Facile dimerization strategy for producing narrowband green multi-resonance delayed fluorescence emitters†
Minlang
Yang‡
a,
Rajendra Kumar
Konidena‡
 b,
So
Shikita
a and
Takuma
Yasuda
b,
So
Shikita
a and
Takuma
Yasuda
 *ab
*ab
aDepartment of Applied Chemistry, Graduate School of Engineering, Kyushu University, Fukuoka, Japan. E-mail: yasuda@ifrc.kyushu-u.ac.jp
bInstitute for Advanced Study, Kyushu University, Fukuoka, Japan
First published on 30th November 2022
Abstract
Establishing a simple molecular design strategy for enabling redshifted emissions while maintaining high color purity in multi-resonance thermally activated delayed fluorescence (MR-TADF) remains a crucial yet challenging task. Herein, we introduce a new design concept based on a dimerization strategy for constructing pure green MR-TADF emitters. Two isomeric MR dimers, namely p-CzB and m-CzB, were developed by tethering two MR fragments through different linking positions. The interconnection mode between the two MR fragments in these dimeric MR-TADF systems plays a vital role in regulating photophysical properties as well as exciton dynamics. Comprehensive photophysical and computational studies revealed that m-CzB exhibits superior green MR-TADF characteristics compared to p-CzB. A m-CzB-based organic light-emitting diode (OLED) delivered pure green electroluminescence with CIE coordinates of (0.20, 0.70), a maximum external quantum efficiency of 23.5%, and alleviated efficiency roll-off.
Introduction
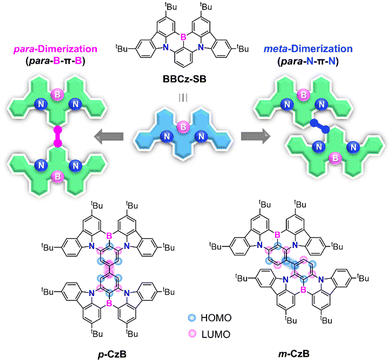
Boron- and nitrogen-embedded polycyclic aromatic hydrocarbons (B,N-PAHs) have recently attracted burgeoning interest,1–9 which is ascribable to their promising use in optoelectronic devices as typified by organic light-emitting diodes (OLEDs). The opposite resonance characteristics of mutually ortho-disposed B and N atoms in B,N-PAHs are capable of inducing the multiple-resonance (MR) effect, leading to the localization and separation of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) on different constituent atoms.5,6 This MR feature endows B,N-PAHs with a small singlet–triplet energy gap (ΔEST) and thermally activated delayed fluorescence (TADF) properties as a consequence.4–9 The biggest advantage of such an MR-TADF emitter is its narrowband emission capability, with an extremely small full width at half maximum (FWHM < 40 nm) enabled by suppressing structural relaxation and vibronic coupling in excited states. Combining the capabilities of full exciton use and narrowband emission leads to ideal emitters for OLED applications, with superior electroluminescence (EL) efficiencies and color purities.Three ultrapure red, green, and blue (RGB) primary emitters are essential for realizing wide-color-gamut OLEDs for use in ultrahigh-definition displays. The advent of sophisticated MR-TADF emitters, as represented by ν-DABNA, has led to the realization of high-efficiency pure blue EL.6,10–15 However, owing to atomically separated HOMOs and LUMOs, these MR-TADF emitters typically possess feeble intramolecular long-range charge-transfer (CT) characteristics; hence, large bathochromic shifts from the blue emission-band region are difficult to achieve. Therefore, new viable design strategies that expand the color gamut of MR-TADF emitters beyond the blue region are in strong demand. In this regard, we focused on BBCz-SB8,16 (Fig. 1) as one of the simplest B,N-based MR frameworks. Peripherally decorating BBCz-SB with either auxiliary donor or acceptor substituents on HOMO- or LUMO-dominant carbons has been reported to stabilize the respective energy levels, resulting in bathochromically shifted emissions.17–23 Our group reported the first deep-red MR-TADF emitter featuring a large fused polycyclic π-system based on para-B-π-B and para-N-π-N conjugation.8 High-efficiency green MR-TADF emitters have recently been developed by extending π-systems via additional ring fusion.9,24–27
Herein, we unveiled a simple but effective dimerization strategy for producing pure green MR-TADF emitters. Two isomeric MR dimers, namely p-CzB and m-CzB, were designed by tethering two BBCz-SB fragments through different linking positions (Fig. 1). This dimerization strategy enables π-systems to be extended while maintaining intrinsic MR characteristics, thereby facilitating conspicuous bathochromic emission-band shifts without compromising narrowband spectral features. The dimerization strategy has previously been proposed for the design of TADF emitters;28,29 however, narrowband emissions with an adequately small FWHM have not been achieved owing to their strong CT character. Indeed, p-CzB and m-CzB exhibited intense green photoluminescence (PL) emissions that peak at 505 and 515 nm in solution, with narrow FWHMs of 34 and 35 nm, respectively. Furthermore, an OLED based on m-CzB as the MR-TADF emitter displayed pure green EL with a high maximum external quantum efficiency (EQEmax) of 23.5% and high color purity.
Results and discussion
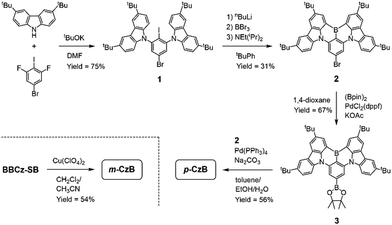
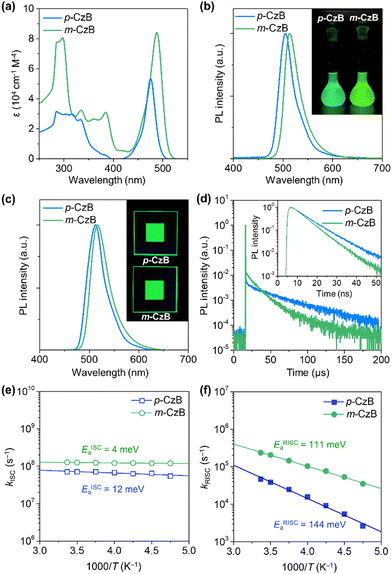
Scheme 1 outlines the route used to synthesize the dimeric MR-TADF emitters; detailed synthesis procedures and characterization data are provided in the ESI.† The para-linked isomer (p-CzB) was finally prepared in 56% yield by Suzuki–Miyaura cross-coupling between precursors 2 and 3. Meanwhile, the meta-linked isomer (m-CzB) was synthesized in 54% yield by the oxidative coupling of BBCz-SB using Cu(ClO4)2·6H2O as the oxidant.30 Since the HOMO of BBCz-SB is largely distributed on the para- and ortho-carbons of the central benzene ring with respect to the N-carbazolyl substituents,8 oxidative coupling proceeded preferentially at this position to afford m-CzB in high yield. m-CzB has a distorted helicene-like structure with a para-N-π-N conjugated linkage. In addition, both dimeric p-CzB and m-CzB were found to be highly thermally stable with 5%-weight-loss decomposition temperatures (Td) of 522 and 511 °C, respectively (ESI†), which are much higher than that of monomeric BBCz-SB (Td = 411 °C).8 The final products were purified by vacuum sublimation for subsequent use.The photophysical properties of p-CzB and m-CzB were investigated as dilute toluene solutions and doped thin films, the results of which are shown in Fig. 2, with relevant photophysical data summarized in Table 1. Both p-CzB and m-CzB exhibit vivid green PL in solution, with emission peaks (λPL) at 504 and 515 nm, respectively, which are redshifted by 15–26 nm compared to that of BBCz-SB (λPL = 489 nm). Despite each dimeric structure being linked through a single bond, p-CzB and m-CzB exhibited small Stokes shifts of 28 and 27 nm and emission FWHMs of 34 and 35 nm, respectively, suggestive of miniscule geometrical changes in their excited states. m-CzB has a higher molar absorptivity (ε) than p-CzB, corresponding to a stronger MR-induced HOMO → LUMO transition. This trend eventually resulted in m-CzB exhibiting a higher PL quantum yield (ΦPL = 90%) than p-CzB (ΦPL = 85%) in solution. Solid thin films of p-CzB and m-CzB embedded in mCBP-CN31 (3,3′-di(carbazol-9-yl)-5-cyano-1,1′-biphenyl), a bipolar host, showed narrowband green PL emissions with λPL = 513 and 517 nm, and ΦPL = 80% and 85%, respectively (Fig. 2c, Table 1, and ESI†). The S1 and T1 excitation energies (ES/ET) of p-CzB and m-CzB were determined to be 2.43/2.29 and 2.40/2.31 eV, respectively, based on the peak wavelengths of their fluorescence and phosphorescence spectra (ESI†). Accordingly, m-CzB exhibited a smaller ΔEST than p-CzB (0.09 vs. 0.14 eV). Moreover, p-CzB underwent serious aggregation-caused emission quenching (ACQ) in the doped films with increasing the dopant concentration, whereas sterically distorted m-CzB exhibited slightly reduced ACQ effect, resulting in higher ΦPL values (ESI†).
| compound | statea | λ PL (nm) | FWHMc (nm/eV) | Φ PL (%) | Φ p (%) | Φ d (%) | τ p (ns) | τ d (μs) | k r (107 s−1) | k RISC (104 s−1) | E S (eV) | E T (eV) | ΔESTj (eV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Sol = deoxygenated toluene solution (10−5 M) at 300 K; film = 1 wt%-doped film in a mCBP-CN host matrix at 300 K under N2. b PL emission maximum. c Full width at half maximum of the PL spectrum given in wavelength and energy. d Absolute PL quantum yield evaluated using an integrating sphere under N2. e Fractional quantum yields for prompt fluorescence (Φp) and delayed fluorescence (Φd); Φp + Φd = ΦPL. f Emission lifetimes for prompt fluorescence (τp) and delayed fluorescence (τd). g Rate constant for fluorescence radiative decay (S1 → S0): kr = Φp/τp. h RISC rate constant (T1 → S1): kRISC = Φd/(kISC·τp·τd·Φp). i Lowest excited singlet (ES) and triplet (ET) energies determined from fluorescence and low-temperature phosphorescence spectral peaks for the doped films. j Singlet–triplet energy gap: ΔEST = ES − ET. | |||||||||||||
| p-CzB | Sol | 505 | 34/0.16 | 85 | 32 | 53 | 9.3 | 58 | 3.3 | 4.2 | 2.46 | 2.29 | 0.17 |
| Film | 513 | 41/0.20 | 80 | 35 | 45 | 9.6 | 41 | 3.7 | 4.6 | 2.43 | 2.29 | 0.14 | |
| m-CzB | Sol | 515 | 35/0.16 | 90 | 23 | 67 | 4.9 | 32 | 4.7 | 12 | 2.42 | 2.29 | 0.13 |
| Film | 517 | 40/0.19 | 85 | 24 | 61 | 6.0 | 15 | 4.1 | 23 | 2.40 | 2.31 | 0.09 | |
The transient PL decay profiles of the doped films of p-CzB and m-CzB show distinct two-component emissions (Fig. 2d). The prompt and delayed fluorescence lifetimes (τp/τd) were determined to be 9.6 ns/32 μs for p-CzB and 5.6 ns/15 μs for m-CzB. Benefiting from their prominent MR electronic features, both materials exhibited radiative decay rates (kr) that far exceed 107 s−1. Moreover, m-CzB (with the smaller ΔEST) exhibited a shorter τd, resulting in a kRISC of 2.3 × 105 s−1 (Table 1), which is three-times higher than that of p-CzB (kRISC = 4.7 × 104 s−1). Notably, the kRISC value of m-CzB is a standout among reported green MR-TADF emitters (ESI†). To further understand exciton thermodynamic behavior, we examined the temperature dependences of kISC and kRISC for the doped films and determined the activation energies for ISC (EISCa) and RISC (ERISCa) by fitting with the Arrhenius equation (Fig. 2e and f). While kISC showed negligibly small temperature dependences, kRISC demonstrated clear positive temperature dependences, to give ERISCa values of 144 and 111 meV for p-CzB and m-CzB, respectively, which are in reasonable agreement with the foregoing ΔEST values. These results support the notion that the smaller ΔEST of m-CzB accounts for its faster RISC.
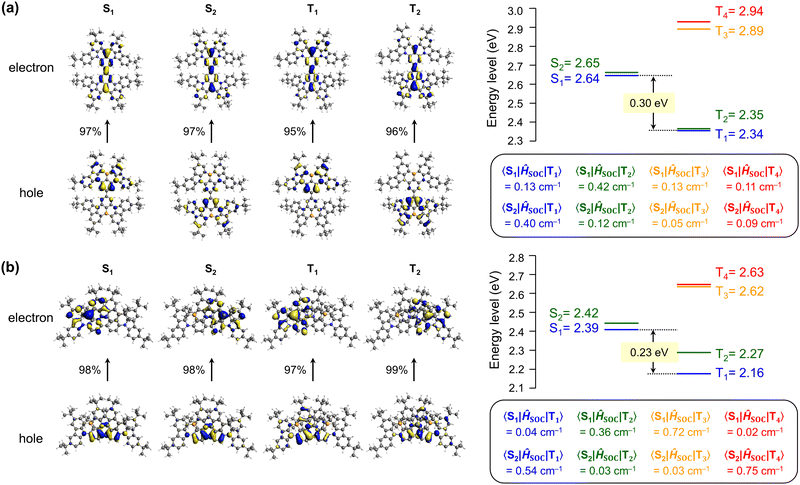
To gain deeper insight into the nature of the excited singlet and triplet states, p-CzB and m-CzB were subjected to natural transition orbital (NTO) analyses using time-dependent density functional theory (TD-DFT) at the B3LYP/DZP level (Fig. 3). p-CzB has quasi-degenerate highest occupied NTOs that reflect its symmetrical dimeric structure, where hole wave functions reside on different B/N-based MR cores and give rise to doubly degenerate singlet (S1 and S2) and triplet (T1 and T2) excited states (Fig. 3a). In this case, the electron wave functions are located across the two MR cores through para-B-π-B conjugation. Meanwhile, m-CzB (with para-N-π-N conjugation) has degenerate lowest unoccupied NTOs; consequently, the electron wave functions are distributed in each of the two MR cores, while hole wave functions are extended to the dimeric MR structure (Fig. 3b). It is worth noting that the linking position significantly affects the resulting dimeric π-conjugated system and excited-state electronic properties.
We next calculated the spin–orbit coupling (SOC) matrix elements (〈Sm|ĤSOC|Tn〉), m = 1, 2 and n = 1–4) for p-CzB and m-CzB (Fig. 3). As predicted by the El-Sayed rule,32 the SOC values for T1–S1 and T2–S2 couples with the same orbital occupations are rather small (0.13 and 0.12 cm−1 for p-CzB and 0.04 and 0.03 cm−1 for m-CzB) because of negligible changes in orbital angular momentum. In contrast, T2–S1 and T1–S2 couples exhibit distinctly larger SOC values that are ascribable to larger changes in orbital angular momentum (0.42 and 0.40 cm−1 for p-CzB and 0.36 and 0.54 cm−1 for m-CzB). We therefore envisage that such quasi-degenerate low-energy excited states induced by these dimeric MR structures contribute to SOC enhancement and offer additional T–S spin-flip channels for RISC.33 Indeed, despite very similar ΔEST values, dimeric p-CzB and m-CzB exhibit RISC rates that are accelerated by factors of more than three and eight, respectively, compared to monomeric BBCz-SB (kRISC = 1.4 × 104 s−1 in toluene).8 Hence, the present dimerization strategy not only modulates the emission color but also favourably impacts excitonic spin conversion crucial for TADF.
To evaluate the actual EL performance of p-CzB and m-CzB, we tested TADF-OLEDs fabricated using the following device structure: indium-tin-oxide (ITO)/HAT-CN (10 nm)/TAPC (50 nm)/mMCP (5 nm)/1 wt%-emitter:mCBP-CN (30 nm)/PPF (5 nm)/B3PyPB (50 nm)/Liq (1 nm)/Al (100 nm);13 the chemical structures of the materials are provided in the ESI.†Fig. 4 shows EL spectra, current density–voltage–luminance (J–V–L) characteristics, and EQE–L plots for the fabricated TADF-OLEDs, and relevant EL data are summarized in Table 2. TADF-OLEDs that incorporate p-CzB and m-CzB display narrowband green EL with emission peaks (λEL) at 511 and 515 nm and FWHMs of 41 and 39 nm, which correspond to CIE chromaticity coordinates of (0.16, 0.66) and (0.20, 0.70), respectively. Notably, the green EL color of the m-CzB-based device fulfils the requirements of the National Television System Committee (NTSC) for pure green (0.21, 0.71) and approaches the coordinates of Rec.2020 pure green (0.17, 0.79)34 (Fig. 4d). Moreover, these OLEDs delivered high maximum EQEs of 20.2% and 23.5%, respectively (Fig. 4c and Table 2). It is worth noting that the m-CzB-based device showed a more alleviated efficiency roll-off and retained high EQEs of 21.0% and 15.0% at luminances of 100 and 1000 cd m−2. The relatively high kRISC of 2.3 × 105 s−1 for m-CzB accounts for the alleviated efficiency roll-off compared to that of p-CzB (4.6 × 104 s−1) and contributes to suppressing detrimental exciton quenching, such as triplet–triplet annihilation (TTA) in operation at high luminance. These different EL characteristics are also reflected in the operational stabilities of the TADF-OLEDs. The m-CzB-based device exhibited an operational lifetime with a 50% luminance reduction (LT50) of 25.5 h at an initial luminance (L0) of 100 cd m−2, which is approximately four-times longer than that of the p-CzB-based device (LT50 = 6.4 h) (ESI†). These device stabilities sill leave much room for improvement; however, isomeric p-CzB and m-CzB reveal that a subtle difference in linking position can greatly impact the efficiency roll-off behavior and operational stability in OLEDs.
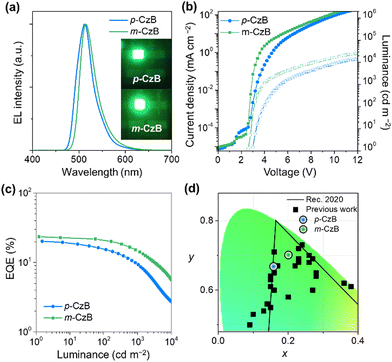 | ||
| Fig. 4 EL characteristics of OLEDs that use p-CzB and m-CzB as MR-TADF emitters: (a) EL spectra and green EL images acquired at 10 mA cm−2, (b) current density–voltage–luminance (J–V–L) characteristics, (c) EQE–L plots. (d) Comparing CIE coordinates for current and previously reported green OLEDs based on MR-TADF emitters (see ESI† for plotted data sets and source references). | ||
| Emitter | p-CzB | m-CzB |
|---|---|---|
| a Turn-on voltage at a luminance above 1 cd m−2. b EL emission maximum at 10 mA cm−2. c Full width at half maximum of the EL spectrum given in wavelength and energy. d Commisssion Internationale de l'Éclairage (CIE) chromaticity coordinates recorded at 10 mA cm−2. e Maximum external EL quantum efficiency. f External EL quantum efficiencies at luminance of 100 and 1000 cd m−2. g Maximum current efficiency. h Maximum power efficiency. | ||
| V on (V) | 3.2 | 2.8 |
| λ EL (nm) | 511 | 515 |
| FWHMc (nm/eV) | 41/0.20 | 39/0.19 |
| CIEd (x, y) | (0.16, 0.66) | (0.20, 0.70) |
| EQEmaxe (%) | 20.2 | 23.5 |
| EQE100/1000f (%) | 15.4/9.3 | 21.0/15.0 |
| CEg (cd A−1) | 67.9 | 85.1 |
| PEh (lm W−1) | 69.9 | 94.7 |
Conclusions
In summary, we introduced a facile dimerization strategy for producing narrowband green MR-TADF emitters. Based on this strategy, we developed para- and meta-linked isomeric MR dimers, p-CzB and m-CzB. The position connecting the two MR cores serves as a conjugation valve that regulates the photophysical properties of the emitter. Both p-CzB and m-CzB exhibited distinct MR-TADF characteristics, concurrently delivering pure green emissions and narrow spectral FWHMs. Notably, the color purity and kRISC associated with the m-CzB emission are standouts among reported green MR-TADF emitters. Consequently, the m-CzB-based green OLED achieved a considerably high EQEmax of 23.5% as well as alleviated efficiency roll-off, outperforming the p-CzB-based device. We expect that these results will expediate new material designs that feature the dimerization strategy for constructing wide-color-gamut MR-TADF systems.Author contributions
M. Y., R. K. K., and T. Y. conceptualized the project. M. Y. and S. S. synthesized materials and performed quantum chemical calculations. M. Y. performed photophysical analysis and device evaluations. M. Y., R. K. K., and T. Y. co-wrote the manuscript. T. Y. supervised the entire research project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by Grant-in-Aid for JSPS KAKENHI (Grant No. JP21H04694 and JP22F21030), JST CREST (Grant No. JPMJCR21O5), and the Mitsubishi Foundation. R. K. K. acknowledges the JSPS Postdoctoral Fellowships for Research in Japan. The authors are grateful for the support provided by the Cooperative Research Program of “Network Joint Center for Materials and Devices” and the computer facilities at the Research Institute for Information Technology, Kyushu University.References
- M. Hirai, N. Tanaka, M. Sakai and S. Yamaguchi, Chem. Rev., 2019, 119, 8291–8331 CrossRef CAS
.
- S. Oda and T. Hatakeyama, Bull. Chem. Soc. Jpn., 2021, 94, 950–960 CrossRef CAS
.
- E. Von Grotthuss, A. John, T. Kaese and M. Wagner, Asian J. Org. Chem., 2018, 7, 37–53 CrossRef CAS
.
- S. M. Suresh, D. Hall, D. Beljonne, Y. Olivier and E. Zysman-Colman, Adv. Funct. Mater., 2020, 30, 1908677 CrossRef
.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS
.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Nat. Photonics, 2019, 13, 678–682 CrossRef CAS
.
- K. Matsui, S. Oda, K. Yoshiura, K. Nakajima, N. Yasuda and T. Hatakeyama, J. Am. Chem. Soc., 2018, 140, 1195–1198 CrossRef CAS PubMed
.
- M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed
.
- H. J. Kim and T. Yasuda, Adv. Opt. Mater., 2022, 10, 2201714 CrossRef CAS
.
- C. Y. Chan, M. Tanaka, Y. T. Lee, Y. W. Wong, H. Nakanotani, T. Hatakeyama and C. Adachi, Nat. Photonics, 2021, 15, 203–207 CrossRef CAS
.
- S. O. Jeon, K. H. Lee, J. S. Kim, S. G. Ihn, Y. S. Chung, J. W. Kim, H. Lee, S. Kim, H. Choi and J. Y. Lee, Nat. Photonics, 2021, 15, 208–215 CrossRef CAS
.
- H. Tanaka, S. Oda, G. Ricci, H. Gotoh, K. Tabata, R. Kawasumi, D. Beljonne, Y. Olivier and T. Hatakeyama, Angew. Chem., Int. Ed., 2021, 60, 17910–17914 CrossRef CAS
.
- I. S. Park, M. Yang, H. Shibata, N. Amanokura and T. Yasuda, Adv. Mater., 2022, 34, 2107951 CrossRef CAS
.
- X. Lv, J. Miao, M. Liu, Q. Peng, C. Zhong, Y. Hu, X. Cao, H. Wu, Y. Yang, C. Zhou, J. Ma, Y. Zou and C. Yang, Angew. Chem., Int. Ed., 2022, 61, e2022015 Search PubMed
.
- K. R. Naveen, H. Lee, R. Braveenth, K. J. Yang, S. J. Hwang and J. H. Kwon, Chem. Eng. J., 2022, 432, 134381–134388 CrossRef
.
- Y. Xu, Z. Cheng, Z. Li, B. Liang, J. Wang, J. Wei, Z. Zhang and Y. Wang, Adv. Opt. Mater., 2020, 8, 1902142 CrossRef CAS
.
- Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Angew. Chem., Int. Ed., 2019, 58, 16912 CrossRef CAS PubMed
.
- Y. Xu, G. Li, Z. Li, Q. Wang, X. Cai, J. Wei and Y. Wang, Angew.
Chem., Int. Ed., 2020, 59, 17442–17446 CrossRef CAS
.
- Y. Qi, W. Ning, Y. Zou, X. Cao, S. Gong and C. Yang, Adv. Funct. Mater., 2021, 31, 2102017 CrossRef CAS
.
- M. Yang, S. Shikita, H. Min, I. S. Park, H. Shibata and T. Yasuda, Angew. Chem., Int. Ed., 2021, 60, 23142–23147 CrossRef CAS
.
- Y. Liu, X. Xiao, Y. Ran, Z. Bin and Y. You, Chem. Sci., 2021, 12, 9408–9412 RSC
.
- Y. Xu, C. Li, Z. Li, J. Wang, J. Xue, Q. Wang, X. Cai and Y. Wang, CCS Chem., 2021, 4, 2065–2079 CrossRef
.
- Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Gui, Z. Bin, G. Li, J. Wei and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 20498–20503 CrossRef CAS PubMed
.
- Y. Zhang, D. Zhang, J. Wei, X. Hong, Y. Lu, D. Hu, G. Li, Z. Liu, Y. Chen and L. Duan, Angew. Chem., Int. Ed., 2020, 59, 17499–17503 CrossRef CAS
.
- Y. Zhang, G. Li, L. Wang, T. Huang, J. Wei, G. Meng, X. Wang, X. Zeng, D. Zhang and L. Duan, Angew. Chem., Int. Ed., 2022, 61, e202202380 CAS
.
- X. F. Luo, H. X. Ni, H. L. Ma, Z. Z. Qu, J. Wang, Y. X. Zheng and J. L. Zuo, Adv. Opt. Mater., 2022, 10, 2102513 CrossRef CAS
.
- Y. Xu, Q. Wang, J. Wei, X. Peng, J. Xue, Z. Wang, S. J. Su and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, e202204652 CAS
.
- Y. J. Cho, S. K. Jeon, B. D. Chin, E. Yu and J. Y. Lee, Angew. Chem., Int. Ed., 2015, 54, 5201–5204 CrossRef CAS PubMed
.
- D. Sun, S. M. Suresh, D. Hall, M. Zhang, C. Si, D. B. Cordes, A. M. Z. Slawin, Y. Olivier, X. Zhang and E. Zysman-Colman, Mater. Chem. Front., 2020, 4, 2018–2022 RSC
.
- S. Shikita, T. Harada and T. Yasuda, Chem. Commun., 2022, 58, 4849–4852 RSC
.
- S. G. Ihn, N. Lee, S. O. Jeon, M. Sim, H. Kang, Y. Jung, D. H. Huh, Y. K. Son, S. Y. Lee, M. Numata, H. Miyazaki, R. Góomez-Bombarelli, J. Aguilera-Iparraguirre, T. Hirzel, A. Aspuru-Guzik, S. Kim and S. Lee, Adv. Sci., 2017, 4, 1600502 CrossRef PubMed
.
- M. A. El-Sayed, Acc. Chem. Res., 1968, 1, 8–16 CrossRef CAS
.
- H. Min, I. S. Park and T. Yasuda, Adv. Opt. Mater., 2022, 10, 2200290 CrossRef CAS
.
- Recommendation ITU-R BT.2020: parameter values for ultrahigh definition television systems for production and international program exchange, International Telecommunication Union, 2012.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc04447a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |