Open Access Article
Open Access ArticleFirst synthesis and aggregation behaviour of periconjugated triazoliumfullerene†
Naohiko
Ikuma
*,
Saori
Inaba
,
Ken
Kokubo
and
Takumi
Oshima
Division of Applied Chemistry, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan. E-mail: ikuma@chem.eng.osaka-u.ac.jp
First published on 22nd October 2013
Abstract
Triazoliumfullerene was first prepared by the [3+2] cycloaddition of in situ generated 1,3-diaza-2-azoniaallene with fullerene and was characterized by the dispersed positive charge over the fullerene sphere associated with periconjugation. This new type of amphiphilic fullerene tended to form vesicles and crystalline aggregates after the casting of THF or MeOH solutions.
Ionic fullerenes1–6 are useful compounds for medical applications such as in antibacterial1f and anti-HIV1g agents, and also for self-assembling nanocarbon materials2,3 owing to the presence of amphiphilic intermolecular ionic, dipole–dipole, π–π and hydrophobic interactions. These ionic and highly polar fullerenes are apt to form vesicle,1h,2,3 nanowire,1h,2,4 and nanosheet5 structures by way of self-assembly. Their aggregation behaviors highly depend on how the introduced ionic (hydrophilic) groups electronically affect the nonpolar (hydrophobic) fullerene cage. Here, it is interesting to know what kind of aggregation occurs if the ionic charge is dispersed on the π-conjugated fullerene cage. Such charge dispersion can be attained for the nitrogen incorporated 60π ionic azafullerene (C59N+).6 However, the extremely low stability and intrinsic activity of reduction7 restricted its usual application as an ionic fullerene.
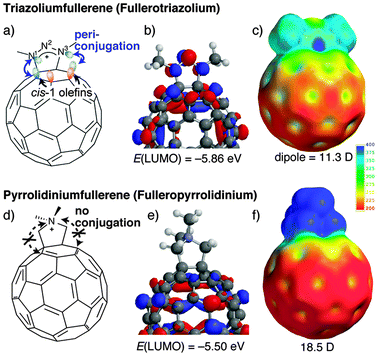
This situation prompted us to design a new type of ionic fullerene with possible conjugation; one promising candidate is through-space periconjugation8 between the outer ionic site and the π-conjugated fullerene cage. We have attempted the introduction of a cationic 1,2,3-triazolium unit9 into the fullerene sphere, because the possible high delocalization of positive charge on the N1–N2–N3 linkage will allow direct electron withdrawal as well as possible periconjugation with the adjacent fullerene cis-1 π-orbital. By DFT calculations,10 it was found that the LUMO of the triazoliumfullerene was delocalized both on the cationic N1–N2–N3 linkage and the fullerene cage (Fig. 1a and b), and the LUMO level was 0.36 eV lower than that of pyrrolidiniumfullerene (Fig. 1d and e),1a–d,2,5a a typical ammonium fullerene. Moreover, in the electrostatic potential (ESP) map of pyrrolidiniumfullerene, the positive site is apparently localized on the pyrrolidinium moiety (blue on the ESP map in Fig. 1f) and the fullerene sphere remains in a less charged state. By contrast, the ESP map of triazoliumfullerene shows a considerable distribution of positive charge over the fullerene cage with an ambiguous boundary (Fig. 1c), whereby its dipole moment is smaller than that of pyrrolidiniumfullerene. Therefore, triazoliumfullerene (as a salt) is expected to behave as a unique amphiphile in contrast to the previously employed ionic fullerenes. In this communication, we report the one-pot first synthesis of triazoliumfullerene (as a PF6 salt), theoretical calculation of the mechanistic pathway, and preliminary DLS/SEM/TEM measurements of its self-aggregation.
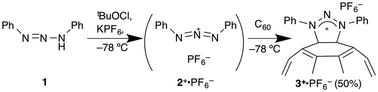
To prepare triazoliumfullerene, we employed [3+2] cycloaddition9 of 1,3-diaza-2-azoniaallene salt 2+ with fullerene as a 2π component. The intermediate 2+ was generated in situ via sequential chlorination and ionization of commercially available diphenyltriazene 1 by tert-butyl hypochlorite and the Lewis acid KPF611 at −78 °C. To avoid spontaneous decomposition of the unstable intermediate 2+, a one-pot reaction was carried out with C60 at −78 °C to give 1,3-diphenyltriazoliumfullerene salt 3+·PF6− (Scheme 1). The 13C-NMR measurement showed ca. 16 peaks of sp2-fullerene carbons (and 4 phenyl peaks, Fig. S1 in the ESI†) and one sp3 peak at 91 ppm, indicating the occurrence of C2v symmetric addition at the 6,6-bond of fullerene. The downfield shift of the sp3 carbon is probably because of the electron-withdrawal by the conjunct positive N1 and N2 atoms, which is in conformity with a computational simulation (96 ppm by a GIAO/B3LYP/6-31G(d) approach with IEFPCM/DMSO, Fig. S2 in the ESI†). Unfortunately, however, such C2v peaks gradually disappeared with the addition of D2O into the NMR DMSO solution (ca. 10% v/v), while the addition of methanol-d4 brought about no change in the spectra. This water degradation may be ascribed to the lower LUMO level of triazoliumfullerene (vs. water persistent pyrrolidiniumfullerene), which would enhance the electrophilic activity.
The enhancement of the electron accepting ability of 3+ was also confirmed by cyclic voltammetry (Fig. S3, ESI†). The higher reducing potential of 3+ (vs. N,N-dimethylpyrrolidiniumfullerene1b,2) is ascribed to the lower LUMO level due to the periconjugation. The irreversibility of the first peak may indicate the instability of the reduced intermediate. This higher electron accepting ability would lead to intensified n-type semiconductibility and π-soft Lewis acidity.12 Moreover, 3+ seems to be a potential candidate for photoinduced electron-transfer materials if some donating counteranions or substituents are introduced.
Although the [3+2] cycloaddition of 1,3-diaza-2-azoniaallene salt with small alkenes was reported to proceed via a concerted pathway,9d,13 it is unclear whether this is also the case for the highly conjugated and strained alkenes like fullerenes. As indicated by DFT calculations10 (BHandHLYP/6-31G(d) with IEFPCM (toluene)),13 the unusually low LUMO level (−3.72 eV) of 2+ is suitable for orbital interaction with the symmetry allowed HOMO (−6.76 eV) of C60 (Fig. 2a and b). TS calculations clarified a concerted transition state with the extremely small activation energy of 1.4 kcal mol−1 (Fig. 2c and d) and almost equivalent C60–N distances (2.3963 and 2.3965 Å). Moreover, the calculation for the product 3+ (Fig. S4 in the ESI†) showed a ca. 1.0 eV lower HOMO level (−7.79 eV) than that of C60, explaining the absence of bisadducts of 2+.14 These theoretical results imply that the present [3+2] reaction of 2+ proceeds via a concerted process like 1,3-dipolar cycloaddition, although such HOMO controlled reactions of C60 are very rare on account of the inherent low LUMO level of fullerene.15
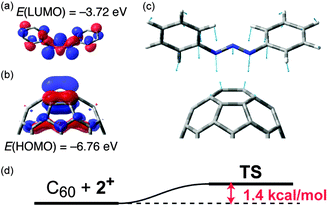 | ||
| Fig. 2 (a) LUMO of 2+ (hydrogen omitted), (b) HOMO (in part) of C60, (c) transition state (TS) geometry and the vibrational vectors of imaginary frequency (νi = −102.3 cm−1), and (d) energy diagram (ZPE corrected) of the reaction. These calculations were carried out with the BHandHLYP/6-31G(d) level of theory with solvent parameters (IEFPCM, toluene).13 | ||
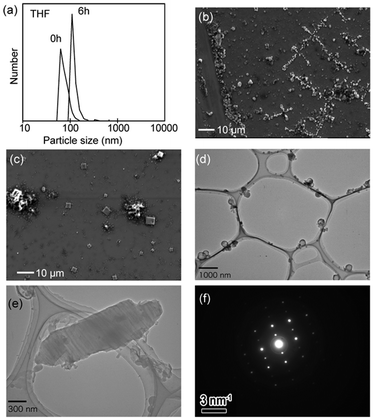
The self-assembly of triazoliumfullerene was assessed by dynamic light scattering (DLS) and scanning and transmission electron microscopy (SEM and TEM). The solvation of its PF6 salt in THF showed slight aggregation (1 × 10−5 M, ca. 100 nm particle size, Fig. 3a). After casting of the solution on a Si-plate and evaporation, SEM images indicated both spherical and nanocrystalline self-assemblies (Fig. 3b and Fig. S5a, ESI†). For the solution with methanol, the ratio of nanocrystals/vesicles seems to increase (Fig. 3c). By TEM analysis, these spherical aggregates seem to be 100–300 nm vesicles with hollow centers (Fig. 3d and Fig. S5b, ESI†), although the present experiments cannot unambiguously certify bilayer formation. Such vesicles have often been observed in ionic fullerenes.2,3 Interestingly, TEM also showed micrometer-scale crystals (Fig. 3e) with a clear diffraction pattern (Fig. 3f). Such microcrystals were occasionally observed in non-ionic supramolecular fullerenes with semiconductivity.16 Crystalline triazoliumfullerene can be useful for electron-transport materials with lower LUMO levels, although the configurational control of counteranions is required to inhibit the possible carrier-trapping.
Although the quantitative evaluation of periconjugative effects requires further experiments (e.g., with varying substituents and counteranions), we consider that the moderate amphiphilicity of 3+ with a small dipole moment (∼11 D) and highly delocalized positive charge would be responsible for the simultaneous formation of vesicles and microcrystals. While pyrrolidiniumfullerene with a larger dipole (∼18 D) preferably aggregates in an antiparallel manner to form bilayers,1i the smaller dipole of triazoliumfullerene can exhibit another type of aggregation mode. In this case, the enhanced π-acceptor ability caused by the delocalized positive charge on the fullerene sphere may promote crystalline aggregation with the aid of counteranions as well as the aryl-substituents.
In conclusion, we first prepared triazoliumfullerene by a one-pot reaction of in situ generated 1,3-diaza-2-azoniaallene with C60 and confirmed the periconjugative delocalization of positive charge into the fullerene cage. This compound is expected to exhibit vesicle/crystalline self-assembly due to its moderate amphiphilic interactions and highly delocalized positive charge.
TEM measurements were carried out by using a piece of equipment in the Research Center for Ultrahigh Voltage Electron Microscopy, Osaka University. We thank Prof. Mikiji Miyata and Dr Ichiro Hisaki for the DLS measurements, Prof. Shu Seki for the SEM measurements, and Dr Keita Kobayashi for the TEM measurements. This work was partly supported by a Grant-in-Aid for Young Scientist (B) (No. 24750039) from JSPS and by Health Labour Sciences Research Grants from MHLW.
Notes and references
-
(a) R. Bullard-Dillard, K. E. Creek, W. A. Scrivens and J. M. Tour, Bioorg. Chem., 1996, 24, 376 CrossRef CAS
; (b) D. M. Guldi, H. Hungerbuhler and K. D. Asmus, J. Phys. Chem. A, 1997, 101, 1783 CrossRef CAS
; (c) A. M. Cassell, W. A. Scrivens and J. M. Tour, Angew. Chem., Int. Ed., 1998, 37, 1528 CrossRef CAS
; (d) K. Kordatos, T. Da Ros, S. Bosi, E. Vázquez, M. Bergamin, C. Cusan, F. Pellarini, V. Tomberli, B. Baiti, D. Pantarotto, V. Georgakilas, G. Spalluto and M. Prato, J. Org. Chem., 2001, 66, 4915 CrossRef CAS PubMed
; (e) S. Bosi, L. Feruglio, D. Milic and M. Prato, Eur. J. Org. Chem., 2003, 4741 CrossRef CAS
; (f) T. Mashino, D. Nishikawa, K. Takahashi, N. Usui, T. Yamori, M. Seki, T. Endo and M. Mochizuki, Bioorg. Med. Chem. Lett., 2003, 13, 4395 CrossRef CAS PubMed
; (g) S. Bosi, T. Da Ros, G. Spalluto, J. Balzarini and M. Prato, Bioorg. Med. Chem. Lett., 2003, 13, 4437 CrossRef CAS PubMed
; (h) D. M. Guldi, F. Zerbetto, V. Georgakilas and M. Prato, Acc. Chem. Res., 2005, 38, 38 CrossRef CAS PubMed
; (i) C. J. Chancellor, A. A. Thorn, C. M. Beavers, M. M. Olmstead and A. L. Balch, Cryst. Growth Des., 2008, 8, 976 CrossRef CAS
.
-
(a) A. M. Cassell, C. L. Asplund and J. M. Tour, Angew. Chem., Int. Ed., 1999, 38, 2403 CrossRef CAS
; (b) V. Georgakilas, F. Pellarini, M. Prato, D. M. Guldi, M. Melle-Franco and F. Zerbetto, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5075 CrossRef CAS PubMed
.
-
(a) S. Zhou, C. Burger, B. Chu, M. Sawamura, N. Nagahama, M. Toganoh, U. E. Hackler, H. Isobe and E. Nakamura, Science, 2001, 291, 1944–1947 CrossRef CAS PubMed
; (b) H. Isobe, T. Homma and E. Nakamura, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 14895 CrossRef CAS PubMed
.
- P. Brough, D. Bonifazi and M. Prato, Tetrahedron, 2006, 62, 2110 CrossRef CAS PubMed
.
-
(a) T. Shiga and T. Motohiro, Thin Solid Films, 2007, 515, 3607 CrossRef CAS PubMed
; (b) K. Masuda, T. Abe, H. Benten, H. Ohkita and S. Ito, Langmuir, 2010, 26, 13472 CrossRef CAS PubMed
; (c) H. Li, M. J. Hollamby, T. Seki, S. Yagai, H. Möhwald and T. Nakanishi, Langmuir, 2011, 27, 7493 CrossRef CAS PubMed
.
-
(a) J. C. Hummelen, B. Knight, J. Pavlovich, R. Gonzalez and F. Wudl, Science, 1995, 269, 1554 CAS
; (b) K. C. Kim, F. Hauke, A. Hirsch, P. D. W. Boyd, E. Carter, R. S. Armstrong, P. A. Lay and C. A. Reed, J. Am. Chem. Soc., 2003, 125, 4024 CrossRef CAS PubMed
; (c) O. Vostrowsky and A. Hirsch, Chem. Rev., 2006, 106, 5191 CrossRef CAS PubMed
.
- Azafullerene is easily reduced to form a dimer, which is a reactive intermediate for further reactions. See
(a) M. Keshavarz-K, R. Gonzalez, R. G. Hicks, G. Srdanov, V. I. Srdanov, T. G. Collins, J. C. Hummelen, C. Bellavia-Lund, J. Pavlovich, F. Wudl and K. Holczer, Nature, 1996, 383, 147 CrossRef CAS
; (b) N. B. Shustova, I. V. Kuvychko, A. A. Popov, M. von Delius, L. Dunsch, O. P. Anderson, A. Hirsch, S. H. Strauss and O. V. Boltalina, Angew. Chem., Int. Ed., 2011, 50, 5537 CrossRef CAS PubMed
.
-
(a) M. Eiermann, R. C. Haddon, B. Knight, Q. C. Li, M. Maggini, N. Martín, T. Ohno, M. Prato, T. Suzuki and F. Wudl, Angew. Chem., Int. Ed. Engl., 1995, 34, 1591 CrossRef CAS
; (b) B. Knight, N. Martín, T. Ohno, E. Ortí, C. Rovira, J. Veciana, J. Vidal-Gancedo, P. Viruela, R. Viruela and F. Wudl, J. Am. Chem. Soc., 1997, 119, 9871 CrossRef CAS
; (c) P. Ceroni, F. Conti, C. Corvaja, M. Maggini, F. Paolucci, S. Roffia, G. Scorrano and A. Toffoletti, J. Phys. Chem. A, 2000, 104, 156 CrossRef CAS
; (d) F. B. Kooistra, T. M. Leuning, E. Maroto-Martinez and J. C. Hummelen, Chem. Commun., 2010, 46, 2097 RSC
.
-
(a) W. Wirschun and J. C. Jochims, Synthesis, 1997, 233 CrossRef CAS PubMed
; (b) W. Wirschun, G. M. Maier and J. C. Jochims, Tetrahedron, 1997, 53, 5755 CrossRef CAS
; (c) W. Wirschun, M. Winkler, K. Lutz and J. C. Jochims, J. Chem. Soc., Perkin Trans. 1, 1998, 1755 RSC
; (d) M. Weng, A. Geyer, A. Friemel, J. C. Jochims and M. Lutz, J. Prakt. Chem., 2000, 342, 486 CrossRef CAS
.
- In this paper, all DFT calculations were carried out with Spartan'08 or Gaussian 09. Full citations are shown in the ESI.†.
- J. Bouffard, B. K. Keitz, R. Tonner, G. Guisado-Barrios, G. Frenking, R. H. Grubbs and G. Bertrand, Organometallics, 2011, 30, 2617 CrossRef CAS PubMed
. Attempts to obtain a SbCl6− salt (see ref. 9) failed probably because of the occurrence of chlorination of C60 by SbCl5.
-
(a) H. Li, C. Risko, J. H. Seo, C. Campbell, G. Wu, J.-L. Brédas and G. C. Bazan, J. Am. Chem. Soc., 2011, 133, 12410 CrossRef CAS PubMed
; (b) J. Iglesias-Sigüenza and M. Alcarazo, Angew. Chem., Int. Ed., 2012, 51, 1523 CrossRef PubMed
.
- S. Y. Yang, X. F. Lin, C. K. Sun and D. C. Fang, THEOCHEM, 2007, 815, 127 CrossRef CAS PubMed
. Our calculation used a rather simplified BHandHLYP/6-31G(d) method to reduce its calculational cost.
- However, the use of a large excess of 1 and KPF6 gave inseparable byproducts, e.g., bi- and multi-adducts or products due to the decomposition of the labile intermediate 2+.
- Most 1,3-dipoar reactions of fullerene proceed with nucleophiles rather than electrophiles, except for the addition of ozone. See
(a) R. Malhotra, S. Kumar and A. Satyam, J. Chem. Soc., Chem. Commun., 1994, 1339 RSC
; (b) D. Heymann, S. M. Bachilo, R. B. Weisman, F. Cataldo, R. H. Fokkens, N. M. M. Nibbering, R. D. Vis and L. P. F. Chibante, J. Am. Chem. Soc., 2000, 122, 11473 CrossRef CAS
.
-
(a) X. Zhang and M. Takeuchi, Angew. Chem., Int. Ed., 2009, 48, 9646 CrossRef CAS PubMed
; (b) X. Zhang, T. Nakanishi, T. Ogawa, A. Saeki, S. Seki, Y. Shen, Y. Yamauchi and M. Takeuchi, Chem. Commun., 2010, 46, 8752 RSC
; (c) T. Nakanishi, Y. Shen, J. Wang, H. Li, P. Fernandes, K. Yoshida, S. Yagai, M. Takeuchi, K. Ariga and D. G. Kurth, et al. , J. Mater. Chem., 2010, 20, 1253 RSC
; (d) S. S. Babu, H. Möhwald and T. Nakanishi, Chem. Soc. Rev., 2010, 39, 4021 RSC
; (e) S. Santhosh Babu, A. Saeki, S. Seki, H. Möhwald and T. Nakanishi, Phys. Chem. Chem. Phys., 2011, 13, 4830 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, 1H/13C NMR charts (with DFT simulation) and magnified SEM/TEM images of 3+, and calculated TS coordinates. See DOI: 10.1039/c3cc45783d |
| This journal is © The Royal Society of Chemistry 2014 |