Polyurethanes synthesized using biomass-derived furan diols as sustainable triboelectric materials†
June Young
Jang‡
a,
Youngmin
Byun‡
 bc,
Sijin
You
a,
Seunghun
Kim
a,
Dong-Min
Lee
bc,
Sang-Woo
Kim
bc,
Sijin
You
a,
Seunghun
Kim
a,
Dong-Min
Lee
bc,
Sang-Woo
Kim
 *bc and
Seung Uk
Son
*bc and
Seung Uk
Son
 *a
*a
aDepartment of Chemistry, Sungkyunkwan University, Suwon 16419, Korea. E-mail: sson@skku.edu
bDepartment of Materials Science and Engineering, Yonsei University, Seoul 03722, Korea. E-mail: kimsw1@yonsei.ac.kr
cCenter for Human-oriented Triboelectric Energy Harvesting, Yonsei University, Seoul 03722, Korea
First published on 24th July 2024
Abstract
This work shows that various polyurethanes (FPUs) prepared using biomass-derived furan diols can be applied as promising tribopositive materials. The elastic FPUs having appropriate glass transition temperature (Tg) could be incorporated into polyethylene terephthalate (PET) fabrics to form FPU/PET films. The optimal FPU-4/PET film showed promising triboelectric performance with output voltages (Vp–p) up to 405 V and a maximum power density (Pmax) of 32 mW cm−2.
Sustaining human societies depends critically on energy. As human societies have rapidly developed, the need for energy has increased.1 At the same time, efforts have been made to improve the efficiency of energy use.1 In this context, the efficient management of small-scale energy in everyday life is becoming increasingly important.2 While many sources of small-scale energy exist, triboelectrification has recently attracted the attention of scientists as a promising energy source.3
Various polymer materials have been used to harvest triboelectric energy.4 To control the triboelectric features of polymer materials, various tailored polymers have been prepared. Most artificial polymers have been produced using petroleum as a chemical resource.4 However, recently, concerns have arisen regarding the impact of using petroleum as a chemical resource on climate change. In this regard, an alternative to petroleum is required for the synthesis of functional polymers.5
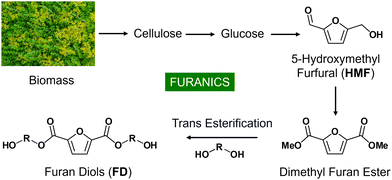
Recently, biomass has emerged as an alternative to petroleum as a chemical resource for the production of functional materials.6 One of the major chemical components of biomass is cell walls that consist of cellulose, hemicellulose, and lignin. Cellulose can be hydrolyzed to glucoses.6 Through the isomerization of glucose to fructose, followed by dehydration, 5-hydroxymethylfurfural (HMF) can be obtained.6 2,5-Dimethyl furan ester can be prepared through the oxidation of HMF to 2,5-furan dicarboxylic acid, followed by esterification with methanol.7 Finally, 2,5-furan diols can be obtained through the transesterification of 2,5-dimethyl furan ester with diols (Fig. 1).8 The 2,5-furan diols (FD) can be utilized as polymer platform materials for the synthesis of polyesters or polyurethanes.9
Our research group has developed heterogeneous catalysts for the synthesis of biomass-derived polymer platform materials.8,10 In addition, we have reported the triboelectric performance of new organic and inorganic materials.11 In this work, we report the synthesis of polyurethanes using FDs (FPUs) and their performance as triboelectric materials.
Fig. 2a shows the synthesis of FPUs through the polymerization of FDs and 1,6-diisocyanatohexane. Through the transesterification of 2,5-dimethyl furan esters with ethylene glycol, 1,3-propanediol, 1,5-pentanediol, diethylene glycol, and 1,4-benzenedimethanol, five FDs (denoted as FD-1, FD-2, FD-3, FD-4, and FD-5) were prepared (Fig. S1 in the ESI†). The FDs were characterized using 1H, 13C nuclear magnetic resonance (NMR), and high resolution mass spectroscopy (HR-MS). Through the polymerization of FDs and 1,6-diisocyanatohexane, five FPUs (denoted as FPU-1, FPU-2, FPU-3, FPU-4, and FPU-5) were prepared (Fig. S2 in the ESI†).
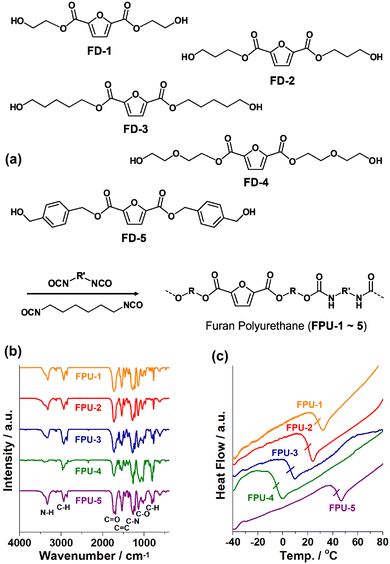 | ||
| Fig. 2 (a) Synthesis of polyurethanes (FPUs) using 2,5-furan diols (FDs). (b) IR absorption spectra and (c) DSC curves of FPU-1–5. | ||
The chemical structures of FPUs were characterized by infrared absorption (IR) and NMR spectroscopy (Fig. 2b and Fig. S2 in the ESI†). In the IR spectra of FPU-1–5, the N–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–N vibration peaks of urethane moieties were observed at 3322–3392, 1692, and 1223–1278 cm−1, respectively (Fig. 2b).9,12 The C
O, and C–N vibration peaks of urethane moieties were observed at 3322–3392, 1692, and 1223–1278 cm−1, respectively (Fig. 2b).9,12 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O vibrations of ester moieties appeared at 1725 and 1135 cm−1, respectively. The aromatic C
O and C–O vibrations of ester moieties appeared at 1725 and 1135 cm−1, respectively. The aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and aliphatic C–H vibrations were observed at 1535–1542 and 2866–2940 cm−1, respectively.9,12 The 1H peaks of furan rings in FPUs appeared at 7.09–7.41 ppm (Fig. S2 in the ESI†).9 Aliphatic 1H peaks were observed at 3.61–4.45 and 1.20–2.94 ppm. The 13C NMR spectra of FPUs showed the C
C and aliphatic C–H vibrations were observed at 1535–1542 and 2866–2940 cm−1, respectively.9,12 The 1H peaks of furan rings in FPUs appeared at 7.09–7.41 ppm (Fig. S2 in the ESI†).9 Aliphatic 1H peaks were observed at 3.61–4.45 and 1.20–2.94 ppm. The 13C NMR spectra of FPUs showed the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks of urethane and ester groups at 156–158 ppm (Fig. S2 in the ESI†). In addition, the 13C peaks of furan rings appeared at 119 and 147 ppm.9 Aliphatic 13C peaks were observed at 40–69 and 22–30 ppm. These observations indicate that FPUs were successfully prepared through the reaction of FDs and 1,6-diisocyanatohexane.
O peaks of urethane and ester groups at 156–158 ppm (Fig. S2 in the ESI†). In addition, the 13C peaks of furan rings appeared at 119 and 147 ppm.9 Aliphatic 13C peaks were observed at 40–69 and 22–30 ppm. These observations indicate that FPUs were successfully prepared through the reaction of FDs and 1,6-diisocyanatohexane.
Differential scanning calorimetric (DSC) curves indicated that as the chain lengths of FDs increased, the glass transition temperature (Tg) of the corresponding FPUs decreased from 28 °C (FPU-1) to 20 (FPU-2) and 5.7 °C (FPU-3), respectively (Fig. 2c). While the Tg of FPU-4 further decreased to −5.3 °C, that of FPU-5 increased to 43 °C, due to the rigid nature of phenyl groups. While the FPU-1–2 and FPU-5 showed a powdery feature, the FPU-3–4 were elastic and soft. Thermogravimetric analysis (TGA) indicated that the FPU-1–5 are thermally stable up to 224–262 °C (Fig. S3 in the ESI†). Using gel permeation chromatography (GPC), the average molecular weights (Mw) of FPU-1–5 were measured to be 1.9, 2.1, 8.8, 8.6, and 2.6 K, respectively. In addition, FPU-1–5 showed a narrow molecular weight distribution with polydispersity index (PDI) values of 1.1, 1.2, 1.2, 1.3, and 1.1, respectively.
Using a polyethylene terephthalate (PET) fabric as a templating material, FPU/PET films were fabricated by dropping a dimethylformamide solution onto PET fabrics (Fig. 3a).
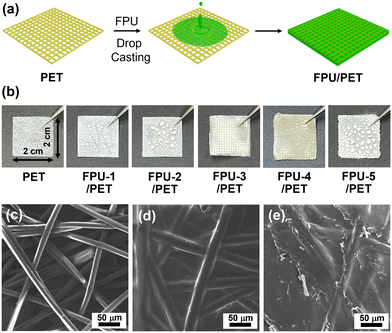 | ||
| Fig. 3 (a) A fabrication process and (b) photographs of FPU/PET films. SEM images of (c) a PET fabric, (d) FPU-3/PET, and (e) FPU-4/PET films. | ||
In the cases of FPU-1–2 and FPU-5, corresponding FPU/PET films could not be obtained to FPU powders on PET fabrics, due to high Tg values (Fig. 3b). In comparison, FPU-3–4/PET films were successfully obtained due to the elastic features of FPU-3–4 (Fig. 3b), whilst FPU-3–4 formed films without PET fabrics, and could not be detached from supports, due to the adhesive feature of PUs. Scanning electron microscopy (SEM) analysis indicated that PET fabrics consist of fibers with diameters of 13–15 μm (Fig. 3c). The SEM images of FPU-3–4/PET films showed that the FPU-3–4 were incorporated into the empty spaces of PET fabrics (Fig. 3d and e). Interestingly, while FPU-3 was significantly adhered to PET fibers to form thickened fibers with diameters of 23–26 μm, FPU-4 mainly filled the empty spaces of PFT fabrics to form a rough surface. While the thickness of PET fabrics was 100 μm, those of FPU-3/PET and FPU-4/PET films decreased to 80–85 μm, due to the FPU-assisted dense packing of PET fibers (Fig. S4 in the ESI†).
According to tensile strength tests, while a PET fabric showed a Young's modulus of 0.20 GPa and a maximum strength of 2.95 MPa, FPU-3–4/PET showed enhanced strengths with Young's moduli of 0.39 and 0.58 GPa and the maximum strengths of 9.16 and 10.88 MPa, respectively (Fig. S5 in the ESI†), indicating that the incorporation of FPU-3–4 improved the strength of PET fabrics.
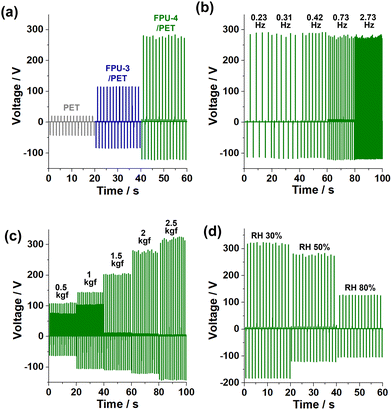
Next, we studied the performance of FPU-3–4/PET films as tribopositive materials (Fig. 4a and Fig. S6 in the ESI†). As a tribonegative material, a perfluoroalkoxyalkane (PFA, a copolymer of tetrafluoroethylene and perfluoroether) film was used.13 While a PET fabric showed an output peak-to-peak voltage (Vp–p) of 64 V (a pushing force of 2 kgf) and an output peak-to-peak current density (Ip–p) of 1.4 μA cm−2, FPU-3–4/PET films showed enhanced Vp–p of 200 and 405 V, respectively. The Ip–p values of FPU-3–4/PET films were measured to be 4.7 and 8.1 μA cm−2 (Fig. S6 in the ESI†). These observations indicate that FPU-4/PET is a better triboelectric material.
The FPU-4/PET film maintained Vp–p of 405–406 V and Ip–p of 7.9–8.2 μA cm−2 in the pushing frequency range of 0.23–2.73 Hz (Fig. 4b and Fig. S6 in the ESI†). As pushing forces gradually increased from 0.5 kgf to 1, 1.5, 2, and 2.5 kgf, Vp–p increased from 175 V with Ip–p of 4.3 μA cm−2 to 250, 315, 405, and 468 V with Ip–p of 5.3, 6.5, 8.1, and 10 μA cm−2, respectively (Fig. 4c and Fig. S6 in the ESI†). At a relative humidity (RH) of 30%, Vp–p and Ip–p further increased to 508 V and 9.4 μA cm−2, respectively (Fig. 4d and Fig. S6 in the ESI†). In comparison, at RH of 80%, Vp–p and Ip–p dropped to 240 V and 4.8 μA cm−2, respectively.
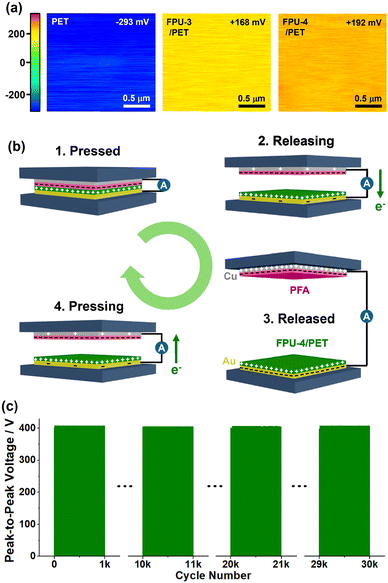
Kelvin probe force microscopy (KPFM) images revealed that surface potentials increased from −293 mV to 168 and 192 mV through the incorporation of FPU-3 and FPU-4 into PET fabrics, respectively (Fig. 5a). We suggest that the superior triboelectric performance of FPU-4/PET is attributable to its electron-rich ether moieties and the efficient triboelectric contact (a surface roughness, Rq of 0.77 μm), compared with FPU-3/PET (Rq of 0.26 μm). Fig. 5b shows the mechanistic process of triboelectric energy harvesting using tribopositive FPU-4/PET and tribonegative PFA films.11,14 Initially, through the contact of two films, electrons transfer from FPU-4/PET to PFA. At a releasing state, electrons move from a Cu electrode with PFA to an Au electrode with FPU-4/PET. After a released state, electrons moved from an Au electrode to a Cu electrode at a pressing state to reach a pressed state. This process is repeated in the triboelectric cycling. Over 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the FPU-4/PET film maintained the original Vp–p values in the range of 405–409 V (Fig. 5c).
000 cycles, the FPU-4/PET film maintained the original Vp–p values in the range of 405–409 V (Fig. 5c).
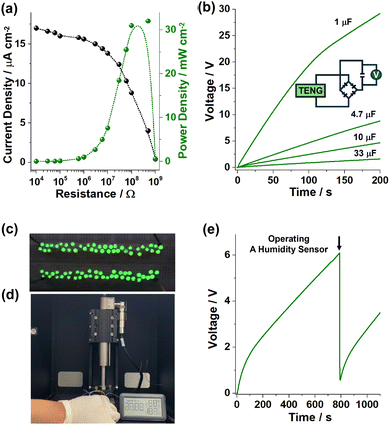
Next, we fabricated spring-assisted triboelectric devices (TENGs) with FPU-4/PET and PFA films to operate electronic devices (Fig. 6 and experimental procedures in the ESI†). First, the TENGs with a FPU-4/PET film showed a maximum power density (Pmax) up to 32 mW cm−2 in the resistance range of 108–109 Ω (Fig. 6a). The TENGs could be applied to charge electrolytic 1, 4.7, 10, and 33 μF capacitors, generating the potentials of 29, 8.7, 4.7, and 1.6 V within 200 s, respectively (Fig. 6b), and to turn on 79 green LED bulbs (Fig. 6c and Movie S1 in the ESI†). Moreover, they could be used to operate a humidity sensor (Fig. 6d and e and Movie S2 in the ESI†).
In conclusion, FPUs were prepared by the reaction of biomass-derived polymer platforms, 2,5-furan diols with 1,6-diisocyanatohexane. The FPUs on PET fabrics could be successfully applied as triboelectric energy harvesting materials. Recently, biomass-derived natural polymers such as chitosan, cellulose, and proteins have been applied as tribopositive materials, showing Vp–p of 50–322 V and Pmax up to 2.0 mW cm−2 (Table S1 in the ESI†).15,16 In addition, the recycled petroleum-derived polymers17 such as polypropylene and polystyrene wastes have been applied as tribopositive materials, showing Vp–p of 68–250 V and Pmax up to 0.547 mW cm−2 (Table S2 in the ESI†).18 In this regard, the triboelectric performance of the optimal FPU-4/PET film, showing Vp–p of 405 V and Pmax up to 32 mW cm−2, is quite promising. Moreover, we believe that various FPUs can be further designed through the scanning of diols for the preparation of new 2,5-furan diol derivatives.
S. U. Son: conceptualization, supervision, writing original draft, and review and editing. S.-W. Kim: supervision, formal analysis, and review. J. Y. Jang, Y. Byun, S. You, S. H. Kim, and D.-M. Lee: investigation and formal analysis.
This work was supported by the National Research Foundation of Korea (NRF) grants (No. RS-2023-00208797) and the Carbon Upcycling Project for Platform Chemicals (No. 2022M3J3A1046019) funded by the Korea government (MSIT).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Guan, J. Yan, Y. Shan, Y. Zhou, Y. Hang, R. Li, Y. Liu, B. Liu, Q. Nie, B. Bruckner, K. Feng and K. Hubacek, Nat. Energy, 2023, 8, 304–316 CrossRef.
- I. Ali, M. Dulal, N. Karim and S. Afroj, Small Struct., 2024, 5, 2300282 CrossRef CAS.
- T. Cheng, J. Shao and Z. L. Wang, Nat. Rev. Methods Primers, 2023, 3, 39 CrossRef CAS.
- X. Tao, X. Chen and Z. L. Wang, Energy Environ. Sci., 2023, 16, 3654–3678 RSC.
- R. Mori, RSC Sustainability, 2023, 1, 179–212 RSC.
- D. J. Aranha and P. R. Gogate, Ind. Eng. Chem. Res., 2023, 62, 3053–3078 CrossRef CAS.
- F. J. A. G. Coumans, Z. Overchenko, J. J. Wiesfeld, N. Kosinov, K. Nakajima and E. J. M. Hensen, ACS Sustainable Chem. Eng., 2022, 10, 3116–3130 CrossRef CAS.
- J. Y. Jang, Y. K. Kim, Y.-J. Ko and S. U. Son, ACS Sustainable Chem. Eng., 2024, 12, 8880–8889 CrossRef CAS.
- H. Chang, E. B. Gilcher, G. W. Huber and J. A. Dumesic, Green Chem., 2021, 23, 4355–4364 RSC.
- J. Y. Jang, G. M. Lee, J. D. Lee and S. U. Son, J. Mater. Chem. A, 2023, 11, 23772–23778 RSC.
- (a) S. I. Park, D.-M. Lee, C. W. Kang, S. M. Lee, H. J. Kim, Y.-J. Ko, S.-W. Kim and S. U. Son, J. Mater. Chem. A, 2021, 9, 12560–12565 RSC; (b) C. W. Kang, D.-M. Lee, J. Park, S. Bang, S.-W. Kim and S. U. Son, Angew. Chem., Int. Ed., 2022, 61, e202209659 CrossRef CAS PubMed; (c) J. Park, I. Hyun, Y. K. Kim, H. Jung, D.-M. Lee, S.-W. Kim and S. U. Son, J. Mater. Chem. A, 2024, 12, 12397–12404 RSC.
- G. Acik, H. R. F. Karabulut, C. Altinkok and A. O. Karatavuk, Polym. Degrad. Stab., 2019, 165, 43–48 CrossRef CAS.
- R. Hinchet, H.-J. Yoon, H. Ryu, M.-K. Kim, E.-K. Choi, D.-S. Kim and S. W. Kim, Science, 2019, 365, 491–494 CrossRef CAS PubMed.
- R. Hinchet, W. Seung and S.-W. Kim, ChemSusChem, 2015, 8, 2327–2344 CrossRef CAS PubMed.
- S. Liu, W. Tong, C. Gao, Y. Liu, X. Li and Y. Zhang, J. Mater. Chem. A, 2023, 11, 9270–9299 RSC.
- (a) K. G. Motora, C.-M. Wu, C. R. M. Jose and G. M. Rani, Adv. Funct. Mater., 2024, 34, 2315069 CrossRef CAS; (b) S. S, A. M. Chandran, S. Varun, M. V. P. Kumar and P. K. S. Mural, ACS Appl. Electron. Mater., 2024, 6, 887–900 CrossRef CAS; (c) Q. Wang, B. Xu, J. Huang and D. Tan, ACS Appl. Mater. Interfaces, 2023, 15, 9182–9192 CrossRef CAS PubMed; (d) B. Tang, Z.-P. Deng, J.-M. Wu, Y.-Y. Zhao, Q.-W. Tan, F. Song, X.-L. Wang and Y.-Z. Wang, J. Mater. Chem. A, 2023, 11, 26716–26726 RSC; (e) Y. Han, Y. Han, X. Zhang, L. Li, C. Zhang, J. Liu, G. Lu, H.-D. Yu and W. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 16442–16450 CrossRef CAS PubMed; (f) H.-Y. Mi, H. Li, X. Jing, P. He, P.-Y. Feng, X. Tao, Y. Liu, C. Liu and C. Shen, Ind. Eng. Chem. Res., 2020, 9, 12399–12408 CrossRef.
- W. Li, Y. Lv, D. Luo and Z. L. Wang, J. Mater. Chem. A, 2023, 11, 9194–9215 RSC.
- (a) S. Roy, P. K. Maji and K.-L. Goh, Chem. Eng. J., 2021, 413, 127409 CrossRef CAS; (b) H. Varghese and A. Chandran, ACS Appl. Mater. Interfaces, 2021, 13, 51132–51140 CrossRef CAS PubMed; (c) S. M. Nawaz, M. Saha, N. Sepay and A. Mallik, Nano Energy, 2022, 104, 107902 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic and experimental procedures, SEM images, 1H and 13C NMR spectra of furan diols and FPUs, TGA curves and SEM images of FPUs, and additional triboelectric performance of FPU-3/PET and FPU-4/PET films. See DOI: https://doi.org/10.1039/d4cc03073g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |