Open Access Article
Open Access ArticleXylochemicals and where to find them
Jonathan Gro߆
a,
Caroline Grundke†
a,
Johannes Rocker†
a,
Anthony J. Arduengo III
 *b and
Till Opatz
*b and
Till Opatz
 *a
*a
aDepartment of Chemistry, Johannes Gutenberg University, Duesbergweg 10-14, 55128, Mainz, Germany. E-mail: opatz@uni-mainz.de
bSchool of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332-0400, USA. E-mail: aj@ajarduengo.net
First published on 27th August 2021
Abstract
This article surveys a range of important platform and high value chemicals that may be considered primary and secondary ‘xylochemicals’. A summary of identified xylochemical substances and their natural sources is provided in tabular form. In detail, this review is meant to provide useful assistance for the consideration of potential synthetic strategies using xylochemicals, new methodologies and the development of potentially sustainable, xylochemistry-based processes. It should support the transition from petroleum-based approaches and help to move towards more sustainability within the synthetic community. This feasible paradigm shift is demonstrated with the total synthesis of natural products and active pharmaceutical ingredients as well as the preparation of organic molecules suitable for potential industrial applications.
Introduction
Humankind's discovery and use of petroleum (from medieval Latin, from Latin petra ‘rock’ (earlier Greek) plus Latin oleum ‘oil’) likely substantially predates recorded history. Some of the earliest known records already contain mentions of “rock oil” in one form or another, with some of the earliest references relating its use as a fuel (light source).1 An early (but, in context a relatively ‘modern’) textual reference to petroleum refining is found in a 1596 translation by J. Frampton of reports by Nicolás Monardes “De Las Drojas De Las Indias”2 As a fuel source, the combustion of petroleum releases heat, light, oxides of carbon, and water. This latter use remained the chief utility (excepting occasional application as a salve or ointment) of petroleum for most of the history of modern humans. Only much later did the science of chemistry – specifically, organic synthesis – develop sufficiently that the very limited structural types found in petroleum could be adequately elaborated into the range of functionality and reactivity required to produce modern materials and pharmaceuticals.3 Previously, contemporaneous biomass provided chemists with a wealth of functionality, reactivity and structural types that were elaborated into man-made materials. A chief disadvantage of biomass is that a suitable starting point (material) must be found in the natural pallet of chemicals. Furthermore, the chemistry developed for one particular natural starting material will likely not be applicable for reaching the same end from a different starting point. Petroleum, though structurally simpler than most natural products, provides a relatively well-defined and abundant starting point, from which more sophisticated chemicals can be assembled. The diversity of structural types that can now be derived from petroleum is a result of the wide variety of synthetic transformation and optimization that have been developed in the most recent 200 years.To date, petrochemical feedstocks such as natural gas, coal and petroleum are the fundament for the majority of all chemical raw materials, that may lead to carbon imbalance in the ecosphere (besides depletion of underground deposits) and ecological risks in terms of production. Some of the potential consequences like alterations in vegetation and soil, changes in the composition of the atmosphere and global water balance may have already emerged in the late 20th century.4 The result has been a paradigm shift recorded in the Rio Declaration on Environment and Development in 1992.5 Based on this, Anastas and Warner developed their well-known 12 principles of green chemistry6,7 in 1998, which evolved as general guidelines for more eco-friendly methodologies, syntheses, technologies and processes over the past 20 years.8–10 Additionally, more metrics and terms have been developed in the early 1990s to describe the extent of sustainability as well as the “greenness” of a given reaction.11 For example, Trost's Atom Economy concept describes the molar mass ratio of the desired product and the total sum of all molecular masses of all the substances produced according to the chemical equation.12–14 This was followed by the Environmental Factor (E-Factor) by Sheldon, indicating the environmental impact of a given process by describing the mass ratio of total waste and product production.15–19 Even though the earliest available sources for pure organic compounds were animals, microorganisms and plants, the 19th and 20th century were dominated by the exploitation of fossil carbon sources for the emerging chemical industry.20,21 Since the last 30 years, the need for renewable resources and especially for alternative carbon atom sources is constantly growing, displaying one of the major aspects of the field of Green Chemistry. One approach within this topic is to use wood as such a renewable alternative (‘Xylochemistry’), as it can be considered a source of atmospherically-bound CO2, and can be counted as CO2–neutral when no further fossil carbon is involved.22,23 With a worldwide production of 5 × 109 m3,24,25 wood provides a broad variety of valuable oxygen-containing functionalities, e.g. hydroxyl or carbonyl groups as well as (enantiomerically pure) building blocks in contrast to fossil fuels, which lost the majority of their heteroatomic functionalities and their stereo-information through the process of kerogenesis.26 Hence, their chemical diversity is limited and functional groups must be reconstructed in cost- and resource-intensive reaction sequences, which leads to additional purification steps, energy consumption and waste production.
In contrast, wood mainly consists of cellulose, hemicellulose and lignin, that affords the opportunity to use already existing waste-streams from paper production or agricultural waste products as well as wood itself. Furthermore, wood-derived materials act as renewable feedstocks for high value and platform chemicals alongside biofuels, while there is no competition with food production.27–33 As there is already a rich body of existing literature concerning the topic of lignin valorization/depolymerization,34–42 the reader shall be referred to this literature to gain a more detailed information about this spacious research field.39,42–46 (Oligo)peptides, (oligo)saccharides as well as (oligo)nucleotides will not be discussed in detail in this context either, as the natural origin of these substances is evident and the criterion of transcendence is not fulfilled in these cases.47–49
Xylochemical synthesis approaches are not only of interest for future industrial scale processes, but have also found their way into laboratory scale synthesis methodologies, particularly in natural product total synthesis. Since the term ‘Xylochemistry‘ was coined in 2015, several natural product total syntheses such as ilicifoline B,23 (−)-oxycodone,50 (−)-thebaine,51 lamellarin G trimethyl ether,52 shancigusin C and bletistrin G53 as well as 2-aminophenoxazinone-type natural products54 have been described. Additionally, antibacterial balsacones have been reported by the Pichette group,55 while some current HIV protease inhibitors56 as well as colorants and polyamides57 have been reported by the Opatz group. The Sperry group demonstrated the use of chitin and chitosan as naturally occurring sources of nitrogen that can be implemented in a variety of N-substituted heterocycles.58,59 Among other groups, the Barta lab worked on the valorization of lignin and its model compounds60 to build up naturally occurring alkaloid scaffolds. Another approach was reported by the Moeller group, who made use of wood waste streams by developing an electrochemical synthesis of value-added building blocks from sawdust.61 One of the most recent examples for the implementation of xylochemical strategies in organic chemistry and its impact on daily consumables is the application of cashew nut shell liquid as a supplier for UV absorbers in sunscreen.62
To the best of our knowledge, there is no current publication that summarizes a large multiplicity of (standard) chemicals and reagents that can be considered primary xylochemicals and additionally demonstrates their natural sources until this date. Furthermore, important secondary xylochemicals are listed, which are accessible via straightforward chemical transformations from primary xylochemicals. Thus, this review article should provide such an overview in tabular form and act as a work of reference.
Registry of xylochemicals
As described by Arduengo and Opatz, xylochemistry uses wood- or plant-based biomass as a source of raw materials for chemical synthesis instead of fossil carbon sources.22,63 The following registry depicts the first 100+ xylochemicals with their molecular structures and lists their corresponding natural origin.21,64 All substances are arranged in ascending order by the number of their carbon atoms and are highlighted either as primary xylochemicals (directly obtainable/isolable from plant/wood extracts, labelled with “P”) or secondary xylochemicals (available through a single transformation such as fermentation from primary xylochemicals, labelled with “S”). Where available, further information such as the yield isolated is provided. However, this information is provisional and it is likely possible to optimize the outcome through improved isolation procedures, specific breeding or genetic engineering in the future (vide supra). Besides compounds that carry a broad variety of oxygen-containing substituents, many hydrocarbons such as benzene, toluene, naphthalene, styrene and other unsaturated compounds have also been obtained from wood by pyrolysis/distillation procedures (see Table 1). Nevertheless, as all of the latter platform chemicals are already accessible with optimized outcomes of more than 99% from petrochemicals, this article will mainly focus on heteroatom-containing and functionalized hydrocarbons.| Compound | Natural source | |
|---|---|---|
 |
 |
wv,65 oxidation of sugars (75%)65,66 |
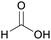
| Formic acid | ||
 |
 |
Dry distillation of wood,67 hydrogenation of lignin68 |
| Methanol | ||
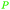 |
 |
wv,65 fmt. of sugars,69 oxidation of carbohydrate biomass (up to 27%),66 wood pyrolysis65 |
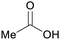
| Acetic acid | ||
 |
 |
Ethanol dehydration (>98%)27,70–72 |
| Ethylene | ||
 |
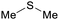 |
From Kraft black liquor with elemental sulfur73 |
| Dimethyl sulfide | ||
 |
 |
ABE-fmt. of sugars27,74,75 |
| Ethanol | ||
 |
 |
Catalytic oxidation of ethanol (up to 96%)76 |
| Ethylene oxide | ||
 |
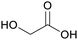 |
Microwave-assisted processes (18%), sugar cane, sugar beets77 |
| Glycolic acid | ||
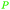 |
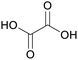 |
Wood-sorrels (Oxalis),78 from sawdust79 |
| Oxalic acid | ||
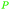 |
 |
wv65 |
| Allyl alcohol | ||
 |
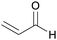 |
Catalytic conversion of glycerol/water mixtures (up to 62%)80 |
| Acrolein | ||
 |
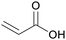 |
Dehydration of 3-hydroxypropionic acid (derived from biomass)81,82 |
| Acrylic acid | ||
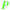 |
 |
wv,65 fmt. of sugars (71.8 g L−1)69,83,84 |
| Propionic acid | ||
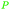 |
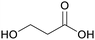 |
From biomass82 |
| 3-Hydroxypropionic acid | ||
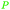 |
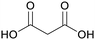 |
Leaves of lucerne and green/growing wheat plants,85 mature Leguminosae leaves, green alfalfa plants86,87 |
| Malonic acid | ||
 |
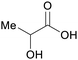 |
fmt. of sugars (up to 90%), corn or sugar beets,75,88 oxidation of carbohydrate biomass66,77 |
| Lactic acid | ||
 |
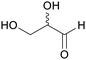 |
Oxidation of D-fructose and L-sorbose (85%)89 |
| Glyceraldehyde | ||
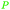 |
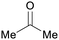 |
ABE-fmt. of sugars,27,90 wood pyrolysis with addition of calcium carbonate65 |
| Acetone | ||
 |
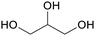 |
Co-product of biodiesel production (50 wt%)91,92 |
| Glycerol | ||
 |
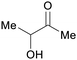 |
fmt. of sugars (>40 g L−1)93 |
| Acetoin | ||
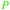 |
 |
Catalytic gas phase synthesis from grain-/potato-based ethanol94 |
| 1,3-Butadiene | ||
 |
 |
ABE-fmt. of sugars (34 wt%)27,74 |
| 1-Butanol | ||
 |
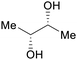 |
fmt. of sugars (up to 25%)95,96 |
| (2R,3R)-butanediol | ||
 |
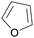 |
Catalytic decarbonylation of furfural (>98%)97 |
| Furan | ||
 |
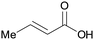 |
wv65 |
| Crotonic acid | ||
 |
 |
fmt. of sugars,75,98–102 dry distillation of amber,103 biochemical transformation of sugar (1.3 mol succinate per mol glucose)104 |
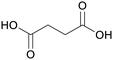
| Succinic acid | ||
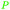 |
 |
Cleavage of ascorbic acid,105 grapes (berries) and wine, Geraniaceae, Vitaceae and Leguminosae families106 |
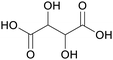
| Tartaric acid | ||
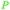 |
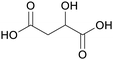 |
Green alfalfa,86 wheat,87 fmt. of sugars (37.9 ± 2.6 g L−1)107 |
| Malic acid | ||
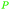 |
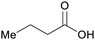 |
wv,65 fmt. of sugars (21 g L−1)69,108 |
| Butanoic acid | ||
 |
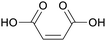 |
Dehydrated malic acid, oxidation of furfural (47%), butanol, levulinic acid, hydroxymethylfurfural109,110 |
| Maleic acid | ||
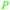 |
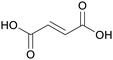 |
fmt. of sugars (16.2 ± 0.2 g L−1),107Fumaria officinalis69 |
| Fumaric acid | ||
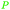 |
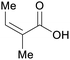 |
wv65 |
| Angelic acid | ||
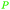 |
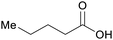 |
wv,65 distillation of valerian root69 |
| Valeric acid | ||
 |
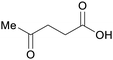 |
Lignocellulose111 (60–70% based on hexose content),112 sugars113,114 |
| Levulinic acid | ||
 |
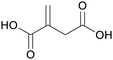 |
fmt. of sugars (glucose),75 by product of the pyrolysis of citric acid115 |
| Itaconic acid | ||
 |
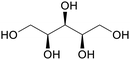 |
Hydrogenation of arabinose from hemicellulose (up to 78%)31,75 |
| Xylitol | ||
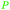 |
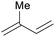 |
Mosses, ferns, trees, polyisoprenes from rubber tree,116 pyrolysis of rubber products117 |
| Isoprene | ||
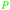 |
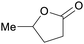 |
From levulinic acid through catalytic hydrogenation (>99%),118 lignocellulose119 |
| γ-Valerolactone | ||
 |
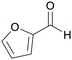 |
Pentose-containing biomass like corn cobs, sugar cane residues,97,109,111,120 wood hydrolysates121 |
| Furfural | ||
 |
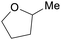 |
Hydrogenation of furfural122 |
| 2-Methyltetrahydrofuran | ||
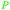 |
 |
wv,65 fractionation of coconut oil69 |
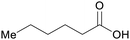
| Caproic acid | ||
 |
 |
Hydrogenation of glucose,75 from cellulose (up to 85%)31,75 |
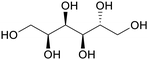
| Sorbitol | ||
 |
 |
Juice of manna-ash (Fraxinus ornus l.), olive trees/leaves,123,124 catalytic hydrogenation of glucose-fructose mixtures or cellulose (up to 85%)75 |
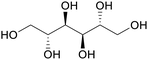
| Mannitol | ||
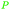 |
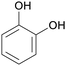 |
Dry distillation of Acacia catechu,125 lignin126,127 |
| Catechol | ||
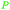 |
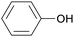 |
Lignin depol.,128 pine wood lignin (9.6 mol%)129 |
| Phenol | ||
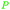 |
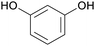 |
Wood tar130 |
| Resorcinol | ||
 |
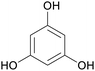 |
From phloretin,131 (apple tree leaves, manchurian apricot)132,133 |
| Phloroglucinol | ||
 |
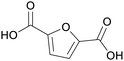 |
Oxidation of hydroxymethylfurfural/methoxymethyl-furfural (59%)134,135 |
| Furan-2,5-dicarboxylic acid | ||
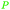 |
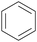 |
Depol. of kraft lignin (175 g kg−1),136 wood combustion137,138 |
| Benzene | ||
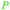 |
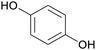 |
Raw wv from Pinus tabulaeformis carr127 |
| Hydroquinone | ||
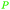 |
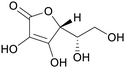 |
Green shell of walnuts,139 citrus fruits140 |
| Ascorbic acid/vitamin C | ||
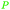 |
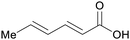 |
wv,65 rowan berries141,142 |
| Sorbic acid | ||
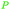 |
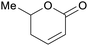 |
Rowan berries (132 mg/100 g),143 cranberry (0.12% of dry plant)144 |
| Parasorbic acid | ||
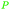 |
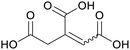 |
Apparent in plants, also available from citric acid87 |
| Aconitic acid | ||
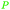 |
 |
Green alfalfa,86 growing wheat,87 industrially through fmt. of sugars (up to 100%),69,145 citrus juice and pineapple waste69 |
| Citric acid | ||
 |
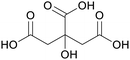
 |
fmt. of sunflower oil (93 g L−1) and purification by esterification146 |
| Isocitric acid | ||
 |
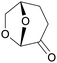 |
Green solvent alternative for DMF or NMP, from cellulose (from larch log, poplar wood, bagasse, corn cob, bilberry presscake),147via hydrogenation of levoglucosenone (up to 100%)147,148 |
| Cyrene | ||
 |
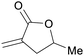 |
From biomass derived levulinic acid and formaldehyde (up to 92%)149 |
| 5-Methyl-3-methylenedihydrofuran-2(3H)-one | ||
 |
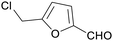 |
From biomass derived glucose, sucrose, cellulose (up to 76%)150,151 |
| 5-(chloromethyl)furfural | ||
 |
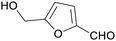 |
Fructose and other sugars (85%)75,114,152–154 |
| 5-(hydroxymethyl)furfural | ||
 |
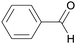 |
Fruit kernels,155 peach leaves,156 from cinnamaldehyde155 |
| Benzaldehyde | ||
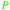 |
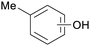 |
Eucalyptus wood tar,157 lignin depol.,128 wood pyrolysis158 |
| Cresoles | ||
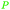 |
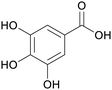 |
Caesalpinia spinosa pods (25% yield),159Rhus chinensis160 |
| Gallic acid | ||
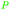 |
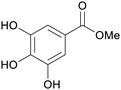 |
Eucalyptus wood extracts (detected via LC-MS),161Terminalia myriocarpa extracts162 |
| Methyl gallate | ||
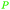 |
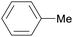 |
Wood combustion,65,163 tolu balsam164,165 |
| Toluene | ||
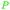 |
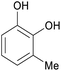 |
Raw wv from Pinus tabulaeformis carr127 |
| 3-Methylcatechol | ||
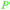 |
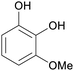 |
Raw wv from Pinus tabulaeformis carr127 |
| 3-Methoxycatechol | ||
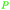 |
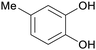 |
Raw wv from Pinus tabulaeformis carr127 |
| 4-Methylcatechol | ||
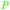 |
 |
Distillation of guaiac resin,65,166 wood tar oil,65 wv,65 bio-oil from lignin pyrolysis (up to 26%)167 |
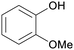
| Guaiacol | ||
 |
 |
From gum benzoin69,103 |
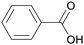
| Benzoic acid | ||
 |
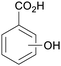 |
From Salicaceae168,169 |
| Hydroxybenzoic acids | ||
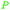 |
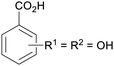 |
From Alchornea cordifolia169 |
| Dihydroxybenzoic acids | ||
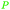 |
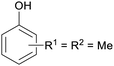 |
wv,65 wood tar170 |
| Xylenoles | ||
 |
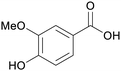 |
Oxidation of vanillin from lignin169,171 |
| Vanillic acid | ||
 |
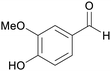 |
Lignin (4.5–7.0%,172 2.8%173),174 aspen wood (0.95–17.5%),175 Eucalyptus wood176 |
| Vanillin | ||
 |
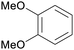 |
Wood pyrolysis (detected via GC)177 |
| Veratrole | ||
 |
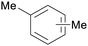 |
Birch wood (combustion)163 |
| Xylenes | ||
 |
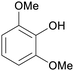 |
Raw wv from Pinus tabulaeformis carr127 |
| 2,6-Dimethoxyphenol | ||
 |
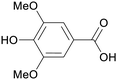 |
Leaves of Syringa vulgaris (2.5% via GC),178Quercus infectoria,179 acai palm oil (1073 ± 62 mg L−1)180 |
| Syringic acid | ||
 |
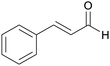 |
Cinnamon bark,181 (1.5% of dried bark),182Pseudocinnamomum183 |
| Cinnamaldehyde | ||
 |
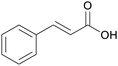 |
Cinnamon bark184 |
| Cinnamic acid | ||
 |
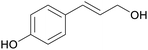 |
Lignin depol.185 |
| p-coumaryl alcohol | ||
 |
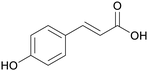 |
Grapevine pruning (0.15%),186 wheat straw (0.66%),187 maize stems (1.08%)187 |
| p-coumaric acid | ||
 |
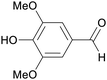 |
Lignin oxidation, poplar lignin (30%),188 aspen wood (0.77–36.2% at temperatures between 100–215 °C),175 maple wood (Klason lignin, 31.8%)189 |
| Syringaldehyde | ||
 |
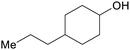 |
Hydrogenation of hardwood lignin (76%)68 |
| 4-Propylcyclohexanol | ||
 |
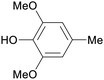 |
Raw wv from Pinus tabulaeformis carr127 |
| 2,6-Dimethoxy-4-methylphenol | ||
 |
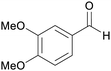 |
From wood of Abies sibirica190 |
| Veratraldehyde | ||
 |
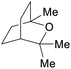 |
Fractional distillation of Eucalyptus oil from leaves (1.0–2.4% of fresh weight)191 |
| 1,8-Cineol | ||
 |
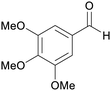 |
Roots of Lactuca sativa var. Angustana cv. (3.7 mg/228 g dried plant),192 leaves of Chloranthus anhuiensis193 and alpinia flabellate (11 mg/800 g dried plant)194 |
| Trimethoxybenzaldehyde | ||
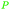 |
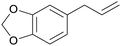 |
Sassafras oil195 (80-93%),196,197Ocotea odorifera oil (42%)198 |
| Safrol | ||
 |
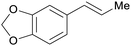 |
From safrol (98%)199 |
| Isosafrol | ||
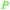 |
 |
Reduction of ferulic acid (68%),200 lignin depol185 |
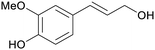
| Coniferyl alcohol | ||
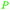 |
 |
Rice straw (0.87%),187 rice bran201 (178.3 μg mg−1),202 grapevine pruning (0.41 mg g−1),186 wheat straw (1.24%)187 |
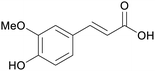
| Ferulic acid | ||
 |
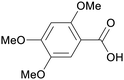 |
Oxidation of asarone (65%)203,204 |
| Asaronic acid | ||
 |
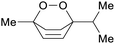 |
Chenopodium oil (67%),205Peumus boldus oil (31%)206 |
| Ascaridole | ||
 |
 |
Sulfate turpentine,207Origanum acutidens oil (ca. 2%),208Chenopodium ambrosioides oil (26%)209 |
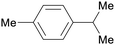
| p-cymene | ||
 |
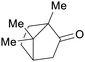 |
Camphor wood69,208 |
| Camphor | ||
 |
 |
Blumea balsamifera leaves (0.5% (−)-borneol, less isoborneol)210 |
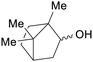
| (iso)borneol | ||
 |
 |
Clove (leaves and buds, 180 mg g−1),211,212 bay leaves, cinnamon bark and leaves213 |
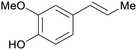
| Eugenol | ||
 |
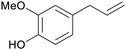 |
Clove (leaves and buds, 180 mg g−1),211,212 bay leaves, cinnamon bark and leaves213 |
| Isoeugenol | ||
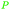 |
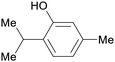 |
Turkish Origanum acutidens,208 thyme oil (up to 50%)69 |
| Thymol | ||
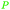 |
 |
Origanum acutidens oil (87%),208Satureja montana extracts (53-66%),214 oils of thyme (up to 60%), majorana (49%), Origanum dictamnus (up to 82%)215 |
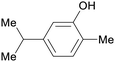
| Carvacrol | ||
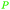 |
 |
Turkish Origanum acutidens,208Cotinus coggygria oil (8.8%),216 or leave distillates (52%)69 |
| Myrcene | ||
 |
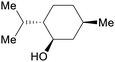 |
Mentha piperita oil (30-55%),217Mentha canadensis oil (63-69%)218 |
| (–)-Menthol | ||
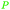 |
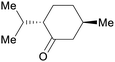 |
Mentha piperita oil (14–32%),217Mentha canadensis oil (8–16%)218 |
| (–)-Menthone | ||
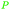 |
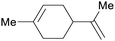 |
(R)-(−)-Limonene: orange oil (92%)219 (3.8 wt% of orange peel),220 lemon, bergamot, dill, mint, among others,219 (S)-(−)-limonene: oaks and pines, Eucalyptus stageriana221 |
| Limonene | ||
 |
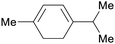 |
Chenopodium ambrosioides oil (63%),209 majoram oil (10%),222 terpene fraction of orange oil,69 american turpentine oil69 |
| α-terpinene | ||
 |
 |
Majoram oil (14%),222 cardamom oil (up to 11%)223 |
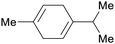
| γ-terpinene | ||
 |
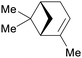 |
Spruce needle oil,224 sulfate turpentine (65%)207 |
| α-pinene | ||
 |
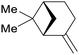 |
From turpentine, from α-pinene69 |
| β-pinene | ||
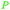 |
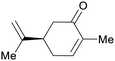 |
(S)-(+)-carvone: Carum carvi oil (50–70%),225 (R)-(−)-Carvone: oil of spearmint (up to 69%)69,226 |
| Carvone | ||
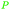 |
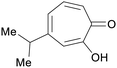 |
Western red cedar (Thuja plicata donn) heartwood 5.8% (w/w) of extractive,227 taiwan hinoki (0.2 mg g−1 sawdust)228 |
| Hinokitiol | ||
 |
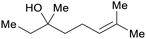 |
Coriandrol ((S)-(+)-linalool): coriander (60–70%), Orthodon linalooliferum (80%) licareol ((R)-(−)-linalool): extracts of Cinnamomum camphora or cajenne rosewood (80–85%)69,229 |
| Linalool | ||
 |
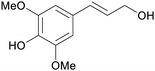 |
Lignin depol.185 |
| Sinapyl alcohol | ||
 |
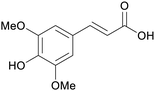 |
Rapeseed hulls (450 mg kg−1),230 mustard meal231 |
| Sinapinic acid | ||
 |
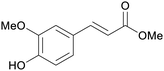 |
Bark of pine trees,232 rice bran oil,233 indonesian sausage fruit234 |
| Methyl ferulate | ||
 |
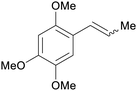 |
Acorus (70% in extract),235,236Asarum237 |
| Asarone | ||
 |
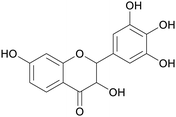 |
Black locust wood (0.5% of dry weight)238 |
| Dihydrorobinetin | ||
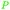 |
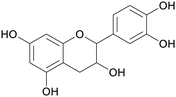 |
Catechu black from Acacia catechu (2–10% catechin),69 grape seed extract (approx. 6% of combined flavanol monomers, including (−)-epicatechin and (+)-catechin), tea extract69 |
| (epi)catechin | ||
 |
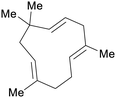 |
Humulus lupulus (40% of volatiles),239 spearmint oil (up to 30%),226 sage oil (13%)240 |
| Humulene | ||
 |
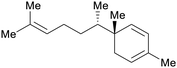 |
Ginger oil (35%)241 |
| Zingiberene | ||
 |
 |
Tall oil242–244 |
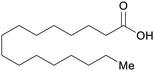
| Palmitic acid | ||
 |
 |
Hydrogenation of palmitic acid (20%)245 |
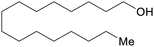
| Cetyl alcohol | ||
 |
 |
Tall oil (45–49%)242–244 |
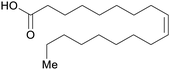
| Oleic acid | ||
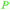 |
 |
Tall oil (45–48%)242–244 |
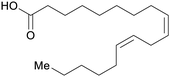
| Linoleic acid | ||
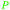 |
 |
Tall oil242–244 |
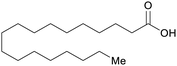
| Stearic acid | ||
 |
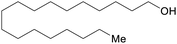 |
Hydrogenation of C18 fatty acids (up to 83%)246,247 |
| Stearyl alcohol | ||
 |
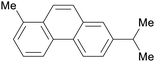 |
Dehydrogenation of abietic acids from resin oils248,249 |
| Retene | ||
 |
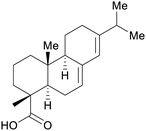 |
Wood rosin250 |
| Abietic acid | ||
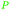 |
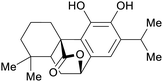 |
Rosemary leaves (1.2% of dry plant)251–253 and sage69,253 |
| Carnosol | ||
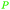 |
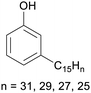 |
Cashew nut shell liquid from Anacardium occidentale (up to 90%)62,254 |
| Cardanol | ||
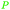 |
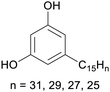 |
Cashew nut shell liquid from Anacardium occidentale62,254 |
| Cardol | ||
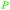 |
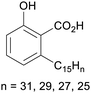 |
Cashew nut shell liquid (82%)255 from Anacardium occidentale, Anacardiaceae, Gingkoaceae, and Myristicaceae62,256 |
| Anacardic acid |
Xylochemical synthesis approaches
One of the numerous issues for the earth's ecosystem is the use of fossil resources for the synthesis of chemical commodities and everyday products. For instance, the UV-absorbers utilized in current sunscreens and photostabilizers are often small organic molecules derived from petroleum. To propose an alternative solution, the groups of Opatz and de Koning opted for a xylochemical synthesis of UV absorbers starting from either cardanol (1) or anarcardic acid,62 both being major components of the bio-renewable and non-edible carbon source cashew nut shell liquid (CNSL).257–260 Starting from these two primary xylochemicals, a series of compounds with promising UV-A and UV-B absorption characteristics belonging to the major commercial classes of UV absorbers (hydroxybenzophenones, triazines, xanthones and flavones) were synthesized (Scheme 1). The color code of all schemes individually traces the origin of the respective atoms.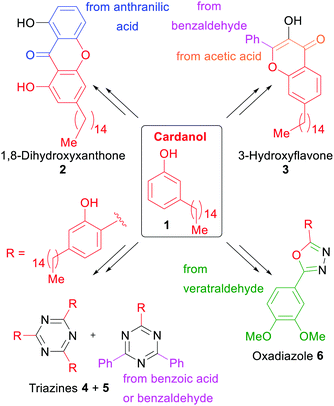 | ||
| Scheme 1 Cashew nut shell liquid derived potential UV-Absorbers synthesized by Opatz and de Koning et al.62 | ||
The first total synthesis of the dimeric alkaloid ilicifoline B (11)261 was reported in 2015 from the groups of Opatz and Arduengo,23 who exclusively utilized wood-derived carbon sources like ferulic acid (7), methanol (8) and veratrole (9). They also reported an asymmetric synthesis of (−)-dihydrocodeine (15) with methyl ferulate (12) and methyl gallate (14) as the staring materials, a xylochemical version of a synthesis developed earlier (Scheme 2).262 Both syntheses demonstrate that the use of wood-derived building blocks can be a sustainable alternative in classical synthetic approaches. In the case of dihydrocodeine, the hitherto most efficient asymmetric synthesis could be surpassed in terms of overall yield even though no carbon input from fossil sources was required with the exception of solvents and reagents.22
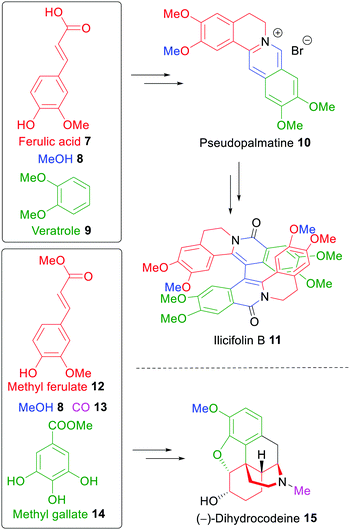 | ||
| Scheme 2 Total Synthesis of ilicifoline B (11) and (−)-dihydrocodeine (15) using a xylochemical approach.23,262 | ||
Another example for the application of xylochemical synthesis strategies was reported in 2019.50 (−)-Oxycodone (19), a naturally occurring263 but mostly semisynthetic opioid related to naturally occurring thebaine,264,265 was synthesized starting from wood-derived methyl gallate (14) and vanillin via the regio- and diastereoselective formation of a 4a-2′-coupled morphinandienone 17 as the key step, followed by Ru-catalyzed Noyori asymmetric transfer hydrogenation (Scheme 3).266,267
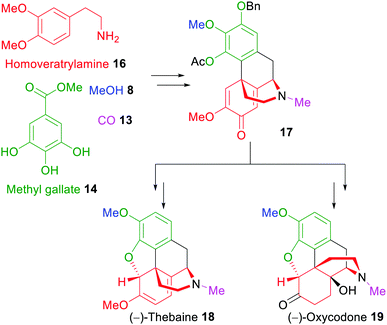 | ||
| Scheme 3 Xylochemical total synthesis of (−)-thebaine (18) and (−)-oxycodone (19).50,51 | ||
Nevertheless, nitrogen-containing fine chemicals remain a challenging task for xylochemical synthesis approaches, as they are not directly attainable from lignocellulosic biomass. To this end, the Sperry group has used chitin (20), the second-most abundant biopolymer, as a cheap natural source of nitrogen, to show a proof-of-concept synthesis of the anticancer alkaloid proximicin A (23) in seven steps.59 Additionally, all of the reagents applied in this synthesis sequence are traceable back towards renewable resources. With this strategy, the group was also able to demonstrate the synthesis of various 3-aminocyclopentanones, 4-aminocyclopentene-1,3-diones and a 4-aminocyclopentenone by applying the chitin-derived furfural 22 in a Piancatelli-like rearrangement (Scheme 4).268
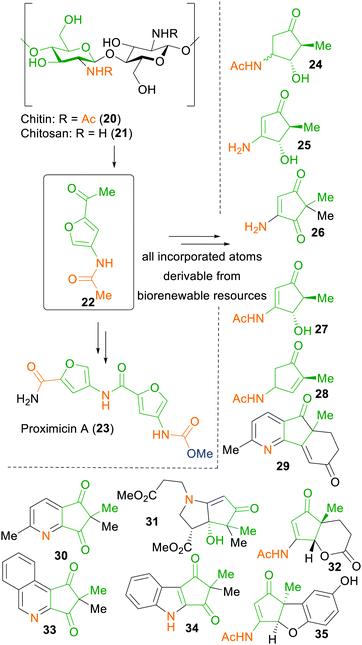 | ||
| Scheme 4 Chitin/Chitosan-derived starting material 22 and products thereof by Sperry et al.59,268 | ||
By applying acidolysis strategies to lignin, the Barta group managed to directly afford three different substance classes of aromatic compounds that can be used as valuable aromatic monomers in further synthesis (Scheme 5). To this end, lignin model compounds 47, representing the β-O-4 linkage in natural lignin, were subjected to strong acids, and the resulting reactive intermediates were converted into more stable products in situ through either reaction with diols furnishing acetals, dehydrogenation to afford the respective diol, or through decarbonylation.269
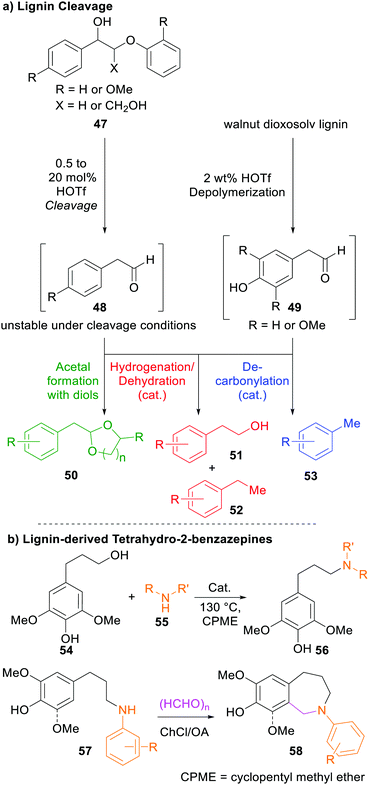 | ||
| Scheme 5 (a) Cleavage pathways of lignin through acidolysis followed by in situ conversion into stable products. (b) Construction of lignin-derived tetrahydro-2-benzazepines 58.60,269 | ||
In 2019, the same group reported the construction of lignin-derived tetrahydro-2-benzazepines (58) through selective catalytic amination followed by cyclization using formaldehyde and choline chloride (ChCl)/oxalic acid (OA) as deep eutectic solvent.60 These substances show promising biological activities270 and represent a scaffold in naturally occurring alkaloids271 such as galanthamine,272 among others.
In 2015, the Moeller group reported the use of sawdust for the electrochemical, sustainable construction of synthetic building blocks bearing electron-rich aromatic rings.61 Solvolysis of the crude sawdust material lead to either cinnamyl ether or aryl aldehyde products, depending on the reaction conditions (Scheme 6). One substance of each class of lignin-derived products was exemplarily converted electrochemically into a series of value-added synthetic substrates, which themselves could act as platform chemicals for the construction of diverse drugs (38a and 38b) and alkaloids (39–41), as monomers for polymer synthesis (42 and 43), as structural elements found in numerous biological systems (44) or as substrates for electrochemical oxidations (45 and 46).
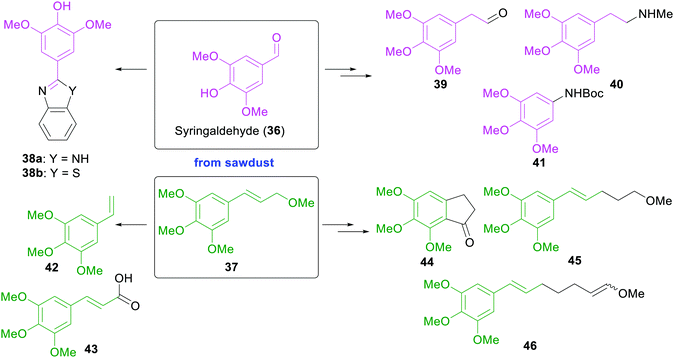 | ||
| Scheme 6 Solvolysis products of sawdust and conversion into electron-rich and value-added synthetic building blocks by the Moeller group.61 | ||
Concluding remarks
This review provides an overview of a variety of platform chemicals that may be obtained from wood-based biomass rather than from petrochemistry. Moreover, a variety of existing syntheses of natural products, drugs and everyday consumer products based on xylochemicals is presented. Nevertheless, there remains room for new discoveries and the improvement of existing technologies to reach the envisioned transition from optimized petroleum-based processes towards sustainable xylochemical approaches. Apart from renewable starting materials, as discussed in this review, the use of alternative, ideally sustainable solvents and reagents as well as work-up procedures, that suit the principles of green chemistry, are a major goal to bear in mind when planning a synthesis. Recommendations on the substitution of carcinogenic, toxic and otherwise undesirable solvents have already been published and adopted on industrial scale but are often disregarded in research laboratories.273 Until alternatives for problematic solvents and reagents are developed, recycling remains the responsible alternative. We hope this article will catalyze thinking and activity in the direction of renewable resources and sustainable chemistry. For the chemical community specifically, and for society in general, it would be advantageous to have access to renewable commodities containing nitrogen (e.g. pyridine, urea, guanidine, aniline, quinoline, phenylenediamine etc.), second row hetero elements (S, P) as well as the industrially and pharmaceutically relevant halogens (F, Cl, Br). The development of industrial processes for the isolation of monomeric building blocks (e.g. ethylene oxide, styrene, ethylene glycol, adipic acid, phthalic anhydride etc.) from woody biomass on an industrial scale would constitute a significant improvement over the current state of the art. In addition, there are numerous important simple substances and platform chemicals for which “green” industrial scale solutions are not yet available on larger scale (e.g. cyclohexanedione and the class of nitriles and isonitriles). Developments in this direction would constitute important additions to the xylochemical toolbox and can be regarded as attractive xylo-targets for future chemical innovation.Conflicts of interest
There are no conflicts to declare.Notes and references
- C. O. Johns, Ind. Eng. Chem., 1923, 15, 446–449 CrossRef CAS.
- N. s. Monardes and J. Frampton, Joyfull News out of the New Founde Worlde, Printed by E. Allde, by the assigne of Bonham Norton, London, 1596 Search PubMed.
- A. D. Hirschfelder, Ind. Eng. Chem., 1923, 15, 455–460 CrossRef CAS.
- G. H. Brundtland, Environ. Conserv., 1987, 14, 291–294 CrossRef.
- M. Eissen, J. O. Metzger, E. Schmidt and U. Schneidewind, Angew. Chem., Int. Ed., 2002, 41, 414–436 CrossRef CAS.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, 29–56 Search PubMed.
- P. T. Anastas and R. L. Lankey, Green Chem., 2000, 2, 289–295 RSC.
- P. T. Anastas and J. B. Zimmerman, Design through the 12 principles of green engineering, ACS Publications, 2003 Search PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- P. T. Anastas, Green Chem., 2003, 5, G29–G34 RSC.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259–281 CrossRef CAS.
- B. M. Trost, Science, 1983, 219, 245 CrossRef CAS PubMed.
- R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233–1246 CAS.
- R. A. Sheldon, Chem. Ind., 1992, 903–906 CAS.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- R. A. Sheldon, J. Chem. Technol. Biotechnol., 1997, 68, 381–388 CrossRef CAS.
- J. Kühlborn, J. Groß and T. Opatz, Nat. Prod. Rep., 2020, 37, 380–424 RSC.
- H. H. Szmant, Organic building blocks of the chemical industry, John Wiley & Sons, 1989 Search PubMed.
- T. Opatz and A. J. Arduengo III, GIT Laborportal, 2016.
- D. Stubba, G. Lahm, M. Geffe, J. W. Runyon, A. J. Arduengo III and T. Opatz, Angew. Chem., Int. Ed., 2015, 54, 14187–14189 CrossRef CAS PubMed.
- FAO, Food Agricult. Organiz. U.N., http://www.fao.org/forestry/en/, accessed on August 13, 2021.
- FAO, Food Agricult. Organiz. U.N., http://www.fao.org/forestry/statistics/80938/en/, accessed on August 13, 2021.
- R. L. Braun and A. K. Burnham, Chemical reaction model for oil and gas generation from type 1 and type 2 kerogen, Lawrence Livermore National Lab., CA United States, 1993 Search PubMed.
- L. Wu, T. Moteki, A. A. Gokhale, D. W. Flaherty and F. D. Toste, Chem, 2016, 1, 32–58 CrossRef CAS PubMed.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- P. L. Rivilli, G. I. Yranzo and J. D. Pérez, BioResources, 2011, 6, 2703–2710 CAS.
- J. Luterbacher, D. M. Alonso and J. Dumesic, Green Chem., 2014, 16, 4816–4838 RSC.
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695 CrossRef CAS PubMed.
- P. B. Thompson, Agriculture, 2012, 2, 339–358 CrossRef.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- X. Wang and R. Rinaldi, ChemSusChem, 2012, 5, 1455–1466 CrossRef CAS PubMed.
- C. K. Nitsos, C. M. Mihailof, K. A. Matis, A. A. Lappas and K. S. Triantafyllidis, The Role of Catalysis for the Sustainable Production of Bio-fuels and Bio-chemicals, Elsevier, 2013, pp. 217–260 Search PubMed.
- R. Behling, S. Valange and G. Chatel, Green Chem., 2016, 18, 1839–1854 RSC.
- E. Feghali, G. Carrot, P. Thuery, C. Genre and T. Cantat, Energy Environ. Sci., 2015, 8, 2734–2743 RSC.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed.
- G. T. Beckham, C. W. Johnson, E. M. Karp, D. Salvachúa and D. R. Vardon, Curr. Opin. Biotechnol, 2016, 42, 40–53 CrossRef CAS PubMed.
- S.-H. Li, S. Liu, J. C. Colmenares and Y.-J. Xu, Green Chem., 2016, 18, 594–607 RSC.
- C. Chio, M. Sain and W. Qin, Renewable Sustainable Energy Rev., 2019, 107, 232–249 CrossRef CAS.
- C. Zhang and F. Wang, Acc. Chem. Res., 2020, 53, 470–484 CrossRef CAS PubMed.
- M. P. Pandey and C. S. Kim, Chem. Eng. Technol., 2011, 34, 29–41 CrossRef CAS.
- T. Q. Yuan, F. Xu and R. C. Sun, J. Chem. Technol. Biotechnol., 2013, 88, 346–352 CrossRef CAS.
- C. Xu, R. A. D. Arancon, J. Labidi and R. Luque, Chem. Soc. Rev., 2014, 43, 7485–7500 RSC.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51, 2564–2601 CrossRef CAS PubMed.
- L. T. Mika, E. Csefalvay and A. Nemeth, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 4464–4480 CrossRef CAS.
- A. Lipp, M. Selt, D. Ferenc, D. Schollmeyer, S. R. Waldvogel and T. Opatz, Org. Lett., 2019, 21, 1828–1831 CrossRef CAS PubMed.
- A. Lipp, D. Ferenc, C. Gütz, M. Geffe, N. Vierengel, D. Schollmeyer, H. J. Schäfer, S. R. Waldvogel and T. Opatz, Angew. Chem., Int. Ed., 2018, 57, 11055–11059 CrossRef CAS PubMed.
- R. Klintworth, C. B. de Koning, T. Opatz and J. P. Michael, J. Org. Chem., 2019, 84, 11025–11031 CAS.
- L. Geske, U. Kauhl, M. E. M. Saeed, A. Schüffler, E. Thines, T. Efferth and T. Opatz, Molecules, 2021, 26, 3224 CrossRef CAS PubMed.
- J. Kühlborn, M. Konhäuser, J. Groß, P. R. Wich and T. Opatz, ACS Sustainable Chem. Eng., 2019, 7, 4414–4419 CrossRef.
- J. Alsarraf, J.-F. Bilodeau, J. Legault, F. Simard and A. Pichette, ACS Sustainable Chem. Eng., 2020, 8, 6194–6199 CrossRef CAS.
- A. Sevenich, G.-Q. Liu, A. J. Arduengo, B. F. Gupton and T. Opatz, J. Org. Chem., 2017, 82, 1218–1223 CrossRef CAS PubMed.
- J. Kühlborn, A.-K. Danner, H. Frey, R. Iyer, A. J. Arduengo and T. Opatz, Green Chem., 2017, 19, 3780–3786 RSC.
- T. T. Pham, G. Gözaydın, T. Söhnel, N. Yan and J. Sperry, Eur. J. Org. Chem., 2019, 1355–1360 CrossRef CAS.
- A. D. Sadiq, X. Chen, N. Yan and J. Sperry, ChemSusChem, 2018, 11, 532–535 CrossRef CAS PubMed.
- S. Elangovan, A. Afanasenko, J. Haupenthal, Z. Sun, Y. Liu, A. K. H. Hirsch and K. Barta, ACS Cent. Sci., 2019, 5, 1707–1716 CrossRef CAS PubMed.
- B. H. Nguyen, R. J. Perkins, J. A. Smith and K. D. Moeller, J. Org. Chem., 2015, 80, 11953–11962 CrossRef CAS PubMed.
- K. J. Ngwira, J. Kühlborn, Q. A. Mgani, C. B. de Koning and T. Opatz, Eur. J. Org. Chem., 2019, 4778–4790 CrossRef CAS.
- A. J. Arduengo III and T. Opatz, Xylochemistry - Commodities, Materials, Pharmaceuticals, & Fuels from renewable wood, https://xylochemistry.com/portal/, accessed on August 13, 2021 Search PubMed.
- In his reference work, Szmant correlates a few materials won from biomass with petrochemical intermediates (see ch. 2 “Principal Sources of Industrial Organic Transformation Products” (p. 29 and Fig. 2.1).
- O. Anselmino and E. Gilg, Kommentar zum Deutschen Arzneibuch 6. Ausgabe 1926, Springer, 1928, pp. 70–73 Search PubMed.
- F. Jin and H. Enomoto, BioResources, 2009, 4, 704–713 CAS.
- P. Klason, G. V. Heidenstam and E. Norlin, Angew. Chem., 1910, 23, 1252–1257 CrossRef CAS.
- E. E. Harris, J. D'Ianni and H. Adkins, J. Am. Chem. Soc., 1938, 60, 1467–1470 CrossRef CAS.
- G. A. Burdock, Fenaroli's handbook of flavor ingredients, CRC press, 2016 Search PubMed.
- A. Mohsenzadeh, A. Zamani and M. J. Taherzadeh, ChemBioEng Rev., 2017, 4, 75–91 CrossRef.
- M. Zhang and Y. Yu, Ind. Eng. Chem. Res., 2013, 52, 9505–9514 CrossRef CAS.
- D. Fan, D.-J. Dai and H.-S. Wu, Materials, 2013, 6, 101–115 CrossRef CAS PubMed.
- M. E. Cisney and J. D. Wethern, US Pat., US2816832A, James River Corp. of Nevada, 1957 Search PubMed.
- A. Kujawska, J. Kujawski, M. Bryjak and W. Kujawski, Renewable Sustainable Energy Rev., 2015, 48, 648–661 CrossRef CAS.
- M. Rose and R. Palkovits, Macromol. Rapid Commun., 2011, 32, 1299–1311 CrossRef CAS PubMed.
- M. J. Lippits and B. E. Nieuwenhuys, Catal. Today, 2010, 154, 127–132 CrossRef CAS.
- D. Carnaroglio, S. Tabasso, B. Kwasek, D. Bogdal, E. C. Gaudino and G. Cravotto, ChemSusChem, 2015, 8, 1342–1349 CrossRef CAS PubMed.
- H. Boerhaave, Elementa Chemiae: Qui continet Operationes Chemicas, Im-Hoff, Basileæ, 1745.
- D. F. Othmer, C. H. Gamer and J. J. Jacobs, Ind. Eng. Chem., 1942, 34, 262–267 CrossRef CAS.
- A. Corma, G. W. Huber, L. Sauvanaud and P. O'Connor, J. Catal., 2008, 257, 163–171 CrossRef CAS.
- J. V. Haveren, E. L. Scott and J. Sanders, Biofuels, Bioprod. Biorefin., 2008, 2, 41–57 CrossRef.
- T. Werpy and G. Petersen, National Renewable Energy Lab, Golden, CO, US, 2004.
- K. Piwowarek, E. Lipińska, E. Hać-Szymańczuk, M. Kieliszek and I. Ścibisz, Appl. Microbiol. Biotechnol., 2018, 102, 515–538 CrossRef CAS PubMed.
- R. A. Gonzalez-Garcia, T. McCubbin, M. S. Turner, L. K. Nielsen and E. Marcellin, Biotechnol. Bioeng., 2020, 117, 167–183 CrossRef CAS PubMed.
- L. Bentley, Nature, 1952, 170, 847–848 CrossRef CAS PubMed.
- W. A. Turner and A. M. Hartman, J. Am. Chem. Soc., 1925, 47, 2044–2047 CrossRef CAS.
- E. Nelson and H. Hasselbring, J. Am. Chem. Soc., 1931, 53, 1040–1043 CrossRef CAS.
- H. Danner and R. Braun, Chem. Soc. Rev., 1999, 28, 395–405 RSC.
- A. Perlin and C. Brice, Can. J. Chem., 1956, 34, 85–88 CrossRef CAS.
- Y. Li, W. Tang, Y. Chen, J. Liu and C.-F. F. Lee, Fuel, 2019, 242, 673–686 CrossRef CAS.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- G. Knothe, J. Krahl and J. Van Gerpen, The biodiesel handbook, Elsevier, 2015 Search PubMed.
- T. Roncal, S. Caballero, M. D. M. Díaz de Guereñu, I. Rincón, S. Prieto-Fernández and J. R. Ochoa-Gómez, Process Biochem., 2017, 58, 35–41 CrossRef CAS.
- G. Pomalaza, P. Arango Ponton, M. Capron and F. Dumeignil, Catal. Sci. Technol., 2020, 10, 4860–4911 RSC.
- C. De Mas, N. B. Jansen and G. T. Tsao, Biotechnol. Bioeng., 1988, 31, 366–377 CrossRef CAS PubMed.
- J.-Y. Dai, P. Zhao, X.-L. Cheng and Z.-L. Xiu, Appl. Biochem. Biotechnol., 2015, 175, 3014–3024 CrossRef CAS PubMed.
- P. Lejemble, A. Gaset and P. Kalck, Biomass, 1984, 4, 263–274 CrossRef CAS.
- I. Bechthold, K. Bretz, S. Kabasci, R. Kopitzky and A. Springer, Chem. Eng. Technol., 2008, 31, 647–654 CrossRef CAS.
- P. Lee, S. Lee, S. Hong, H. N. Chang and S. Park, Biotechnol. Lett., 2003, 25, 111–114 CrossRef CAS PubMed.
- P. C. Lee, W. G. Lee, S. Y. Lee, H. N. Chang and Y. K. Chang, Biotechnol. Bioprocess Eng., 2000, 5, 379–381 CrossRef CAS.
- J. Akhtar, A. Idris and R. A. Aziz, Appl. Microbiol. Biotechnol., 2014, 98, 987–1000 CrossRef CAS PubMed.
- H. Choudhary, S. Nishimura and K. Ebitani, Appl. Catal., A, 2013, 458, 55–62 CrossRef CAS.
- R. Burrell, J. Chem. Educ., 1937, 14, 520 CrossRef CAS.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- F. A. Loewus, Phytochemistry, 1999, 52, 193–210 CrossRef CAS.
- C. Hale, Nature, 1962, 195, 917–918 CrossRef CAS.
- S. Dörsam, J. Fesseler, O. Gorte, T. Hahn, S. Zibek, C. Syldatk and K. Ochsenreither, Biotechnol. Biofuels, 2017, 10, 242 CrossRef PubMed.
- Z. Xiao, C. Cheng, T. Bao, L. Liu, B. Wang, W. Tao, X. Pei, S.-T. Yang and M. Wang, Biotechnol. Biofuels, 2018, 11, 164 CrossRef PubMed.
- Y. Rodenas, R. Mariscal, J. Fierro, D. M. Alonso, J. Dumesic and M. L. Granados, Green Chem., 2018, 20, 2845–2856 RSC.
- R. Wojcieszak, F. Santarelli, S. Paul, F. Dumeignil, F. Cavani and R. V. Gonçalves, Sustainable Chem. Processes, 2015, 3, 9 CrossRef.
- S. W. Fitzpatrick, US Pat., US4897497A, Biofine Technologies LLC Biofine Tech LLC, 1990 Search PubMed.
- S. W. Fitzpatrick, US Pat., US5608105A, Biofine Technologies LLC Biofine Tech LLC, 1997 Search PubMed.
- M. Mascal and E. B. Nikitin, ChemSusChem, 2009, 2, 859–861 CrossRef CAS PubMed.
- M. Mascal and E. B. Nikitin, Green Chem., 2010, 12, 370–373 RSC.
- M. G. Steiger, N. Wierckx, L. M. Blank, D. Mattanovich and M. Sauer, Industrial biotechnology, products and processes, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2017 Search PubMed.
- T. D. Sharkey and S. Yeh, Annu. Rev. Plant Biol., 2001, 52, 407–436 CrossRef CAS PubMed.
- T. D. Sharkey, Endeavour, 1996, 20, 74–78 CrossRef CAS PubMed.
- W. Luo, M. Sankar, A. M. Beale, Q. He, C. J. Kiely, P. C. Bruijnincx and B. M. Weckhuysen, Nat. Commun., 2015, 6, 1–10 Search PubMed.
- Z. Yu, X. Lu, C. Liu, Y. Han and N. Ji, Renewable Sustainable Energy Rev., 2019, 112, 140–157 CrossRef CAS.
- K. J. Zeitsch, The chemistry and technology of furfural and its many by-products, Elsevier, 2000 Search PubMed.
- N. Galeotti, F. Jirasek, J. Burger and H. Hasse, Chem. Eng. Technol., 2018, 41, 2331–2336 CrossRef CAS PubMed.
- H. Pringsheim and H. Noth, Ber. Dtsch. Chem. Ges., 1920, 53, 114–118 CrossRef.
- E. Oddo, F. Saiano, G. Alonzo and E. Bellini, Ann. Bot., 2002, 90, 239–243 CrossRef CAS PubMed.
- S. M. Ghoreishi and R. G. Shahrestani, J. Food Eng., 2009, 93, 474–481 CrossRef CAS.
- Repertorium für die Pharmacie, Bei J. L. Schrag, 1839.
- S. Jeenpadiphat, I. Mongkolpichayarak and D. N. Tungasmita, J. Anal. Appl. Pyrolysis, 2016, 121, 318–328 CrossRef CAS.
- X. Liu, H. Sun, P. Gao, C. Liu, X. Ding, M. Huang, D. Li and L. Zhang, J. Wood Chem. Technol., 2018, 38, 313–323 CrossRef CAS.
- D. T. A. P. J. Huibers and J. Hugh, US Pat., US4420644A, Hydrocarbon Research, Inc., 1983 Search PubMed.
- X. Ouyang, X. Huang, M. D. Boot and E. J. M. Hensen, ChemSusChem, 2020, 13, 1705–1709 CrossRef CAS PubMed.
- D. E. Hruza, M. Van Praag and H. Heinsohn, J. Agric. Food Chem., 1974, 22, 123–126 CrossRef CAS.
- H. Hlasiwetz, Liebigs Ann. Chem., 1855, 96, 118–123 CrossRef.
- A. Picinelli, E. Dapena and J. J. Mangas, J. Agric. Food Chem., 1995, 43, 2273–2278 CrossRef CAS.
- L. Min, W. Daoqing and P. Cheng, CN Pat., CN102701938A, Chengdu University of Traditional Chinese Medicine, 2012 Search PubMed.
- C. M. de Diego, W. P. Schammel, M. A. Dam and G. J. M. Gruter, EP2486027A2, Furanix Technologies BV, 2013.
- E. de Jong, M. Dam, L. Sipos and G.-J. Gruter, Biobased monomers, polymers, and materials, ACS Publications, 2012, pp. 1–13 Search PubMed.
- X.-P. Wu, M.-H. Fan and Q.-X. Li, Chin. J. Chem. Phys., 2017, 30, 479–486 CrossRef CAS.
- A. M. Niziolek, O. Onel, Y. A. Guzman and C. A. Floudas, Energy Fuels, 2016, 30, 4970–4998 CrossRef CAS.
- D. Congcong, W. Peilun, Z. Qianqian, M. Jialao, Z. Zhao and W. Daohong, CN106045805A, Beijing Shenwu Environment Energy Technology Group Co Ltd China, 2016.
- A. A. Klose, J. B. Stark, G. G. Purvis, J. Peat and H. L. Fevold, Ind. Eng. Chem., 1950, 42, 387–391 CrossRef CAS.
- A. Szent-Györgyi, Biochem. J., 1928, 22, 1387–1409 CrossRef PubMed.
- A. W. Hofmann, Liebigs Ann. Chem., 1859, 110, 129–140 CrossRef.
- U. Brunner, J. Biol. Educ., 1985, 19, 41–47 CrossRef.
- W. Diemair and K. Franzen, Z. Lebensm.-Unters. Forsch., 1959, 109, 373–378 CrossRef CAS.
- J. H. Cardellina and J. Meinwald, Phytochemistry, 1980, 19, 2199–2200 CrossRef CAS.
- M. Pazouki and T. Panda, Bioprocess Eng., 1998, 19, 435–439 CrossRef CAS.
- P. Heretsch, F. Thomas, A. Aurich, H. Krautscheid, D. Sicker and A. Giannis, Angew. Chem., Int. Ed., 2008, 47, 1958–1960 CrossRef CAS PubMed.
- J. E. Camp, ChemSusChem, 2018, 11, 3048–3055 CrossRef CAS PubMed.
- J. Sherwood, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- L. E. Manzer, Appl. Catal., A, 2004, 272, 249–256 CrossRef CAS.
- I. Rinkes, Org. Synth., 1934, 14, 62–63 CrossRef.
- M. Mascal, in Production of Platform Chemicals from Sustainable Resources, ed. Z. Fang, J. R. L. Smith and X. Qi, Springer, Singapore, Singapore, 2017, pp. 123–140 Search PubMed.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef PubMed.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr, Green Chem., 2010, 12, 1855–1860 RSC.
- X. Tong, Y. Ma and Y. Li, Appl. Catal., A, 2010, 385, 1–13 CrossRef CAS.
- G. Feron, P. Bonnarme and A. Durand, Trends Food Sci. Technol., 1996, 7, 285–293 CrossRef CAS.
- R. S. Verma, R. C. Padalia, V. R. Singh, P. Goswami, A. Chauhan and B. Bhukya, Int. J. Food Prop., 2017, 20, 1259–1263 CAS.
- C. Amen-Chen, H. Pakdel and C. Roy, Biomass Bioenergy, 1997, 13, 25–37 CrossRef CAS.
- F. A. Agblevor, S. Beis, O. Mante and N. Abdoulmoumine, Ind. Eng. Chem. Res., 2010, 49, 3533–3538 CrossRef CAS.
- J. M. G. Galvez, B. Riedl and A. H. Conner, Holzforschung, 1997, 51, 235–243 CrossRef.
- O. Djakpo and W. Yao, Phytother. Res., 2010, 24, 1739–1747 CrossRef CAS PubMed.
- S. A. O. Santos, C. Vilela, C. S. R. Freire, C. P. Neto and A. J. D. Silvestre, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 938, 65–74 CrossRef CAS PubMed.
- M. S. A. Marzouk, S. A. A. El-Toumy, F. A. Moharram, N. M. M. S. Nagwa and N. A. E. A. Amany, Planta Med., 2002, 68, 523–527 CrossRef CAS PubMed.
- E. Hedberg, A. Kristensson, M. Ohlsson, C. Johansson, P.-Å. Johansson, E. Swietlicki, V. Vesely, U. Wideqvist and R. Westerholm, Atmos. Environ., 2002, 36, 4823–4837 CrossRef CAS.
- A. W. Hofmann, Liebigs Ann. Chem., 1845, 55, 200–205 CrossRef.
- C. Wiegand, Angew. Chem., 1948, 60, 109–111 CrossRef.
- O. Unverdorben, Ann. Phys., 1829, 92, 369–376 CrossRef.
- Y. Cui, W. Wang and J. Chang, Materials, 2019, 12, 1609 CrossRef CAS PubMed.
- J. A. Buchner and B. J. L. Schrag, Repertorium für die Pharmacie, 1828 Search PubMed.
- R. k. Ibrahim and G. H. N. Towers, Arch. Biochem. Biophys., 1960, 87, 125–128 CrossRef CAS PubMed.
- S. Preiss, J. Prakt. Chem., 1955, 1, 157–171 CrossRef CAS.
- K. Kürschner, Mikrochemie, 1925, 3, 1–20 CrossRef.
- M. Zirbes, L. L. Quadri, M. Breiner, A. Stenglein, A. Bomm, W. Schade and S. R. Waldvogel, ACS Sustainable Chem. Eng., 2020, 8, 7300–7307 CrossRef CAS.
- F. Stecker, A. Fischer, A. Kirste, S. Waldvogel, C. Regenbrecht and D. Schmitt, US Pat., US20140034508A1, BASF SE, Johannes Gutenberg Universitaet Mainz, 2014.
- D. Schmitt, C. Regenbrecht, M. Hartmer, F. Stecker and S. R. Waldvogel, Beilstein, J. Org. Chem., 2015, 11, 473–480 CAS.
- K. Kavanagh and J. Pepper, Can. J. Chem., 1955, 33, 24–30 CrossRef CAS.
- Y. Wang, S. Sun, F. Li, X. Cao and R. Sun, Ind. Crops Prod., 2018, 116, 116–121 CrossRef CAS.
- M. B. Polk and M. Phingbodhipakkiya, Nucl. Chem. Waste Manage., 1980, 1, 111–118 CrossRef CAS.
- S. Hwang, D. Shin and K. Kim, Korean J. Weed Sci., 1997, 17, 334–344 Search PubMed.
- C. Srinivasulu, M. Ramgopal, G. Ramanjaneyulu, C. M. Anuradha and C. Suresh Kumar, Biomed. Pharmacother., 2018, 108, 547–557 CrossRef CAS PubMed.
- L. A. Pacheco-Palencia, S. Mertens-Talcott and S. T. Talcott, J. Agric. Food Chem., 2008, 56, 4631–4636 CrossRef CAS PubMed.
- M. T. Tunç and İ. Koca, J. Food Process Eng., 2021, 44, e13635 Search PubMed.
- M. Y. A.-M. Y. C. Wong and W. A. Wan-Nurdiyana, Orient. J. Chem., 2014, 30, 37–47 CrossRef.
- C.-Y. Lin, S.-S. Cheng, C.-L. Wu and S.-T. Chang, Wood Sci. Technol., 2020, 54, 237–247 CrossRef CAS.
- H.-G. Lee, Y. Jo, K. Ameer and J.-H. Kwon, Food Sci. Biotechnol., 2018, 27, 1607–1617 CrossRef CAS PubMed.
- X. Liu, F. P. Bouxin, J. Fan, V. L. Budarin, C. Hu and J. H. Clark, ChemSusChem, 2020, 13, 4296–4317 CrossRef CAS PubMed.
- B. Max, A. M. Torrado, A. B. Moldes, A. Converti and J. M. Domínguez, Biochem. Eng. J., 2009, 43, 129–134 CrossRef CAS.
- R.-C. Sun, X.-F. Sun and S.-H. Zhang, J. Agric. Food Chem., 2001, 49, 5122–5129 CrossRef CAS PubMed.
- R. Sun, J. Tomkinson, X. F. Sun and N. J. Wang, Polymer, 2000, 41, 8409–8417 CrossRef CAS.
- R. H. J. Creighton, J. L. McCarthy and H. Hibbert, J. Am. Chem. Soc., 1941, 63, 312 CrossRef CAS.
- N. A. Tyukavkina, S. A. Medvedeva and L. N. Ermolaeva, Chem. Nat. Compd., 1970, 6, 126 CrossRef.
- R. Hamir Singh and M. L. N. Leo, Green Pesticides Handbook, CRC Press, 2017 Search PubMed.
- K. Michalska, O. Michalski and A. Stojakowska, Phytochem. Lett., 2017, 20, 425–428 CrossRef CAS.
- B. Wu, L. Gan and H. Qu, J. Nat. Prod., 2010, 73, 1069–1074 CrossRef CAS PubMed.
- H. Kikuzaki, S. Tesaki, S. Yonemori and N. Nakatani, Phytochemistry, 2001, 56, 109–114 CrossRef CAS PubMed.
- M. Carlson and R. Thompson, J. AOAC Int., 2020, 80, 1023–1028 CrossRef.
- D. P. Kamdem and D. A. Gage, Planta Med., 1995, 61, 574–575 CrossRef CAS PubMed.
- M. J. Hickey, J. Org. Chem., 1948, 13, 443–446 CrossRef CAS PubMed.
- A. J. Mossi, C. A. Zanella, G. Kubiak, L. A. Lerin, R. L. Cansian, F. S. Frandoloso, V. D. Prá, M. A. Mazutti, J. A. V. Costa and H. Treichel, Renewable Agric. Food Syst., 2014, 29, 161–166 CrossRef.
- E. J. Barreiro and M. E. F. Lima, J. Pharm. Sci., 1992, 81, 1219–1222 CrossRef CAS PubMed.
- H. Amer, V. Mimini, D. Schild, U. Rinner, M. Bacher, A. Potthast and T. Rosenau, Holzforschung, 2020, 74, 197–202 CAS.
- W. Wang, J. Guo, J. Zhang, J. Peng, T. Liu and Z. Xin, Food Chem., 2015, 171, 40–49 CrossRef CAS PubMed.
- H.-I. Jun, J.-W. Shin, G.-S. Song and Y.-S. Kim, J. Food Sci., 2015, 80, C262–C268 CrossRef CAS PubMed.
- R. Fabinyi and T. Széki, Ber. Dtsch. Chem. Ges., 1906, 39, 3679–3685 CrossRef.
- B. D. W. Luff, W. H. Perkin and R. Robinson, J. Chem. Soc., Trans., 1910, 97, 1131–1140 RSC.
- A. Halpern, J. Am. Pharm. Assoc., 1948, 37, 161–165 CrossRef CAS PubMed.
- D. S. B. de Castro, D. B. da Silva, J. D. Tibúrcio, M. E. G. Sobral, V. Ferraz, A. G. Taranto, J. E. Serrão, J. M. de Siqueira and S. N. Alves, Exp. Parasitol., 2016, 171, 84–90 CrossRef PubMed.
- J. A. Linnekoski, M. Asikainen, H. Heikkinen, R. K. Kaila, J. Räsänen, A. Laitinen and A. Harlin, Org. Process Res. Dev., 2014, 18, 1468–1475 CrossRef CAS.
- S. Kordali, A. Cakir, H. Ozer, R. Cakmakci, M. Kesdek and E. Mete, Bioresour. Technol., 2008, 99, 8788–8795 CrossRef CAS PubMed.
- M. S. Owolabi, L. Lajide, M. O. Oladimeji, W. N. Setzer, M. C. Palazzo, R. A. Olowu and A. Ogundajo, Nat. Prod. Commun., 2009, 4, 989–992 CrossRef CAS PubMed.
- Y. Wang, A. Wang, H. Tian, H. Wang and C. Zou, Asian J. Chem., 2014, 26, 997–1001 CrossRef CAS.
- A. A. Khalil, U. Rahman, M. R. Khan, A. Sahar, T. Mehmood and M. Khan, RSC Adv., 2017, 7, 32669–32681 RSC.
- M. C. Raja, V. Srinivasan, S. Selvaraj and S. Mahapatra, Pharm. Anal. Acta, 2015, 6, 367 Search PubMed.
- Y. Orihara, T. Furuya, N. Hashimoto, Y. Deguchi, K. Tokoro and T. Kanisawa, Phytochemistry, 1992, 31, 827–831 CrossRef CAS PubMed.
- J. Vladić, Z. Zeković, S. Jokić, S. Svilović, S. Kovačević and S. Vidović, J. Supercrit. Fluids, 2016, 117, 89–97 CrossRef.
- M. De Vincenzi, A. Stammati, A. De Vincenzi and M. Silano, Fitoterapia, 2004, 75, 801–804 CrossRef CAS PubMed.
- N. P. Bahadirli, Int. J. Agric. For. Life Sci., 2020, 4, 111–114 Search PubMed.
- K. Skalicka-Woźniak and M. Walasek, Phytochem. Lett., 2014, 10, xciv–xcviii CrossRef.
- V. D. Zheljazkov, C. L. Cantrell and T. Astatkies, Agron. J., 2010, 102, 1652–1656 CrossRef.
- Y. Amanzadeh, M. Ashrafi and F. Mohammadi, Iran. J. Pharm. Sci., 2006, 2, 87–90 Search PubMed.
- M. Pourbafrani, G. Forgács, I. S. Horváth, C. Niklasson and M. J. Taherzadeh, Bioresour. Technol., 2010, 101, 4246–4250 CrossRef CAS PubMed.
- A. Thomas and Y. Bessiere, Nat. Prod. Rep., 1989, 6, 291–309 RSC.
- S. B. Waller, M. B. Cleff, C. B. de Mattos, C. C. da Silva, C. Giordani, D. F. Dalla Lana, A. M. Fuentefria, R. A. Freitag, E. S. Viegas Sallis, J. R. B. de Mello, R. O. de Faria and M. C. A. Meireles, Nat. Prod. Res., 2019, 1–5 Search PubMed.
- R. A. Bernhard, R. O. B. Wijesekera and C. O. Chichester, Phytochemistry, 1971, 10, 177–184 CrossRef CAS.
- J. Bertram and H. Walbaum, Arch. Pharm., 1893, 231, 290–305 CrossRef.
- C. C. C. R. de Carvalho and M. M. R. da Fonseca, Food Chem., 2006, 95, 413–422 CrossRef CAS.
- S. S. Chauhan, O. Prakash, R. C. Padalia, V. Vivekanand, A. K. Pant and C. S. Mathela, Nat. Prod. Commun., 2011, 6, 1373–1378 CrossRef CAS PubMed.
- R. J. Chedgy, C. R. Daniels, J. Kadla and C. Breuil, Holzforschung, 2007, 61, 190–194 CAS.
- J. Hu, Y. Shen, S. Pang, Y. Gao, G. Xiao, S. Li and Y. Xu, J. Environ. Sci., 2013, 25, S32–S35 CrossRef.
- A. C. Aprotosoaie, M. Hăncianu, I.-I. Costache and A. Miron, Flavour Fragrance J., 2014, 29, 193–219 CrossRef CAS.
- L. Quinn, S. G. Gray, S. Meaney, S. Finn, O. Kenny and M. Hayes, Ir. J. Agric. Food Res., 2017, 56, 104–119 CAS.
- U. Thiyam, H. Stöckmann, T. Zum Felde and K. Schwarz, Eur. J. Lipid Sci. Technol., 2006, 108, 239–248 CrossRef CAS.
- T. Suga, S. Ohta, K. Munesada, N. Ide, M. Kurokawa, M. Shimizu and E. Ohta, Phytochemistry, 1993, 33, 1395–1401 CrossRef CAS.
- A. Tanaka, A. Kato and T. Tsuchiya, J. Am. Oil Chem. Soc, 1971, 48, 95–97 CrossRef CAS.
- A. Ilmiawati, D. Anggraini, G. Syahbirin, D. U. C. Rahayu and P. Sugita, AIP Conf. Proc., 2020, 2243, 030009 CrossRef CAS.
- H. Zhu, I. Ali, H. Hussain, M. Hussain, X.-B. Wang, X. Song, G. Luo, Z. Zhang, Z. Wang and D. Wang, J. Chromatogr. A, 2021, 1643, 462080 CrossRef CAS PubMed.
- L. J. McGaw, A. K. Jäger, J. van Staden and J. N. Eloff, S. Afr. J. Bot., 2002, 68, 31–35 CrossRef CAS.
- R. Oprean, M. Tamas and L. Roman, J. Pharm. Biomed. Anal., 1998, 18, 227–234 CrossRef CAS PubMed.
- E. Destandau, J.-P. Charpentier, S. Bostyn, S. Zubrzycki, V. Serrano, J.-M. Seigneuret and C. Breton, Separations, 2016, 3, 23 CrossRef.
- S. T. Katsiotis, C. R. Langezaal and J. J. C. Scheffer, Planta Med., 1989, 55, 634 CrossRef.
- S. Bouajaj, A. Benyamna, H. Bouamama, A. Romane, D. Falconieri, A. Piras and B. Marongiu, Nat. Prod. Res., 2013, 27, 1673–1676 CrossRef CAS PubMed.
- Y. Wang, A. L. Du and A. Q. Du, Adv. Mater. Res., 2012, 550–553, 1666–1670 CAS.
- R. H. Anderson and D. H. Wheeler, Oil Soap, 1945, 22, 137–141 CAS.
- E. O. Barnes, R. H. Potts and F. B. White, J. Am. Oil Chem. Soc., 1959, 36, 158–163 CrossRef CAS.
- R. L. Logan, J. Am. Oil Chem. Soc., 1979, 56, 777A–779A CrossRef CAS.
- M. I. Szynkowska, A. Jakubowska, E. Lesniewska and J. Goralski, Przem. Chem., 2014, 93, 1414–1417 CAS.
- J. Wang, R. Nie, L. Xu, X. Lyu and X. Lu, Green Chem., 2019, 21, 314–320 RSC.
- H. J. Jeon, J. S. Lee and Y. W. Kim, US Pat., US9845280B2, SK Innovation Co Ltd, SK Lubricants Co Ltd, 2017.
- D. E. Adelson and M. T. Bogert, Chem. Rev., 1939, 24, 135–176 CrossRef CAS.
- A. Bernas, T. Salmi, D. Y. Murzin, J.-P. Mikkola and M. Rintola, Top. Catal., 2012, 55, 673–679 CrossRef CAS.
- G. C. H. T. F. Sanderson, Org. Synth., 1952, 32, 1 CrossRef.
- M. T. Huang, C. T. Ho, Z. Y. Wang, T. Ferraro, Y. R. Lou, K. Stauber, W. Ma, C. Georgiadis, J. D. Laskin and A. H. Conney, Cancer Res., 1994, 54, 701–708 CAS.
- A.-H. Lo, Y.-C. Liang, S.-Y. Lin-Shiau, C.-T. Ho and J.-K. Lin, Carcinogenesis, 2002, 23, 983–991 CrossRef CAS PubMed.
- C. H. Brieskorn, A. Fuchs, J. B.-S. Bredenberg, J. D. McChesney and E. Wenkert, J. Org. Chem., 1964, 29, 2293–2298 CrossRef CAS.
- F. Jaillet, E. Darroman, A. Ratsimihety, B. Boutevin and S. Caillol, Green Mater., 2015, 3, 59–70 CrossRef.
- J. T. Sullivan, C. S. Richards, H. A. Lloyd and G. Krishna, Planta Med., 1982, 44, 175–177 CrossRef CAS PubMed.
- B. Prithiviraj, U. Singh, M. Manickam and A. Ray, Can. J. Bot., 1997, 75, 207–211 CrossRef CAS.
- J. Tyman, Chem. Soc. Rev., 1979, 8, 499–537 RSC.
- R. Paramashivappa, P. P. Kumar, P. Vithayathil and A. S. Rao, J. Agric. Food Chem., 2001, 49, 2548–2551 CrossRef CAS PubMed.
- J. Julis, S. A. Bartlett, S. Baader, N. Beresford, E. J. Routledge, C. S. Cazin and D. J. Cole-Hamilton, Green Chem., 2014, 16, 2846–2856 RSC.
- P. H. Gedam and P. S. Sampathkumaran, Prog. Org. Coat., 1986, 14, 115–157 CrossRef CAS.
- V. Fajardo, C. Cárcamo and B. R. Moreno, Heterocycles, 1996, 5, 949–951 CrossRef.
- M. Geffe and T. Opatz, Org. Lett., 2014, 16, 5282–5285 CrossRef CAS PubMed.
- A. Jakubska, D. Przado, M. Steininger, J. Aniol-Kwiatkowska and M. Kadej, Appl. Ecol. Environ. Res., 2005, 3, 29–38 CrossRef.
- S. Berenyi, C. Csutoras and A. Sipos, Curr. Med. Chem., 2009, 16, 3215–3242 CrossRef CAS PubMed.
- T. Hudlicky, Can. J. Chem., 2015, 93, 492–501 CrossRef CAS.
- N. Uematsu, A. Fujii, S. Hashiguchi, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1996, 118, 4916–4917 CrossRef CAS.
- G. J. Meuzelaar, M. C. A. van Vliet, L. Maat and R. A. Sheldon, Eur. J. Org. Chem., 1999, 2315–2321 CrossRef CAS.
- T. T. Pham, X. Chen, T. Söhnel, N. Yan and J. Sperry, Green Chem., 2020, 22, 1978–1984 RSC.
- P. J. Deuss, M. Scott, F. Tran, N. J. Westwood, J. G. de Vries and K. Barta, J. Am. Chem. Soc., 2015, 137, 7456–7467 CrossRef CAS PubMed.
- B. P. Feuston, J. C. Culberson, M. E. Duggan, G. D. Hartman, C.-T. Leu and S. B. Rodan, J. Med. Chem., 2002, 45, 5640–5648 CrossRef CAS PubMed.
- J. Jin and S. M. Weinreb, J. Am. Chem. Soc., 1997, 119, 2050–2051 CrossRef CAS.
- C. Guillou, J.-L. Beunard, E. Gras and C. Thal, Angew. Chem., Int. Ed., 2001, 40, 4745–4746 CrossRef CAS PubMed.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy and D. A. Perry, Green Chem., 2008, 10, 31–36 RSC.
Footnote |
| † Contributed equally, ordered alphabetically by last name. |
| This journal is © The Royal Society of Chemistry 2021 |