Can you break the oxo-wall? A multiconfigurational perspective
Abstract
The concept of the “oxo-wall” was conceived about 60 years ago by Harry B. Gray, and has been found to be related to the non-existence of high-valent M–oxo species in the +IV oxidation state in a tetragonal geometry beyond group 8 in the periodic table. Several efforts have been made in the past decades to test and find examples that violate this theory. Several claims of violation in the past were attributed to the difference in the geometries/coordination number and, therefore, these are not examples of true violation. In recent years, substantial efforts have been undertaken to synthesise a true CoIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species with various ligand architectures. CoIV
O species with various ligand architectures. CoIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and CoIII–O˙ are electromers and, while they are interchangeably used in the literature; the former violates the oxo-wall while the latter does not. The possibility that these two species could exist in various proportions similar to resonating structures has not been considered in detail in this area. Furthermore, there have been no attempts to quantify such mixing. In this direction, we have employed density functional theory (DFT) and ab initio CASSCF/NEVPT2 methods to quantify the co-existence of CoIV
O and CoIII–O˙ are electromers and, while they are interchangeably used in the literature; the former violates the oxo-wall while the latter does not. The possibility that these two species could exist in various proportions similar to resonating structures has not been considered in detail in this area. Furthermore, there have been no attempts to quantify such mixing. In this direction, we have employed density functional theory (DFT) and ab initio CASSCF/NEVPT2 methods to quantify the co-existence of CoIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and CoIII–O˙ isomeric species. By thoroughly studying six different metal-oxo species, we affirm that the nature of such electromeric mixing is minimal/negligible for FeIV
O and CoIII–O˙ isomeric species. By thoroughly studying six different metal-oxo species, we affirm that the nature of such electromeric mixing is minimal/negligible for FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and MnIV
O and MnIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species – both are pre-oxo-wall examples. By studying four different ligand architectures with Co–oxo species, our results unveil that the mixing of CoIV
O species – both are pre-oxo-wall examples. By studying four different ligand architectures with Co–oxo species, our results unveil that the mixing of CoIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ↔ CoIII–O˙ is substantial in all geometries, with dominant CoIV
O ↔ CoIII–O˙ is substantial in all geometries, with dominant CoIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species favourable for the S = 3/2 intermediate spin state. The percentage of the CoIII–O˙ species is enhanced substantially for the S = 1/2 low-spin state. We have attempted to develop a tool to estimate the percentage of the CoIII–O˙ species using various structural parameters. Among those tested, a linear relationship between % of CoIII–O˙ and a bond length based ratio is found (
O species favourable for the S = 3/2 intermediate spin state. The percentage of the CoIII–O˙ species is enhanced substantially for the S = 1/2 low-spin state. We have attempted to develop a tool to estimate the percentage of the CoIII–O˙ species using various structural parameters. Among those tested, a linear relationship between % of CoIII–O˙ and a bond length based ratio is found (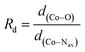 , where d(Co–O) and d(Co–Nax) are the axial Co–O and Co–Nax bond lengths in Å, respectively). It is found that the higher the Rd, the greater the CoIII–O˙ character will be and the geometrically portable correlation developed offers a way to qualitatively compute the % of CoIII–O˙ character for unknown geometries.
, where d(Co–O) and d(Co–Nax) are the axial Co–O and Co–Nax bond lengths in Å, respectively). It is found that the higher the Rd, the greater the CoIII–O˙ character will be and the geometrically portable correlation developed offers a way to qualitatively compute the % of CoIII–O˙ character for unknown geometries.

- This article is part of the themed collection: Natural and artificial metalloenzymes


 Please wait while we load your content...
Please wait while we load your content...