Catalytic dinitrogen reduction to hydrazine and ammonia using Cr(N2)2(diphosphine)2 complexes†
Charles H.
Beasley
 a,
Olivia L.
Duletski
a,
Olivia L.
Duletski
 a,
Ksenia S.
Stankevich
a,
Ksenia S.
Stankevich
 a,
Navamoney
Arulsamy
a,
Navamoney
Arulsamy
 b and
Michael T.
Mock
b and
Michael T.
Mock
 *a
*a
aDepartment of Chemistry and Biochemistry, Montana State University, Bozeman, MT 59717, USA. E-mail: michael.mock@montana.edu
bDepartment of Chemistry, University of Wyoming, Laramie, WY 82071, USA
First published on 26th March 2024
Abstract
The synthesis, characterization of trans-[Cr(N2)2(depe)2] (1) is described. 1 and trans-[Cr(N2)2(dmpe)2] (2) catalyze the reduction of N2 to N2H4 and NH3 in THF using SmI2 and H2O or ethylene glycol as proton sources. 2 produces the highest total fixed N for a molecular Cr catalyst to date.
Motivated by the desire to understand and control the challenging multi-proton, multi-electron reaction of N2 reduction to NH3, researchers have intensely studied the reactivity of molecular transition metal dinitrogen complexes.1 Well-defined molecular systems offer a high degree of electronic and structural control to regulate chemical reactivity of N2.2 When combined with effective strategies to form N–H bonds, such as proton-coupled electron transfer (PCET) reagents,3i.e. SmI2 and a proton source, tens-of-thousands of equivalents of NH3 can be generated.4 The valuable information obtained from these studies includes the identification of viable M–NxHy reaction intermediates from spectroscopic data that can be used to delineate the mechanistic steps of a putative catalytic cycle. Such studies can aid in the understanding of the mechanistically complex biological N2 fixation processes carried out by nitrogenase enzymes,5 as well as heterogeneous Haber–Bosch catalysts.6
Group 6 N2 complexes bearing monodentate phosphine ligands, especially with Mo and W, were among the first molecular systems to generate stoichiometric quantities of N2-derived NH3 from protonolysis reactions with strong acids nearly 50 years ago.7 Recently, a renaissance of examining structurally similar [M(N2)2(P–P)2], (M = Mo, W; P–P = diphosphine) systems has begun, elevating these simple complexes as catalysts for N2 reduction to NH3, or other remarkable reactions such as cleavage of the N2 triple bond.8 Masuda and co-workers reported spontaneous N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond cleavage upon one-electron oxidation of trans-[Mo(N2)2(depe)2] (depe = Et2PCH2CH2PEt2) to form [Mo(N)(depe)2]+.9 Chirik and co-workers developed a photocatalytic strategy to form NH3 from [Mo(N)(depe)2]+ and H2.10 Electrocatalytic N2 fixation with Mo and W-phosphine complexes was described by Peters and co-workers using a tandem catalysis approach.11 Nishibayashi and co-workers showed simple Mo-phosphine complexes catalyzed N2 reduction to NH3 using SmI2 and various proton sources.12
N bond cleavage upon one-electron oxidation of trans-[Mo(N2)2(depe)2] (depe = Et2PCH2CH2PEt2) to form [Mo(N)(depe)2]+.9 Chirik and co-workers developed a photocatalytic strategy to form NH3 from [Mo(N)(depe)2]+ and H2.10 Electrocatalytic N2 fixation with Mo and W-phosphine complexes was described by Peters and co-workers using a tandem catalysis approach.11 Nishibayashi and co-workers showed simple Mo-phosphine complexes catalyzed N2 reduction to NH3 using SmI2 and various proton sources.12
While these examples highlight new discoveries using [M(N2)2(P–P)2] (M = Mo, W) complexes, catalytic N2 reduction with analogous Cr compounds are limited. Recent reports highlighted the utility of molecular Cr complexes using a variety of ligand architectures for N2 activation,8a,13 functionalization,14 or catalytic N2 silylation.15 However, molecular Cr complexes that catalyze the direct reduction of N2 to NH3 are rare. In 2022, Nishibayashi and co-workers reported a Cr complex bearing a PCP pincer ligand that catalyzed direct N2 reduction to NH3 and N2H4 at −78 °C to rt. KC8 and phosphonium salts as H+ sources were required for turnover, and this system was not catalytic using SmI2.16 Herein we prepared and characterized trans-[Cr(N2)2(depe)2] (1), and report catalytic N2 reduction to NH3 and N2H4 with 1 and trans-[Cr(N2)2(dmpe)2]17 (2) (dmpe = Me2PCH2CH2PMe2) at room temperature using SmI2 and ethylene glycol or H2O as proton sources.
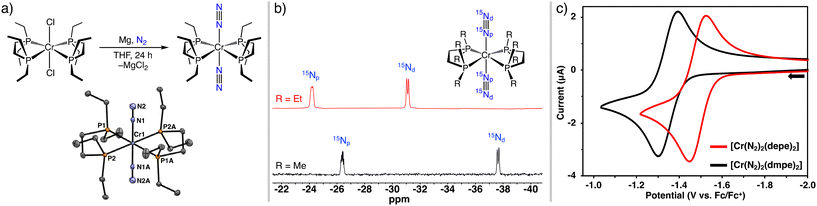
Vigorous stirring of yellow trans-[CrCl2(depe)2]18 (1-Cl) in THF with excess Mg powder under a N2 atmosphere for 24 h furnished 1 as a dark red solid in 70% yield. Isolation of 1 allowed for a comparison of the structural and spectroscopic data with 2 that was reported in 1983.17a The structure of 1, determined by single crystal X-ray diffraction, shows Cr with four phosphorus atoms of the chelates on the equatorial plane and two axial end-on bound N2 ligands, Fig. 1, panel a. The average Cr–N, Cr–P, and N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond distances are 1.904 ± 0.005 Å, 2.334 ± 0.007 Å, and 1.104 ± 0.004 Å, respectively. The corresponding Cr–N, and Cr–P, bond distances in 2 (see ESI†), are slightly shorter at 1.8862(17) Å, and 2.294 ± 0.005 Å, and the N
N bond distances are 1.904 ± 0.005 Å, 2.334 ± 0.007 Å, and 1.104 ± 0.004 Å, respectively. The corresponding Cr–N, and Cr–P, bond distances in 2 (see ESI†), are slightly shorter at 1.8862(17) Å, and 2.294 ± 0.005 Å, and the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N distance is 1.110(2) Å.19 The ligand bite angles for 1 and 2, i.e. P1–Cr–P2, are 81.6° and 83.5°, respectively, and the P–Cr–N angles are near 90°.
N distance is 1.110(2) Å.19 The ligand bite angles for 1 and 2, i.e. P1–Cr–P2, are 81.6° and 83.5°, respectively, and the P–Cr–N angles are near 90°.
The 31P{1H} NMR spectrum of 1 in THF-d8, displays a singlet at 79.9 ppm (68.8 ppm for 2) consistent with four magnetically equivalent P atoms. Complexes 1 and 2 were characterized by 15N NMR spectroscopy to augment the cumulative library of tabulated 15N NMR data of phosphine-supported group 6 N2 complexes.13h The 15N2-labelled complexes 115N and 215N, were prepared by mixing the respective Cr–N2 complexes in THF-d8 under 1 atm 15N2. The 15N NMR spectra were collected after mixing for 24 h. The 15N{1H} NMR spectra contain two resonances; a doublet (JNN = 7.0 Hz) and a multiplet (∼2.5 Hz 31P coupling) (115N: −31.1 ppm, −24.2 ppm, and 215N: −37.6 ppm, −26.4 ppm), assigned as the distal (Nd) and proximal (Np) nitrogen atoms, respectively, (Fig. 1, panel b).13i
Cyclic voltammetry (CV) experiments established the redox behaviour of the Cr(0)-N2 complexes. Voltammograms were recorded using a glassy carbon working electrode at 0.1 V s−1 in THF. The voltammogram for each complex displays a reversible, one-electron CrI/0 wave with the half-wave potential (E1/2) of −1.49 V and −1.34 V (vs. Cp2Fe+/0) for 1 and 2, respectively (Fig. 1, panel c). The electrochemically reversible CrI/0 couples indicate N2 dissociation does not occur upon oxidation to Cr(I) during the CV experiments. The reversibility of the waves for 1 and 2 contrasts other cis- or trans-[Cr(N2)2(P4)] complexes measured by CV that exhibit quasi-reversible or irreversible CrI/0 waves due to rapid N2 loss upon oxidation.13b,c,i In the current study, an irreversible anodic wave was assigned to the CrII/I redox feature at Epa = −0.48 V and Epa = −0.63 V, for 1 and 2, respectively, due to N2 dissociation at more positive potentials, (Fig. S17 and S18 ESI†). The CV results suggest a one-electron chemical oxidation to form trans-[Cr(N2)2(P-P)2]+ should be possible; however, our attempts to isolate such a species have been unsuccessful. Owing to the more electron-rich metal centre of 1, the νNN band in the infrared spectrum at 1906 cm−1 (THF) appears at lower energy than the νNN band for 2 at 1917 cm−1 (THF).
Complexes 1 and 2 were examined as catalysts for the direct reduction of N2 to NH3 and N2H4. The catalysis studies were performed in THF at room temperature using the PCET reagent SmI2 and ethylene glycol and/or water as proton donors. A typical catalytic run used 583 equiv. SmI2, 1166 equiv. ROH per Cr centre and was stirred for 48 h. Quantification of NH3, N2H4 and H2 (see ESI for details†) products assessed the total fixed N generated in each reaction. Selected catalytic data are listed in Table 1 (see ESI for all tabulated results†).
| Entry | Cr cat. | ROH | NH3 equiv./Cra | N2H4 equiv./Crb | Total fixed N | Time (h) |
|---|---|---|---|---|---|---|
Experiments performed using 0.6 μmol catalyst in 15.0 mL THF at 25 °C under 1 atm N2, with 583 equiv. of SmI2, and with 1166 equiv. ROH unless otherwise specified.a Determined by acidification and NH4+ quantification using 1H NMR spectroscopy (see ESI†).b Determined by colormetric p-dimethylaminobenzaldehyde method (see ESI†).c 1000 equiv. H2O/Cr.d 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 equiv. H2O/Cr.e 25 ppm of H2O.f 250 ppm of H2O.g 583 equiv. (CH2OH)2, 583 equiv. H2O.h Average of two or more trials. H2 quantification by gas chromatography, values are tabulated in ESI.† 000 equiv. H2O/Cr.e 25 ppm of H2O.f 250 ppm of H2O.g 583 equiv. (CH2OH)2, 583 equiv. H2O.h Average of two or more trials. H2 quantification by gas chromatography, values are tabulated in ESI.† |
||||||
| 1 | None | (CH2OH)2 | 0 | 0 | 0 | 48 |
| 2 | 1 | (CH2OH)2 | 3.7 ± 0.9 | 1.4 ± 0.8 | 4.9h ± 1.5 | 48 |
| 3 | 1 | (CH2OH)2 | 4.6 ± 0.6 | 4.0 ± 1.7 | 8.6h ± 2.1 | 100 |
| 4c | 1 | H2O | 1.4 | 0.7 | 2.1 | 48 |
| 5d | 1 | H2O | 3.2 | 0.6 | 3.8 | 28 |
| 6 | 1-Cl | (CH2OH)2 | 1.2 | 0.9 | 2.1 | 48 |
| 7 | 2 | (CH2OH)2 | 14.6 ± 1.6 | 5.9 ± 2.9 | 20.5h ± 3.8 | 48 |
| 8e | 2 | (CH2OH)2 | 6.2 ± 0.5 | 6.4 ± 0.8 | 12.6h ± 0.3 | 48 |
| 9f | 2 | (CH2OH)2 | 4.4 ± 0.9 | 6.6 ± 0.6 | 11h ± 0.4 | 48 |
| 10g | 2 | (CH2OH)2 | 1.1 | 5.7 | 6.8 | 48 |
| 11d | 2 | H2O | 5.1 | 5.9 | 11 | 3 |
| 12 | 2-Cl | (CH2OH)2 | 13.5 ± 2.8 | 5.9 ± 0.6 | 19.4h ± 3.4 | 48 |
Analysis of the catalysis results provides insights about the performance of 1 and 2 under identical reaction conditions. 2 afforded more total fixed N than 1 in all catalytic trials. For example, 1 generated up to 5 equiv. of NH3 and 5 equiv. N2H4 per Cr center using ethylene glycol as the proton donor after >100 h. Under identical conditions, 2 produced up to 16 equiv. NH3 and 10 equiv. N2H4 in 48 h. Furthermore, ethylene glycol worked more effectively as the proton donor affording higher total fixed N than using H2O. The deliterious effect of H2O on catalysis was noted in reactions with 2 using ethylene glycol as the primary proton source. As the amount of H2O added to the reaction increased, NH3 production declined, while the N2H4 formed stayed relatively constant. We postulate the Cr complexes may simply be more prone to degradation in the presence of H2O. Separately, 2 was treated with 500 equiv. H2O or ethylene glycol in THF-d8. Free dmpe from complex degradation appeared more rapidly using H2O, as assessed by 31P NMR spectroscopy. Catalysis performed with 2 under an atmosphere of 15N2 afforded 15NH4+ as a doublet at 7.1 ppm (J15N-1H = 71 Hz) in the 1H NMR spectrum, identifying 15N2 as the source of 15NH3.
Catalytic trials using trans-[CrCl2(dmpe)2] (2-Cl) and ethylene glycol generated comparable amounts of NH3 and N2H4 as using 2 as the precatalyst. 1-Cl did not catalyze N2 reduction, affording only 1 equiv. of NH3 and N2H4 per Cr center. SmI2 and ethylene glycol may be ineffective at reducing the Cr(II) center of 1-Cl to Cr(0) where N2 is strongly activated. Treatment of 2-Cl with 2 equiv. SmI2 and 2 equiv. ethylene glycol rapidly generated 2 (see ESI†). However, the same reaction of 1-Cl and SmI2 with ethylene glycol additive did not form 1 (E1/2 = −1.49 V, vide supra). 1 or 2 could not be generated from 1-Cl or 2-Cl using excess SmI2(THF) alone (E° of SmI2(THF) = −1.41 ± 0.08 V20vs. Fc/Fc+). A Cr(I) species could be accessible, but N2 activation and subsequent functionalization steps may be moderated at Cr(I), limiting catalysis.
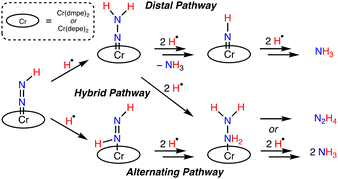
The mixed N2 reduction selectivity to form NH3 and N2H4 provides preliminary evidence for a catalytic cycle that follows, at least in part, an alternating N2 reduction mechanism, Fig. 2, bottom. A purely distal N2 reduction pathway, Fig. 2, top, would be selective for NH3 formation. In a 1986 report, the reaction of 2 with CF3SO3H was postulated to form a Cr-hydrazido product, [Cr(NNH2)(dmpe)2][CF3SO3]2.21 A recent study by Wei, Yi, Xi, and co-workers examining early stage N2 functionalization of [Cp*Cr0(depe)(N2)]− (Cp* = η5-C5(CH3)5) using a variety of electrophiles (H+, Me3Si+, Me+) also revealed the selective formation of Cr-hydrazido products, consistent with a distal pathway. Contrary to these reaction patterns, protonation studies of related cis- or trans-[Cr(N2)2(P4)] complexes we examined using strong acids or H+/e− reagents, as well as the catalytic Cr[PCP] system16 generated NH3 and N2H4.13c,i,15a Considering all these examples, and that N2 reduction mechanisms are sensitive to reaction conditions, (i.e. identity of the H+ and e− reagents, solvent, temperature), a hybrid N2 reduction pathway22 where the third and fourth N–H bonds are formed at the proximal N atom of a Cr-hydrazido intermediate, Fig. 2, middle, cannot be excluded for the current systems. Further studies are warranted to understand the N2 reduction pathways with Cr.
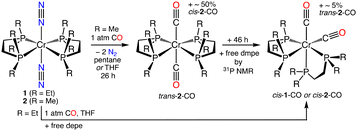
The proclivity for N2 ligand substitution in 1 and 2 was evaluated as a metric that could reflect catalyst stability and influence catalytic performance. We examined reactions of 1 and 2 with CO to assess the rate of ligand exchange, Fig. 3. Ligand substitution in these six-coordinate complexes is expected to be a dissociative process; a result of Cr–N or Cr–P bond dissociation. Wilkinson, Hursthouse, and co-workers noted 2 did not react with 7 atm CO for several hours except under u.v. irradiation (in light petroleum) to form cis-[Cr(CO)2(dmpe)2] (cis-2-CO).17b This account was surprising, and the unreactive nature toward N2/CO exchange seemed uncharacteristic of a complex with terminally bound N2 ligands. We reacted 2 with 1 atm CO at 25 °C in pentane or THF without u.v. irradiation and monitored the reaction by in situ IR spectroscopy, or 31P NMR spectroscopy (see ESI†). In both solvents the reaction was slow, but 2 was not unreactive. In THF, after 26 h ∼85% of 2 converted to a ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of cis-2-CO and trans-[Cr(CO)2(dmpe)2] (trans-2-CO). trans-2-CO converts to ∼95% cis-2-CO (and ∼5% free dmpe) after additional 46 h by 31P NMR spectroscopy. In THF, 1 converts directly to cis-[Cr(CO)2(depe)2] cis-1-CO (νCO = 1829, 1768 cm−1) in ∼3 h by in situ IR spectroscopy (see ESI†). The vastly different rates of N2/CO ligand exchange underscore the greater kinetic stability of 2 toward Cr–L dissociative processes that could ultimately curtail catalyst deactivation pathways (i.e. ligand loss) improving catalyst performance for N2 reduction compared to 1.
1 mixture of cis-2-CO and trans-[Cr(CO)2(dmpe)2] (trans-2-CO). trans-2-CO converts to ∼95% cis-2-CO (and ∼5% free dmpe) after additional 46 h by 31P NMR spectroscopy. In THF, 1 converts directly to cis-[Cr(CO)2(depe)2] cis-1-CO (νCO = 1829, 1768 cm−1) in ∼3 h by in situ IR spectroscopy (see ESI†). The vastly different rates of N2/CO ligand exchange underscore the greater kinetic stability of 2 toward Cr–L dissociative processes that could ultimately curtail catalyst deactivation pathways (i.e. ligand loss) improving catalyst performance for N2 reduction compared to 1.
In conclusion, we present a contemporary advancement in the use of trans-[Cr(N2)2(P–P)2] complexes (1 and 2) for direct catalytic reduction of N2 to form NH3 and N2H4 using the PCET reagent SmI2 and H2O and/or ethylene glycol as proton donors. A new complex, trans-[Cr(N2)2(depe)2], was presented herein. Despite having similar electronic structures, we posit 2 is a better catalyst than 1 (using the presented conditions), due to a less negative CrI/0 redox couple and greater kinetic stability from Cr–L dissociative processes.
Author contributions
C. Beasley, investigation, methodology, writing, editing; O. L. Duletski, investigation; K. S. Stankevich, investigation; N. Arulsamy, investigation, writing; M. T. Mock, conceptualization, methodology, supervision, writing, editing, funding acquisition.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank Dr. Bernhard Linden and Mathias Linden for LIFDI-MS analysis. This material is based upon work supported by the National Science Foundation (NSF) under Grant No. CHE-1956161 and CHE-2247748. Support for MSU's NMR Center has been provided by the NSF (Grant No. NSF-MRI: CHE-2018388 and DBI-1532078), the Murdock Charitable Trust Foundation (2015066:MNL), and MSU's office of the Vice President for Research and Economic Development. The authors gratefully acknowledge financial support for the X-ray diffractometer from the NSF (CHE-0619920) and a Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (Grant # 2P20GM103432).References
- (a) Y. Tanabe and Y. Nishibayashi, Chem. Soc. Rev., 2021, 50, 5201–5242 RSC; (b) Y. Tanabe and Y. Nishibayashi, Coord. Chem. Rev., 2022, 472, 214783 CrossRef CAS; (c) Transition Metal−Dinitrogen Complexes: Preparation and Reactivity, ed. Y. Nishibayashi, Wiley-VCH, Weinheim, 2019 Search PubMed.
- M. J. Chalkley, M. W. Drover and J. C. Peters, Chem. Rev., 2020, 120, 5582–5636 CrossRef CAS PubMed.
- (a) Y. Ashida, K. Arashiba, K. Nakajima and Y. Nishibayashi, Nature, 2019, 568, 536–540 CrossRef CAS PubMed; (b) N. G. Boekell and R. A. Flowers II, Chem. Rev., 2022, 122, 13447–13477 CrossRef CAS PubMed; (c) E. A. Boyd and J. C. Peters, J. Am. Chem. Soc., 2022, 144, 21337–21346 CrossRef CAS PubMed.
- Y. Ashida, T. Mizushima, K. Arashiba, A. Egi, H. Tanaka, K. Yoshizawa and Y. Nishibayashi, Nat. Synth., 2023, 2, 635–644 CrossRef.
- C. Van Stappen, L. Decamps, G. E. Cutsail III, R. Bjornsson, J. T. Henthorn, J. A. Birrell and S. DeBeer, Chem. Rev., 2020, 120, 5005–5081 CrossRef CAS PubMed.
- C. M. Goodwin, P. Lömker, D. Degerman, B. Davies, M. Shipilin, F. Garcia-Martinez, S. Koroidov, J. Katja Mathiesen, R. Rameshan, G. L. S. Rodrigues, C. Schlueter, P. Amann and A. Nilsson, Nature, 2024, 625, 282–286 CrossRef CAS PubMed.
- J. Chatt, A. J. Pearman and R. L. Richards, Nature, 1975, 253, 39–40 CrossRef CAS.
- (a) F. A. Darani, G. P. A. Yap and K. H. Theopold, Organometallics, 2023, 42, 1324–1330 CrossRef CAS; (b) S. J. K. Forrest, B. Schluschass, E. Y. Yuzik-Klimova and S. Schneider, Chem. Rev., 2021, 121, 6522–6587 CrossRef CAS PubMed; (c) C. E. Laplaza and C. C. Cummins, Science, 1995, 268, 861–863 CrossRef CAS PubMed.
- A. Katayama, T. Ohta, Y. Wasada-Tsutsui, T. Inomata, T. Ozawa, T. Ogura and H. Masuda, Angew. Chem., Int. Ed., 2019, 58, 11279–11284 CrossRef CAS PubMed.
- (a) S. Kim, Y. Park, J. Kim, T. P. Pabst and P. J. Chirik, Nat. Synth., 2022, 1, 297–303 CrossRef; (b) M. T. Mock, Nat. Synth., 2022, 1, 262–263 CrossRef.
- P. Garrido-Barros, J. Derosa, M. J. Chalkley and J. C. Peters, Nature, 2022, 609, 71–76 CrossRef CAS PubMed.
- Y. Ashida, K. Arashiba, H. Tanaka, A. Egi, K. Nakajima, K. Yoshizawa and Y. Nishibayashi, Inorg. Chem., 2019, 58, 8927–8932 CrossRef CAS PubMed.
- (a) A. J. Kendall and M. T. Mock, Eur. J. Inorg. Chem., 2020, 1358–1375 CrossRef CAS; (b) M. T. Mock, S. Chen, R. Rousseau, M. J. O'Hagan, W. G. Dougherty, W. S. Kassel, D. L. DuBois and R. M. Bullock, Chem. Commun., 2011, 47, 12212–12214 RSC; (c) M. T. Mock, S. Chen, M. O'Hagan, R. Rousseau, W. G. Dougherty, W. S. Kassel and R. M. Bullock, J. Am. Chem. Soc., 2013, 135, 11493–11496 CrossRef CAS PubMed; (d) M. Fritz, S. Demeshko, C. Würtele, M. Finger and S. Schneider, Eur. J. Inorg. Chem., 2023, 26, e202300011 CrossRef CAS; (e) W. H. Monillas, G. P. A. Yap, L. A. MacAdams and K. H. Theopold, J. Am. Chem. Soc., 2007, 129, 8090–8091 CrossRef CAS PubMed; (f) W. H. Monillas, G. P. A. Yap and K. H. Theopold, Inorg. Chim. Acta, 2011, 369, 103–119 CrossRef CAS; (g) X. Wang, Y. Wang, Y. Wu, G. X. Wang, J. Wei and Z. Xi, Inorg. Chem., 2023, 62, 18641–18648 CrossRef CAS PubMed; (h) M. T. Mock, A. W. Pierpont, J. D. Egbert, M. O'Hagan, S. Chen, R. M. Bullock, W. G. Dougherty, W. S. Kassel and R. Rousseau, Inorg. Chem., 2015, 54, 4827–4839 CrossRef CAS PubMed; (i) J. D. Egbert, M. O'Hagan, E. S. Wiedner, R. M. Bullock, N. A. Piro, W. S. Kassel and M. T. Mock, Chem. Commun., 2016, 52, 9343–9346 RSC; (j) I. Vidyaratne, J. Scott, S. Gambarotta and P. H. M. Budzelaar, Inorg. Chem., 2007, 46, 7040–7049 CrossRef CAS PubMed.
- (a) J. Yin, J. Li, G. X. Wang, Z. B. Yin, W. X. Zhang and Z. Xi, J. Am. Chem. Soc., 2019, 141, 4241–4247 CrossRef CAS PubMed; (b) G. X. Wang, X. Wang, Y. Jiang, W. Chen, C. Shan, P. Zhang, J. Wei, S. Ye and Z. Xi, J. Am. Chem. Soc., 2023, 145, 9746–9754 CrossRef CAS PubMed; (c) G. X. Wang, Z. B. Yin, J. Wei and Z. Xi, Acc. Chem. Res., 2023, 56, 3211–3222 CrossRef CAS PubMed; (d) Z. B. Yin, B. Wu, G. X. Wang, J. Wei and Z. Xi, J. Am. Chem. Soc., 2023, 145, 7065–7070 CrossRef CAS PubMed; (e) T. Shima, J. Yang, G. Luo, Y. Luo and Z. Hou, J. Am. Chem. Soc., 2020, 142, 9007–9016 CrossRef CAS PubMed; (f) Y. Kokubo, K. Tsuzuki, H. Sugiura, S. Yomura, Y. Wasada-Tsutsui, T. Ozawa, S. Yanagisawa, M. Kubo, T. Takeyama, T. Yamaguchi, Y. Shimazaki, S. Kugimiya, H. Masuda and Y. Kajita, Inorg. Chem., 2023, 62, 5320–5333 CrossRef CAS PubMed.
- (a) A. J. Kendall, S. I. Johnson, R. M. Bullock and M. T. Mock, J. Am. Chem. Soc., 2018, 140, 2528–2536 CrossRef CAS PubMed; (b) M. C. Eaton, B. J. Knight, V. J. Catalano and L. J. Murray, Eur. J. Inorg. Chem., 2020, 1519–1524 CrossRef CAS PubMed; (c) J. Li, J. Yin, G. X. Wang, Z. B. Yin, W. X. Zhang and Z. Xi, Chem. Commun., 2019, 55, 9641–9644 RSC.
- Y. Ashida, A. Egi, K. Arashiba, H. Tanaka, T. Mitsumoto, S. Kuriyama, K. Yoshizawa and Y. Nishibayashi, Chem. – Eur. J., 2022, 28, e202200557 CrossRef CAS PubMed.
- (a) G. S. Girolami, J. E. Salt, G. Wilkinson, M. Thornton-Pett and M. B. Hursthouse, J. Am. Chem. Soc., 1983, 105, 5954–5956 CrossRef CAS; (b) J. E. Salt, G. S. Girolami, G. Wilkinson, M. Motevalli, M. Thornton-Pett and M. B. Hursthouse, J. Chem. Soc., Dalton Trans., 1985, 685–692 RSC.
- D. M. Halepoto, D. G. L. Holt, L. F. Larkworthy, G. J. Leigh, D. C. Povey and G. W. Smith, J. Chem. Soc., Chem. Commun., 1989, 1322–1323 RSC.
- Structural metrics from XRD data of 2 collected here at 100 K. Data from ref. 17 at 295 K.
- (a) M. L. Kuhlman and R. A. Flowers II, Tetrahedron Lett., 2000, 41, 8049–8052 CrossRef CAS; (b) R. J. Enemærke, K. Daasbjerg and T. Skrydstrup, Chem. Commun., 1999, 343–344 RSC.
- J. E. Salt, G. Wilkinson, M. Motevalli and M. B. Hursthouse, J. Chem. Soc., Dalton Trans., 1986, 1141–1154 RSC.
- (a) J. Rittle and J. C. Peters, J. Am. Chem. Soc., 2016, 138, 4243–4248 CrossRef CAS PubMed; (b) N. B. Thompson, P. H. Oyala, H. T. Dong, M. J. Chalkley, J. Zhao, E. E. Alp, M. Hu, N. Lehnert and J. C. Peters, Inorg. Chem., 2019, 58, 3535–3549 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, crystallographic details, and additional spectroscopic and electrochemical data. CCDC 2330754 (1) and 2330755 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt00702f |
| This journal is © The Royal Society of Chemistry 2024 |