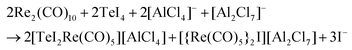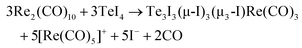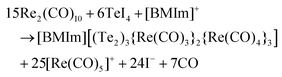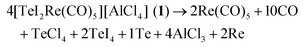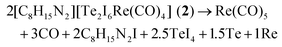Open Access Article
Open Access ArticleIonic-liquid-based synthesis of tellurium–rhenium carbonyls with specific reaction control†‡
Silke
Wolf
 and
Claus
Feldmann
and
Claus
Feldmann
 *
*
Institute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, 76131 Karlsruhe, Germany. E-mail: claus.feldmann@kit.edu
First published on 11th July 2019
Abstract
The novel tellurium rhenium carbonyls [TeI2Re(CO)5][AlCl4] (1), [BMIm][Te2I4(μ-I)2Re(CO)4] (2), {Te3I2(μ-I)3(μ3-I)}Re(CO)3 (3) and [BMIm][(Te2)3{Re(CO)3}2{Re(CO)4}3] (4) were prepared by reacting TeI4 and Re2(CO)10 in ionic liquids (ILs). [TeI2Re(CO)5][AlCl4] (1) was obtained in a mixture of [BMIm]Cl (BMIm: 1-butyl-3-methylimidazolium) and AlCl3 (ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and contains a [TeI2Re(CO)5]+ cation. Increasing the amount of AlCl3 ([BMIm]Cl
1) and contains a [TeI2Re(CO)5]+ cation. Increasing the amount of AlCl3 ([BMIm]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3 = 1
AlCl3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) results in [BMIm][Te2I4(μ-I)2Re(CO)4] (2) with the anion [Te2I4(μ-I)2Re(CO)4]−. At a [BMIm]Cl to AlCl3 ratio of 1
2) results in [BMIm][Te2I4(μ-I)2Re(CO)4] (2) with the anion [Te2I4(μ-I)2Re(CO)4]−. At a [BMIm]Cl to AlCl3 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, {Te3I2(μ-I)3(μ3-I)}Re(CO)3 (3) was realized with a Te3I3 ring and μ3-coordinating iodine. Finally, [BMIm][(Te2)3{Re(CO)3}2{Re(CO)4}3] (4) was prepared in [BMIm][OTf] (OTf: triflate) and contains the ufosan-like anion [(Te2)3{Re(CO)3}2{Re(CO)4}3]− with three Te22− and two Re(CO)3+ units that establish a distorted heterocuban-like cage. The compounds were characterized by single-crystal structure analysis, energy dispersive X-ray (EDX) analysis, thermogravimetry (TG), and infrared spectroscopy (FT-IR). The course of the reaction and the formation of the four novel tellurium-rhenium carbonyls can be directly correlated to the reaction conditions and especially to the acidity of the IL.
3, {Te3I2(μ-I)3(μ3-I)}Re(CO)3 (3) was realized with a Te3I3 ring and μ3-coordinating iodine. Finally, [BMIm][(Te2)3{Re(CO)3}2{Re(CO)4}3] (4) was prepared in [BMIm][OTf] (OTf: triflate) and contains the ufosan-like anion [(Te2)3{Re(CO)3}2{Re(CO)4}3]− with three Te22− and two Re(CO)3+ units that establish a distorted heterocuban-like cage. The compounds were characterized by single-crystal structure analysis, energy dispersive X-ray (EDX) analysis, thermogravimetry (TG), and infrared spectroscopy (FT-IR). The course of the reaction and the formation of the four novel tellurium-rhenium carbonyls can be directly correlated to the reaction conditions and especially to the acidity of the IL.
Introduction
Due to their high redox stability, ionic liquids (ILs) turned out as suitable reaction media for the synthesis of metal clusters and reactive carbonyl compounds.1–4 A fascinating structure, for instance, relates to the guest-free germanium clathrate □24Ge136 (□ indicates non-occupied lattice sites) representing a novel element modification of germanium.5 In view of carbonyls, especially compounds that are neither electronically nor sterically stabilized by alkyl or aryl ligands were obtained by IL-based synthesis. Such non-stabilized carbonyls were denoted as extremely labile and highly sensitive.6 Carbonyls without alkyl/aryl stabilization but with even more destabilizing halide ligands were actually considered as non-producible. Here, ILs reveal their full potential as they are exactly suitable to prepare such reactive carbonyl compounds.2,3 Beside excellent redox stability, the weekly coordinating properties and the stabilization via inherent cation–anion interactions turned out as further merits of ILs.1–4 By now, however, many IL-based syntheses predominately remain explorative and without specific IL-based control and variability of the resulting compound. Only recently, first attempts to understand the decisive reaction parameters and studies to steer the course of the reaction were reported.4 Especially, Lewis-acidic ILs such as [Cation][AlX4] (X = Cl, Br) seem to allow specific control over the product composition.4,7,8Based on our previous studies, we could already realize several novel, reactive carbonyl compounds such as the adamantane-like Fe4Sn6 cluster in [{Fe(CO)3}4{SnI}6I4]2−,9 the ufosane-like Te6Mn2 in [(Te2)3{Mn(CO)3}2{Mn(CO)4}3]−,10 or the anti-(WCl2)6-type anion [(Pb6I8){Mn(CO)5}6]2−.11 All of them were prepared via IL-based synthesis. Aiming at novel carbonyls, we have yet addressed the system TeI4/Re2(CO)10. Here, all already known compounds are essentially stabilized by organic ligands. Re(CO)3{TeIPh}3(μ3-I),12 for instance, exhibits a central Re atom that is distorted octahedrally coordinated by three Te atoms and three CO ligands. Additionally, Te is connected to iodine and phenyl (Ph) ligands. IRe(CO)4Te(CH3)2, Re(CO)3I(TePh2)2 and {Re(CO)3I}2Te2Ph2 also exhibit Te–Re bonds with iodine coordinated to Re.13 Furthermore, several compounds contain Te–Re–Te or Re–Te–Re strings.13b,c,14 Some examples exhibit four-membered Te2Re2 rings such as in [{Re(CO)4}2{μ-TePh}2].15 Few more sophisticated arrangements were described, including a trigonal planar Te with three Re(CO)5 units in [Te{Re(CO)5}3][BF4]16 or two Re(CO)3 groups bridged by three Te atoms in [{Re(CO)3}2(μ-TePh)3]−.17 Finally, the largest Te–Re arrangements were reported for the distorted heterocubane [Re4Te4(CN)12]4−,18 as well as for (Cp*)3Re3Te8,19 [Re6Te8(CN)6]4−,20 or [Re6Te8(TeI2)]I2,21 in which Re6 octahedra are capped by Te atoms.
For the first time, we here use IL-based synthesis to study the system TeI4/Re2(CO)10. As a result, four new Te–Re carbonyl compounds can be identified, including [TeI2Re(CO)5][AlCl4] (1), [BMIm][Te2I4(μ-I)2Re(CO)4] (2), {Te3I2(μ-I)3(μ3-I)}Re(CO)3 (3), and [BMIm][(Te2)3{Re(CO)3}2{Re(CO)4}3] (4). In addition to the identification of the novel compounds, specific control of the resulting carbonyl is possible depending on the anion and acidity of the applied IL.
Results and discussion
[TeI2Re(CO)5][AlCl4] (1)
[TeI2Re(CO)5][AlCl4] was prepared by reacting TeI4 and Re2(CO)10 in a mixture of [BMIm]Cl and AlCl3 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The synthesis results in red crystals of 1 with two minor by-phases: colorless crystals of excess Re2(CO)10 and yellow needles of [{Re(CO)5}2I][Al2Cl7]. The presence of [{Re(CO)5}2I][Al2Cl7] was validated by crystal structure analysis (ESI: Table S1 and Fig. S1, S2‡), cation and anion, however, are well-known with, e.g., [{Re(CO)5}2I]+ in [{Re(CO)5}2I][AsF6].221 crystallizes with monoclinic symmetry in P21/c and contains [TeI2Re(CO)5]+ cations and [AlCl4]− anions (Table 1 and Fig. 1a). By comparison with similar compounds, an oxidation of Re0 to Re+I and a reduction of Te+IV to Te+II seems reasonable.13 Accordingly, the synthesis can be rationalized by the following equation:
1 ratio. The synthesis results in red crystals of 1 with two minor by-phases: colorless crystals of excess Re2(CO)10 and yellow needles of [{Re(CO)5}2I][Al2Cl7]. The presence of [{Re(CO)5}2I][Al2Cl7] was validated by crystal structure analysis (ESI: Table S1 and Fig. S1, S2‡), cation and anion, however, are well-known with, e.g., [{Re(CO)5}2I]+ in [{Re(CO)5}2I][AsF6].221 crystallizes with monoclinic symmetry in P21/c and contains [TeI2Re(CO)5]+ cations and [AlCl4]− anions (Table 1 and Fig. 1a). By comparison with similar compounds, an oxidation of Re0 to Re+I and a reduction of Te+IV to Te+II seems reasonable.13 Accordingly, the synthesis can be rationalized by the following equation:| Data | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sum formula | C5O5AlCl4I2TeRe | C12H15N2O4I6Te2Re | C3O3I7Te3Re | C26H15N2O18Te6Re5 |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Triclinic |
| Space group | P21/c | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| Lattice parameters | a = 1634.8(3) pm | a = 970.9(2) pm | a = 853.8(2) pm | a = 1152.1(2) pm |
| b = 1728.7(3) pm | b = 1353.9(3) pm | b = 996.1(2) pm | b = 1193.0(2) pm | |
| c = 1386.3(3) pm | c = 2297.6(6) pm | c = 1322.9(2) pm | c = 1925.5(4) pm | |
| α = 90° | α = 90° | α = 84.3(1)° | α = 74.8(1)° | |
| β = 108.1(1)° | β = 108.6(1)° | β = 72.8(1)° | β = 73.3(1)° | |
| γ = 90° | γ = 90° | γ = 86.0(1)° | γ = 64.6(1)° | |
| Cell volume | V = 3724.1 × 106 pm3 | V = 2862.2 × 106 pm3 | V = 1068.6 × 106 pm3 | V = 2260.5 × 106 pm3 |
| Formula units per cell | Z = 8 | Z = 4 | Z = 2 | Z = 2 |
| Calculated density | ρ = 3.126 g cm−3 | ρ = 3.374 g cm−3 | ρ = 4.790 g cm−3 | ρ = 3.438 g cm−3 |
| Measurement limits | −22 ≤ h ≤ 22, −23 ≤ k ≤ 21, −18 ≤ l ≤ 19 | −13 ≤ h ≤ 13, −18 ≤ k ≤ 0, −31 ≤ l ≤ 12 | −11 ≤ h ≤ 10, −13 ≤ k ≤ 13, −18 ≤ l ≤ 18 | −15 ≤ h ≤ 15, −16 ≤ k ≤ 16, −26 ≤ l ≤ 26 |
| Theta range for data collection | 3.5 to 58.8° | 3.0 to 58.5° | 4.1 to 58.6° | 3.8 to 58.8° |
| Linear absorption coefficient | μ = 12.00 mm−1 | μ = 12.73 mm−1 | μ = 19.81 mm−1 | μ = 17.21 mm−1 |
| Number of reflections | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854 (14 854 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 047 independent) 047 independent) |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 (24 083 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244 independent) 244 independent) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 458 (5317 independent) 458 (5317 independent) |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749 (12 749 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235 independent) 235 independent) |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Merging | R int = 0.149 | R int = 0.072 | R int = 0.058 | R int = 0.107 |
| Number of parameters | 343 | 264 | 154 | 515 |
| Residual electron density | 2.94 to −1.50 e−·10−6 pm−3 | 1.67 to −1.74 e−·10−6 pm−3 | 1.91 to −1.87 e−·10−6 pm−3 | 1.57 to −2.30 e−·10−6 pm−3 |
| Figures of merit | R 1 (I ≥ 4σI) = 0.049 | R 1 (I ≥ 4σI) = 0.032 | R 1 (I ≥ 4σI) = 0.033 | R 1 (I ≥ 4σI) = 0.038 |
| R 1 (all data) = 0.145 | R 1 (all data) = 0.041 | R 1 (all data) = 0.092 | R 1 (all data) = 0.12 | |
| wR2 (all data) = 0.096 | wR2 (all data) = 0.093 | wR2 (all data) = 0.055 | wR2 (all data) = 0.068 | |
| GooF = 0.728 | GooF = 0.956 | GooF = 0.705 | GooF = 0.731 |
Beside single crystal structure analysis, the composition of 1 was verified by EDX analysis. The observed Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I
I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re ratio of 0.87
Re ratio of 0.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.63 (scaled on I) fits well with expected ratio (1
0.63 (scaled on I) fits well with expected ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
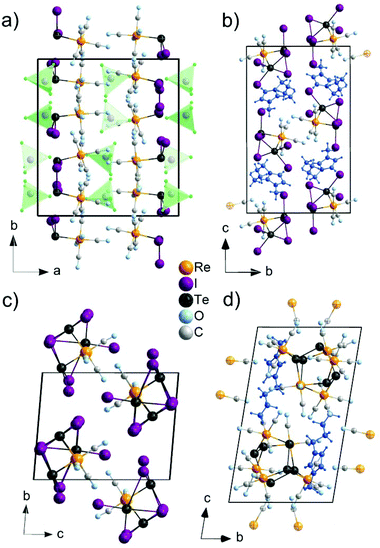
The [TeI2Re(CO)5]+ cation exhibits Te–Re distances of 275.9(1) (Re1–Te1) and 275.7(1) pm (Re2–Te2) (Fig. 2a). These distances are very similar to those in 2, 3, 4 and also compare to literature data, e.g., 275.5–276.3 pm in Re(CO)3{TeIC6H5}3(μ3-I)12 or 272.9–274.1 pm in [Re(CO)3Cl{o-C6H4(TeMe)2}] (Table 2).14 In comparison to [Re6Te8(TeI2)]I2,21 in which TeI2 units are coordinated to each Re atom of a Re6 octahedron (Te–Re 266.4–267.2 pm), the distances in the title compound are slightly longer, which can be ascribed to the different bonding situation and valence state of Re. Furthermore, the Te atom is coordinated like a distorted trigonal pyramid and exhibits two Te–I bonds with distances of 269.4(2) (Te2–I3) to 272.6(2) pm (Te2–I4) in addition to the Te–Re bond and the remaining lone pair. Again, these distances fit well with literature, e.g., 275.4–278.8 pm for terminally bond I atoms in the Te4I16 building unit of TeI4.23
| Te–Re/pm | Te–Te/pm | Te–I/pm | |
|---|---|---|---|
| 1 | 275.8(1)–275.9(1) | — | 269.4(2)–272.6(2) |
| 2 | 276.7(1)–276.8(1) | — | μ1: 275.9(1)–292.1(1) |
| μ2: 300.3(1)–314.8(1) | |||
| 3 | 272.2(1)–274.0(1) | — | μ1: 276.2(2)–281.7(2) |
| μ2/short: 274.4–276.0 | |||
| μ2/long: 349.6–379.8 | |||
| μ3: 318.7–331.5 | |||
| 4 | 276.3(1)–280.1(1) | 278.7(1)–279.5(1) |
The [TeI2Re(CO)5]+ cation, finally, exhibits an almost octahedrally coordinated Re atom with five carbonyl ligands and a tellurium atom (Fig. 1a). The Re–(CO) distances range from 193(2) (Re2–C10) to 206(2) pm (Re1–C3). These values relate to known Re–(CO) distances, e.g., 192.9–200.7 pm in Re2(CO)10.24
[BMIm][Te2I4(μ-I)2Re(CO)4] (2)
[BMIm][Re(CO)4Te2I2(μ-I)2] was obtained with shiny black crystals by a similar reaction as 1, but with an increased amount of AlCl3, thus, with an [BMIm]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3 ratio of 1
AlCl3 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The compound 2 crystallizes in the monoclinic space group P21/c and consists of [BMIm]+ cations, stemming from the IL, and [Te2I4(μ-I)2Re(CO)4]− anions (Table 1 and Fig. 1b). The synthesis of 2 can be rationalized based on a similar redox reaction as for 1:
2. The compound 2 crystallizes in the monoclinic space group P21/c and consists of [BMIm]+ cations, stemming from the IL, and [Te2I4(μ-I)2Re(CO)4]− anions (Table 1 and Fig. 1b). The synthesis of 2 can be rationalized based on a similar redox reaction as for 1:The [Te2I4(μ-I)2Re(CO)4]− anion consists of a Te2I4(μ-I)2 unit that is bond to a Re(CO)4+ group (Fig. 2b). With 276.7(1) (Te2–Re1) and 276.8(1) pm (Te1–Re1), the Te–Re distances are similar to 1 and compare with literature data as well (Table 2).12,14 Both Te atoms are interlinked by two I atoms with distances of 300.3(1) (Te1–I3) to 343.3(1) pm (Te2–I3). These distances are slightly longer compared to the bridging I atoms in the Te4I16 building unit of TeI4 (293.2–327.8 pm).23 In addition, each Te atom is terminally coordinated to two I atoms. The respective Te–I distances (Te2–I1: 275.9(1), Te1–I6: 292.1(1) pm) are also in accordance with literature (Table 2).23 Taken together, each Te atom is coordinated like a distorted square pyramid with four iodine atoms forming a square and one Re atom as top of the pyramid (Fig. 2b). Together with the remaining lone pair a pseudo octahedron around Te is formed. The respective angles range from 83.4 (Re1–Te2–I3) to 99.7° (Re1–Te2–I2). Similar to 1, Re is distorted octahedrally coordinated by four CO ligands and two Te atoms. The Re–(CO) distances (Re1–C4: 194.2(8) to Re1–C3: 201.8(7) pm) compare to those in 1 and Re2(CO)10.24 Finally, the composition of 2 was validated by EDX analysis resulting a Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I
I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re ratio of 2.1
Re ratio of 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (scaled on I), which is well in accordance with the expectation (2
1.0 (scaled on I), which is well in accordance with the expectation (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Te3I3(μ-I)3(μ3-I)Re(CO)3 (3)
Following the synthesis strategy of 1 and 2, Te3I3(μ-I)3(μ3-I)Re(CO)3 was obtained upon further increasing the amount of AlCl3 resulting in a [BMIm]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3 ratio of 1
AlCl3 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. 3 formed shiny black crystals and crystallizes triclinically with the space group PN (Table 1 and Fig. 1c). EDX analysis confirms the chemical composition with a Te
3. 3 formed shiny black crystals and crystallizes triclinically with the space group PN (Table 1 and Fig. 1c). EDX analysis confirms the chemical composition with a Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I
I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re of 2.7
Re of 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 (scaled on I, expected: 3
0.9 (scaled on I, expected: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The synthesis can be ascribed to the following redox reaction:
1). The synthesis can be ascribed to the following redox reaction:Te3I3(μ-I)3(μ3-I)Re(CO)3 contains a Te3I3(μ-I)3(μ3-I) unit that is bond to a Re(CO)3 unit (Fig. 2c and d). The Te–Re distances range from 272.2(1) (Te3–Re1) to 274.0(1) pm (Te2–Re1) and fit well to the distances observed in 1 and 2 (Table 2). Moreover, each Te atom is terminally bond to one iodine atom with comparable distances (Te3–I7: 276.2(2) to Te1–I5: 281.7(2) pm) as observed in 1, 2 or the terminally bond I in TeI4.23 In addition, one iodine atom (I1) is μ3-coordinated to all Te atoms (Fig. 2d). The observed distances of 318.7 (Te1–I1) to 331.5 (Te2–I1) pm are in agreement with Te–I distances of μ3-coordinated iodine of the Te4I16 building unit in TeI4 (319.8–324.1 pm).23 Such μ3-coordination of iodine to tellurium is yet only known for {(C6H5)TeI4Te(C6H5)SCN2H4}2 and o-C6H4(CH2TeMe2I)2 as additional examples.25 Finally, three iodine atoms (I2, I3, I4) bridge two Te atoms, leading to a Te3I3 ring (Fig. 2c). These μ2-I atoms exhibit a short distance to one Te atom (274.4–276.0 pm) and comparably long distances to the other Te atom (349.6–379.8 pm) (Fig. 2d). Thus, bonding of iodine is significantly stronger to one of the Te atoms, which is similarly observed for Te–I distances of the dimer {(C6H5)TeI4Te(C6H5)SCN2H4}2.25
Re is again coordinated like a distorted octahedron. In 3, however, Re is only bond by three carbonyl ligands in addition to three Te atoms (Fig. 2c and d). The Re–(CO) distances range from 192(6) (Re1–C2) to 195(1) pm (Re1–C1) and compare to those in 1, 2 or Re2(CO)10.24
[BMIm][(Te2)3{Re(CO)3}{Re(CO)4}3] (4)
In difference to 1, 2, and 3, the forth Te–Re carbonyl compound [BMIm][(Te2)3{Re(CO)3}{Re(CO)4}3] was prepared in [BMIm][OTf] as the IL instead of [BMIm]Cl/AlCl3. In this regard, [BMIm][OTf] can be considered as non-acidic IL. Again, TeI4 and Re2(CO)10 were used as starting materials and reacted at 130 °C. The synthesis of 4 can be ascribed to a reduction of Te+IV to Te−I with oxidation of Re0 to Re+I according to the following equation:This sequence of reaction, in fact, compares to the reaction of TeI4 and Mn2(CO)10 to [BMIm][(Te2)3{Mn(CO)3}{Mn(CO)4}3], which we reported previously.10
4 crystallizes triclinically (space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Table 1) and contains the anion [(Te2)3{Re(CO)3}{Re(CO)4}3]− and the IL-cation [BMIm]+ (Fig. 1d). The [(Te2)3{Re(CO)3}{Re(CO)4}3]− anion exhibits three Te22− ditelluride units and two Re(CO)3+ fragments that establish a distorted heterocubane-like Te6Re2 unit with angles of 72.9(1) to 112.9(1)° (Fig. 2e and f). Three edges of this distorted cube are additionally bridged by Re(CO)4+ fragments (Fig. 2f). The Te–Te distances in the Te22− units with 278.7(1) (Te1–Te2) to 279.5(1) pm (Te5–Te6) can be considered as single bonds and fit with [Mn4(CO)13(Te2)3]2− (272.3–277.7 pm)26 or [(Te2)3{Mn(CO)3}2{Mn(CO)4}3]− (277.0–278.3 pm)10 as reference compounds.
, Table 1) and contains the anion [(Te2)3{Re(CO)3}{Re(CO)4}3]− and the IL-cation [BMIm]+ (Fig. 1d). The [(Te2)3{Re(CO)3}{Re(CO)4}3]− anion exhibits three Te22− ditelluride units and two Re(CO)3+ fragments that establish a distorted heterocubane-like Te6Re2 unit with angles of 72.9(1) to 112.9(1)° (Fig. 2e and f). Three edges of this distorted cube are additionally bridged by Re(CO)4+ fragments (Fig. 2f). The Te–Te distances in the Te22− units with 278.7(1) (Te1–Te2) to 279.5(1) pm (Te5–Te6) can be considered as single bonds and fit with [Mn4(CO)13(Te2)3]2− (272.3–277.7 pm)26 or [(Te2)3{Mn(CO)3}2{Mn(CO)4}3]− (277.0–278.3 pm)10 as reference compounds.
The intermolecular distances between different Te22− groups in the heterocubane range from 381.1(1) (Te2–Te6) to 383.6(1) pm (Te1–Te4), which is still below the doubled van der Waals radius (418 pm),27 indicating at least weak attractive interactions. Finally, Te22− groups are bridged by Re(CO)4+ units with Te–Re distances of 277.9(1) (Te2–Re3) to 280.1(2) pm (Te5–Re5), which is very similar to the respective distances in 1, 2, and 3 (276.3(1)–276.6(1) pm) (Table 1). Finally, EDX was again used to validate the chemical composition of 4, resulting in a Re![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te ratio of 4.7
Te ratio of 4.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (scaled on Te), which fits with the expectation (5
6 (scaled on Te), which fits with the expectation (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6).
6).
In regard of structure and connectivity of [(Te2)3{Mn(CO)3}2{Mn(CO)4}3]−, the Te6Re5 arrangement can be compared to the so-called ufosane anion P113− (Fig. 2e).28 Beside the similar shape, moreover, the electronic situation can be considered comparable. Thus, the electron deficient fragments Re(CO)3+ (Re: 12e−) and Re(CO)4+ (Re: 14e−) form three and two additional bonds. In contrast to P113− with each phosphor atom contributing one electron to each P–P bond, coordinative bonding is observed in 4 between the electron deficient Re(CO)3+/Re(CO)4+ fragments and the electron-rich Te22− units.
Comparison of synthesis conditions and compounds
The synthesis of the title compounds 1–4 can be obviously controlled by the conditions of the reaction. To avoid an unrulable variation of conditions, several parameters were kept constant to allow direct comparison. Thus, temperature (130 °C), duration of heating (96 h) and the amount of TeI4 (0.126 mmol) and Re2(CO)10 (1.718 mmol) with a Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re ratio of 1
Re ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 were used to obtain all Te-Re carbonyls. In this regard, it needs to be noticed that the Te
27 were used to obtain all Te-Re carbonyls. In this regard, it needs to be noticed that the Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Re ratio of 1, 2 and 3 is identical (1
Re ratio of 1, 2 and 3 is identical (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) anyway. Excessive Re2(CO)10 is nevertheless essential due to the underlying redox reaction and the formation of [Re(CO)5]+ as a by-product of all syntheses. In regard of the stability of 1–4, moreover, appreciable intermolecular interactions are lacking, and therefore, not decisive for the obtained compounds. Essential changes of the experimental conditions relate to the increasing amount of AlCl3, and thus, the increasing acidity of the IL in the direction 1 ([BMIm]Cl
1) anyway. Excessive Re2(CO)10 is nevertheless essential due to the underlying redox reaction and the formation of [Re(CO)5]+ as a by-product of all syntheses. In regard of the stability of 1–4, moreover, appreciable intermolecular interactions are lacking, and therefore, not decisive for the obtained compounds. Essential changes of the experimental conditions relate to the increasing amount of AlCl3, and thus, the increasing acidity of the IL in the direction 1 ([BMIm]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3 at 1
AlCl3 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to 3 ([BMIm]Cl
1) to 3 ([BMIm]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3 at 1
AlCl3 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and the use of the acid–base neutral IL [BMIm][OTf] for 4.
3) and the use of the acid–base neutral IL [BMIm][OTf] for 4.
The course of the reaction can be clearly arranged by focusing on the starting materials and the additional products besides the title compounds 1–4 as shown below:
| 1TeI4 + 1Re2(CO)10 + [AlCl4]− → 1 + 1[Re(CO)5]+ + 2I− |
| 2TeI4 + 2Re2(CO)10 + [BMIm]+ → 2 + 3[Re(CO)5]+ + 2I− + 1CO |
| 3TeI4 + 3Re2(CO)10 → 3 + 5[Re(CO)5]+ + 5I− + 2CO |
| 6TeI4 + 15Re2(CO)10 + [BMIm]+ → 4 + 25[Re(CO)5]+ + 24I− + 7CO |
In contrast to other metal carbonyls that we obtained by IL-based synthesis,9–11 only a minor amount of CO (in relation to the CO present in the starting materials) was released. Whereas the formation of, for instance, the Fe4Sn6 cluster in [{Fe(CO)3}4{SnI}6I4]2− is clearly driven by CO release,9 the entropic effect is limited here. For 1–3, in general, the number of remaining CO ligands decreases (5 in 1, 4 in 2, 3 in 3), whereas the number of Te–Re is increased (1 in 1, 2 in 2, 3 in 3). This variation can be directly related to the amount of AlCl3 and the Lewis acidity of the applied IL. Since the formation of [AlCl4]− is limited at [BMIm]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3 ratios <1, excess AlCl3 tends to coordinate I− stemming from TeI4 and thereby supports the formation of Te–Re bonds and the occurrence of μ2- or μ3-brided I atoms. Finally, it needs to be noticed that the solubility of TeI4 and Re2(CO)10 is poor in [BMIm]Cl/AlCl3, especially at 1
AlCl3 ratios <1, excess AlCl3 tends to coordinate I− stemming from TeI4 and thereby supports the formation of Te–Re bonds and the occurrence of μ2- or μ3-brided I atoms. Finally, it needs to be noticed that the solubility of TeI4 and Re2(CO)10 is poor in [BMIm]Cl/AlCl3, especially at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Thus, excess AlCl3 not only influences the type of product, but also supports the solubility of the starting materials. [BMIm][OTf] used for the synthesis of 4 is much less polar than [BMIm]Cl/AlCl3, resulting in a good solubility of TeI4 and Re2(CO)10. The higher concentration of dissolved reactants, on the one hand, obviously supports the redox reaction and results in the oxidation of rhenium with formation of [Re+I(CO)5]+ as observed for all title compounds. Whereas Te+IV was reduced to Te+II in the case of 1–3, the reduction proceeds to Te−I only for 4, resulting in Te–Te bonds and the total absence of Te–I bonds.
1 ratio. Thus, excess AlCl3 not only influences the type of product, but also supports the solubility of the starting materials. [BMIm][OTf] used for the synthesis of 4 is much less polar than [BMIm]Cl/AlCl3, resulting in a good solubility of TeI4 and Re2(CO)10. The higher concentration of dissolved reactants, on the one hand, obviously supports the redox reaction and results in the oxidation of rhenium with formation of [Re+I(CO)5]+ as observed for all title compounds. Whereas Te+IV was reduced to Te+II in the case of 1–3, the reduction proceeds to Te−I only for 4, resulting in Te–Te bonds and the total absence of Te–I bonds.
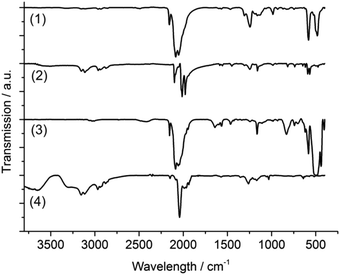
For further characterization and comparison of the Te–Re carbonyl compounds 1–4, Fourier-transformed (FT-IR) spectroscopy and thermogravimetry (TG) were performed. According to FT-IR, all compounds show characteristic CO vibrations (Fig. 3), which can be assigned to strong ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and weaker δ(Re–C
O) and weaker δ(Re–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) vibrations.29 By tendency, the C
O) vibrations.29 By tendency, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration is shifted to higher wavenumbers with increasing number of electronegative Te atoms coordinated to rhenium (1 → 2 → 3/4) (Table 3). Moreover, the CO vibrations broaden with the number of non-symmetry equivalent CO groups (1 → 2/3 → 4). Additional vibrations of 2 and 4 stem from the [BMIm]+ cation (ν(C–H): 3150–2900 cm−1, fingerprint area: 1600–1100 cm−1, Fig. 3). Moreover, certain vibrations relate to the inert oil (perfluoroalkylether), in which the crystals were embedded for stabilization (1308, 1244, 1185, 1162, 985 cm−1). The observed CO vibrations are well in agreement with terminal carbonyl ligands as observed, for instance, in Re2(CO)10 or Re(CO)5Cl (Table 3).24,29
O vibration is shifted to higher wavenumbers with increasing number of electronegative Te atoms coordinated to rhenium (1 → 2 → 3/4) (Table 3). Moreover, the CO vibrations broaden with the number of non-symmetry equivalent CO groups (1 → 2/3 → 4). Additional vibrations of 2 and 4 stem from the [BMIm]+ cation (ν(C–H): 3150–2900 cm−1, fingerprint area: 1600–1100 cm−1, Fig. 3). Moreover, certain vibrations relate to the inert oil (perfluoroalkylether), in which the crystals were embedded for stabilization (1308, 1244, 1185, 1162, 985 cm−1). The observed CO vibrations are well in agreement with terminal carbonyl ligands as observed, for instance, in Re2(CO)10 or Re(CO)5Cl (Table 3).24,29
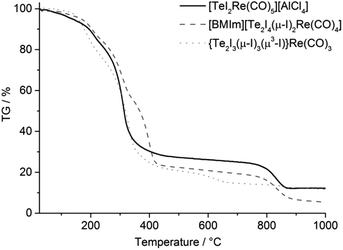
TG was used to compare the thermal stability of the Te–Re carbonyls 1, 2 and 3 (Fig. 4). Since 4 was only obtained with minor quantities, TG of this phase could not be performed. Generally, all compounds show two-step decomposition with slow release starting already at room temperature. At temperatures higher than 180 °C, the decomposition becomes very fast and results in a major release (70–80 wt%) of gaseous compounds up to 450 °C. A second decomposition step (about 15 wt%) occurred for 1 and 2 at 750–850 °C (Fig. 4). Although the thermal decomposition is generally very similar, the neutral compound 3 shows the lowest decomposition temperature, which can be ascribed to the lacking lattice energy in comparison to the ionic compounds 1 and 2. All in all, the thermal decomposition can be ascribed to the following reactions:
| Te3I7Re(CO)3 (3) → 3CO + 3.5I2 + 3Te + Re |
Thus, elemental rhenium remains as thermal residue, which is in accordance with a black, metallic film on the crucible surface. Whereas the first massive decomposition step can be explained by more-or-less simultaneous release of Re(CO)5, CO, TeCl4, TeI4 and C8H15N2I, the second decomposition step relates to the evaporation of Al2Cl6 for 1 and the evaporation of tellurium for 2.
Conclusions
The four novel Te–Re carbonyls [TeI2Re(CO)5][AlCl4] (1), [BMIm][Re(CO)4Te2I4(μ-I)2] (2), Re(CO)3{Te3I2(μ-I)3(μ3-I)} (3) and [BMIm][(Te2)3{Re(CO)3}2{Re(CO)4}3] (4) are presented for the first time. The title compounds were prepared by reaction of TeI4 and Re2(CO)10 in [BMIm]Cl/AlCl3 or [BMIm][OTf]. All of them turned out as highly reactive and sensitive to air and moisture, which can be ascribed to the absence of stabilizing alkyl and aryl ligands.All title compounds were characterized via single-crystal structure analysis, EDX and FT-IR spectroscopy. Thus, 1 contains a [TeI2Re(CO)5]+ cation, 2 the anion [Te2I4(μ-I)2Re(CO)4]−. 3 exhibits a remarkable Te3I3 ring and μ3-coordinating iodine. 4 contains the ufosan-like anion [(Te2)3{Re(CO)3}2{Re(CO)4}3]− with three Te22− and two Re(CO)3+ units that establish a distorted heterocubane-like cage.
IL-based synthesis turned out as a versatile strategy to obtain reactive metal carbonyl compounds. The formation of the compounds 1–4 can be steered by the type and acidity of the IL. Thus, depending on the amount of AlCl3 applied in the synthesis, the number of CO ligands decreases from 5 (1) via 4 (2) to 3 (3) and the number of Te–Re bonds increases from 1 (1) via 2 (2) to 3 (3). In addition, 1 exhibits only terminally bond I atoms, 2 bridging (μ2) and terminally coordinated I atoms and 3 bridging (μ2-, μ3) and terminally coordinated I atoms. Hence, the connectivity of iodine can be as well related to the amount of AlCl3 and the acidity of the IL. In the case of the acid–base neutral IL [BMIm][OTf] for the synthesis of 4, the significantly increased solubility of TeI4 and Re2(CO)10 is decisive and results in a different redox reaction with Te+IV reduced to Te−I, whereas the reduction is limited to Te+II for 1–3. Taken together, IL-based synthesis allows preparing four different Te–Re carbonyls in the system TeI4/Re2(CO)10 with good control due to specific experimental conditions. This observation leaves plenty of room for reaction control and the realization of numerous novel compounds in other systems.
Experimental
Synthesis
In alternative to the above recipe with [BMIm]Cl and AlCl3, 2 can be also prepared using [BMIm][OTf] as the IL. This route, however, resulted in a mixture of 2 and 4, whereas the reaction with [BMIm]Cl and AlCl3 as IL yields 2 as sole Te–Re compound.
Analytical equipment
Single-crystal X-ray structure analysis. For single crystal structure analysis, suitable crystals were selected manually, covered by inert-oil (perfluoropolyalkylether, ABCR), and placed in a glass capillary (Hilgenberg) that was sealed immediately. Data collection was performed at 200 K on an IPDS II image-plate diffractometer (Stoe, Darmstadt) using Mo-Kα radiation (λ = 0.71073 Å, graphite monochromator). Data reduction and numerical absorption correction were conducted by the X-AREA software package (version 1.26).30 Due to the high sensitivity of the title compounds and the resulting limited crystal quality (especially for 1), proper correction of absorption effects was difficult. Space group determination based on systematic absence of reflections, structure solution by direct methods and refinement were performed by XPREP and SHELXTL (version 6.14, SHELXS-97).31 All non-hydrogen atoms were refined anisotropically. Detailed information on crystal data and structure refinement is listed in Table 1. DIAMOND was used for all illustrations.32 Further details of the crystal structure investigation may be obtained from the joint CCDC/FIZ Karlsruhe deposition service on quoting the depository numbers CCDC 1913799–1913802.‡Fourier-transformed infrared (FT-IR) spectra were recorded on a Bruker Vertex 70 FT-IR spectrometer (Bruker). The samples were measured as pellets in KBr. Thus, 300 mg of dried KBr and 0.5–1.0 mg of the sample were carefully pestled together and pressed to a thin pellet.
Thermogravimetry (TG) was carried out with a Netzsch STA 449 F3 Jupiter device using α-Al2O3 as crucible material and reference. Buoyancy effects were corrected by baseline subtraction of a blank measurement. The samples were measured under dried nitrogen up to 800 °C with a heating rate of 10 K min−1.
Energy dispersive X-ray (EDX) analysis was performed using an Ametec EDAX mounted on a Zeiss SEM Supra 35 VP scanning electron microscope. The samples were prepared in the glove-box by selecting single crystals that were fixed on a conductive carbon pad on an aluminum sample holder. The samples were handled under inert conditions during transport and sample preparation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Deutsche Forschungsgemeinschaft (DFG) for funding in the Priority Program SPP1708 “Synthesis near room temperature”.Notes and references
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2008 Search PubMed.
- D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050 CrossRef CAS PubMed (Review).
- E. Ahmed and M. Ruck, Dalton Trans., 2011, 40, 9347 RSC (Review).
- M. F. Groh, A. Wolff, M. A. Grasser and M. Ruck, Int. J. Mol. Sci., 2016, 17, 1452 CrossRef.
- A. M. Guloy, R. Ramlau, Z. Tang, W. Schnelle, M. Baitinger and Y. Grin, Nature, 2006, 443, 320 CrossRef CAS.
- (a) P. Braunstein, L. A. Oro and P. R. Raithby, Metal Clusters in Chemistry, Wiley-VCH, Weinheim, 2008 Search PubMed; (b) G. Frenking and N. Froehlich, Chem. Rev., 2000, 100, 717 CrossRef CAS.
- (a) M. F. Groh, A. Isaeva, U. Müller, P. Gebauer, M. Knies and M. Ruck, Eur. J. Inorg. Chem., 2016, 2016, 880 CrossRef CAS; (b) M. F. Groh, J. Breternitz, E. Ahmed, A. Isaeva, A. Efimova, P. Schmidt and M. Ruck, Z. Anorg. Allg. Chem., 2015, 641, 388 CrossRef CAS; (c) E. Ahmed, A. Isaeva, A. Fiedler, M. Haft and M. Ruck, Chem. – Eur. J., 2011, 17, 6847 CrossRef CAS; (d) E. Ahmed, J. Breternitz, M. F. Groh, A. Isaeva and M. Ruck, Eur. J. Inorg. Chem., 2014, 2014, 3037 CrossRef.
- (a) S. Wolf and C. Feldmann, Z. Anorg. Allg. Chem., 2017, 643, 25 CrossRef CAS; (b) S. Wolf, W. Klopper and C. Feldmann, Chem. Commun., 2018, 54, 1217 RSC.
- S. Wolf, F. Winter, R. Pöttgen, N. Middendorf, W. Klopper and C. Feldmann, Chem. – Eur. J., 2012, 18, 13600 CrossRef CAS.
- S. Wolf and C. Feldmann, Z. Anorg. Allg. Chem., 2012, 638, 1787 CrossRef CAS.
- S. Wolf, K. Reiter, F. Weigend, W. Klopper and C. Feldmann, Inorg. Chem., 2015, 54, 3989 CrossRef CAS.
- Y. V. Torubaev, A. A. Pasynskii and P. Mathur, Russ. J. Coord. Chem., 2009, 35, 807 CrossRef CAS.
- (a) P. Jeitner and W. Winder, Inorg. Chim. Acta, 1987, 134, 201 CrossRef; (b) W. Hieber, W. Opavsky and W. Rohm, Chem. Ber., 1968, 101, 2244 CrossRef CAS; (c) F. Calderazzo, D. Vitali, R. Poli, J. L. Atwood, R. D. Rogers, J. M. Cummings and I. Bernal, J. Chem. Soc., Dalton Trans., 1981, 1004 RSC.
- W. Levason, S. D. Orchard and G. Reid, Organometallics, 1999, 18, 1275 CrossRef CAS.
- J. Muthukumaran, M. Kannan, A. Vanitha, B. Manimaran and R. Krishnaa, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, m558 CrossRef CAS.
- W. Beck, W. Sacher and U. Nagel, Angew. Chem., 1986, 98, 280 CrossRef CAS.
- A. A. Pasynskii, S. S. Shapovalov, I. V. Skabitsky, O. G. Ikhonova and T. A. Krishtop, Russ. J. Coord. Chem., 2015, 41, 75 Search PubMed.
- Y. V. Mironov, A. V. Virovets, W. S. Sheldrick and V. E. Fedorov, Polyhedron, 2001, 20, 969 CrossRef CAS.
- G.-X. Jin, Y. Arikawa and K. Tatsumi, J. Am. Chem. Soc., 2001, 123, 735 CrossRef CAS.
- Y. V. Mironov, J. A. Cody, T. E. Albrecht-Schmitt and J. A. Ibers, J. Am. Chem. Soc., 1997, 119, 493 CrossRef CAS.
- V. P. Fedin, V. E. Fedorov, H. Imoto and T. Saito, Polyhedron, 1997, 16, 1615 CrossRef CAS.
- R. Mews, Angew. Chem., 1976, 88, 28 CrossRef.
- (a) V. Paulat and B. Krebs, Angew. Chem., 1976, 88, 28 CrossRef CAS; (b) B. Krebs and V. Paulat, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1976, 32, 1470 CrossRef.
- M. R. Churchill, K. N. Amoh and H. J. Wasserman, Inorg. Chem., 1981, 20, 1609 CrossRef CAS.
- (a) E. Schulz Lang, G. A. Casagrande, G. Manzoni de Oliveira, G. N. Ledesma, S. S. Lemos, E. E. Castellano and U. Abram, Eur. J. Inorg. Chem., 2006, 958 CrossRef; (b) N. J. Hill, W. Levason, G. Reid and A. J. Ward, J. Organomet. Chem., 2002, 642, 186 CrossRef CAS.
- (a) M. A. El-Sayed and H. D. Kaesz, J. Mol. Spectrosc., 1962, 9, 310 CrossRef CAS; (b) E. W. Abel, G. B. Hargreaves and G. Wilkinson, J. Chem. Soc., 1958, 3149 RSC; (c) S. D. Huang, C. P. Lai and C. L. Barnes, Angew. Chem., Int. Ed. Engl., 1997, 36, 1854 CrossRef CAS.
- M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806 CrossRef CAS.
- (a) W. Wichelhaus and H. G. von Schnering, Naturwissenschaften, 1973, 60, 104 CrossRef CAS; (b) H. G. von Schnering and W. Hönle, Chem. Rev., 1988243 Search PubMed.
- N. Flitcroft, D. K. Huggins and H. D. Kaesz, Inorg. Chem., 1964, 3, 1123 CrossRef CAS.
- X-RED32, Data Reduction Program, Version 1.01, Stoe, Darmstadt, 2001 Search PubMed.
- (a) SHELXTL – Structure Solution and Refinement Package. Version 5.1, Bruker AXS, Karlsruhe, 1998 Search PubMed; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS.
- DIAMOND Version 4.2.2. – Crystal and Molecular Structure Visualization, Crystal Impact GbR, Bonn, 2016 Search PubMed.
Footnotes |
| † Dedicated to Professor Annie K. Powell on the occasion of her 60th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1913799–1913802. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9dt01897b |
| This journal is © The Royal Society of Chemistry 2019 |