Research highlights: microfluidics and magnets
Ivan
Pushkarsky
,
Peter
Tseng
,
Coleman
Murray
and
Dino
Di Carlo
*
Department of Bioengineering, California NanoSystems Institute, Jonsson Comprehensive Cancer Center, University of California Los Angeles, 420 Westwood Plaza, 5121 Engineering V, Box 951600, Los Angeles, California 90095, USA. E-mail: dicarlo@ucla.edu
First published on 2nd July 2014
Abstract
In this highlight we present a snapshot of recent work using magnetic forces and particles to perform lab on a chip operations. Magnetic micro- & nanoparticles have been widely used for separations in cell biology & clinical diagnostics and as solid phase supports for reactions and chemical assays. Microscale approaches to control and manipulate magnetic particles can enable new functionality; allowing parallel and complex automation of assays, manipulation of fluids themselves, and precise separations based on small differences in magnetic properties. Here we discuss recent work demonstrating advances in these three areas.
An integrated circuit approach to magnetophoretic particle manipulation
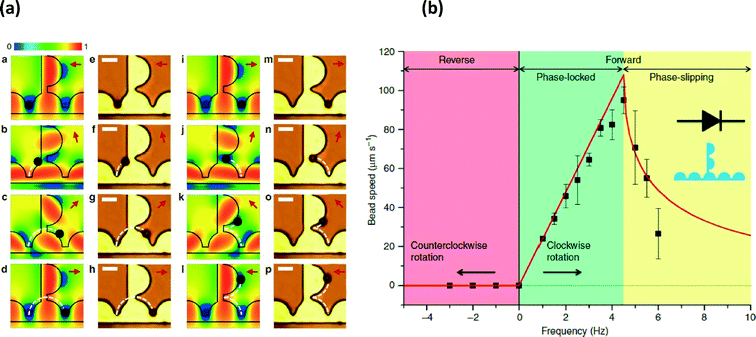
Lab on a chip technology has enabled manipulation of fluid droplets, particles or single-cells. In particular, there is a need for tools to organize and evaluate dynamic responses of cellular populations with single-cell resolution. Following the same design architectures as integrated circuits, Lim et al.1 have developed an integrated magnetic particle manipulation system consisting of current carrying lines, thin permalloy films and an external rotating magnetic field to perform functions such as particle manipulation, rectification, capacitance and transistor functions, which could enable the arrayed study of biological processes.Using standard lithographic and metal evaporation techniques, the authors created bridged semicircular geometries of permalloy (Fig. 1a). By introducing a counter clockwise rotating magnetic field with a field strength of 15.9 kA m−1, magnetic particles could be trapped and then manipulated in shifting potential energy wells (shown in blue) provided by the permalloy geometries when externally magnetized in different directions. The transport of particles through the permalloy geometries, corresponding with the shifting external field direction, possessed a frequency-dependent behavior where particle velocity correlated linearly with the externally applied magnetic field frequency until viscous effects on the particles dampened particle transport (Fig. 1b) at approximately 4.5 Hz. However, in the phase locked frequency regime, particle transport was highly linear with driving frequency. Interestingly permalloy geometries were designed that exhibit rectification behavior where a reversal of the rotating field direction would prevent reverse particle motion (Fig. 1a & b).
Additionally, the authors developed closed trapping geometries to hold particles and combined geometries with gold electrodes to enable current-gated control of the transit of a magnetic particle along a lane, what they refer to as a particle “transistor”. Combining these design elements the authors were able to perform additional functions on magnetically-labeled cells including trapping and storing magnetically labeled T and B cells, selecting magnetically labeled cells from unbound magnetic beads and generating arrays of magnetic particles.
In summary, the authors were able to apply integrated circuit design logic to the manipulation of magnetic particles. This technology presents a promising option, allowing application of widely used design concepts to a new regime. Next steps should apply these systems to complex automation of biological experiments.
Full-range manipulation of magnetic droplets
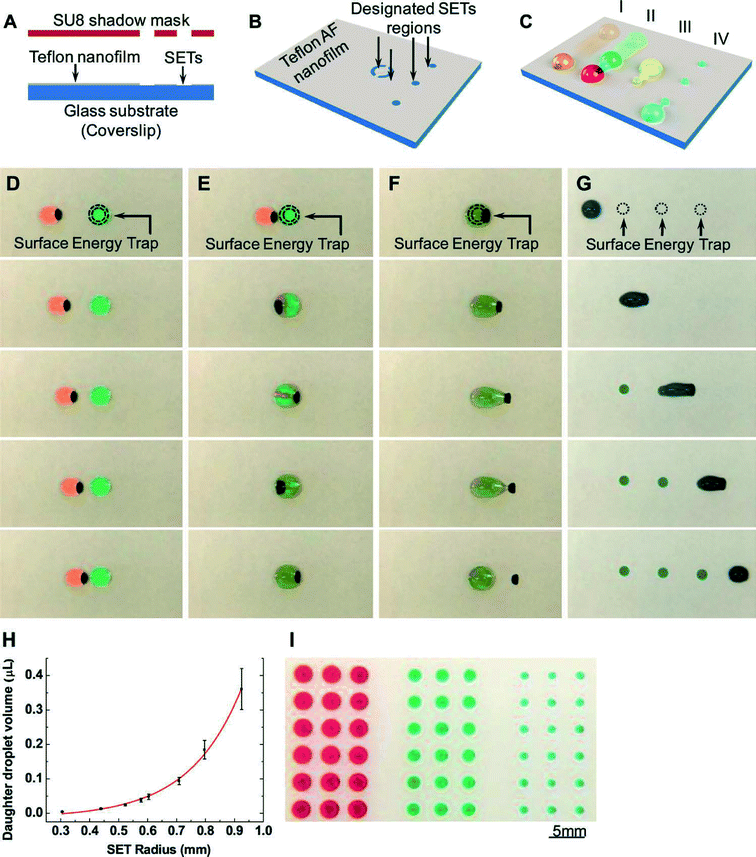
In addition to manipulation of solid-phase magnetic particles, we highlight an approach to manipulate fluids using magnetic forces. Droplet-based microfluidic systems are a subset of lab-on-a-chip devices that enable a different set of functionalities compared to continuous flow microfluidics.2 Applications range from the synthesis of chemicals, to chemical analysis, diversity generation, and single-cell assays. These systems invariably require spatial manipulation of small volume droplets, to eventually enable dispensing, coalescence, mixing, and sorting of droplet populations, each containing engineered chemistries. For droplets on surfaces, electrowetting is the most widely used mechanism of droplet actuation, although many additional mechanisms have been utilized, including magnetic fields, photo-actuation, acoustic waves, and dielectrophoresis. Magnetic actuation, which relies on the manipulation of magnetic particles mixed into the fluid, possesses some conceptual advantages over its electrowetting counterpart, including the ability to manipulate physical structures of the droplet, scalable volume handling, and fundamental additional functionality due to the capability of particles to act as a mobile substrate for particle-based chemistries. In general, however, magnetic actuation only performs a limited subset of the standard fluidic operations, and has not traditionally been able to dispense or split droplets.In order to resolve the limited actuation capability of magnetic-based droplet manipulations, Zhang et al. utilized surface energy traps (or SETs) in combination with magnetic droplet manipulation to enable the full-range of droplet manipulations.3 They utilized this approach to quantitatively study a variety of droplet operations on this platform, including droplet transport, fusion, dispensing, and mixing, alongside particle extraction from droplets. As a functional demonstration of their platform, the researchers prepared a multiplexed structure containing SETs, magnetic particles, and PCR reagents including washing and lysis buffers. They utilized this to prepare and generate samples for PCR.
Surface energy traps were generated on Teflon thin films via oxygen plasma etching through a reusable SU-8 stencil. These oxygen plasma generated hydrophilic regions served to trap smaller droplets of water as a larger magnetically-manipulated droplet was actuated through the region via an underlying magnet (Fig. 2). Traditionally, magnetic particles can be extracted from droplets through careful engineering of the interplay of magnetic and frictional forces. Here, researchers found that by directly varying the SET size, droplets could be encouraged to either dispense (for smaller SET size), or particles could be extracted (for larger SET sizes that pin the entire droplet). Notably, the volumes of dispensed daughter droplets were directly proportional to the size of the SETs, thus providing a simple and effective way of generating microarrays of droplets with individually controllable contents. The authors qualitatively described their observed phenomena as a tug-of-war between the magnetic particles and the SETs, and quantitatively extracted plots detailing the modes with which either droplet dispensing, particle extraction, or magnetic disengagement occur as a function of SET diameter and the mass of input magnetic particles.
The authors finally generated a multiplexed device to prepare analyte for subsequent PCR detection. Human blood was incubated with silica magnetic particles inside a lysis/binding buffer, in order to lyse cells and adsorb DNA onto the silica surface. Particles containing DNA were then washed in washing buffer droplets, and incubated in elution buffer to detach DNA, and finally mixed with PCR reagents for subsequent quantification.
In summary, the researchers enabled the full-range of fluidic operations using magnetic actuation, including droplet transport, fusion, dispensing, and particle extraction. The use of engineered surface energy traps, similar to previous work that used changes in channel dimension to create traps and guide droplets,4 enables a simple and effective method of handling small, precision quantities of fluids on chip, allowing straightforward dispensing of droplets, and extraction of magnetic particles. These structures can potentially broaden magnetic droplet platforms to a variety of applications in chemistry and life sciences.
Marker-free CTC isolation from whole blood
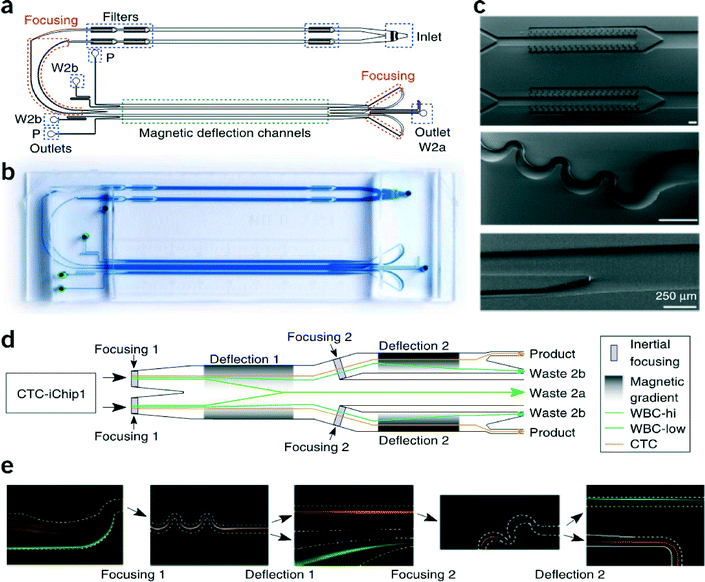
Magnetic particles have a long history of use to isolate biomolecules or cells. Recently, these approaches have been applied in microfluidic chips to isolate or remove specific cell populations based on surface expression of antigens, with applications in isolating very rare cells like circulating tumor cells (CTCs). Depleting other populations of cells within blood besides CTCs is advantageous in that the technique is independent of potentially unknown surface expression of CTCs. Karabacak et al. developed an on-chip system for the isolation of CTCs from whole blood using magnetophoretic-based negative selection that achieves a 97% rare cell yield.5Overall, the system consists of two serially connected microfluidic chips that first separate nucleated cells from erythrocytes, platelets, plasma (and free magnetic beads) and then deplete leukocytes tagged with functionalized magnetic beads using magnetophoresis, allowing CTCs, alone, to be collected downstream. To start the process, the target sample is first mixed with biotinylated anti-CD45 (leukocyte-specific) and anti-CD66b (granulocyte-specific) antibodies and then with streptavidin-functionalized magnetic beads. This mixture is then injected into the serial two-chip system.
The first chip, called CTC-iChip1, uses arrays of specifically shaped posts in the laminar flow regime to deflect particles with a hydrodynamic diameter greater than 4 μm, i.e., nucleated cells, towards one outlet while allowing the remainder of the blood's contents to exit through the others. The nucleated cells proceed directly into the second chip, CTC-iChip2. This second chip uses magnetic elements to deflect magnetically-tagged cells based on their magnetic load. To accomplish this, the nucleated cells entering CTC-iChip2 are inertially focused to a single stream using asymmetric serpentine channels in the inertial flow regime (finite Reynolds number), so that all cells are subjected to roughly identical magnetic fields.6 The focused cells then flow through the deflection region where they experience magnetic forces and are deflected, or not, into waste collection outlets (Fig. 3). This process is repeated, whereby the undeflected cells (CTCs and leukocytes with few beads) are refocused and subjected to a second round of magnetic separation with a longer residence time in the deflection region and also a larger magnetic field gradient.
In summary, the CTC-iChip system reports a sorting rate of 107 cells s−1 with 3.8-log depletion of leukocytes from whole blood at a sample processing rate of 8 mL h−1 and the magnetic separation parameters have been optimized to achieve a sensitivity down to ~2 beads per cell. By using negative selection of CTCs via depletion of leukocytes, rather than positive selection using antibodies against common CTC surface markers, the isolated CTC population more accurately represents the true molecular heterogeneity present in a given patient's CTCs. Additionally, by avoiding the use of antibodies against CTCs, which may invoke intracellular molecular pathways, the original state of the CTCs is more likely preserved, which is important for downstream analysis. Heterogeneity in size is also well-represented since inertial focusing using asymmetric serpentine channels operates effectively across the needed size range. Although concentration of the CTCs to small volumes is not accomplished with the chip, the same group developed a closed centrifugation system called Spintrap, which layers the CTCs onto an adhesive surface where they can be fixed and stained. Negative depletion, in general, also leads to lower purity samples, which may limit some downstream assays.
References
- B. Lim, V. Reddy, X. Hu, K. Kim, M. Jadhav, R. Abedini-Nassab, Y. W. Noh, Y. T. Lim, B. B. Yellen and C. Kim, Magnetophoretic circuits for digital control of single particles and cells, Nat. Commun., 2014, 5, 3846, DOI:10.1038/ncomms4846.
- S. Y. Teh, R. Lin, L. H. Hung and A. P. Lee, Droplet microfluidics, Lab Chip, 2008, 8(2), 198–220, 10.1039/b715524g.
- Y. Zhang and T. H. Wang, Full-Range Magnetic Manipulation of Droplets via Surface Energy Traps Enables Complex Bioassays, Adv. Mater., 2013, 25, 2903–2908 CrossRef CAS PubMed.
- P. Abbyad, R. Dangla, A. Alexandrou and C. N. Baroud, Rails and anchors: guiding and trapping droplet microreactors in two dimensions, Lab Chip, 2011, 11(5), 813–821, 10.1039/c0lc00104j.
- N. M. Karabacak, et al., Microfluidic, marker-free isolation of circulating tumor cells from blood samples, Nat. Protoc., 2014, 9, 694–710 CrossRef CAS PubMed.
- D. Di Carlo, D. Irimia, R. G. Tompkins and M. Toner, Continuous inertial focusing, ordering, and separation of particles in microchannels, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 18892–18897 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |