Effect of temperature on the gas-phase reaction of CH3CN with OH radicals: experimental (T = 11.7–177.5 K) and computational (T = 10–400 K) kinetic study†
Abstract
Acetonitrile (CH3CN) is present in the interstellar medium (ISM) in a variety of environments. However, at the ultracold temperatures of the ISM, radical-molecule reactions are not widely investigated because of the experimental handicap of getting organic molecules in the gas phase by conventional techniques. The CRESU (French acronym for Reaction Kinetics in a Uniform Supersonic Flow) technique solves this problem. For this reason, we present in this work the kinetic study of the gas-phase reaction of CH3CN with one of the most ubiquitous radicals, the hydroxyl (OH) radical, as a function of temperature (11.7–177.5 K). The kinetic technique employed to investigate the CH3CN + OH reaction was the pulsed laser photolysis-laser induced fluorescence. The rate coefficient for this reaction k(T) has been observed to drastically increase from 177.5 K to 107.0 K (about 2 orders of magnitude), while the increase in k(T) from 107.0 K to 11.7 K was milder (around 4 times). The temperature dependent expressions for k(T) are provided in the two distinct T-ranges, excluding the upper limit obtained for k(177.5 K): 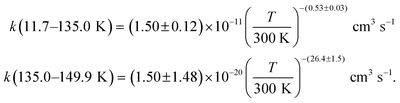 In addition, the rate coefficients estimated by the canonical competitive unified statistical (CCUS) theory show a similar behaviour to the experimental results, when evaluated within the high-pressure limit. This is consistent with the experimentally observed independence of k(T) with total gas density at selected temperatures. Astrochemical networks, such as the KIDA database or UMIST, do not include the CH3CN + OH reaction as a potential depletion process for acetonitrile in the ISM because the current studies predict very low rate coefficients at IS temperatures. According to the model (T = 10 K), the impact of the titled reaction on the abundances of CH3CN appears to be negligible in dark molecular clouds of the ISM (∼1% of the total depletion reactions included in UMIST network). With respect to the potential formation of the CH2CN radical in those environments, even in the most favourable scenario, where this radical could be formed in a 100% yield from the CH3CN + OH reaction, this route would only contribute around 2% to the current assumed formation routes by the UMIST network.
In addition, the rate coefficients estimated by the canonical competitive unified statistical (CCUS) theory show a similar behaviour to the experimental results, when evaluated within the high-pressure limit. This is consistent with the experimentally observed independence of k(T) with total gas density at selected temperatures. Astrochemical networks, such as the KIDA database or UMIST, do not include the CH3CN + OH reaction as a potential depletion process for acetonitrile in the ISM because the current studies predict very low rate coefficients at IS temperatures. According to the model (T = 10 K), the impact of the titled reaction on the abundances of CH3CN appears to be negligible in dark molecular clouds of the ISM (∼1% of the total depletion reactions included in UMIST network). With respect to the potential formation of the CH2CN radical in those environments, even in the most favourable scenario, where this radical could be formed in a 100% yield from the CH3CN + OH reaction, this route would only contribute around 2% to the current assumed formation routes by the UMIST network.

- This article is part of the themed collection: Molecular Dynamics in the Gas Phase


 Please wait while we load your content...
Please wait while we load your content...