Indoor air: sources, chemistry and health effects
Delphine K.
Farmer
 *a and
Marina E.
Vance
*a and
Marina E.
Vance
 b
b
aDepartment of Chemistry, Colorado State University, Fort Collins, CO, USA 80523. E-mail: delphine.farmer@colostate.edu
bDepartment of Mechanical Engineering, University of Colorado, Boulder, CO, USA 80309
An array of molecules are present in the gas phase in indoor environments, many with higher concentrations indoors than outdoors. Oxidants drive chemical transformations in the outdoor atmosphere, but their indoor concentrations are poorly understood. Young et al. (DOI: 10.1039/c9em00111e) review the literature on indoor oxidants and their precursors, highlighting the need for further measurements. While outdoor air is a dominant source of ozone, humans can introduce oxidants such as reactive nitrogen oxides (NO and NO2) through the use of gas stoves, or chlorine compounds through the use of bleach – and buildings themselves can even be sources of oxidants such as formaldehyde. Both Zhou et al. (DOI: 10.1039/c9em00129h) and Liu et al. (DOI: 10.1039/c9em00194h) provide critical measurements of oxidants and their precursors. Zhou et al. describe NO, 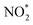 (NO2 + HONO), and O3 measurements in three US indoor environments across spring and winter, noting the influence of door opening and cooking on levels and suggesting that the relatively high indoor NO concentrations in residences (relative to outdoor concentrations) contribute to low ozone (O3) levels. Liu et al. measured NOx, HONO and light levels in a home in China – similarly noting the role of cooking in elevated NOx. That study highlights the potential for photolysis from sunlight entering homes through windows to produce substantial OH radicals from HONO photolysis.
(NO2 + HONO), and O3 measurements in three US indoor environments across spring and winter, noting the influence of door opening and cooking on levels and suggesting that the relatively high indoor NO concentrations in residences (relative to outdoor concentrations) contribute to low ozone (O3) levels. Liu et al. measured NOx, HONO and light levels in a home in China – similarly noting the role of cooking in elevated NOx. That study highlights the potential for photolysis from sunlight entering homes through windows to produce substantial OH radicals from HONO photolysis.
Duncan et al. (DOI: 10.1039/c9em00105k) investigate water soluble organic gases in the indoor environment through residential measurements, finding high concentrations dominated by organic acids. Interestingly, multiple water soluble gases included reduced organic nitrogen moieties, suggesting that nitrogen-containing molecules such as amines and imidazoles – and their interfacial chemistry – warrant further attention. Assessing the health impact of these and other VOCs is a clear challenge for the indoor chemistry community as each emitted molecule can chemically transform into a myriad of daughter products. To this end, Carslaw and Shaw (DOI: 10.1039/c9em00140a) describe a new metric, the Secondary Product Creation Potential (SPCP), that utilizes a novel modeling approach to rank precursors based on the potential health impact of their potential oxidative products. Isocyanic acid (HNCO) represents one particularly toxic molecule in the indoor environment. Hems et al. (DOI: 10.1039/c9em00107g) investigate the role of smoking and subsequent gas–particle partitioning in controlling indoor levels of HNCO.
Sources of indoor aerosols are diverse, ranging from the infiltration of ambient particles to indoor activities, such as cooking and the use of consumer and office products, to mere human presence. Shin et al. (DOI: 10.1039/c9em00015a) investigated airborne particles released during paper printing and shredding processes and evaluated their cytotoxicity using in vitro human lung epithelial cell models. Printing caused substantial release of particles smaller than 300 nm, primarily in the form of metal granules and graphite. Avery et al. (DOI: 10.1039/c9em00097f) demonstrate the impact of human presence on the composition of indoor aerosol in a classroom environment by showing aerosol mass enhancements of ion families consistent with squalene, a compound naturally present in human skin oil, and its oxidized products from reactions with ozone.
From an air quality and chemistry standpoint, one of the most important differences between indoor and outdoor environments is the relative prevalence of surfaces, which is significantly higher indoors. Manuja et al. (DOI: 10.1039/c9em00157c) quantitatively characterized the physical characteristics of 10 bedrooms, nine kitchens, and three offices, including surface area, volume, shape, and material of objects with a spatial resolution of ∼1 cm. They found that the presence of contents (e.g., furniture, window coverings, books, clothing) increases the surface area of rooms by 50% and decreases their volume by 10%, compared to empty rooms. These surfaces, along with insulation and other building materials, can act as a reservoir for molecules and site for chemical reactions with indoor and incoming outdoor air. Chin et al. (DOI: 10.1039/c9em00024k) investigate primary VOC emissions, O3 reaction probabilities, and O3 reaction byproduct formation yields from eight insulation materials commonly used in building enclosures.
Surface dynamics are especially important for semi-volatile organic compounds (SVOC). Uhde et al. (DOI: 10.1039/c9em00121b) monitored the concentration profiles of five SVOCs in the air and on the surfaces inside a test house over a period of six months and show that the operation of an air purifier caused an ∼50% reduction in their concentrations in air, but these quickly returned to steady-state after the purifier was switched off. Yang et al. (DOI: 10.1039/c9em00200f) measured emission characteristics of di(2-ethylhexyl)phthalate (DEHP), an SVOC commonly used as a plasticizer, from vehicle cabin materials. An often ignored type of indoor surface is dust. Bope et al. (DOI: 10.1039/c9em00050j) investigated the degradation of phthalates in dust collected from a home and found that, at elevated relative humidity, both biotic and abiotic mechanisms contributed to degradation.
Many of these above studies utilize sophisticated instrumentation, but new detection systems are providing the opportunity for greater spatial coverage in indoor measurements. For example, Tryner et al. (DOI: 10.1039/c9em00234k) describe a low-cost sensor for PM2.5 that combines a light scattering detector with an active filter sampler. This combination of optical and filter sampling enables both real-time particle concentration measurements and time-integrated chemical analysis.
While most indoor studies focus on a subset of gas or particle measurements, Farmer et al. (DOI: 10.1039/c9em00228f) describe a different approach. In HOMEChem (the House Observations of Microbial and Environmental Chemistry study), over thirteen research groups from numerous institutions worked collaboratively to provide a comprehensive chemical picture of cooking, cleaning and occupancy in a test house. Cooking is a key source of indoor particles, which are generally dominated by hydrocarbon-like organic aerosol with low oxygen content – similar to the chemical composition of indoor organic aerosol and surface films.
The chemical information that is potentially available to researchers studying indoor air is vast. Models provide the opportunity to simplify systems, fill in measurement gaps, and enable generalization and investigation of chemical mechanisms. Model development is an active area of research, and Shiraiwa et al. (DOI: 10.1039/c9em00123a) describe a consortium of modeling activities aimed at synthesizing gas, particle and surface chemistry across spatial scales, from molecular dynamics to computational fluid dynamics.
The field of indoor chemistry is rapidly growing, and this themed issue highlights the breadth and vibrance of ongoing research – and the continued need for high quality chemical measurements in the indoor environment coupled to careful modeling and consideration of building parameters. We hope that the readers of this themed issue find the work as exciting as we did! We also hope that readers will be inspired to consider the chemistry of their everyday lives in a new light (albeit one with fewer photons than outdoors!), and think about how their actions influence the composition of the air they breathe. We thank the authors who contributed manuscripts, the reviewers who ensured the high quality of the work, and the editors and staff at the Royal Society of Chemistry who managed the process.
| This journal is © The Royal Society of Chemistry 2019 |
