][Tf2N], whereas these composites were prepared from solution casting. Additionally, the effects of C(2)–Me for the 6FDA analogues were minor, only resulting in small permeability decreases (12–25%) for CO2, CH4, and N2, which may signify that other structural features dominate chain organization in these systems. Although other factors contribute to the gas separation ability, the permeabilities and d-spacing range were higher for [6FDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N] than [PMDA-2MeAPI(pXy)][Tf2N] + [C4mim][Tf2N]. Thus, the correlation between d-spacing and permeability holds true for these membranes, as seen for other similar ionic polymer systems.17,18,52,53 It should be noted that H2 permeabilities were still lower than CO2 when IL was present in the polymer matrix, as the permselectivity of CO2/H2 is >1.
The QM calculations and MD simulations validate these gas permeation results and the changes within the d-spacings. The MD simulations indicate that many conformations exist for these molecules, and thus a broad range of interchain spacings would be expected for these amorphous composites. As stated in the discussion of QM simulations, both 6FDA-2MeAPI and PMDA-2MeAPI molecules exhibited altered charges, potentially demonstrating increased interactions between the C(2)-position and the O atoms in Tf2N or CO2. However, the MD simulations indicate increased intramolecular interactions between polymer chains due to the increased H-bonding sites, which may cause the observed decrease in permeability when comparing the API and 2MeAPI derivatives. Factors like the increase in H-bonding between the cationic chains and introduction of free IL may supersede the modified electrostatic interactions from C(2)-methylation. The effects further are supported in the XRD results, as well as the gas permeation data, which these simulations prove that the addition of the –CH3 on the C(2) position has substantial influence over the neat materials, but is not a determinant effect on the structuring and performance of these IL-composite systems.
Selectivity values were also calculated from permeabilities, s reported in Table 3. Based on our prior work with ionenes and ILs, CO2 selectivity is driven by solubility (e.g., condensability, quadrupole) whereas H2 selectivity is favored by diffusion (i.e., smallest gas)54 Permselectivities of the IL-composite membranes coincided with the inverse relationship of permeability/selectivity. The relationship is demonstrated by Robeson's Upper Bound when plotting CO2 permeability versus CO2 selectivity.55,56 Robeson plots with similar ionic gas separation membranes are provided as Fig. S19 and S20.† The materials were below the upper bound and within the bulk of other materials when comparing CO2/N2 and CO2/CH4 separations.
| Pair | [PMDA-2MeAPI(pXy)][Tf2N] + IL | [6FDA-2MeAPI(pXy)][Tf2N] + IL |
|---|---|---|
| CO2/N2 | 41.0 | 35.4 |
| CO2/CH4 | 33.1 | 35.5 |
| H2/N2 | 20.0 | 24.7 |
| H2/CH4 | 16.1 | 24.8 |
| CO2/H2 | 2.05 | 1.43 |
4. Conclusions
This study computationally and experimentally explored the effects of C(2)-methylation of the imidazole ring in high-performance, imidazolium ionenes. This work reports the synthesis and modeling of two new polyimide ionenes for comparison with previous analogues, one of few examples of imidazolium ionenes with substitution rather than a proton at the acidic C(2) site. 2MeAPI was reacted with two common dianhydrides (planar PMDA and bent 6FDA) through a condensation reaction to form two new bis(imidazole) diimide monomers. These monomers were then polymerized via the Menshutkin reaction, followed by anion metathesis to exchange the halide with [Tf2N−]. The materials were characterized via NMR and FT-IR to ensure the structure of the material, and the thermal properties of [PMDA-2MeAPI(pXy)][Tf2N] and [6FDA-2MeAPI(pXy)][Tf2N] were also measured. The XRD profiles demonstrated that the C(2)–Me ionenes experienced a significant decrease in the regularity of chain packing and morphology of these materials. The glassy neat materials were impregnated with a stoichiometric equivalent of IL, which resulted in amorphous composites with increased membrane flexibility, altered chain ordering/structure, and increased PCO2. The gas transport behaviors were tested for two polyimide ionene composites with [C4mim][Tf2N], which demonstrated that the C(2)-methylation and IL caused the diffusivity to significantly decrease while slightly increasing the solubility. These materials exhibited lowered gas permeabilities than their C(2)–H counterparts, which was supported by computational insights, indicative of increased intrachain interactions due to additional H-bonding sites along these cationic ionenes and decreased intermolecular and electrostatic interactions between the positively-charged backbone and paired anions or CO2. Due to the ionic nature of the films, it was still observed that these membranes have an affinity for polar vs. non-polar gases and maintained distinct CO2 selectivities, particularly against N2 and CH4. The correlations between the QM and MD simulations and the structural changes and performance of these materials as gas separation membranes were summarized.Author contributions
Manuscript preparation (GPD, KEO, JEB), writing (GPD), manuscript revision and editing (GPD, KEO), material synthesis (KEO), material characterizations (KEO, EMJ), gas permeation studies (GPD), funding acquisition and project management (JEB, CHT), computational studies and analysis (XL, CHT). All authors contributed to this manuscript and approved its final form.Conflicts of interest
There are no conflicts to declare.Acknowledgements
JEB and CHT acknowledge support from the U.S. National Science Foundation (CBET-1605411). JEB and EMJ acknowledge support from NASA Marshall Space Flight Center (80MSFC19M0048). KEO acknowledges a GAANN Fellowship from the United States Department of Education (P200A180056).References
- K. C. O'Brien, W. J. Koros and G. R. Husk, Polym. Eng. Sci., 1987, 27, 211–217 CrossRef.
- Y. Hirayama, T. Yoshinaga, Y. Kusuki, K. Ninomiya, T. Sakakibara and T. Tamari, J. Membr. Sci., 1996, 111, 169–182 CrossRef CAS.
- S. A. Stern, Y. Liu and W. A. Feld, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 939–951 CrossRef CAS.
- S. R. Williams and T. E. Long, Prog. Polym. Sci., 2009, 34, 762–782 CrossRef CAS.
- E. B. Anderson and T. E. Long, Polymer, 2010, 51, 2447–2454 CrossRef CAS.
- K. E. O'Harra and J. E. Bara, Polym. Int., 2021, 70, 944–950 CrossRef.
- J. E. Bara and K. E. O'Harra, Macromol. Chem. Phys., 2019, 220, 1900078 CrossRef.
- J. S. Lee, A. Hocken and M. D. Green, Mol. Syst. Des. Eng., 2021, 6, 334–354 RSC.
- C. F. Gibbs, E. R. Littmann and C. S. Marvel, J. Am. Chem. Soc., 1933, 55, 753–757 CrossRef CAS.
- M. T. Bogert, Science, 1933, 77, 197–198 CrossRef CAS PubMed.
- P. Scovazzo, J. Kieft, D. A. Finan, C. Koval, D. DuBois and R. Noble, J. Membr. Sci., 2004, 238, 57–63 CrossRef CAS.
- K. Friess, P. Izák, M. Kárászová, M. Pasichnyk, M. Lanč, D. Nikolaeva, P. Luis and J. C. Jansen, Membranes, 2021, 11, 97 CrossRef CAS PubMed.
- P. Li, Q. Zhao, J. L. Anderson, S. Varanasi and M. R. Coleman, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4036–4046 CrossRef CAS.
- C. Zhang, B. Cao, M. R. Coleman and P. Li, J. Appl. Polym. Sci., 2016, 133, 43077 Search PubMed.
- P. Li and M. R. Coleman, Eur. Polym. J., 2013, 49, 482–491 CrossRef CAS.
- M. S. Mittenthal, B. S. Flowers, J. E. Bara, J. W. Whitley, S. K. Spear, J. D. Roveda, D. A. Wallace, M. S. Shannon, R. Holler, R. Martens and D. T. Daly, Ind. Eng. Chem. Res., 2017, 56, 5055–5069 CrossRef CAS.
- K. E. O’Harra, I. Kammakakam, D. M. Noll, E. M. Turflinger, G. P. Dennis, E. M. Jackson and J. E. Bara, Membranes, 2020, 10, 51 CrossRef PubMed.
- K. E. O'Harra, I. Kammakakam, E. M. Devriese, D. M. Noll, J. E. Bara and E. M. Jackson, Membranes, 2019, 9, 79 CrossRef PubMed.
- P. Bonhôte, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef PubMed.
- P. A. Hunt, J. Phys. Chem. B, 2007, 111, 4844–4853 CrossRef CAS PubMed.
- T. Endo, T. Kato and K. Nishikawa, J. Phys. Chem. B, 2010, 114, 9201–9208 CrossRef CAS PubMed.
- K. Fumino, A. Wulf and R. Ludwig, Angew. Chem., Int. Ed., 2008, 47, 8731–8734 CrossRef CAS PubMed.
- K. Noack, P. S. Schulz, N. Paape, J. Kiefer, P. Wasserscheid and A. Leipertz, Phys. Chem. Chem. Phys., 2010, 12, 14153–14161 RSC.
- S. Zahn, G. Bruns, J. Thar and B. Kirchner, Phys. Chem. Chem. Phys., 2008, 10, 6921–6924 RSC.
- B. Lin, H. Dong, Y. Li, Z. Si, F. Gu and F. Yan, Chem. Mater., 2013, 25, 1858–1867 CrossRef CAS.
- K. Fumino, T. Peppel, M. Geppert-Rybczynska, D. H. Zaitsau, J. K. Lehmann, S. P. Verevkin, M. Kockerling and R. Ludwig, Phys. Chem. Chem. Phys., 2011, 13, 14064–14075 RSC.
- T. K. Carlisle, J. E. Bara, A. L. Lafrate, D. L. Gin and R. D. Noble, J. Membr. Sci., 2010, 359, 37–43 CrossRef CAS.
- X. He and T. H. Chan, Org. Lett., 2007, 9, 2681–2684 CrossRef CAS PubMed.
- J. Demarteau, K. E. O'Harra, J. E. Bara and H. Sardon, ChemSusChem, 2020, 13, 3122–3126 CrossRef CAS PubMed.
- T. J. Schwan, J. Heterocycl. Chem., 1967, 4, 633–634 CrossRef CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
- J. G. Brandenburg, C. Bannwarth, A. Hansen and S. Grimme, J. Chem. Phys., 2018, 148, 064104 CrossRef PubMed.
- C. M. Breneman and K. B. Wiberg, J. Comput. Chem., 1990, 11, 361–373 CrossRef CAS.
- A. V. Marenich, S. V. Jerome, C. J. Cramer and D. G. Truhlar, J. Chem. Theory Comput., 2012, 8, 527–541 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- R. Dennington, T. Keith and J. Millam, GaussView, Version 5, 2009 Search PubMed.
- T. Lu, OfakeG program, Version 1.0.6, https://sobereva.com/soft/OfakeG, (accessed May, 2020) Search PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- J. S. Murray, T. Brinck, P. Lane, K. Paulsen and P. Politzer, J. Mol. Struct.: THEOCHEM, 1994, 307, 55–64 CrossRef.
- R. F. Bader, M. T. Carroll, J. R. Cheeseman and C. Chang, J. Am. Chem. Soc., 1987, 109, 7968–7979 CrossRef CAS.
- S. Spicher and S. Grimme, Angew. Chem., Int. Ed., 2020, 59, 15665–15673 CrossRef CAS PubMed.
- S. Grimme, C. Bannwarth and P. Shushkov, J. Chem. Theory Comput., 2017, 13, 1989–2009 CrossRef CAS PubMed.
- B. Martin and B. Kirchner, J. Chem. Inf. Model., 2011, 51, 2007–2023 CrossRef PubMed.
- M. Brehm, M. Thomas, S. Gehrke and B. Kirchner, J. Chem. Phys., 2020, 152, 164105 CrossRef CAS PubMed.
- K. E. O'Harra, I. Kammakakam, J. E. Bara and E. M. Jackson, Polym. Int., 2019, 68, 1547–1556 CrossRef.
- A. Shimazu, T. Miyazaki and K. Ikeda, J. Membr. Sci., 2000, 166, 113–118 CrossRef CAS.
- K. E. O'Harra, I. Kammakakam, E. M. Devriese, D. M. Noll, J. E. Bara and E. M. Jackson, Membranes, 2019, 9, 79 CrossRef PubMed.
- T. H. Kim, W. J. Koros and G. R. Husk, Sep. Sci. Technol., 1988, 23, 1611–1626 CrossRef CAS.
- T. H. Kim, W. J. Koros, G. R. Husk and K. C. O'Brien, J. Membr. Sci., 1988, 37, 45–62 CrossRef CAS.
- I. Kammakakam, K. E. Oharra, J. E. Bara and E. M. Jackson, ACS Omega, 2019, 4, 3439–3448 CrossRef CAS PubMed.
- I. Kammakakam, K. E. O'Harra, G. P. Dennis, E. M. Jackson and J. E. Bara, Polym. Int., 2019, 68, 1123–1129 CrossRef CAS.
- M. S. Mittenthal, B. S. Flowers, J. E. Bara, J. W. Whitley, S. K. Spear, J. D. Roveda, D. A. Wallace, M. S. Shannon, R. Holler, R. Martens and D. T. Daly, Ind. Eng. Chem. Res., 2017, 56, 5055–5069 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165–185 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3lp00092c |
| This journal is © The Royal Society of Chemistry 2023 |