Implementing a sulfur-substitution approach toward a high-performance recyclable polythioester†
Si-Qi
Wang
,
Long-Hai
Liu
,
Kun
Li
,
Wei
Xiong
,
Hua-Zhong
Fan
,
Qing
Cao
,
Zhongzheng
Cai
 * and
Jian-Bo
Zhu
* and
Jian-Bo
Zhu
 *
*
National Engineering Laboratory of Eco-Friendly Polymeric Materials (Sichuan), College of Chemistry, Sichuan University, Chengdu 610064, People's Republic of China. E-mail: zzcai@scu.edu.cn; jbzhu@scu.edu.cn
First published on 16th January 2025
Abstract
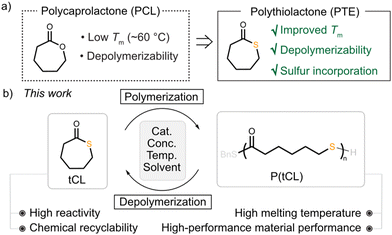
Developing new chemically recyclable polymers is highly demanded for a circular plastic economy. Herein, we implement a sulfur-substitution approach for improving the chemical recyclability and material performance of polycaprolactone (PCL). This thiocaprolactone (tCL) displayed excellent reactivity towards ring-opening polymerization, affording P(tCL) products with high thermal stability (Td = 330 °C), high air stability, high crystallinity, and outstanding mechanical and transport properties (σB = ∼20 MPa, εB = ∼600%, PO2 = 0.38 Barrer) comparable to commercial low-density polyethylene. Impressively, chemical recycling of P(tCL) to its monomer could be accomplished with excellent yield and purity, thus establishing its circular life cycle.
Introduction
The dramatic growth in global plastic production and consumption has caused the massive accumulation of plastic waste due to the non-biodegradability of non-renewable fossil-based plastics, leading to severe environmental problems.1–3 The development of chemically recyclable polymers with a closed-loop economy has served as an attractive approach for alleviating plastic pollution.4–16 Polycaprolactone (PCL) produced via the ring-opening polymerization (ROP) of ε-caprolactone (CL) has attracted increasing attention for use in packaging materials and medical devices owing to its outstanding mechanical properties and promising biocompatibility and degradability.17–20 However, its low melting transition temperature (∼60 °C) limits its further applications as a plastic product. In addition, chemical recycling of PCL still requires harsh reaction conditions and is accompanied by the formation of side oligomer products because of its high ring strain.21Considering the greater van der Waals radius of a sulfur atom than that of an oxygen atom, the elongated thioester bond could release the ring strain of thiolactone monomers. In addition, the soft thioester is a more reactive nucleophile in comparison with the ester bond, which could facilitate the depolymerization process with a more favourable thermodynamic tendency.22–25 The sulfur-substitution strategy has been proved to have a significant influence on the chemical recyclability of the resulting polymers. Elegant examples from Tao and coworkers have demonstrated that an O-to-S substitution of six-membered dilactone could decrease ring strain and improve its depolymerizability.26,27 Moreover, bridged bicyclic thiolactone monomers designed by Lu and Chen's research groups were reported to give rise to polythioesters with full recyclability.28,29 Gutekunst and our laboratory have showcased the polymerization thermodynamics of 4-dithian-2-one.30,31 A systematic study related to heteroatom-incorporated PTEs was continuously reported by Wang and Zhang's research groups, manifesting the effect of the S-substitution strategy.32,33
Despite significant advances in chemically recyclable PTEs, current polymer systems have mainly focused on low ring-strain six-membered-based thiolactones which generally suffer from low polymerization conversions at room temperature and poor thermal stability. Seven-membered thiolactones remain to be explored for chemical recycling. More importantly, the introduction of sulfur into polymers endowed the resulting materials with distinct material properties.34–54
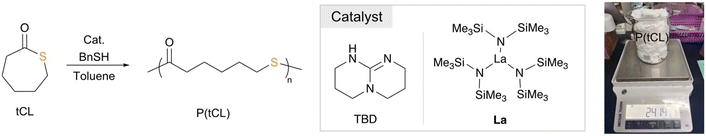
In this context, we reasoned that a sulfur-substitution approach would improve the chemical recyclability and material performance of PCL, accessing high-performance chemically recyclable plastics (Fig. 1a). Specifically, we have investigated the ROP of ε-thiocaprolactone (tCL) with organic bases (1,5,7-triazabicyclo[4.4.0]dec-5-ene) or an La complex (tris[N,N-bis(trimethylsilyl)amide]lanthanum) as catalysts. The produced P(tCL) exhibited high air stability, high thermal stability, good barrier properties to oxygen and water, and polyolefin-like mechanical properties (σB = 19.99 ± 3.53 MPa and εB = 597 ± 71%). Intriguingly, chemical recycling of P(tCL) to monomers could be carried out under mild conditions with excellent yields, establishing a closed-loop economy (Fig. 1b). Notably, P(tCL) could be converted to pristine monomers with high yield (90%) and purity (>99%) from a mixture of plastic waste. This comprehensive study of P(tCL) illustrated that it is a promising candidate for the next generation of high-performance real world recyclable polymers.
Results and discussion
Monomer synthesis
To prove our concept, ε-thiocaprolactone (tCL) was prepared on a gram scale to explore its polymerization performance. In fact, a modest yield of tCL was previously reported via an inefficient cyclization of 6-mercaptohexanoic acid.55 To circumvent this synthetic obstacle, we proposed that it was obtainable from commercially available CL. By the employment of S-substitution, CL could transform to ε-thionocaprolactone,56 which subsequentially underwent O/S isomerization polymerization to form poly(ε-thiocaprolactone) (Scheme S1†). Ultimately, pure tCL on a 12 g scale with a total yield of 51% could be obtained via the bulk depolymerization of poly(ε-thiocaprolactone).Ring-opening polymerization
Initial polymerization screening of tCL was performed with TBD and La as catalysts and benzyl mercaptan (BnSH) as the initiator at room temperature (Table 1, entries 1–5). In fact, the TBD-mediated ROP of tCL was previously reported at a low [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD] ratio of 50
[TBD] ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the produced P(tCL) displayed limited molecular weight (∼6 kDa).55 Herein, we aim to prepare high-molecular-weight P(tCL) to explore its material performance. To our delight, tCL exhibited excellent polymerizability at a [tCL]
1 and the produced P(tCL) displayed limited molecular weight (∼6 kDa).55 Herein, we aim to prepare high-molecular-weight P(tCL) to explore its material performance. To our delight, tCL exhibited excellent polymerizability at a [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnSH] ratio of 100
[BnSH] ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, approaching 88% conversion within 10 min. The resulting product P(tCL) displayed a number-average molecular weight (Mn) value of 13.9 kDa and a dispersity (Đ) of 1.33 based on size exclusion chromatography (SEC) analysis. Increasing the [tCL]
1, approaching 88% conversion within 10 min. The resulting product P(tCL) displayed a number-average molecular weight (Mn) value of 13.9 kDa and a dispersity (Đ) of 1.33 based on size exclusion chromatography (SEC) analysis. Increasing the [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnSH] ratio to 500
[BnSH] ratio to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 contributed to an increase in Mn to 42.1 kDa. These experimental Mn values were in good agreement with the corresponding theoretical values (Mn,calcd) calculated from the [tCL]
1 contributed to an increase in Mn to 42.1 kDa. These experimental Mn values were in good agreement with the corresponding theoretical values (Mn,calcd) calculated from the [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnSH] ratios and conversions. This TBD-mediated polymerization showed high activity even at a [tCL]
[BnSH] ratios and conversions. This TBD-mediated polymerization showed high activity even at a [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnSH] ratio of 1000
[BnSH] ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaching 60% conversion in 4 h. However, SEC analysis revealed that the Mn value of the resulting product P(tCL) was significantly lower than its Mn,calcd (43.3 kDa vs. 78.1 kDa). Improving the [tCL]
1, reaching 60% conversion in 4 h. However, SEC analysis revealed that the Mn value of the resulting product P(tCL) was significantly lower than its Mn,calcd (43.3 kDa vs. 78.1 kDa). Improving the [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnSH] ratio to 2000
[BnSH] ratio to 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at an elevated temperature of 60 °C led to a further decrease in Mn. We speculated that the inevitable chain transfer in this PTE system was responsible for the decreased Mn. Because the conjugation of the sulfur p-orbital was not as strong as that of oxygen with the π-orbital of carbonyl, thioesters are well known as more reactive to nucleophiles than esters, which led to chain transfer in the polymerization system (Fig. S3†). To further probe the microstructure of the obtained P(tCL), a P(tCL) sample prepared with [tCL]
1 at an elevated temperature of 60 °C led to a further decrease in Mn. We speculated that the inevitable chain transfer in this PTE system was responsible for the decreased Mn. Because the conjugation of the sulfur p-orbital was not as strong as that of oxygen with the π-orbital of carbonyl, thioesters are well known as more reactive to nucleophiles than esters, which led to chain transfer in the polymerization system (Fig. S3†). To further probe the microstructure of the obtained P(tCL), a P(tCL) sample prepared with [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] = 50
[I] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was examined by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry. Its MALDI-TOF mass spectrum consisted of two series of molecular ion peaks, which presumably corresponded to a linear P(tCL) with BnSH as the chain ends (BnS-[tCL]n-H) and a cyclic polymer [tCL]n with no chain ends (Fig. S9†). These results further supported our hypothesis that the presence of a cyclic polymer might lead to decreased Mn.
1 was examined by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry. Its MALDI-TOF mass spectrum consisted of two series of molecular ion peaks, which presumably corresponded to a linear P(tCL) with BnSH as the chain ends (BnS-[tCL]n-H) and a cyclic polymer [tCL]n with no chain ends (Fig. S9†). These results further supported our hypothesis that the presence of a cyclic polymer might lead to decreased Mn.
| Entry | Cat. | [M]/[Cat.]/[I] | Time (h) | Conv.b (%) |
M
n,calcd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kg mol−1) c (kg mol−1) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (kDa) d (kDa) |
Đ |
|---|---|---|---|---|---|---|---|
| a Conditions: [tCL] = 1.1 M, initiator (I) = benzyl mercaptan, RT. b Monomer conversion measured by 1H NMR of the quenched solution. c Calculated from [tCL]0/[BnSH]0 × conv. × MWM + MWBnSH. d Number-average molecular weight (Mn) and dispersity index (Đ = Mw/Mn), determined by size exclusion chromatography (SEC) at 40 °C in THF. e Temp. = 60 °C. | |||||||
| 1 | TBD | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 min | 88 | 11.5 | 13.9 | 1.33 |
| 2 | TBD | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 65 | 42.3 | 42.1 | 1.32 |
| 3 | TBD | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 60 | 78.1 | 43.3 | 1.31 |
| 4e | TBD | 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5 | 74 | 144 | 39.2 | 1.33 |
| 5e | TBD | 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 | 82 | 214 | 31.3 | 1.35 |
| 6 | La | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2 min | 95 | 41.2 | 52.0 | 2.01 |
| 7 | La | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1.5 min | 95 | — | 99.2 | 1.94 |
| 8 | La | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4 min | 94 | — | 103 | 1.67 |
Instead, La promoted a robust polymerization of tCL in a more controlled fashion. tCL reached 95% conversion in 2 min with a [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [La]
[La]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BnSH] ratio of 1000
[BnSH] ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and it produced P(tCL) with a Mn of 52.0 kDa and a Đ of 2.01. Interestingly, in the absence of the initiator BnSH, the La-mediated ROP of tCL reached 94% conversion rapidly within 4 min and gave rise to P(tCL) with a Mn of 103 kDa and a dispersity (Đ) of 1.67 (Table 1, entry 8). Attempts to conduct MALDI-TOF mass spectrometry experiments for these samples failed due to the high Mn value. Considering that no chain-end groups were observed in its 1H NMR spectrum (Fig. S4†), we speculated that the P(tCL) obtained with [tCL]
3 and it produced P(tCL) with a Mn of 52.0 kDa and a Đ of 2.01. Interestingly, in the absence of the initiator BnSH, the La-mediated ROP of tCL reached 94% conversion rapidly within 4 min and gave rise to P(tCL) with a Mn of 103 kDa and a dispersity (Đ) of 1.67 (Table 1, entry 8). Attempts to conduct MALDI-TOF mass spectrometry experiments for these samples failed due to the high Mn value. Considering that no chain-end groups were observed in its 1H NMR spectrum (Fig. S4†), we speculated that the P(tCL) obtained with [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [La] = 50
[La] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was a cyclic structure. A large-scale polymerization on 25 g was carried out within 5 min to furnish 24 g of P(tCL) with a Mn of 59.4 kDa and a dispersity (Đ) of 1.73.
1 was a cyclic structure. A large-scale polymerization on 25 g was carried out within 5 min to furnish 24 g of P(tCL) with a Mn of 59.4 kDa and a dispersity (Đ) of 1.73.
Polymer characterization
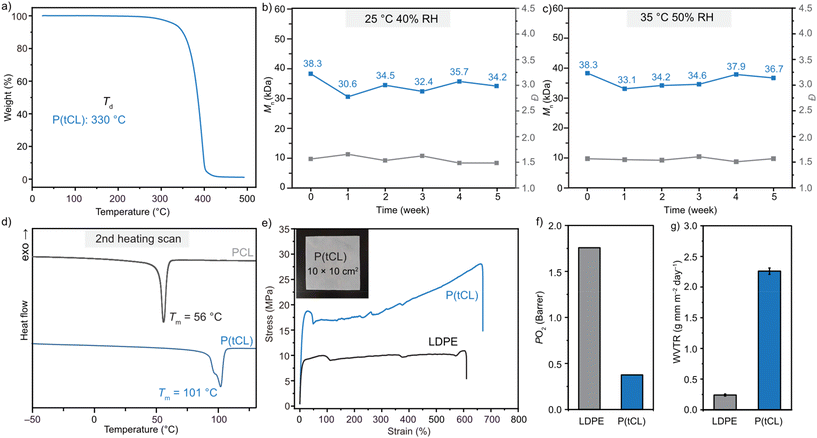
Thermal gravimetric analysis (TGA) was employed to evaluate the thermal stability of P(tCL). P(tCL) demonstrated outstanding thermal stability with a decomposition temperature (Td, defined by the temperature of 5% weight loss) of 330 °C (Fig. 2a). This value was significantly higher than those of previously reported chemically recyclable polythioesters (<250 °C).57 The air stability was examined by placing a P(tCL) sample (Mn = 38.3 kDa, Đ = 1.57) inside an incubator (25 °C and 35 °C) at 40% and 50% relative humidity (RH) to monitor their weight and Mn change over 5 weeks (Fig. S19, 20† and Fig. 2b and c). After 5 weeks at 25 °C and 40% RH and 35 °C and 50% RH, the polymer samples showed identical 1H NMR spectra to those of the pristine material, and no significant change in Mn and Đ was observed. These experiments underscored the air and moisture stability of P(tCL). In contrast, a significant Mn loss from 77.3 to 18.8 kDa was reported for PPDO stored under similar conditions.58 In regard to the differential scanning calorimetry (DSC) study, P(tCL) exhibited a melting-transition temperature (Tm) of 101 °C on the second heating scan (Fig. 2d), highlighting a remarkable improvement of Tm in comparison with PCL (∼60 °C). This Tm value is consistent with the previous literature report.59 Meanwhile, it provided a wide thermal processing window of ∼230 °C for semicrystalline P(tCL) (between the Tm and Td). The PXRD study also confirmed that P(tCL) is a semi-crystalline polymer by the observation of several sets of strong diffraction peaks at 19° and 24° (Fig. S14†).Motivated by the superior thermal behaviour of P(tCL), we set out to investigate its mechanical performance. The dog-bone-shaped opaque P(tCL) specimens were prepared by melt pressing at 102 °C. SEC analysis of the resulting P(tCL) film revealed that no obvious degradation was observed, confirming their excellent thermal stability (Table S2†). These P(tCL) specimens were subjected to uniaxial extension experiments and exhibited outstanding mechanical performance. Its stress–strain curve is presented in Fig. 2e. Impressively, P(tCL) (Mn = 63.7 kDa, Đ = 1.68) displayed a tensile strength at break (σB) of 19.99 ± 3.53 MPa, an elongation at break (εB) of 597 ± 71%, and Young's modulus, E, of 0.20 ± 0.04 GPa. This tough and ductile mechanical performance was comparable to the commercialized low-density polyethylene LDPE (σB = 8.71 ± 0.23 MPa and εB = 569 ± 33%). It is worth noting that the polymer molecular weight played a critical role in its tensile performance. Low-molecular-weight P(tCL) (28.9 kDa) exhibited poor mechanical properties with a σB of only 9.00 ± 0.59 MPa at 47.84 ± 10.65% strain (εB) (Table S4 and Fig. S17†).
Considering the superior mechanical performance of P(tCL) to LDPE, we next investigated its transport properties to evaluate its potential as a barrier material for food packaging applications. Oxygen permeability measurements revealed that P(tCL) exhibited a remarkably low oxygen permeability value (PO2) of 0.38 Barrer, which is critical to maintaining the safety and quality of the food product (Fig. 2f). Meanwhile, P(tCL) also displayed a moderate water vapor transmission rate (WVTR) of 2.26 g mm per m2 per day (Fig. 2g), which might have a beneficial effect on fresh food respiration and transpiration.60 As a reference, the commercial LDPE films examined under the same conditions showed a PO2 of 1.76 Barrer and a WVTR of 0.24 g mm per m2 per day. Collectively, P(tCL) combined an excellent gas barrier and appropriate water permeability with robust material properties to serve as a chemically recyclable polymer that could be attractive for food packaging applications.
Chemical recycling to monomers
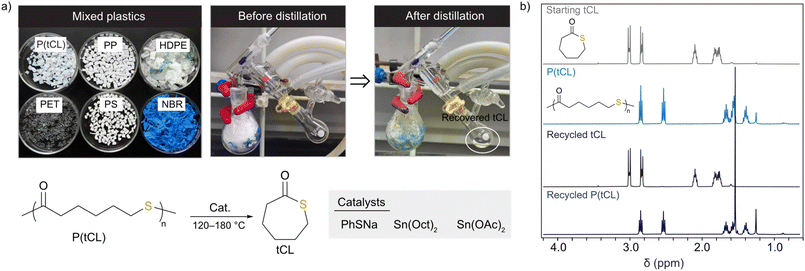
The good stability and promising tensile strength of P(tCL) encouraged us to further study its chemical recyclability (Fig. 3). The ceiling temperature (Tc) of these polymer systems was determined by monitoring the polymerization equilibrium changes over a temperature range of 60 to 120 °C. Their standard-state thermodynamic parameters of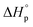 and
and 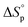 were calculated according to the van't Hoff plot (Table S8†). P(tCL) demonstrated
were calculated according to the van't Hoff plot (Table S8†). P(tCL) demonstrated 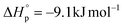 ,
, 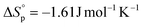 , and a relatively high Tc of 5379 °C at [tCL]0 = 1.0 M, which is in good agreement with previously reported values. Catalysts including PhSNa, Sn(Oct)2 and Sn(OAc)2 were initially examined for thermal bulk depolymerization of P(tCL) at 120–180 °C (Table S9†). It was found that PhSNa facilitated the most efficient depolymerization of P(tCL) to recover quantitatively tCL in 2 h at 180 °C. To establish the closed-loop economy of P(tCL), the recycled tCL was subjected to repolymerization with TBD as the catalyst and BnSH as the initiator. To our delight, it went through repolymerization at an [tCL]/[TBD]/[I] ratio of 100/1/1 without a decrease in polymerization reactivity, approaching 85% conversion within 10 min (Table S10†). In line with the results from original P(tCL), the recycled P(tCL) displayed similar Mn values, highlighting the feasibility and efficiency for chemical recycling. More impressively, P(tCL) was able to depolymerize back to tCL from a mixed plastic stream to obviate the intricate sorting and separation process (Fig. 3a). P(tCL) and commercial plastic products PP, HDPE, PET, PS, and NBR were premixed and subjected to vacuum distillation at 180 °C in the presence of 5% PhSNa. Ultimately, 2.7 g of tCL was isolated and collected in 90% yield (>99% purity) (Fig. S24†). The recovered monomer tCL underwent repolymerization at a [tCL]
, and a relatively high Tc of 5379 °C at [tCL]0 = 1.0 M, which is in good agreement with previously reported values. Catalysts including PhSNa, Sn(Oct)2 and Sn(OAc)2 were initially examined for thermal bulk depolymerization of P(tCL) at 120–180 °C (Table S9†). It was found that PhSNa facilitated the most efficient depolymerization of P(tCL) to recover quantitatively tCL in 2 h at 180 °C. To establish the closed-loop economy of P(tCL), the recycled tCL was subjected to repolymerization with TBD as the catalyst and BnSH as the initiator. To our delight, it went through repolymerization at an [tCL]/[TBD]/[I] ratio of 100/1/1 without a decrease in polymerization reactivity, approaching 85% conversion within 10 min (Table S10†). In line with the results from original P(tCL), the recycled P(tCL) displayed similar Mn values, highlighting the feasibility and efficiency for chemical recycling. More impressively, P(tCL) was able to depolymerize back to tCL from a mixed plastic stream to obviate the intricate sorting and separation process (Fig. 3a). P(tCL) and commercial plastic products PP, HDPE, PET, PS, and NBR were premixed and subjected to vacuum distillation at 180 °C in the presence of 5% PhSNa. Ultimately, 2.7 g of tCL was isolated and collected in 90% yield (>99% purity) (Fig. S24†). The recovered monomer tCL underwent repolymerization at a [tCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TBD]
[TBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] ratio of 100
[I] ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 without a decrease in its polymerizability (Table S12†). These results established a circular chemical recycling of P(tCL) from many commodity plastic streams.
1 without a decrease in its polymerizability (Table S12†). These results established a circular chemical recycling of P(tCL) from many commodity plastic streams.
Experimental
Materials and methods
All synthesis and manipulations of air- and moisture-sensitive materials were carried out in an argon-filled glovebox. All reagents from Adamas-beta and Energy Chemical were used as received unless otherwise stated.1H NMR and 13C NMR spectroscopy
1H and 13C NMR spectra were recorded on an Agilent 400-MR DD2 or a Bruker Advance 400 spectrometer (1H: 400 MHz, 13C: 100 MHz). Chemical shifts (δ) for 1H and 13C NMR spectra are given in ppm relative to TMS. The residual solvent signals were used as references for 1H and 13C NMR spectra and the chemical shifts were converted to the TMS scale (CDCl3: δH = 7.26 ppm, δC = 77.00 ppm). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. All spectra were processed with MestReNova 14.1.1 software, and coupling constants were reported as observed.Size exclusion chromatography (SEC)
Measurements of polymer absolute weight-average molecular weight (Mw), number average molecular weight (Mn), and molecular weight distributions or dispersity indices (Đ = Mw/Mn) were performed via size exclusion chromatography (SEC). The SEC instrument consisted of an Agilent LC system equipped with one guard column and two PL gel 5 μm mixed-C gel permeation columns and coupled with an Agilent G7162A 1260 Infinity II RI detector. The analysis was performed at 40 °C using THF as the eluent at a flow rate of 1.0 mL min−1. The instrument was calibrated with nine polystyrene standards, and chromatograms were processed with Agilent OpenLab CDS Acquisition 2.5 molecular weight characterization software.Differential scanning calorimetry (DSC)
Melting-transition temperature (Tm) and glass-transition temperature (Tg) of purified and thoroughly dried polymer samples were measured by differential scanning calorimetry (DSC) on a TRIOS DSC25, TA Instruments. All Tg values were obtained from a second scan after the thermal history was removed from the first scan.Thermo-gravimetric analysis (TGA)
Decomposition temperatures (Td) and maximum rate decomposition temperatures (Tmax) of the polymers were measured by thermal gravimetric analysis (TGA) on a TGA55 analyzer, TA Instruments. Polymer samples were heated from ambient temperature to 500 °C at a heating rate of 10 °C min−1. Values of Tmax were obtained from derivative (wt%/°C) vs. temperature (°C) plots and defined by the peak values, while Td values were obtained from wt% vs. temperature (°C) plots and defined by the temperature of 5% weight loss.Conclusions
In conclusion, a sulfur-substitution approach has been implemented for improving the chemical recyclability and material performance of PCL. This thiocaprolactone (tCL) went through robust polymerization to afford semicrystalline P(tCL) products with an Mn of up to 103 kDa. Impressively, P(tCL) exhibited an enhanced Tm of 101 °C in comparison with PCL. In contrast to the typical poor thermal stability of PTEs (Td < 250 °C), P(tCL) presented exceptional thermal stability (Td = 330 °C) and excellent air stability. Mechanical characterization demonstrated that P(tCL) exhibited comparable tensile strength and ductility with respect to LDPE. Film barrier properties showcased promising results for food packaging with low permeability to O2 (0.38 Barrer) and suitable water vapor (2.26 g mm per m2 per day). More importantly, P(tCL) could efficiently and selectively convert back to tCL which underwent repolymerization to establish a closed-loop lifecycle. Collectively, this sulfur-substitution approach paved the pathway towards the development of next-generation plastics.Data availability
The data supporting this article have been included as part of the ESI.† Data are available upon reasonable request from the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFA1501700), the National Natural Science Foundation of China (22071163, 22301197, and 22371194), and the Fundamental Research Funds from Sichuan University (2023SCUNL103 and 2024SCUQJTX005). We would like to thank Peng-Chi Deng from the Analytical & Testing Center of Sichuan University and Dongyan Deng and Jing Li from the College of Chemistry at Sichuan University for compound testing.References
- M. MacLeod, H. P. H. Arp, M. B. Tekman and A. Jahnke, Science, 2021, 373, 61–65 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, 25–29 Search PubMed.
- K. L. Law and R. Narayan, Nat. Rev. Mater., 2022, 7, 104–116 CrossRef CAS.
- C. Shi, L. T. Reilly, V. S. Phani Kumar, M. W. Coile, S. R. Nicholson, L. J. Broadbelt, G. T. Beckham and E. Y. X. Chen, Chem, 2021, 7, 2896–2912 CAS.
- G. W. Coates and Y. D. Y. L. Getzler, Nat. Rev. Mater., 2020, 5, 501–516 CrossRef CAS.
- Q. Bo and Z. Xi, CCS Chem., 2024, 6, 297–312 CrossRef.
- C. M. Plummer, L. Li and Y. Chen, Macromolecules, 2023, 56, 731–750 CrossRef CAS PubMed.
- X.-L. Li, K. Ma, F. Xu and T.-Q. Xu, Chem. – Asian J., 2023, 18, e202201167 CrossRef CAS PubMed.
- G. Xu and Q. Wang, Green Chem., 2022, 24, 2321–2346 RSC.
- C. Shi, E. C. Quinn, W. T. Diment and E. Y.-X. Chen, Chem. Rev., 2024, 124, 4393–4478 CrossRef CAS PubMed.
- Z. Li, Y. Shen and Z. Li, Macromolecules, 2024, 57, 1919–1940 CrossRef CAS.
- F. M. Haque, J. S. A. Ishibashi, C. A. L. Lidston, H. Shao, F. S. Bates, A. B. Chang, G. W. Coates, C. J. Cramer, P. J. Dauenhauer, W. R. Dichtel, C. J. Ellison, E. A. Gormong, L. S. Hamachi, T. R. Hoye, M. Jin, J. A. Kalow, H. J. Kim, G. Kumar, C. J. LaSalle, S. Liffland, B. M. Lipinski, Y. Pang, R. Parveen, X. Peng, Y. Popowski, E. A. Prebihalo, Y. Reddi, T. M. Reineke, D. T. Sheppard, J. L. Swartz, W. B. Tolman, B. Vlaisavljevich, J. Wissinger, S. Xu and M. A. Hillmyer, Chem. Rev., 2022, 122, 6322–6373 CrossRef CAS PubMed.
- Y. Sun, Z. An, Y. Gao, R. Hu, Y. Liu, H. Lu, X.-B. Lu, X. Pang, A. Qin, Y. Shen, Y. Tao, Y.-Z. Wang, J. Wang, G. Wu, G.-P. Wu, T.-Q. Xu, X.-H. Zhang, Y. Zhang, Z. Zhang, J.-B. Zhu, M. Hong and Z. Li, Sci. China: Chem., 2024, 67, 2803–2841 CrossRef CAS.
- S. Yang, S. Du, J. Zhu and S. Ma, Chem. Soc. Rev., 2024, 53, 9609–9651 RSC.
- Z. Cai, Y. Liu, Y. Tao and J.-B. Zhu, Acta Chim. Sin., 2022, 80, 1165–1182 CrossRef CAS.
- D. Sathe, S. Yoon, Z. Wang, H. Chen and J. Wang, Chem. Rev., 2024, 124, 7007–7044 CrossRef CAS PubMed.
- P. Mandal and R. Shunmugam, J. Macromol. Sci., Part A, 2020, 58, 111–129 CrossRef.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC.
- R. Pawar, A. Pathan, S. Nagaraj, H. Kapare, P. Giram and R. Wavhale, Polym. Adv. Technol., 2023, 34, 3296–3316 CrossRef CAS.
- J. S. Lyu, J. S. Lee and J. Han, Sci. Rep., 2019, 9, 1–11 CrossRef PubMed.
- Y.-M. Tu, F.-L. Gong, Y.-C. Wu, Z. Cai and J.-B. Zhu, Nat. Commun., 2023, 14, 3198 CrossRef CAS PubMed.
- W. Xiong and H. Lu, Sci. China: Chem., 2023, 66, 725–738 CAS.
- H. Li, S. M. Guillaume and J.-F. Carpentier, Chem. – Asian J., 2022, 17, e202200641 CrossRef CAS.
- N. Illy and E. Mongkhoun, Polym. Chem., 2022, 13, 4592–4614 RSC.
- Y. Sun, C. Zhang and X. Zhang, Chem. – Eur. J., 2024, 30, e202401684 CrossRef CAS PubMed.
- Y. Wang, M. Li, J. Chen, Y. Tao and X. Wang, Angew. Chem., Int. Ed., 2021, 60, 22547–22553 CrossRef CAS PubMed.
- Y. Wang, Y. Zhu, W. Lv, X. Wang and Y. Tao, J. Am. Chem. Soc., 2023, 145, 1877–1885 CrossRef CAS PubMed.
- J. Yuan, W. Xiong, X. Zhou, Y. Zhang, D. Shi, Z. Li and H. Lu, J. Am. Chem. Soc., 2019, 141, 4928–4935 CrossRef CAS PubMed.
- C. Shi, M. L. McGraw, Z. C. Li, L. Cavallo, L. Falivene and E. Y. X. Chen, Sci. Adv., 2020, 6, 1–12 Search PubMed.
- J. Dai, W. Xiong, M.-R. Du, G. Wu, Z. Cai and J.-B. Zhu, Sci. China: Chem., 2023, 66, 251–258 CrossRef CAS.
- K. A. Stellmach, M. K. Paul, M. Xu, Y.-L. Su, L. Fu, A. R. Toland, H. Tran, L. Chen, R. Ramprasad and W. R. Gutekunst, ACS Macro Lett., 2022, 11, 895–901 CrossRef CAS PubMed.
- M. Wang, Z. Ding, X. Shi, Z. Ma, B. Wang and Y. Li, Macromolecules, 2024, 57, 869–879 CrossRef CAS.
- D. Zhang, X. Wang, Z. Zhang and N. Hadjichristidis, Angew. Chem., Int. Ed., 2024, 63, e202402233 CrossRef CAS PubMed.
- L.-Y. Wang and W.-M. Ren, in Sulfur–Containing Polymers, 2021, pp. 171–190 Search PubMed.
- A. Kausar, S. Zulfiqar and M. I. Sarwar, Polym. Rev., 2014, 54, 185–267 CrossRef CAS.
- Y.-L. Su, W. Xiong, L. Yue, M. K. Paul, K. S. Otte, J. Bacsa, H. J. Qi and W. R. Gutekunst, J. Am. Chem. Soc., 2024, 146, 18074–18082 CrossRef CAS PubMed.
- Y.-L. Su, L. Yue, H. Tran, M. Xu, A. Engler, R. Ramprasad, H. J. Qi and W. R. Gutekunst, J. Am. Chem. Soc., 2023, 145, 13950–13956 CrossRef CAS PubMed.
- J. M. M. Pople, T. P. Nicholls, L. N. Pham, W. M. Bloch, L. S. Lisboa, M. V. Perkins, C. T. Gibson, M. L. Coote, Z. Jia and J. M. Chalker, J. Am. Chem. Soc., 2023, 145, 11798–11810 CrossRef CAS PubMed.
- L. S. Kariyawasam, J. Rolsma and Y. Yang, Angew. Chem., Int. Ed., 2023, 62, e202303039 CrossRef CAS PubMed.
- K. Li, J.-L. Cheng, M.-Y. Wang, W. Xiong, H.-Y. Huang, L.-W. Feng, Z. Cai and J.-B. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202405382 CrossRef CAS PubMed.
- H.-Z. Fan, X. Yang, J.-H. Chen, Y.-M. Tu, Z. Cai and J.-B. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202117639 CrossRef CAS PubMed.
- C.-J. Zhang, H.-L. Wu, Y. Li, J.-L. Yang and X.-H. Zhang, Nat. Commun., 2018, 9, 2137 CrossRef.
- T. Tian, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2018, 140, 6156–6163 CrossRef CAS PubMed.
- V. Puchelle, Y. Latreyte, M. Girardot, L. Garnotel, L. Levesque, O. Coutelier, M. Destarac, P. Guégan and N. Illy, Macromolecules, 2020, 53, 5188–5198 CrossRef CAS.
- H. Li, J. Ollivier, S. M. Guillaume and J.-F. Carpentier, Angew. Chem., Int. Ed., 2022, 61, e202202386 CrossRef CAS PubMed.
- H. Mutlu, E. B. Ceper, X. Li, J. Yang, W. Dong, M. M. Ozmen and P. Theato, Macromol. Rapid Commun., 2019, 40, 1–51 CrossRef PubMed.
- W. Xiong, J. Dai, Z. Cai and J.-B. Zhu, Polymer, 2024, 290, 126515 CrossRef CAS.
- V. B. Purohit, M. Pięta, J. Pietrasik and C. M. Plummer, Polym. Chem., 2022, 13, 4858–4878 RSC.
- Z. Sun, H. Huang, L. Li, L. Liu and Y. Chen, Macromolecules, 2017, 50, 8505–8511 CrossRef CAS.
- J.-Z. Zhao, T.-J. Yue, B.-H. Ren, X.-B. Lu and W.-M. Ren, Nat. Commun., 2024, 15, 3002 CrossRef CAS PubMed.
- L. Zhou, L. T. Reilly, C. Shi, E. C. Quinn and E. Y.-X. Chen, Nat. Chem., 2024, 16, 1357–1365 CrossRef CAS PubMed.
- M. Hirata, T. Yoshimatsu, S. Matsuoka, S. Kawauchi and M. Suzuki, Polym. J., 2024, 56, 711–723 CrossRef CAS.
- Y. Zhu, M. Li, Y. Wang, X. Wang and Y. Tao, Angew. Chem., Int. Ed., 2023, 62, e202302898 CrossRef CAS PubMed.
- Y. Xia, P. Yuan, Y. Zhang, Y. Sun and M. Hong, Angew. Chem., Int. Ed., 2023, 62, e202217812 CrossRef CAS PubMed.
- T. J. Bannin and M. K. Kiesewetter, Macromolecules, 2015, 48, 5481–5486 CrossRef CAS PubMed.
- K. C. Nicolaou, D. G. McGarry, P. K. Somers, B. H. Kim, W. W. Ogilvie, G. Yiannikouros, C. V. C. Prasad, C. A. Veale and R. R. Hark, J. Am. Chem. Soc., 1990, 112, 6263–6276 CrossRef CAS.
- W. Xiong, W. Chang, D. Shi, L. Yang, Z. Tian, H. Wang, Z. Zhang, X. Zhou, E.-Q. Chen and H. Lu, Chem, 2020, 6, 1831–1843 CAS.
- Z.-H. Yang, G.-Q. Tian, S.-C. Chen, G. Wu and Y.-Z. Wang, Acta Polym. Sin., 2022, 53, 236–242 CAS.
- C. G. Overberger and J. K. Weise, J. Am. Chem. Soc., 1968, 90, 3533–3537 CrossRef CAS.
- D. Turan, Food Eng. Rev., 2021, 13, 54–65 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py01425a |
| This journal is © The Royal Society of Chemistry 2025 |