Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atelocollagen-templated fabrication of tangled fibrous silica†
Tatsuya
Nojima
 *a,
Seiya
Suzuki
b and
Tomokazu
Iyoda
ab
*a,
Seiya
Suzuki
b and
Tomokazu
Iyoda
ab
aIyoda Supra-integrated Material Project Exploratory Research for Advanced Technology (ERATO), Japan Science and Technology Agency (JST), 4259 Nagatsuta-cho, Midori-ku, Yokohama, Kanagawa 226-8503, Japan. E-mail: nojima.t.aa@m.titech.ac.jp
bChemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama, Kanagawa 226-8503, Japan
First published on 6th September 2016
Abstract
Protein-templated structured silica and titania are fabricated via a biomimetic method based on the synergistic effect of amine/carboxyl complexes under ambient conditions. Atelocollagen-templated silica showed a tangled fibrous structure with a smooth surface. The number of carboxyl groups of a protein is an important factor for homogeneous silica growth.
The templating approach is a recent important technique for the controlled fabrication of structured and functional materials. Proteins have well-defined three-dimensional nano-scale structures dependent on the amino acid sequence. The fine structure of a protein is a useful natural resource for the creation of a new class of nano-materials. Protein-templated inorganic minerals are promising as such nano materials, because they would be stable under the harsh conditions for the native protein. The mineralization of proteins should be performed under mild conditions at which the protein is not denatured.
Silica is one of the most abundant minerals and has a wide range of applications. In particular, nano- to meso-structured silica has attracted considerable attention due to their potential applications in nanotechnology and biotechnology. The sol–gel process using silicon alkoxides as the precursors is widely used for template-based synthesis of structured silica, for example, in surfactant-directed synthesis of mesoporous silica.1 While the traditional sol–gel method requires harsh conditions, biomimetic silica synthesis proceeds under gentle conditions. In nature, aquatic life such as diatoms and sponges (phylum Porifera) synthesize structured silica using biocatalysts such as silaffin peptide, silicatein enzyme and polyamines under ambient conditions.2–11 Inspired by them, the mimicking organic molecules have been studied for biomimetic silica synthesis.12–18
Biomimetic protein-templated silica synthesis was pioneered by Shinkai et al.19 In their research, the collagen protein was soaked into a tetraethoxysilane (TEOS) solution for one month, and rod shaped silica was obtained. Collagen-templated silica has been synthesized under various conditions: with different kinds of precursors (TEOS, tetramethoxysilane and Na2SiO3), with or without a catalyst for the hydrolysis of the precursor and varying states of collagen (solution or gelled).20–22 However, the reported methods are time consuming and have shown limited structural reproducibility and dispersion of the fibrous silica.
In this study, we used collagen as the model template protein. Collagen is a rod-like protein with a triple helical structure with a diameter of 1.5 nm, composed of three polypeptide chains with a length of 300 nm and a high aspect ratio (−200). Under physiological conditions, collagen molecules self-assemble into insoluble fibrils. Unlike the heterogeneous reaction dispersing collagen fibrils in the reported methods, we used atelocollagen (AC), pretreated by a proteolytic removal of telopeptides on both ends to suppress fibril formation,23,24 as the homogeneous template for the primary step of such biomineralization processes. The triple helical rod-like fibrous structure of collagen is retained in AC (Fig. S1, ESI†).
It is well known that amine groups are essential for biomimetic silica formation.12,17,18 Ionic complexes of amine groups with carboxyl groups are known to improve silicification activity.25 Jin et al. fabricated chiral silica from an ionic complex of polyethyleneimine (PEI) with chiral tartaric acid under ambient conditions.26 The PEI/tartaric acid complex catalyzes the hydrolysis of TEOS eliminating the need for pre-hydrolysis of the precursor by an acid or base catalyst. This hydrolysis activity of amine–carboxyl complexes would offer two good advantages for our protein-templated silica synthesis. Firstly, the precursor silicon alkoxide is typically pre-hydrolyzed by a strong acid or base catalyst, which has an adverse effect on the native protein. Secondly, since the hydrolyzed reactive species is generated on the surface of the complex, the polycondensation of silica proceeds in the vicinity of the complex, resulting in the formation of the protein-templated silica. We tried to apply a strategy utilizing this amine–carboxyl ionic complex for the fabrication of protein-templated silica, because soluble proteins typically have exposed carboxyl groups on the surface. The combination of protein and PEI as a template for biomimetic silica synthesis was reported by Jackson et al. and spherical silica particles were obtained; however, they used pre-hydrolyzed TEOS with HCl as the precursor, and the catalytic activity of the amine–carboxyl complex was not mentioned at all.27
Instead of polymeric amines such as PEI, we used ethylenediamine (EDA) as the amine group donor for uniform association onto the protein. In addition to AC, we used succinylated AC (SC-AC), whose side-chain amines of the lysine residues were succinylated. Because of succinylation, the number of carboxyl groups increased from 340 in the AC to 440 in SC-AC. The effect of carboxy groups on silicification was examined by comparing the results for AC and SC-AC.
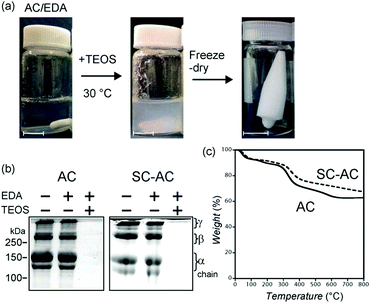
The collagen-templated silica was fabricated as follows (Fig. 1a). Solutions of AC and SC-AC (0.1 wt%, 3.3 μM) were prepared and the pH of each solution was adjusted to 6.5. Then a solution of EDA–HCl was added, to maintain the ratio of the number of amine groups of EDA to that of the carboxyl groups of AC or SC-AC at 20. TEOS was directly added to each solution and stirred at 30 °C for 20 hours, and a white insoluble precipitate was generated. AC and SC-AC were not detected from the resultant reaction mixtures in the SDS-PAGE analysis (Fig. 1b). The result of SDS-PAGE analysis (AC or SC-AC was absent) indicated that collagen molecules were not absorbed on the white precipitate but were completely incorporated inside the product. The precipitate was collected via centrifugation, washed with water and ethanol, and freeze-dried to obtain a white product. TGA showed that the product contained 10% of retained water, 20–25% of organic matter and 65–70% of inorganic matter (Fig. 1c). We also calcined the product at 500 °C in air.
The product was characterized using EDX, XPS and IR (Fig. S2, ESI† and Fig. 2). The EDX analysis detected silicon. The XPS spectrum showed a Si 2p peak at 103 eV, implying that silicon was present as Si4+. The IR spectrum showed several absorption peaks. The peak at 1085 cm−1 corresponds to siloxane (Si–O–Si) stretching. The two peaks at 1650 and 1550 cm−1 are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (amide I) and C–N stretching in combination with N–H bending (amide II) of the peptide bond of the protein, respectively. In the calcined samples, a siloxane linkage was confirmed, whereas amide I and amide II groups were lost. From the above analytical results, the formation of silica with incorporated protein was confirmed.
O stretching (amide I) and C–N stretching in combination with N–H bending (amide II) of the peptide bond of the protein, respectively. In the calcined samples, a siloxane linkage was confirmed, whereas amide I and amide II groups were lost. From the above analytical results, the formation of silica with incorporated protein was confirmed.
 | ||
| Fig. 2 Identification of the silica. (a) XPS spectrum of AC-templated silica. (b) IR spectrum of AC-templated silica before (lower) and after (upper) calcination. | ||
The formation of silica did not occur in the absence of EDA. In the presence of 1 M NaCl, which prevents the electrostatic complexation of protein and EDA, silica was not formed (Fig. S3, ESI†). These results revealed that the formation of silica, with TEOS as the precursor, was catalyzed by the ionic complexes of AC/EDA and SC-AC/EDA.
The morphological structures of the AC- and SC-AC-templated silica were observed using SEM (Fig. 3). The AC-templated silica showed a tangled fibrous network structure (Fig. 3a). The surface of the fibers was smooth and ledge-free, showing uniform silica formation on the surface of the AC. The length and width of the fiber were >500 nm and 50 nm, respectively, which are larger than the molecular dimensions of collagen, indicating that multiple AC molecules were bundled by the intermolecular bridging of EDA. The bundled AC worked as a catalytic template for the formation of long silica with a high aspect ratio. The characteristic structure of AC-templated silica was retained after calcination, showing that the structure originating from AC was fixed as an inorganic material (Fig. 3b).
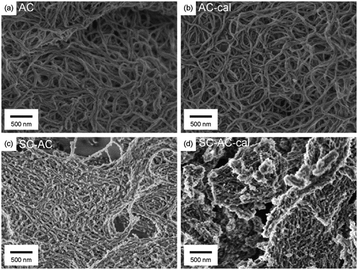 | ||
| Fig. 3 SEM image of AC (a and b) and SC-AC (c and d) templated silica before (a and c) and after (b and d) calcination. | ||
In the case wherein the starting AC solution (pH = 3.7) was not neutralized by NaOH, the addition of EDA and TEOS also resulted in the formation of silica. However, the silica sample thus obtained was not fibrous, but it had a sheet-like structure (Fig. S4, ESI†).
The SC-AC-templated silica also showed a network structure before and after calcination (Fig. 3c and d). In contrast to the AC-templated silica, the network was densely connected, the surface of the silica fibers was rough and non-fibrous silica was also observed. The structural roughness of SC-AC-templated silica was suggested to be caused by an increased number of carboxyl groups. The SC-AC/EDA complex had more catalytic sites than the AC/EDA complex, and so reactive species were generated in excess, resulting in heterogeneous growth of silica.
We investigated the effect of rod-like fibrous structure of collagen as the template material on the formation of fibrous silica. The use of heat-denatured SC-AC with EDA also resulted in the formation of silica. However, the morphological structure of the obtained silica was not tangled fibrous, instead, it consisted of aggregated particles (Fig. S5, ESI†).
For further structural characterization, the nitrogen adsorption–desorption isotherm was obtained (Fig. 4). The AC-templated silica showed a type II/III isotherm, characteristic of nonporous or macroporous (>50 nm) structures, indicating a uniform growth of the silica layer on the AC/EDA complex. The BET surface area was 218 m2 g−1. The calcined AC-templated silica also showed a nonporous isotherm (type II), and the BET surface area increased to 667 m2 g−1. The SC-AC-templated silica exhibited a type II/IV isotherm, indicating macroporous and mesoporous structures, and the corresponding BET surface area was of 280 m2 g−1. The difference in mesoporosity is consistent with the different surface roughness observed using SEM. The calcined SC-AC-templated silica also showed a type II/IV isotherm and the corresponding BET surface area was 640 m2 g−1.
Finally, we also applied the present method for titania as a functional metal oxide (Fig. S6, ESI†). By using the AC–EDA complex and the water-stable precursor titanium(IV) bis(ammonium lactato)-dihydroxide, tangled fibrous titania was formed at 4 °C after 20 hours.
Conclusions
In this study, we demonstrated that the atelocollagen used in this study plays roles in both the template and supply of inherently equipped carboxyl groups, which are paired with the added amine to work as catalysts for mild silicification. AC-templated silica shows finer and smoother structures than the SC-AC counterparts. Thus, we concluded that a number of carboxyl groups of the protein have the potential to adopt any protein shape to form silica and other functional metal oxide nanomaterials such as titania. The tangled fibrous AC and SC-AC templated silica materials constitute a novel class of porous materials with rod-like structures. Such materials have potential applications such as high-performance adsorbent carriers, such as “monolithic columns”.28 The method, based on the use of an amine–carboxyl complex, is simple and can be easily applied to other proteins for the fabrication of a new class of nano-structured materials.Acknowledgements
This work was partially supported by JSPS KAKENHI Grant Number 26810087. The authors acknowledge Y. Akimoto and S. Hirashima for their support with SEM observations, and J. Nomura and H. Yamazaki for their support with nitrogen adsorption–desorption isotherm measurements.References
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- N. Kroger, R. Deutzmann and M. Sumper, Science, 1999, 286, 1129–1132 CrossRef CAS PubMed.
- N. Kroger, S. Lorenz, E. Brunner and M. Sumper, Science, 2002, 298, 584–586 CrossRef PubMed.
- N. Kroger, G. Lehmann, R. Rachel and M. Sumper, Eur. J. Biochem., 1997, 250, 99–105 CrossRef CAS PubMed.
- N. Kroger, R. Deutzmann, C. Bergsdorf and M. Sumper, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 14133–14138 CrossRef CAS PubMed.
- N. Poulsen, M. Sumper and N. Kroger, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12075–12080 CrossRef CAS PubMed.
- M. Sumper and E. Brunner, ChemBioChem, 2008, 9, 1187–1194 CrossRef CAS PubMed.
- K. Shimizu, J. Cha, G. D. Stucky and D. E. Morse, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 6234–6238 CrossRef CAS.
- J. N. Cha, K. Shimizu, Y. Zhou, S. C. Christiansen, B. F. Chmelka, G. D. Stucky and D. E. Morse, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 361–365 CrossRef CAS.
- W. E. G. Muller, X. H. Wang, F. Z. Cui, K. P. Jochum, W. Tremel, J. Bill, H. C. Schroder, F. Natalio, U. Schlossmacher and M. Wiens, Appl. Microbiol. Biotechnol., 2009, 83, 397–413 CrossRef PubMed.
- H. C. Schroder, D. Brandt, U. Schlossmacher, X. Wang, M. N. Tahir, W. Tremel, S. I. Belikov and W. E. Muller, Naturwissenschaften, 2007, 94, 339–359 CrossRef PubMed.
- K. M. Roth, Y. Zhou, W. J. Yang and D. E. Morse, J. Am. Chem. Soc., 2005, 127, 325–330 CrossRef CAS PubMed.
- J. N. Cha, G. D. Stucky, D. E. Morse and T. J. Deming, Nature, 2000, 403, 289–292 CrossRef CAS PubMed.
- F. Rodriguez, D. D. Glawe, R. R. Naik, K. P. Hallinan and M. O. Stone, Biomacromolecules, 2004, 5, 261–265 CrossRef CAS PubMed.
- M. M. Tomczak, D. D. Glawe, L. F. Drummy, C. G. Lawrence, M. O. Stone, C. C. Perry, D. J. Pochan, T. J. Deming and R. R. Naik, J. Am. Chem. Soc., 2005, 127, 12577–12582 CrossRef CAS PubMed.
- A. Bernecker, R. Wieneke, R. Riedel, M. Seibt, A. Geyer and C. Steinem, J. Am. Chem. Soc., 2010, 132, 1023–1031 CrossRef CAS PubMed.
- S. V. Patwardhan, Chem. Commun., 2011, 47, 7567–7582 RSC.
- R. H. Jin and J. J. Yuan, Chem. Commun., 2005, 1399–1401, 10.1039/b417351a.
- Y. Ono, Y. Kanekiyo, K. Inoue, J. Hojo, M. Nango and S. Shinkai, Chem. Lett., 1999, 475–476 CrossRef CAS.
- S. Heinemann, H. Ehrlich, C. Knieb and T. Hanke, Int. J. Mater. Res., 2007, 98, 603–608 CrossRef CAS.
- M. F. Desimone, C. Helary, G. Mosser, M. M. Giraud-Guille, J. Livage and T. Coradin, J. Mater. Chem., 2010, 20, 666–668 RSC.
- C. J. Garcia-Valdes, G. Hernandez-Padron, M. V. Garcia-Garduno and V. M. Castano, E-Polymers, 2009, 9, 886–892, DOI:10.1515/epoly.2009.9.1.886.
- K. H. Stenzel, T. Miyata and A. L. Rubin, Annu. Rev. Biophys. Bio., 1974, 3, 231–253 CrossRef CAS PubMed.
- D. L. Helseth, Jr. and A. Veis, J. Biol. Chem., 1981, 256, 7118–7128 Search PubMed.
- A. F. Wallace, J. J. DeYoreo and P. M. Dove, J. Am. Chem. Soc., 2009, 131, 5244–5250 CrossRef CAS PubMed.
- H. Matsukizono and R. H. Jin, Angew. Chem., Int. Ed., 2012, 51, 5862–5865 CrossRef CAS PubMed.
- E. Jackson, M. Ferrari, C. Cuestas-Ayllon, R. Fernandez-Pacheco, J. Perez-Carvajal, J. M. de la Fuente, V. Grazu and L. Betancor, Langmuir, 2015, 31, 3687–3695 CrossRef CAS PubMed.
- F. Svec and Y. Lv, Anal. Chem., 2015, 87, 250–273 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figure and experimental section. See DOI: 10.1039/c6tb01770c |
| This journal is © The Royal Society of Chemistry 2016 |