Hypersensitive ratiometric temperature sensing in a bimetallic lanthanide metal–organic framework†
Jing-Wen
Hu
a,
Xue-Yan
Wang
a,
Jun
Xu
 a,
Xi-Yan
Dong
a,
Xi-Yan
Dong
 *a and
Chong
Zhang
*b
*a and
Chong
Zhang
*b
aCollege of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo 454000, China. E-mail: dongxiyan0720@hpu.edu.cn
bCollege of Materials Science and Engineering, Henan University of Technology, Zhengzhou 450001, China. E-mail: zhangcqy@haut.edu.cn
First published on 26th September 2023
Abstract
Ratiometric luminescent temperature sensing based on a single matrix is highly desirable at present. However, the adjustments of the temperature response range and sensitivity are still a huge challenge. Herein, a novel series of isostructural three-dimensional (3D) lanthanide metal–organic frameworks (Ln-MOFs) {[Ln4(ebdc)6(DMF)2.75(H2O)7]}n (HPU-99, where Ln = Eu and EuxTb1−x, H2ebdc = 5-ethynyl-isophthalic acid) are synthesized by a solvothermal method using a 5-ethynyl-isophthalic acid ligand (H2ebdc). Notably, a series of Ln-MOFs EuxTb1−x-HPU-99 (x = 0.05, 0.1, 0.2) are obtained by adjusting the proportion of Eu3+/Tb3+ in the bimetallic materials, which exhibit temperature dependent luminescent behavior with a fluorescence color gradient and demonstrate that chemical components would affect the ratiometric luminescent temperature sensing performance. Furthermore, the luminescence of HPU-99 is altered after the argentophilic alkynyl groups anchor the silver nanoclusters (Ag NCs). This work showcases that bimetallic Ln-MOFs have great potential in luminescent sensing at an atomically precise level.
Temperature, a very crucial physical parameter, is tightly connected with human production and life, which can be seen in many aspects of the atmosphere, oceans, medicine, chemical production and so on.1–3 Therefore, developing and optimizing the sensitivity of fluorescent temperature sensing materials is especially important for temperature quantification.4–6 Lanthanide metal–organic frameworks (Ln-MOFs), as an important branch of metal–organic frameworks with enormous potential, have attracted considerable attention due to the inherent characteristics and remarkable luminescence properties of lanthanide ions.7–17 Eu3+ and Tb3+ ions are the most widely used lanthanide ions in photoluminescence research,18–26 which can emit strong characteristic red or green luminescence when excited by energy transfer from ligands to lanthanide ions under ultraviolet radiation. Because of the similarity of both chemical properties and electronic structures, the lanthanide ions can be easily replaced with each other to obtain hybrid Ln-MOFs,27 in which each lanthanide ion can maintain its own characteristic emission. Based on the excellent optical properties of lanthanide ions, hybrid Ln-MOF materials have tremendous performance advantages in ratiometric fluorescence temperature detection.28–30
Ln-MOF-based fluorescence sensing could be accomplished by temperature-related fluorescence parameters, such as luminescence intensity, quantum yield, fluorescence lifetime, and wavelength shift.31–34 Among them, quantum yield and wavelength shift are both associated with temperature to some extent, but the cost is very high.
At present, the temperature dependent fluorescence parameters are fluorescence peak intensity and fluorescence lifetime. Remarkably, fluorescence lifetime-based thermometers require more sophisticated instruments. In comparison, a ratiometric fluorescence thermometer designed based on the relationship between fluorescence emission intensity and temperature is currently the comparatively cost-effective fluorescence MOF temperature sensor in the field.31,35–37
Although some Eu–Tb based MOF fluorescent temperature sensing materials have been reported,29,35,38–40 relatively little attention has been paid to the relationship between chemical composition and temperature response sensitivity. Research on the relationships is of great significance to the guidance of optimizing and regulating the luminescent temperature sensing performance of Eu3+/Tb3+ bimetallic MOF materials. Obviously, the molar ratio of Ln3+ can be effectively controlled in the initial stages of synthesis.
Herein, a series of Ln-MOFs, {[Ln4(ebdc)6(DMF)2.75(H2O)7]}n, were synthesized by using the H2ebdc ligand. And the chemical composition of EuxTb1−x-HPU-99 was successfully correlated with the temperature measurement properties, demonstrating that the Eu3+/Tb3+ ratio can affect the energy transfer between Ln3+ emission centers, thus adjusting the fluorescence sensing performance. In addition, the Ag NCs were successfully anchored by the uncoordinated alkynyl group on the HPU-99, whose fluorescence properties were further studied.
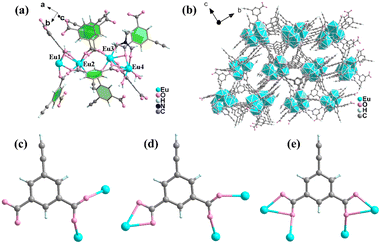
The Ln-MOF HPU-99 was synthesized by a solvothermal method in a mixture of europium nitrate hexahydrate, H2O and dimethylformamide (DMF). Subsequently, a series of isomorphic mixed Ln-MOFs, EuxTb1−x-HPU-99 (x = 0.05, 0.1, 0.2, 0.3) were prepared at the same temperature by adjusting the molar ratio of Eu3+/Tb3+. Single crystal X-ray diffraction (SC-XRD) analysis shows that HPU-99 belongs to the P21/n space group of the monoclinic system. The asymmetric unit contains 4 crystallographically independent Eu3+ ions, 6 ligands, 2.75 DMF molecules and 7 H2O molecules, and its chemical formula is {[Eu4(ebdc)6(DMF)2.75(H2O)7]}n, as shown in Fig. 1(a). The amount of solvent molecules was determined by comprehensive analysis of the SC-XRD, elemental analysis and thermogravimetric results. Eu1 combines with nine oxygen atoms, eight of which are from five different ligands and the remaining one is from a water molecule, forming a tetrakaidecahedron configuration. Eu2 is coordinated with seven carboxyl oxygen atoms from six different ligands and one oxygen atom from a water molecule to form an eight-coordination with dodecahedron geometry. Eu3 also adopts eight-coordination to bind to eight carboxyl oxygen atoms from six different ligands. Eu4 is bonded to six carboxyl oxygen atoms on four different ligands, two oxygen atoms from two water molecules and one oxygen atom from DMF to form a tetrakaidecahedron geometry (Fig. S1, ESI†).
In the crystal structure of HPU-99, ebdc2− ligand anions possess three coordinated modes: one is coordinated with two Eu3+ ions in the mode μ2-η1,η1, another is connected with three Eu3+ ions in the mode μ3-η1,η1,η2, and the other is combined with four Eu3+ ions in the manner μ4-η1,η2,η1,η2 (Fig. 1(c)–(e)). Based on the above coordination modes, the tetranuclear europium units are further connected to form a 3D network (Fig. 1(b)). The crystallographic data and main bond length data for HPU-99 are listed in Tables S1 and S2 in the ESI.†
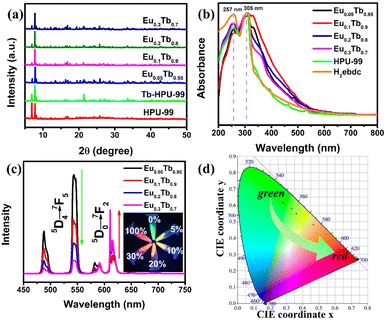
The powder X-ray diffraction (PXRD) patterns show that the HPU-99 owns good phase purity and is isostructural to EuxTb1−x-HPU-99 (x = 0, 0.05, 0.1, 0.2, 0.3), as shown in Fig. S2 (ESI†) and Fig. 2(a). In addition, the Fourier transform infrared (FT-IR) spectra of EuxTb1−x-HPU-99 show similar vibration characteristics, which also confirms this point (Fig. S3, ESI†). The mole ratios of Eu3+/Tb3+ in experimental EuxTb1−x-HPU-99 (x = 0.05, 0.1, 0.2, 0.3) were measured by using inductively coupled plasma (ICP), which match well with the feed ratios, as described in Table S3 (ESI†). As shown in Fig. S4 (ESI†), the morphology of the bimetallic samples EuxTb1−x-HPU-99 (x = 0.05, 0.1, 0.2, 0.3) was observed by scanning electron microscope (SEM) and the EDX mapping images of EuxTb1−x-HPU-99 clearly show that Eu3+ and Tb3+ ions exhibit great dispersity, respectively.
Thermogravimetric (TG) analysis was performed in a nitrogen atmosphere to explore the thermal stability of the Ln-MOFs HPU-99 and EuxTb1−x-HPU-99 (x = 0, 0.05, 0.1, 0.2, 0.3). As shown in Fig. S5 (ESI†), the TG trend of HPU-99 coincides well with EuxTb1−x-HPU-99. Therefore, only HPU-99 is taken as an example for analysis. According to the TG curve of HPU-99, there are two obvious stages of weightlessness. The first stage of weightlessness occurs in the range of 28–150 °C, which is mainly due to the loss of coordination water molecules. The sample weight loss was about 6.71%, which is close to the theoretical value of 6.10%. The main framework of HPU-99 can be maintained within the range of 150–360 °C. The major structure of HPU-99 gradually collapses with increasing temperature, and eventually converts to metallic oxide.
Subsequently, the optical properties of the above bimetallic Ln-MOFs have been systematically studied. In Fig. 2(b), the ultraviolet-visible spectra indicated that the locations of the strong absorption peaks of the HPU-99 and EuxTb1−x-HPU-99 are basically consistent with those of the ligands in the range of 250–350 nm. However, compared with the free H2ebdc ligands, the strong absorption peak at 257 nm in the Ln-MOFs is weakened, possibly due to the coordination of the carboxyl group with the metal Eu3+ or Tb3+ ions.41
The Ln-MOFs were characterized by the steady-state fluorescence spectra at room temperature, as shown in Fig. 2(c). The bimetallic Ln-MOFs EuxTb1−x-HPU-99 (x = 0.05, 0.1, 0.2, 0.3) all showcase the characteristic luminescence of both Tb3+ and Eu3+ ions with clear and well-separated emission peaks when excited by 317 nm. For all bimetallic Ln-MOFs, the characteristic emission peaks of Eu3+ can be observed at 582, 591, 611, 615, and 701 nm, corresponding to transitions of 5D0–7FJ (J = 0–4), respectively. Meanwhile, the composites also exhibit 5D4–7FJ (J = 6–5) transitions from Tb3+ at 488 and 543 nm, and the remaining emission bands overlap those of Eu3+. As the molar proportion of Eu3+ in the bimetallic EuxTb1−x-HPU-99 increases from 5% to 30%, the energy transfer process in the system will be redistributed, resulting in the changes of the emission peak intensity ratio between Eu3+ and Tb3+ ions. When the proportion of Eu3+ is 20%, the emission intensity of the two ions is basically the same, while when the proportion of Eu3+ is low (5% and 10%), the fluorescence spectrum is dominated by Tb3+. It can be clearly seen from Fig. 2(c) inset that the luminescence colors of EuxTb1−x-HPU-99 (x = 0, 0.05, 0.1, 0.2, 0.3, 1) change in a gradient from green, to yellow-green, to yellow, orange and then to red, which can be achieved by simply adjusting the molar ratio of Eu3+/Tb3+.
To better shed light on the mechanism of the energy transfer process, the lifetimes of the excited states 5D4 (Tb3+) at about 543 nm and 5D0 (Eu3+) at 611 nm in EuxTb1−x-HPU-99 (x = 0, 0.05, 0.1, 0.2, 0.3, 1) were monitored. As Table S4 (ESI†) illustrates, the lifetimes of 5D4 (Tb3+) and 5D0 (Eu3+) in the bimetal EuxTb1−x-HPU-99 (x = 0.05, 0.1, 0.2, 0.3) are all shorter than those of Tb-HPU-99 and longer than those of HPU-99, suggesting that there is energy transfer from Tb3+ to Eu3+ ions. By comparison, this series of isostructural MOFs shows different luminescence behaviors. The quantum yield of Tb-HPU-99 at 543 nm is as high as 54.03%, while that of HPU-99 at 611 nm is only 17.60%, indicating that the same ligand sensitized Tb3+ more efficiently than Eu3+, which is also highlighted by the shorter fluorescence lifetime of HPU-99 compared to Tb-HPU-99. In particular, the emission of Eu3+ ions in bimetallic lanthanide Ln-MOFs can be efficiently sensitized not only by organic ligands, but also by Tb3+ within the identical framework.
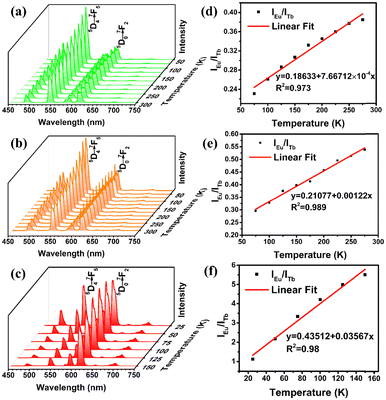
From above, different lanthanide ion doping seems to be seen as an efficient strategy of constructing ratiometric fluorescence thermometer materials. Considering the bimetallic emission central characteristics at room temperature, the photoluminescence emission properties of Eu0.05Tb0.95-HPU-99, Eu0.1Tb0.9-HPU-99 and Eu0.2Tb0.8-HPU-99 at low temperature were investigated. As anticipated, the emission peak of Eu3+ centered at 611 nm and that of Tb3+ at 543 nm in Eu0.05Tb0.95-HPU-99 exhibits significantly different temperature-dependent luminescence behaviors, respectively, as shown in Fig. 3(a). The emission intensity of Tb3+ decreased with increasing temperature, which was quenched by 49% when the temperature reached 300 K, and the decline rate exhibited a trend of fast first and slow afterwards. Meanwhile, the emission intensity of Eu3+ increases gradually in the low temperature range of 50–175 K, and decreases somewhat in the range of 175–300 K, but is always higher than that at 50 K (Fig. S6(a), ESI†).
The variable temperature emission spectra of Eu0.05Tb0.95-HPU-99 indicated the ratio of the characteristic emission intensity of Eu3+ to that of Tb3+ have a good linear relationship in the range of 75–275 K. The fitted standard curve of IEu/ITb with temperature was obtained in Fig. 3(d), and the correlation coefficient of the standard curve reached 0.973.
The formula of the corresponding fitting curve is as follows:
| Δ = 0.18633 + 7.66712 × 10−4T | (1) |
To evaluate the temperature detection performance of the material, the relative thermal sensitivity (SR) of Eu0.05Tb0.95-HPU-99 is 0.210% K−1, as calculated by the formula (2).42
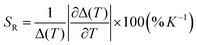 | (2) |
The luminescence of the Eu0.1Tb0.9-HPU-99 shows a similar temperature dependence in the range of 75–275 K, with a relative thermal sensitivity of 0.168% K−1 in Fig. 3(b) and (e). As the Eu3+ content in the composition rises to 20%, the sample Eu0.2Tb0.8-HPU-99 displays different characteristics of temperature sensing performance compared with the others. As illustrated in Fig. 3(c) and(f), the temperature-dependent emission spectra of Eu0.2Tb0.8-HPU-99 showed that the ratio of the characteristic emission intensity of Eu3+ (IEu) to Tb3+(ITb) exhibited a good linear relationship within the temperature range from 25–150 K with a correlation coefficient of 0.98 and the relative thermal sensitivity was 0.055% K−1. The working range of Eu0.2Tb0.8-HPU-99 moves in the lower temperature direction but the sensitivity is increased. The comparison results of three sets of samples show that the ratio of Eu3+/Tb3+ in the lanthanide metal ion mixed system can indeed impact the energy transfer between metal centers, which influences the luminescence temperature detection performance.
To better illustrate the fatigue resistance of the luminescence temperature sensing of the three bimetallic EuxTb1−x-HPU-99 (x = 0.05, 0.1, 0.2), the reversibility of the sample cycling with temperature changes was measured, taking Eu0.05Tb0.95-HPU-99 as an example. In Fig. S8 (ESI†), the alterations of the ratio of the characteristic emission intensity of Eu3+ to that of Tb3+ with temperature are reversible in the process of three temperature cycles. As shown in Fig. S9 (ESI†), the diffraction peaks of PXRD of the Eu0.05Tb0.95-HPU-99 corresponded well before and after the alternative thermo-cycles, showing a good stability.
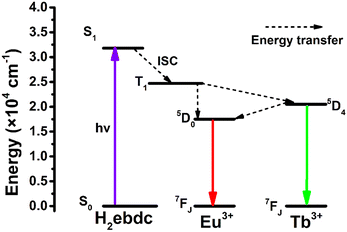
To better understand the energy transfer process from Tb3+ to Eu3+, the fluorescence lifetimes of Eu0.05Tb0.95-HPU-99, Eu0.1Tb0.9-HPU-99 and Eu0.2Tb0.8-HPU-99 at Eu3+ (5D0) and Tb3+ (5D4) were measured and calculated. Then, according to the energy transfer efficiency formula (S1) between donor and acceptor,43 as listed in the ESI,† we successively calculated the energy transfer efficiency of double luminescent center materials from donor Tb3+ to acceptor Eu3+ at different temperature points, and the maximum energy transfer efficiency of 9.01%, 18.31% and 76.54%, respectively, as shown in Fig. S7 (ESI†). Furthermore, in the range of 25–300 K, the energy transfer efficiency from Tb3+ to Eu3+ in the three groups of dual-emission center Ln-MOFs improves with the increase of the molar content of Eu3+, which may be attributed to the control of the Förster transfer mechanism.44 Moreover, the Gaussian09 program was used to calculate the electronic state energy levels of the ligand. As shown in Fig. 4, theoretical calculations show that the singlet level and triplet level of ligand H2ebdc are 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 cm−1 and 24
760 cm−1 and 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 cm−1, respectively, and the energy level difference between the two levels is 7032 cm−1, which is higher than 5000 cm−1, indicating that there is an effective intersystem crossing process in the ligand. Besides, the triplet state (T1) of the ligand matches well with the 5D0 (17
728 cm−1, respectively, and the energy level difference between the two levels is 7032 cm−1, which is higher than 5000 cm−1, indicating that there is an effective intersystem crossing process in the ligand. Besides, the triplet state (T1) of the ligand matches well with the 5D0 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) of Eu3+ and 5D4 (20
500 cm−1) of Eu3+ and 5D4 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) of the Tb3+ levels, which can sensitize Eu3+ and Tb3+ luminescence effectively.
500 cm−1) of the Tb3+ levels, which can sensitize Eu3+ and Tb3+ luminescence effectively.
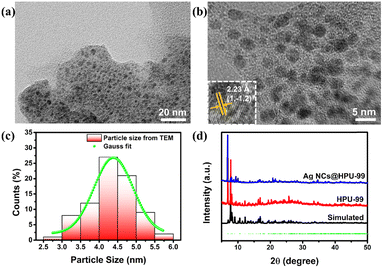
Furthermore, considering that the alkynyl group, the benzene ring on the HPU-99 structure, is still an active group, the Ag NCs@HPU-99 composite was prepared by using an uncoordinated alkynyl group as the binding site of the guest Ag NCs. The fine structure of Ag NCs in the matrix HPU-99 was clearly observed by transmission electron microscopy (TEM). In Fig. 5(a), Ag NCs were confined and evenly distributed in the host HPU-99. From Fig. 5(b), high-resolution TEM images show that the lattice fringes of Ag NCs@Eu-MOF-2 are clearly arranged with lattice spacing of 0.223 nm, corresponding to the (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2) crystal face. As shown in Fig. 5(c), the average diameter of the Ag NCs is about 4.3 nm by Gaussian fitting. PXRD analysis of Fig. 5(d) shows that Ag NCs@HPU-99 is consistent with the main structure of HPU-99, and its main diffraction peaks correspond well with the original crystal simulated data, indicating that the introduction of Ag NCs will not damage the structural integrity of HPU-99. To better illustrate the presence of Ag element in the host HPU-99, X-ray photoelectron spectroscopy (XPS) was conducted on Ag NCs@HPU-99, as shown in Fig. S10 (ESI†). The binding energy peaks of Ag element appear in the XPS spectra of the composite after successfully confining the Ag NCs. In addition, SEM images of Ag NCs@HPU-99 and EDX mapping images of the corresponding metal elements show the uniform distribution of Ag in the HPU-99 matrix more directly, as shown in Fig. S11 (ESI†). Inductively coupled plasma (ICP) analyses results showed that the Ag element content of Ag NCs@HPU-99 was 4.53% (Table S5, ESI†). Thermogravimetric analysis (TGA) of HPU-99 and Ag NCs@HPU-99 was performed. As shown in Fig. S12 (ESI†), the weight loss trends of HPU-99 and Ag NCs@ HPU-99 are basically similar. Above 600 °C, the silver in Ag NCs@HPU-99 generates additional silver oxide, so the residual amount of Ag NCs@HPU-99 is greater than that of HPU-99, which corresponds to the results of the ICP test.
2) crystal face. As shown in Fig. 5(c), the average diameter of the Ag NCs is about 4.3 nm by Gaussian fitting. PXRD analysis of Fig. 5(d) shows that Ag NCs@HPU-99 is consistent with the main structure of HPU-99, and its main diffraction peaks correspond well with the original crystal simulated data, indicating that the introduction of Ag NCs will not damage the structural integrity of HPU-99. To better illustrate the presence of Ag element in the host HPU-99, X-ray photoelectron spectroscopy (XPS) was conducted on Ag NCs@HPU-99, as shown in Fig. S10 (ESI†). The binding energy peaks of Ag element appear in the XPS spectra of the composite after successfully confining the Ag NCs. In addition, SEM images of Ag NCs@HPU-99 and EDX mapping images of the corresponding metal elements show the uniform distribution of Ag in the HPU-99 matrix more directly, as shown in Fig. S11 (ESI†). Inductively coupled plasma (ICP) analyses results showed that the Ag element content of Ag NCs@HPU-99 was 4.53% (Table S5, ESI†). Thermogravimetric analysis (TGA) of HPU-99 and Ag NCs@HPU-99 was performed. As shown in Fig. S12 (ESI†), the weight loss trends of HPU-99 and Ag NCs@ HPU-99 are basically similar. Above 600 °C, the silver in Ag NCs@HPU-99 generates additional silver oxide, so the residual amount of Ag NCs@HPU-99 is greater than that of HPU-99, which corresponds to the results of the ICP test.
To illustrate the fluorescence changes of host HPU-99 after confining Ag NCs, the solid-state photoluminescence excitation and emission spectra of HPU-99 and Ag NCs@HPU-99 were tested under the same conditions and compared at room temperature. As shown in Fig. S13(a) (ESI†), the characteristic luminescence intensity of Eu3+ (5D0–7F2) in Ag NCs@HPU-99 is higher than that of HPU-99 at the same position, indicating that the guest Ag NCs do make a difference to the luminescence of the parent HPU-99. Moreover, it is found that after the HPU-99 anchor guest Ag NCs, its own colour changed from white to yellow, and the fluorescence colour also changed from bright red to dark red under a 365 nm UV lamp, as shown in Fig. S13(b) (ESI†). The effect of Ag NCs on the luminescence of Ag NCs@HPU-99 was also evaluated based on the fluorescence lifetime decay time of Eu3+ (611 nm). As illustrated in Fig. S13(c) and (d) (ESI†), the luminescence lifetime of Ag NCs@HPU-99 significantly increases from 547.45 μs to 608.26 μs after anchoring Ag NCs, which is in good agreement with the fluorescence spectra. This study experimentally proved the feasibility of using the modified functional group on the structure of MOFs as the binding site to confine guest Ag NCs, and also confirmed that anchoring Ag NCs would affect the photophysics of host Ln-MOFs.
Conclusions
In summary, a series of novel isostructural 3D Ln-MOFs were prepared by a solvothermal method using H2ebdc as an organic ligand. And the chemical composition was successfully associated with the temperature detection properties, confirming that the Eu3+/Tb3+ ratio would affect the energy transfer between the Ln3+ emission centers, thus adjusting the ratiometric luminescent temperature sensing performance, of which the highest relative thermal sensitivity is 0.055% K−1. In addition, Ag NCs were confined into the HPU-99 matrix by a simple in situ growth method and the changes of its luminescence properties were studied. This work has enriched the research on the application of dual luminescent center Ln-MOFs as self-calibrated luminescent temperature sensing, and provided guidance for optimizing the ratiometric luminescent temperature sensing performance and adjusting their relative sensitivity and response temperature region.Author contributions
X. Y. D. and C. Z. conceived and supervised the project. J. W. H. conducted the syntheses and measurements. J. W. H. drew the pictures in the manuscript. X. Y. D., C. Z. and J. W. H. analyzed the experimental results. J. W. H. and C. Z. completed the crystallographic data collection and refinement of the structure. X. Y. D., C. Z., J. X., and X. Y. W. helped to revise the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21975065, U21A20277, 22305067) and the Excellent Youth Foundation of Henan Scientific Committee (232300421022).Notes and references
- J. J. Zhou, B. del Rosal, D. Jaque, S. Uchiyama and D. Y. Jin, Nat. Methods, 2020, 17, 967–980 CrossRef CAS PubMed.
- K. M. N. de Souza, R. N. Silva, J. A. B. Silva, C. D. S. Brites, B. Francis, R. A. S. Ferreira, L. D. Carlos and R. L. Longo, Adv. Opt. Mater., 2022, 10, 2200770 CrossRef CAS.
- M. Quintanilla and L. M. Liz-Marzán, Nano Today, 2018, 19, 126–145 CrossRef CAS.
- G. F. Feng, H. Z. Zhang, X. H. Zhu, J. H. Zhang and J. Fang, Biomater. Sci., 2022, 10, 1855–1882 RSC.
- Y. Y. Ding, Y. T. Lu, K. L. Yu, S. Wang, D. Zhao and B. L. Chen, Adv. Opt. Mater., 2021, 9, 2100945 CrossRef CAS.
- K. Xue, C. Wang, J. X. Wang, S. Y. Lv, B. Y. Hao, C. L. Zhu and B. Z. Tang, J. Am. Chem. Soc., 2021, 143, 14147–14157 CrossRef CAS PubMed.
- F. Saraci, V. Quezada-Novoa, P. R. Donnarumma and A. J. Howarth, Chem. Soc. Rev., 2020, 49, 7949–7977 RSC.
- H. R. Guan, M. X. Qi, L. F. Shi, W. S. Liu, L. Z. Yang and W. Dou, ACS Appl. Mater. Interfaces, 2023, 15, 18114–18124 CrossRef CAS PubMed.
- Y. J. Cui, J. Zhang, H. J. He and G. D. Qian, Chem. Soc. Rev., 2018, 47, 5740–5785 RSC.
- C. Viravaux, O. Oms, A. Dolbecq, E. Nassar, L. Busson, C. Mellot-Draznieks, R. Dessapt, H. Serier-Brault and P. Mialane, J. Mater. Chem. C, 2021, 9, 8323–8328 RSC.
- M. C. Jia, X. Chen, R. R. Sun, D. Wu, X. J. Li, Z. F. Shi, G. Y. Chen and C. X. Shan, Nano Res., 2023, 16, 2949–2967 CrossRef CAS.
- S. Y. Wu, H. Min, W. Shi and P. Cheng, Adv. Mater., 2020, 32, 1805871 CrossRef CAS PubMed.
- X. G. Yang, J. H. Qin, Y. D. Huang, Z. M. Zhai, L. F. Ma and D. P. Yan, J. Mater. Chem. C, 2020, 8, 17169–17175 RSC.
- Y. Zang, L. K. Li and S. Q. Zang, Coord. Chem. Rev., 2021, 440, 213955 CrossRef CAS.
- P. Jia, L. Gao, Y. Zheng, X. Zheng, C. Wang, C. L. Yang, Y. B. Li and Y. L. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 33546–33556 CrossRef CAS PubMed.
- P. Jia, Z. H. Wang, Y. F. Zhang, D. Zhang, W. C. Gao, Y. Su, Y. B. Li and C. L. Yang, Spectrochim. Acta, Part A, 2020, 230, 118084 CrossRef CAS PubMed.
- C. L. Yang, J. Xu, J. Y. Li, M. G. Lu, Y. B. Li and X. L. Wang, Sens. Actuators, B, 2014, 196, 133–139 CrossRef CAS.
- X. Y. Zhai, P. F. Feng, N. Song, G. D. Zhao, Q. Y. Liu, L. L. Liu, M. Tang and Y. Tang, Inorg. Chem. Front., 2022, 9, 1406–1415 RSC.
- D. H. Chen, R. Haldar and C. Wöll, ACS Appl. Mater. Interfaces, 2023, 15, 19665–19671 CrossRef CAS PubMed.
- S. A. Younis, N. Bhardwaj, S. K. Bhardwaj, K.-H. Kim and A. Deep, Coord. Chem. Rev., 2021, 429, 213620 CrossRef CAS.
- C. Zhang, Z. S. Li, X. Y. Dong, Y. Y. Niu and S. Q. Zang, Adv. Mater., 2022, 34, 2109496 CrossRef CAS PubMed.
- C. Zhang, Z. P. Yan, X. Y. Dong, Z. Han, S. Li, T. Fu, Y. Y. Zhu, Y. X. Zheng, Y. Y. Niu and S. Q. Zang, Adv. Mater., 2020, 32, 2002914 CrossRef CAS PubMed.
- H. Y. Li, Y. L. Wei, X. Y. Dong, S. Q. Zang and T. C. W. Mak, Chem. Mater., 2015, 27, 1327–1331 CrossRef CAS.
- B. Li, J. P. Dong, Z. Zhou, R. Wang, L. Y. Wang and S. Q. Zang, J. Mater. Chem. C, 2021, 9, 3429–3439 RSC.
- L. H. Cao, F. Shi, W. M. Zhang, S. Q. Zang and T. C. W. Mak, Chem. – Eur. J., 2015, 21, 15705–15712 CrossRef CAS PubMed.
- Y. Su, J. H. Yu, Y. B. Li, S. F. Z. Phua, G. F. Liu, W. Q. Lim, X. Z. Yang, R. Ganguly, C. Dang, C. L. Yang and Y. L. Zhao, Commun. Chem., 2018, 1, 12 CrossRef.
- Y. Pan, H. Q. Su, E. L. Zhou, H. Z. Yin, K. Z. Shao and Z. M. Su, Dalton Trans., 2019, 48, 3723–3729 RSC.
- Y. J. Cui, H. Xu, Y. F. Yue, Z. Y. Guo, J. C. Yu, Z. X. Chen, J. K. Gao, Y. Yang, G. D. Qian and B. L. Chen, J. Am. Chem. Soc., 2012, 134, 3979–3982 CrossRef CAS PubMed.
- X. T. Rao, T. Song, J. K. Gao, Y. J. Cui, Y. Yang, C. D. Wu, B. L. Chen and G. D. Qian, J. Am. Chem. Soc., 2013, 135, 15559–15564 CrossRef CAS PubMed.
- Y. J. Huang, W. X. Feng, Z. P. Zhou, H. Z. Zheng, Y. Zhao, H. X. Yan and X. Q. Lü, J. Mater. Chem. C, 2022, 10, 7586–7593 RSC.
- T. Y. Qin, B. Liu, K. N. Zhu, Z. J. Luo, Y. Y. Huang, C. J. Pan and L. Wang, TrAC, Trends Anal. Chem., 2018, 102, 259–271 CrossRef CAS.
- Z. H. Wang, L. J. Qu, L. Gao, X. Zheng, Y. Zheng, Y. Y. Zhu, J. Xia, Y. F. Zhang, C. Wang, Y. B. Li and C. L. Yang, Adv. Opt. Mater., 2022, 10, 2200481 CrossRef CAS.
- Y. F. Zhang, X. H. Chen, J. R. Xu, Q. L. Zhang, L. Gao, Z. H. Wang, L. J. Qu, K. T. Wang, Y. B. Li, Z. X. Cai, Y. L. Zhao and C. L. Yang, J. Am. Chem. Soc., 2022, 144, 6107–6117 CrossRef CAS PubMed.
- Y. Zheng, Q. Zhou, Y. Yang, X. H. Chen, C. Wang, X. Zheng, L. Gao and C. L. Yang, Small, 2022, 18, 2201223 CrossRef CAS PubMed.
- V. Trannoy, A. N. Carneiro Neto, C. D. S. Brites, L. D. Carlos and H. Serier-Brault, Adv. Opt. Mater., 2021, 9, 2001938 CrossRef CAS.
- D. Zhao, H. Z. Wang and G. D. Qian, CrystEngComm, 2018, 20, 7395–7400 RSC.
- J. W. Liu, X. Han, Y. T. Lu, S. Wang, D. Zhao and C. X. Li, Inorg. Chem., 2021, 60, 4133–4143 CrossRef CAS PubMed.
- W. N. Miao, B. Liu, H. Li, S. J. Zheng, H. Jiao and L. Xu, Inorg. Chem., 2022, 61, 14322–14332 CrossRef CAS PubMed.
- L. Chen, D. H. Liu, J. Peng, Q. Z. Du and H. He, Coord. Chem. Rev., 2020, 404, 213113 CrossRef CAS.
- Y. J. Cui, F. L. Zhu, B. L. Chen and G. D. Qian, Chem. Commun., 2015, 51, 7420–7431 RSC.
- Y. Liu, Y. Yang, G. D. Qian, Z. Y. Wang and M. Q. Wang, Mater. Sci. Eng., B, 2007, 137, 74–79 CrossRef CAS.
- A. Bednarkiewicz, L. Marciniak, L. D. Carlos and D. Jaque, Nanoscale, 2020, 12, 14405–14421 RSC.
- M. Xiao and P. R. Selvin, J. Am. Chem. Soc., 2001, 123, 7067–7073 CrossRef CAS PubMed.
- Y. Liu, G. D. Qian, Z. Y. Wang and M. Q. Wang, Appl. Phys. Lett., 2005, 86, 071907 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental and spectroscopic details. CCDC 2292095. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tc03179a |
| This journal is © The Royal Society of Chemistry 2023 |