Open Access Article
Open Access ArticleFacile synthesis of highly conductive PEDOT:PSS via surfactant templates
Phimchanok Sakunpongpitiporna,
Katesara Phasuksoma,
Nophawan Paradeeb and
Anuvat Sirivat *a
*a
aThe Conductive and Electroactive Polymers Research Unit, The Petroleum and Petrochemical College, Chulalongkorn University, Bangkok 10330, Thailand. E-mail: anuvat.s@chula.ac.th
bDepartment of Chemistry, Faculty of Science, King Mongkut's University of Technology Thonburi, Bangkok, 10140, Thailand
First published on 21st February 2019
Abstract
Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) nanoparticles in powder form with high electrical conductivity were synthesized via chemical oxidative polymerization. In addition, the effects of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio, EDOT
PSS weight ratio, EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, and surfactant concentration and type, namely hexadecyltrimethylammonium bromide (CTAB), sodium dodecylsulfate (SDS), and polyoxyethylene octyl phenyl ether (Triton X-100) on the properties of PEDOT:PSS were investigated. For the effect of EDOT
Na2S2O8 mole ratio, and surfactant concentration and type, namely hexadecyltrimethylammonium bromide (CTAB), sodium dodecylsulfate (SDS), and polyoxyethylene octyl phenyl ether (Triton X-100) on the properties of PEDOT:PSS were investigated. For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio, at the EDOT
PSS weight ratio, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the EDOT
1, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 was the optimal condition to obtain electrical conductivity of 999.74 ± 10.86 S cm−1 due to the high amount of PSS− and SO42− available to interact with the PEDOT chain with a low % PSSNa. For the effect of EDOT
11 was the optimal condition to obtain electrical conductivity of 999.74 ± 10.86 S cm−1 due to the high amount of PSS− and SO42− available to interact with the PEDOT chain with a low % PSSNa. For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, at the EDOT
Na2S2O8 mole ratio, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, the EDOT
11, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was the best condition as it provided the highest dopant (PSS− and SO42−) amount, while the % PSSNa was relatively low. For the effect of surfactant type and concentration, at the EDOT
2 was the best condition as it provided the highest dopant (PSS− and SO42−) amount, while the % PSSNa was relatively low. For the effect of surfactant type and concentration, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Triton X-100 at 2.5CMC provided electrical conductivity higher than with CTAB and SDS. The thermal stability of PEDOT:PSS obtained from various conditions was investigated, and PEDOT:PSS without surfactant showed the highest thermal stability since it produced the highest char yield. In this study, the highest electrical conductivity of PEDOT:PSS, which was obtained in the presence of Triton X-100 to reduce the PSSNa amount, was 1879.49 ± 13.87 S cm−1, the highest value reported to date.
2, Triton X-100 at 2.5CMC provided electrical conductivity higher than with CTAB and SDS. The thermal stability of PEDOT:PSS obtained from various conditions was investigated, and PEDOT:PSS without surfactant showed the highest thermal stability since it produced the highest char yield. In this study, the highest electrical conductivity of PEDOT:PSS, which was obtained in the presence of Triton X-100 to reduce the PSSNa amount, was 1879.49 ± 13.87 S cm−1, the highest value reported to date.
1. Introduction
Conductive polymers (CPs) are organic polymers that are different from typical polymers because they can conduct electricity through their conjugated structures, consisting of alternating double (π) bonds and single (σ) bonds along the CP chains.1 CPs have sp2 hybridization in their backbones, in which the p-orbital in each atom is perpendicular to the plane of the polymer chain but parallel to each other, allowing electron delocalization along the polymer chains.2 The first conductive polyacetylene was discovered in 1977,3 and since then, many CPs have been intensively investigated. Some common and interesting CPs include polypyrrole (PPy), polyaniline (PANI), polythiophene (PT), trans-polyacetylene, poly(p-phenylene vinylene), and poly(3,4-ethylenedioxythiophene) (PEDOT).4 CPs have many advantageous properties such as chemical diversity, low density, flexibility, corrosion resistance, controllable morphology, and tunable conductivity.5,6 Therefore, CPs are utilized in many applications, such as polymer light-emitting diodes,7 organic transistors,8 actuators,9 anti-static coatings, sensors, batteries, solar cells,10 and drug delivery.11Poly(3,4-ethylenedioxythiophene) (PEDOT) is one of most studied conductive polymers owing to its relatively high electrical conductivity and electro-optical properties. PEDOT can be synthesized via both chemical oxidative polymerization and electrochemical polymerization. However, chemical oxidative polymerization provides a higher yield with no special setup required.12 Although PEDOT has high electrical conductivity, it is insoluble in water, making it difficult to process. This problem is overcome by using a polyelectrolyte, poly(styrenesulfonic acid) (PSS), which acts as a dopant and stabilizer for PEDOT through charge balance.13
Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) is a PEDOT derivative, which has higher electrical conductivity as compared to other CPs.14 Moreover, it has other useful properties, such as high transparency,15 low thermal conductivity, low density, good flexibility, and high thermal stability.16 In general, PEDOT:PSS is used in various electrical and optical devices, such as thin film transistors, light-emitting diodes, sensors, and photovoltaics.17
The electrical conductivity of PEDOT:PSS can be enhanced by solvent treatment,18 adding surfactant,19 and varying the PSS concentration.20 Ouyang et al. investigated the effect of organic solvent treatment using secondary dopants, such as acetonitrile (ACN), 4-methoxyphenol, N,N-dimethylacetamide (DMAc), N-methyl-2-pyrrolidone (NMP), ethylene glycol (EG), and dimethyl sulfoxide (DMSO) to increase the electrical conductivity of the PEDOT:PSS film. The highest electrical conductivity of a PEDOT:PSS film treated with DMSO was 200 S cm−1.18 Oh et al. studied the effect of Triton X-100 (nonionic surfactant) on the electrical conductivity of PEDOT:PSS films, which increased from 0.85 ± 0.08 to 882 ± 75 S cm−1 at a Triton X-100 concentration of 1.0 wt%.19 Horri et al. studied the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio on the electrical conductivity of PEDOT:PSS films, and an EDOT
PSS weight ratio on the electrical conductivity of PEDOT:PSS films, and an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 provided the highest electrical conductivity of 700 S cm−1.20
2.3 provided the highest electrical conductivity of 700 S cm−1.20
Alternatively, PEDOT:PSS can be prepared in powder form, which can be subsequently modified and processed for various applications, such as proton exchange membrane fuel cells,21 actuators,22 and sensors.23 Lefebvre et al. studied the effect of the PEDOT:PSS weight ratio in the range of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 to 1
2.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5, where the electrical conductivity of the PEDOT:PSS powder varied from 0.3 to 1.3 S cm−1.24 Wichaiansee et al. synthesized PEDOT:PSS powder via chemical oxidative polymerization at room temperature using sodium persulfate (Na2S2O8) as the oxidizing agent and ferric sulphate (Fe2(SO4)3) as the catalyst. The synthesized PEDOT:PSS powder possessed electrical conductivity of 27.5 ± 0.6 S cm−1.22 Chanthanont et al. synthesized PEDOT:PSS powder using Na2S2O8 as the oxidizing agent and Fe2(SO4)3 as the catalyst in aqueous solution at room temperature, and the electrical conductivity of the PEDOT:PSS powder was determined to be 11.69 ± 0.006 S cm−1.23 In summary, it can be noted that PEDOT:PSS in the powder form has relatively lower electrical conductivity than that of its films, as previously reported.
7.5, where the electrical conductivity of the PEDOT:PSS powder varied from 0.3 to 1.3 S cm−1.24 Wichaiansee et al. synthesized PEDOT:PSS powder via chemical oxidative polymerization at room temperature using sodium persulfate (Na2S2O8) as the oxidizing agent and ferric sulphate (Fe2(SO4)3) as the catalyst. The synthesized PEDOT:PSS powder possessed electrical conductivity of 27.5 ± 0.6 S cm−1.22 Chanthanont et al. synthesized PEDOT:PSS powder using Na2S2O8 as the oxidizing agent and Fe2(SO4)3 as the catalyst in aqueous solution at room temperature, and the electrical conductivity of the PEDOT:PSS powder was determined to be 11.69 ± 0.006 S cm−1.23 In summary, it can be noted that PEDOT:PSS in the powder form has relatively lower electrical conductivity than that of its films, as previously reported.
Herein, we report the synthesis of PEDOT:PSS nanoparticles with high electrical conductivity via chemical oxidative polymerization in aqueous solution at room temperature. The effects of the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio, EDOT
PSS weight ratio, EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, and surfactant type and concentration on the properties of PEDOT:PSS were systematically investigated. The PEDOT:PSS powder samples were characterized via Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy (Raman), and wide-angle X-ray spectroscopy (XRD) to determine their chemical structure, X-ray photoelectron spectroscopy (XPS) to analyze their element contents, UV-Vis spectrophotometry (UV-Vis) to determine their doping state, and field-emission scanning electron microscopy (FE-SEM) to determine their particle shape and size. Thermogravimetric analysis (TG-DTA) was used to determine their thermal stability. It was found that the PEDOT:PSS powder with the EDOT
Na2S2O8 mole ratio, and surfactant type and concentration on the properties of PEDOT:PSS were systematically investigated. The PEDOT:PSS powder samples were characterized via Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy (Raman), and wide-angle X-ray spectroscopy (XRD) to determine their chemical structure, X-ray photoelectron spectroscopy (XPS) to analyze their element contents, UV-Vis spectrophotometry (UV-Vis) to determine their doping state, and field-emission scanning electron microscopy (FE-SEM) to determine their particle shape and size. Thermogravimetric analysis (TG-DTA) was used to determine their thermal stability. It was found that the PEDOT:PSS powder with the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, EDOT
11, EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and Triton X-100 at the concentration of 2.5CMC possessed the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 with the corresponding spherical particle size of 56.77 ± 5.54 nm, values not previously obtained to date.
2, and Triton X-100 at the concentration of 2.5CMC possessed the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 with the corresponding spherical particle size of 56.77 ± 5.54 nm, values not previously obtained to date.
2. Experimental
2.1 Materials
3,4-Ethylenedioxythiophene (EDOT, 97% purity) monomer, poly(styrenesulfonate) (PSS, 99% purity) with Mw of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, hexadecyltrimethylammonium bromide (CTAB, ≥96% purity), and sodium persulfate (Na2S2O8, ≥98% purity) were purchased from Sigma Aldrich. Sodium dodecylsulfate (SDS, ≥99% purity) and polyoxyethylene octyl phenyl ether (Triton X-100, ≥99% purity) were purchased from Omnipur. Methanol (>99.8% purity) and acetone (99.5% purity) were purchased from RCI Labscan. Distillated water was used as the solvent in the synthesis. All reagents were of analytical reagent grade.
000 g mol−1, hexadecyltrimethylammonium bromide (CTAB, ≥96% purity), and sodium persulfate (Na2S2O8, ≥98% purity) were purchased from Sigma Aldrich. Sodium dodecylsulfate (SDS, ≥99% purity) and polyoxyethylene octyl phenyl ether (Triton X-100, ≥99% purity) were purchased from Omnipur. Methanol (>99.8% purity) and acetone (99.5% purity) were purchased from RCI Labscan. Distillated water was used as the solvent in the synthesis. All reagents were of analytical reagent grade.
2.2 Synthesis of PEDOT:PSS
To investigate the effect of the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio, 0.5 g of EDOT was mixed with PSS ranging from 0.5 g to 6.5 g (weight ratios of 1–13), where the Na2S2O8 content as the oxidant was fixed at 0.8335 g to give the EDOT
PSS weight ratio, 0.5 g of EDOT was mixed with PSS ranging from 0.5 g to 6.5 g (weight ratios of 1–13), where the Na2S2O8 content as the oxidant was fixed at 0.8335 g to give the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ingredients were dissolved in 100 mL of DI water, then the solution was stirred for 24 h at room temperature. The precipitate was collected by centrifugation at 9000 rpm, rinsed with a mixed solution of acetone
1. The ingredients were dissolved in 100 mL of DI water, then the solution was stirred for 24 h at room temperature. The precipitate was collected by centrifugation at 9000 rpm, rinsed with a mixed solution of acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol with a volume ratio of 3
methanol with a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20,25 and then dried in an oven at 60 °C for 24 h.
20,25 and then dried in an oven at 60 °C for 24 h.
To determine the effect of the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, the synthesis procedure was the same as before. 0.83, 1.25, 1.67, 2.08, 2.50, and 3.33 g of Na2S2O8 were added to 0.5 g of EDOT to give the EDOT
Na2S2O8 mole ratio, the synthesis procedure was the same as before. 0.83, 1.25, 1.67, 2.08, 2.50, and 3.33 g of Na2S2O8 were added to 0.5 g of EDOT to give the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios of 1
Na2S2O8 mole ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 1
2.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and the EDOT
4, and the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio was fixed at 1
PSS weight ratio was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (0.5 g
11 (0.5 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 g).
5.5 g).
To study the effect of surfactant type, namely CTAB, SDS, and Triton X-100, and concentration, each surfactant (0.084 g, 0.721 g, 0.141 g, respectively) was added to 100 mL of distilled water and stirred for 1 h to from a surfactant solution at 2.5CMC. Then 0.5 g of EDOT was added in the surfactant solution, which was continuously stirred for 1 h. Then PSS (5.5 g) was added to the above solution, and it was stirred for 1 h before the Na2S2O8 oxidant (1.67 g) was added. The EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio was fixed at 1
PSS weight ratio was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and the EDOT
11 and the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio was 1
Na2S2O8 mole ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The solution was stirred continuously for 24 h at room temperature, and the color of the solution changed from clear to dark blue. The precipitate was centrifuged at 9000 rpm and then washed with a solution of acetone
2. The solution was stirred continuously for 24 h at room temperature, and the color of the solution changed from clear to dark blue. The precipitate was centrifuged at 9000 rpm and then washed with a solution of acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol at the volume ratio of 3
methanol at the volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Finally, the precipitate was dried in an oven at 60 °C for 24 h. Next, the PEDOT:PSS precipitate was ground in a mortar by hand for 3 min to obtain the PEDOT:PSS powder with smaller particle sizes, which was further modified for characterization.26 Lislie et al. reported that grinding PPy with a mortar and pestle for 10 min did not significantly change its electrical conductivity.26 However, Fufang et al. reported that the electrical conductivity of PPy decreased by 21% and the particle size decreased by 26% after grinding in a mortar for 1 h.27
20. Finally, the precipitate was dried in an oven at 60 °C for 24 h. Next, the PEDOT:PSS precipitate was ground in a mortar by hand for 3 min to obtain the PEDOT:PSS powder with smaller particle sizes, which was further modified for characterization.26 Lislie et al. reported that grinding PPy with a mortar and pestle for 10 min did not significantly change its electrical conductivity.26 However, Fufang et al. reported that the electrical conductivity of PPy decreased by 21% and the particle size decreased by 26% after grinding in a mortar for 1 h.27
2.3 Characterization of PEDOT:PSS nanoparticles
The critical micelle concentration (CMC) of PSS was determined by measuring its surface tension using a tensiometer (Kruss/Easydyne tensiometer, K20) with the Wilhelmy plate mode at 23 °C. PSS was dissolved in deionized water at various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios. The CMC of PSS was found to be 7 × 10−5 mol L−1.
PSS weight ratios. The CMC of PSS was found to be 7 × 10−5 mol L−1.
The functional group analysis was carried out via Fourier transformed infrared spectroscopy, FTIR (Thermo Nicolet, Nexus 670). All spectra were recorded in the wavenumber range of 400–4000 cm−1, with 32 scans and a resolution of 4 cm−1. The PEDOT:PSS samples were mixed with background KBr powder and compressed using a hydraulic press machine.
The chemical and structural information of the PEDOT:PSS powder were identified via Raman spectroscopy (Bruker, Ram II) with a laser source at 1064 nm and power of 22 mW. All spectra were recorded in the wavenumber range of 400–4000 cm−1, with 800 scans and a resolution of 4 cm−1.
The PEDOT:PSS crystalline structure was identified via wide-angle X-ray spectroscopy, XRD (Rigaku/Smartlab), at a scan step of 0.02° and scan speed of 5° min−1 in the 2θ range of 5° to 70°. The Cu-Kα radiation source was operated at 40 kV/30 mA. The PDLX 2 software was used to analyze the PEDOT:PSS XRD patterns.
The element analysis was carried out via X-ray photoelectron spectroscopy, XPS (Kratos, Axis Ultra DLD), using a monochromatized Al Kα radiation source and recorded at the analyzer pass energy of 160 eV for the survey scan, and at 40 eV for the high-resolution scan. All spectra were corrected by using the reference C 1s (binding energy of 284.8 eV). The Casa-XPS software was used for the interpretation of the XPS spectra.
The doping state and optical band gap of PEDOT:PSS was identified via UV-Vis spectrophotometry, UV-Vis (Tecan, The Infinite® 200 PRO NanoQuant). The PEDOT:PSS powders were dissolved in deionized water and filtered using a nylon filter. Deionized water was used as the reference. The i-Control software was used to determine the UV adsorption of the PEDOT:PSS solutions. The band gap energy was calculated using the Tauc eqn (1):28
| αhν = A(hν − Eg)n | (1) |
| α = 2.303Ab/I | (2) |
The morphology of PEDOT:PSS was identified via field-emission scanning electron microscopy, FE-SEM (Hitachi, S-4800), operating at 5 kV/10 μA at a magnification of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Each sample was distributed on the sample holder with a carbon adhesive tape and coated with a thin layer of platinum before the measurement.
000. Each sample was distributed on the sample holder with a carbon adhesive tape and coated with a thin layer of platinum before the measurement.
The thermal analysis was carried out using a thermogravimetric analyzer, TG-DTA (Perkin Elmer, TGA model 7). 4 to 8 mg of each sample was loaded into an aluminum pan. The sample was scanned from 30 °C to 800 °C at a heating rate of 10 °C min−1 under a nitrogen flow.
The electrical conductivity was measured using an electrometer (Keithley, model 17A) at room temperature in air. A custom-built two-point probe was used as a fixture for each PEDOT:PSS pellet. The graph between I (y-axis) and V (x-axis) was plotted to obtain the I–V slope, which was used to calculate the electrical conductivity according to eqn (3):30
| σ = I/KVt = (I–V) slope/Kt | (3) |
3. Results and discussion
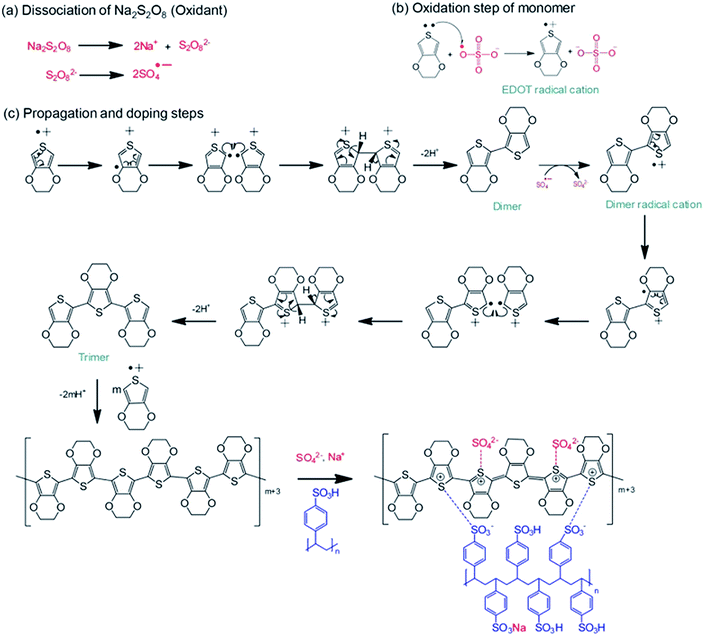
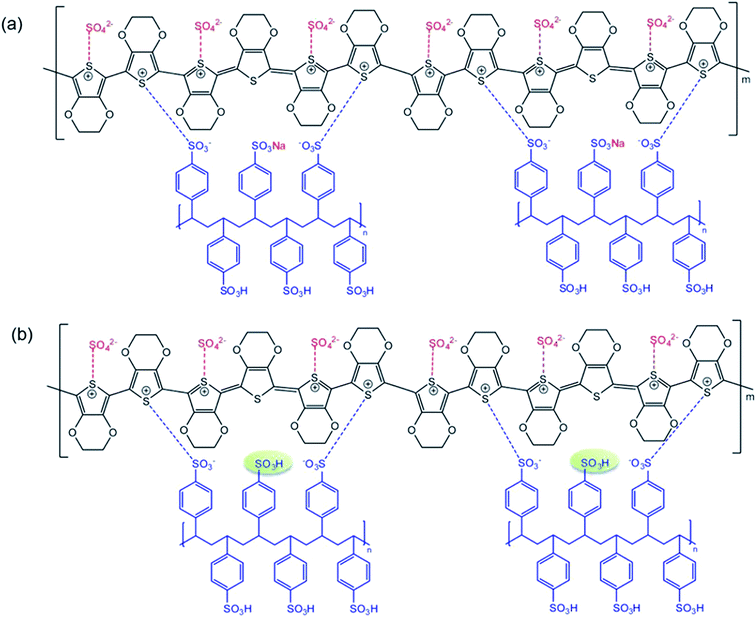
The PEDOT:PSS powder was synthesized via chemical oxidative polymerization following the procedure shown in Scheme 1. In the initial step, Na2S2O8 as the oxidant dissociates into sodium ions and a persulfate ion, where the persulfate ion can homolytically dissociate into sulfate radicals in aqueous solution, as shown in Scheme 1(a).31 In the oxidation step, an EDOT monomer is oxidized to an EDOT radical cation by the sulfate radical, leading to the formation of sulfate anions, as shown in Scheme 1(b).32 For the propagation and doping step, the coupling of EDOT radical cations generates two protons, which are removed in this step. Simultaneously, the PSS and sulfate ions acting as dopants can interact with the oxidized PEDOT chains during the polymerization. The sodium ion from the oxidant can also react with the PSS chains, as illustrated in Scheme 1(c).3.1 Structural conformation of PEDOT:PSS
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 shows peaks at 1640 and 1518 cm−1, which are assigned to the C
2 shows peaks at 1640 and 1518 cm−1, which are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in the aromatic rings of PSS33 and the C
C stretching in the aromatic rings of PSS33 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in the thiophene ring of PEDOT,34 respectively. The peak at 1340 cm−1 indicates the C–C stretching from the thiophene ring of PEDOT.34 The symmetric stretching and antisymmetric stretching of S
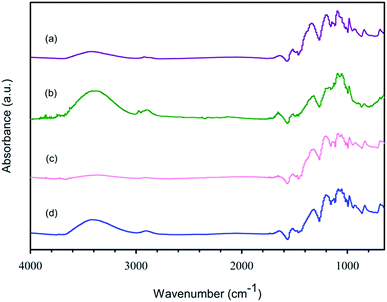
C stretching in the thiophene ring of PEDOT,34 respectively. The peak at 1340 cm−1 indicates the C–C stretching from the thiophene ring of PEDOT.34 The symmetric stretching and antisymmetric stretching of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O can be seen at 1198 and 1055 cm−1, respectively, belonging to PSS and SO42− from the oxidant.34 The peaks at 1145 and 983 cm−1 can be attributed to the S–O stretching in PSS and SO42− from the oxidant and the S–phenyl bond in PSS, respectively.35 The three peaks at 936, 840, and 691 cm−1 correspond to the C–S stretching of the thiophene ring in PEDOT.35 The two peaks at 1144 and 1092 cm−1 are correspond to the C–O stretching of PEDOT.23 The peaks at 3415, and 2921 cm−1 correspond to the O–H stretching of PSS and C–H stretching of PEDOT and PSS, respectively, resulting from PSS incorporated in the PEDOT chain.34 For the PEDOT:PSS synthesized without surfactant at various EDOT
O can be seen at 1198 and 1055 cm−1, respectively, belonging to PSS and SO42− from the oxidant.34 The peaks at 1145 and 983 cm−1 can be attributed to the S–O stretching in PSS and SO42− from the oxidant and the S–phenyl bond in PSS, respectively.35 The three peaks at 936, 840, and 691 cm−1 correspond to the C–S stretching of the thiophene ring in PEDOT.35 The two peaks at 1144 and 1092 cm−1 are correspond to the C–O stretching of PEDOT.23 The peaks at 3415, and 2921 cm−1 correspond to the O–H stretching of PSS and C–H stretching of PEDOT and PSS, respectively, resulting from PSS incorporated in the PEDOT chain.34 For the PEDOT:PSS synthesized without surfactant at various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios and EDOT
PSS weight ratios and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios, their FTIR spectra showed the same functional groups as that with the EDOT
Na2S2O8 mole ratios, their FTIR spectra showed the same functional groups as that with the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The FTIR spectra of the synthesized PEDOT:PSS (Fig. 1(a)) are similar to the PEDOT:PSS spectrum reported by Chanthanont and Sirivat, 2013.23
2. The FTIR spectra of the synthesized PEDOT:PSS (Fig. 1(a)) are similar to the PEDOT:PSS spectrum reported by Chanthanont and Sirivat, 2013.23
The FTIR spectrum of PEDOT:PSS synthesized in CTAB (Fig. 1(b)) shows peaks at 2973 and 2898 cm−1, indicating the CH3–N+ anti-symmetric stretching and C–H stretching, respectively, which confirm the incorporation of CTAB in PEDOT:PSS.36 In the case of PEDOT:PSS prepared with SDS (Fig. 1(c)), the O–H stretching peak shifts from 3415 to 3339 cm−1 because SDS can repel the O–H of PSS by electro-repulsive forces.37 For the system of Triton X-100, the FTIR spectrum of PEDOT:PSS (Fig. 1(d)) is the same as that without surfactant, and it does not show any characteristic peaks of Triton X-100. Thus, Triton X-100 was not incorporated in PEDOT:PSS.
For the PEDOT:PSS synthesized at various CTAB concentrations, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the spectra show the CH3–N+ anti-symmetric stretching of CTAB at 2973 cm−1 and the C–H stretching peak at 2898 cm−1,36 where the intensity of the former increased with an increase in CTAB concentration. In the case of various SDS concentrations, the intensity of the O–H stretching peak of SDS located at 3339 cm−1 (ref. 38) increased with an increase in SDS concentration. Under various Triton X-100 concentrations, the FT-IR spectra of PEDOT:PSS were nearly the same with an increase in Triton X-100 concentration.
2, the spectra show the CH3–N+ anti-symmetric stretching of CTAB at 2973 cm−1 and the C–H stretching peak at 2898 cm−1,36 where the intensity of the former increased with an increase in CTAB concentration. In the case of various SDS concentrations, the intensity of the O–H stretching peak of SDS located at 3339 cm−1 (ref. 38) increased with an increase in SDS concentration. Under various Triton X-100 concentrations, the FT-IR spectra of PEDOT:PSS were nearly the same with an increase in Triton X-100 concentration.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios of 1
PSS weight ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, and 1
11, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and EDOT
13 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio fixed at 1
Na2S2O8 mole ratio fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were characterized using the FT Raman technique. The FT Raman spectra showed the dominant peak at 1424 cm−1, indicative of the Cα–Cβ stretching vibration mode of the quinoid structure in PEDOT.39 The quinoid structure represents the linear structure or the expanded-coil of the PEDOT:PSS chain, which lies on the same plane. Hence, the conjugated π-electrons can delocalize in the PEDOT chain.40 The PEDOT:PSS synthesized at the EDOT
1 were characterized using the FT Raman technique. The FT Raman spectra showed the dominant peak at 1424 cm−1, indicative of the Cα–Cβ stretching vibration mode of the quinoid structure in PEDOT.39 The quinoid structure represents the linear structure or the expanded-coil of the PEDOT:PSS chain, which lies on the same plane. Hence, the conjugated π-electrons can delocalize in the PEDOT chain.40 The PEDOT:PSS synthesized at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton-X 100 (2.5CMC) was also characterized via FT-Raman spectroscopy and it was observed that the FT-Raman spectrum with the surfactant was similar to that without surfactant, indicating the same quinoid structure in the PEDOT chain.
2 with Triton-X 100 (2.5CMC) was also characterized via FT-Raman spectroscopy and it was observed that the FT-Raman spectrum with the surfactant was similar to that without surfactant, indicating the same quinoid structure in the PEDOT chain.3.2 X-ray diffraction
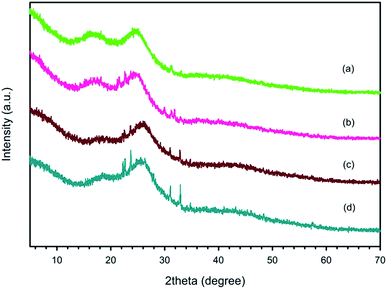
The XRD patterns of PEDOT:PSS with different EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios at the EDOT
PSS weight ratios at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are shown in Fig. 2. The two main peaks at 2θ = 17.7° and 25.8° refer to the amorphous halo diffraction of PSS and the inter-chain packing of PEDOT, respectively.41 At the EDOT
1 are shown in Fig. 2. The two main peaks at 2θ = 17.7° and 25.8° refer to the amorphous halo diffraction of PSS and the inter-chain packing of PEDOT, respectively.41 At the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios ranging from 1
PSS weight ratios ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to 1
9 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, the peaks in the XRD patterns show a shift from 25.5° to 25.8°, which is consistent with the smaller d-spacings between the PEDOT chains ranging from 3.48 Å to 3.45 Å. However, the intensities of the crystalline peaks of EDOT
11, the peaks in the XRD patterns show a shift from 25.5° to 25.8°, which is consistent with the smaller d-spacings between the PEDOT chains ranging from 3.48 Å to 3.45 Å. However, the intensities of the crystalline peaks of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS at the weight ratio of 1
PSS at the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 were lower than that of the other EDOT
11 were lower than that of the other EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios, suggesting that the PEDOT:PSS at this condition possessed a lower PSSNa content. PSSNa is a salt that reduces the available doping sites on PSS, and hence induces charge screening between PEDOT and PSS.42 Zotti et al., 2003, reported that the electrical conductivity of PEDOT:PSS was reduced due the presence of PSSNa in PEDOT:PSS.43
PSS weight ratios, suggesting that the PEDOT:PSS at this condition possessed a lower PSSNa content. PSSNa is a salt that reduces the available doping sites on PSS, and hence induces charge screening between PEDOT and PSS.42 Zotti et al., 2003, reported that the electrical conductivity of PEDOT:PSS was reduced due the presence of PSSNa in PEDOT:PSS.43
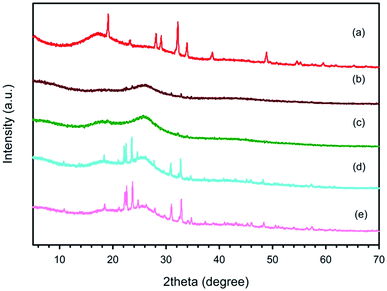
Fig. 3 illustrates the XRD patterns of commercial PSSNa and PEDOT:PSS synthesized using various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios at the EDOT
Na2S2O8 mole ratios at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11. The PSSNa XRD pattern showed crystalline peaks at 19.1°, 23.2°, 28.1°, 29.1°, 32.2°, 34.0°, 38.7° and 48.9°.44 The two main peaks of PEDOT:PSS are evident at 2θ = 17.7° and 2θ = 25.8°, similar to the results in Fig. 2. However, the intensities of the crystalline peak of PSSNa incorporated in PEDOT:PSS tended to increase with an increase in EDOT
11. The PSSNa XRD pattern showed crystalline peaks at 19.1°, 23.2°, 28.1°, 29.1°, 32.2°, 34.0°, 38.7° and 48.9°.44 The two main peaks of PEDOT:PSS are evident at 2θ = 17.7° and 2θ = 25.8°, similar to the results in Fig. 2. However, the intensities of the crystalline peak of PSSNa incorporated in PEDOT:PSS tended to increase with an increase in EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio because sodium ions from the oxidant are more prone to interact with PSS molecules by electrostatic interaction.44 The increase in PSSNa with an increase in EDOT
Na2S2O8 mole ratio because sodium ions from the oxidant are more prone to interact with PSS molecules by electrostatic interaction.44 The increase in PSSNa with an increase in EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio was further investigated by XPS.
Na2S2O8 mole ratio was further investigated by XPS.
The PEDOT:PSS synthesized using various surfactant types and concentrations at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were also investigated via XRD. The obtained XRD patterns were nearly the same as that without surfactant, suggesting the surfactants did not affect the crystallinity of PEDOT:PSS.
2 were also investigated via XRD. The obtained XRD patterns were nearly the same as that without surfactant, suggesting the surfactants did not affect the crystallinity of PEDOT:PSS.
3.3 X-ray photoelectron spectroscopy
The element analysis and chemical bonding of the synthesized PEDOT:PSS were characterized by XPS. The wide-scan XPS spectra of PEDOT:PSS revealed the peaks of Na 1s, O 1s, C 1s, and S 2p located at 1066.0, 527.0, 280.0, and 163.0 eV, respectively. The existence of Na in PSSNa44 is due to the interaction of PSS− and Na+ from the oxidant. The high-resolution scan XPS spectra of S 2p showed the deconvolution of S 2p of PEDOT:PSS, which indicated the C–S of PEDOT was located at 163.22 and 164.46 eV,45 S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of SO42− from the oxidant and S
O of SO42− from the oxidant and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of SO3− from PSS at 168.21 and 169.10 eV,46,47 respectively, and S
O of SO3− from PSS at 168.21 and 169.10 eV,46,47 respectively, and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O from PSSNa at 169.81 and 170.59 eV.48,49 The element and chemical bonding of PEDOT (without PSS) was also investigated via XPS for comparison with PEDOT:PSS. The wide-scan XPS spectrum of pure PEDOT showed the elements C 1s, O 1s, and S 2p without Na. Thus, it can be confirmed that PSS is the main component inducing the formation of PSSNa. The deconvolution of S 2p indicated the C–S of the pure PEDOT at 163.22 and 164.46 eV,50 and S
O from PSSNa at 169.81 and 170.59 eV.48,49 The element and chemical bonding of PEDOT (without PSS) was also investigated via XPS for comparison with PEDOT:PSS. The wide-scan XPS spectrum of pure PEDOT showed the elements C 1s, O 1s, and S 2p without Na. Thus, it can be confirmed that PSS is the main component inducing the formation of PSSNa. The deconvolution of S 2p indicated the C–S of the pure PEDOT at 163.22 and 164.46 eV,50 and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of SO42− from the oxidant at 167.53 and 168.59 eV,47 suggesting that SO42− from the oxidant acts as a dopant by interacting with PEDOT through electrostatic interaction.12 The S
O of SO42− from the oxidant at 167.53 and 168.59 eV,47 suggesting that SO42− from the oxidant acts as a dopant by interacting with PEDOT through electrostatic interaction.12 The S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of PSSNa was not found in the S 2p deconvolution of the pure PEDOT (without PSS), while it was present for PEDOT:PSS. The atom percentages of the sulphur species from PEDOT, PSS−, SO42− and PSSNa are tabulated in Table 1, where both PSS− and SO42− act as dopants for the oxidized PEDOT chain.
O of PSSNa was not found in the S 2p deconvolution of the pure PEDOT (without PSS), while it was present for PEDOT:PSS. The atom percentages of the sulphur species from PEDOT, PSS−, SO42− and PSSNa are tabulated in Table 1, where both PSS− and SO42− act as dopants for the oxidized PEDOT chain.
| Sample code | % PEDOT | % PSS− and % SO42− | % PSSNa |
|---|---|---|---|
| Effect of PSS (no surfactant) | |||
| Pure PEDOT (without PSS) | 85.41 | 14.59 | — |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and EDOT 1 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
62.96 | 21.23 | 15.89 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31.47 | 45.57 | 22.96 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and EDOT 13 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
36.43 | 39.89 | 23.68 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Effect of oxidant (no surfactant) | |||
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
31.47 | 45.57 | 22.96 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
29.05 | 50.59 | 20.36 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
19.56 | 35.27 | 45.13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Effect of surfactant | |||
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at CMC) 2 with Triton X-100 (at CMC) |
27.80 | 49.78 | 22.42 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 2.5CMC) 2 with Triton X-100 (at 2.5CMC) |
45.40 | 37.52 | 17.08 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 5CMC) 2 with Triton X-100 (at 5CMC) |
42.53 | 37.42 | 20.05 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 10CMC) 2 with Triton X-100 (at 10CMC) |
47.81 | 36.46 | 19.22 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with CTAB (at 2.5CMC) 2 with CTAB (at 2.5CMC) |
15.34 | 30.57 | 54.09 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with SDS (at 2.5CMC) 2 with SDS (at 2.5CMC) |
45.82 | 34.32 | 19.87 |
For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios, at the EDOT
PSS weight ratios, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Table 1 shows that the % PSS− and % SO42− tended to increase with an increase in EDOT
1, Table 1 shows that the % PSS− and % SO42− tended to increase with an increase in EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio because PSS− acts as a dopant. However, at the EDOT
PSS weight ratio because PSS− acts as a dopant. However, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, the decrease in % PSS− and % SO42− was due to the over-doping or excessive PSS.51 In the present work, the PEDOT:PSS synthesized at the EDOT
13, the decrease in % PSS− and % SO42− was due to the over-doping or excessive PSS.51 In the present work, the PEDOT:PSS synthesized at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 possessed the highest % PSS− and % SO42− compared to other EDOT
11 possessed the highest % PSS− and % SO42− compared to other EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios and with a low % PSSNa. Thus, the high amount of dopants (% PSS− and % SO42−) is available to provide a high number of charge carriers.52 On the other hand, PSSNa reduces the number of doping sites on PSS, and thus induces charge screening between the PEDOT and PSS chains.42 Therefore, EDOT
PSS weight ratios and with a low % PSSNa. Thus, the high amount of dopants (% PSS− and % SO42−) is available to provide a high number of charge carriers.52 On the other hand, PSSNa reduces the number of doping sites on PSS, and thus induces charge screening between the PEDOT and PSS chains.42 Therefore, EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS at the weight ratio of 1
PSS at the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 is optimal for the synthesis of PEDOT:PSS.
11 is optimal for the synthesis of PEDOT:PSS.
For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, at the EDOT
Na2S2O8 mole ratio, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, the EDOT
11, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 possessed the highest % PSS− and % SO42− (50.59%), while the % PSSNa was relatively low (20.36%), as shown in Table 1. The increase in % PSS− and % SO42− is because the SO42− ions from the oxidant prefer to interact with the PEDOT chain. However, at a higher EDOT
2 possessed the highest % PSS− and % SO42− (50.59%), while the % PSSNa was relatively low (20.36%), as shown in Table 1. The increase in % PSS− and % SO42− is because the SO42− ions from the oxidant prefer to interact with the PEDOT chain. However, at a higher EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, the PSSNa content tends to increase as Na+ ions from the oxidant largely interact with PSS− to form PSSNa.44 This suggests that the PEDOT:PSS interaction is reduced by the existence of PSSNa via a reduction in doping sites and its screening. Thus, the EDOT
Na2S2O8 mole ratio, the PSSNa content tends to increase as Na+ ions from the oxidant largely interact with PSS− to form PSSNa.44 This suggests that the PEDOT:PSS interaction is reduced by the existence of PSSNa via a reduction in doping sites and its screening. Thus, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is optimal since it provided the highest dopant content (% PSS− and % SO42−), while % PSSNa is relatively low.
2 is optimal since it provided the highest dopant content (% PSS− and % SO42−), while % PSSNa is relatively low.
For the effect of surfactant type, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and the EDOT
11 and the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, each surfactant differently affected the presence of sulphur species in PEDOT:PSS. The PEDOT:PSS synthesized with CTAB (2.5CMC) possessed a lower % PSS− and % SO42− than that with SDS and Triton X-100, respectively, as shown in Table 1. CTAB is a cationic surfactant, which can interact with PSS− (negatively charged) via electrostatic interaction, and thus reduces the interaction between PEDOT and PSS.53 For the SDS system (2.5CMC), SDS is an anionic surfactant, which prefers to interact with PEDOT instead of PSS−,37 resulting in a higher % PEDOT than that without surfactant. For the Triton X-100 system (2.5CMC), the PEDOT:PSS possessed a higher % PSS− and % SO42− and a lower % PSSNa than that with the other two surfactants.
2, each surfactant differently affected the presence of sulphur species in PEDOT:PSS. The PEDOT:PSS synthesized with CTAB (2.5CMC) possessed a lower % PSS− and % SO42− than that with SDS and Triton X-100, respectively, as shown in Table 1. CTAB is a cationic surfactant, which can interact with PSS− (negatively charged) via electrostatic interaction, and thus reduces the interaction between PEDOT and PSS.53 For the SDS system (2.5CMC), SDS is an anionic surfactant, which prefers to interact with PEDOT instead of PSS−,37 resulting in a higher % PEDOT than that without surfactant. For the Triton X-100 system (2.5CMC), the PEDOT:PSS possessed a higher % PSS− and % SO42− and a lower % PSSNa than that with the other two surfactants.
For effect of Triton X-100 concentration, as shown in Table 1, Triton X-100 forms micelles at its CMC,30 but they are unstable. At high Triton X-100 concentrations of 2.5CMC, 5CMC and 10CMC, the % PSSNa was lower, while % PSS− and % SO42− decreased with an increase in concentration. Triton X-100 is a non-ionic surfactant that can interact with both PEDOT and PSS. However, the resulting TX-PSS complex can be easily removed by washing with methanol,54 resulting in the simultaneous removal of PSS− as a dopant and PSSNa as a salt. This suggests that Triton X-100 at higher concentrations than 2.5CMC reduces the PEDOT and PSS interaction as well as the PSSNa amount.
3.4 UV-visible spectroscopy
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
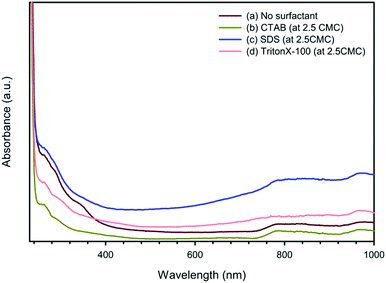
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with surfactants (at 2.5CMC) and without surfactant are shown in Fig. 4. All the UV spectra show a UV absorbance at 258 nm, which can be assigned to the substituted phenyl groups in PSS.55 The broad bands between 600 and 900 nm and between 700 to 1000 nm can be ascribed to the polaron and bipolaron states of PEDOT:PSS, respectively, indicative of the characteristic doped state of PEDOT:PSS.25
2 with surfactants (at 2.5CMC) and without surfactant are shown in Fig. 4. All the UV spectra show a UV absorbance at 258 nm, which can be assigned to the substituted phenyl groups in PSS.55 The broad bands between 600 and 900 nm and between 700 to 1000 nm can be ascribed to the polaron and bipolaron states of PEDOT:PSS, respectively, indicative of the characteristic doped state of PEDOT:PSS.25
For the effects of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio and EDOT
PSS weight ratio and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, the characteristics of the PEDOT:PSS UV-spectra were nearly the same with an increase in EDOT
Na2S2O8 mole ratio, the characteristics of the PEDOT:PSS UV-spectra were nearly the same with an increase in EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio or increase in EDOT
PSS weight ratio or increase in EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, consistent with previous work of Khan and Narula, 2016.25
Na2S2O8 mole ratio, consistent with previous work of Khan and Narula, 2016.25
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios, the band gap energy of PEDOT:PSS prepared at the EDOT
PSS weight ratios, the band gap energy of PEDOT:PSS prepared at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (2.92 eV) WAS higher than that for the EDOT
5 (2.92 eV) WAS higher than that for the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (2.67 eV). This indicates that at a higher PSS content, a larger number of SO3− groups from PSS is available to interact with PEDOT.13 However, at the EDOT
11 (2.67 eV). This indicates that at a higher PSS content, a larger number of SO3− groups from PSS is available to interact with PEDOT.13 However, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, the band gap energy is higher at 2.72 eV due to the over-doping.51
13, the band gap energy is higher at 2.72 eV due to the over-doping.51
| Sample code | Eg (eV) |
|---|---|
| Effect of PSS (no surfactant) | |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and EDOT 5 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.92 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.67 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and EDOT 13 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.72 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Effect of oxidant (no surfactant) | |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.67 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1.90 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2.95 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Effect of surfactant | |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at CMC) 2 with Triton X-100 (at CMC) |
2.39 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 2.5CMC) 2 with Triton X-100 (at 2.5CMC) |
1.80 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 3.5CMC) 2 with Triton X-100 (at 3.5CMC) |
2.83 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with CTAB (at 2.5CMC) 2 with CTAB (at 2.5CMC) |
3.50 |
EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with SDS (at 2.5CMC) 2 with SDS (at 2.5CMC) |
3.00 |
For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, at the EDOT
Na2S2O8 mole ratio, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, the band gap energy of the EDOT
11, the band gap energy of the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 possessed the lowest value of 1.90 eV. This is due to the SO42− ions from the oxidant interacting with the PEDOT chains as a dopant. This result is consistent with the increments in % PSS− and % SO42− determined by XPS. The doping process generally reduces the band gap energy between the HOMO and the LUMO to a level between 1–4 eV, as reported by Kar et al., 2013.2 However, the band gap energy for the EDOT
2 possessed the lowest value of 1.90 eV. This is due to the SO42− ions from the oxidant interacting with the PEDOT chains as a dopant. This result is consistent with the increments in % PSS− and % SO42− determined by XPS. The doping process generally reduces the band gap energy between the HOMO and the LUMO to a level between 1–4 eV, as reported by Kar et al., 2013.2 However, the band gap energy for the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 is larger because a large number of Na+ ions from the oxidant can interact with the PSS− chains to form PSSNa,44 which reduces the amount of doping sites on PSS. This result is consistent with the XPS result, indicating an increase in PSSNa, and the XRD result showing the highly crystalline peaks of PSSNa.
4 is larger because a large number of Na+ ions from the oxidant can interact with the PSS− chains to form PSSNa,44 which reduces the amount of doping sites on PSS. This result is consistent with the XPS result, indicating an increase in PSSNa, and the XRD result showing the highly crystalline peaks of PSSNa.
For the effect of surfactant type on the band gap energy of PEDOT:PSS, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 utilizing Triton X-100 at 2.5CMC, this condition provided a band gap energy of 1.80 eV, which is lower than that of 3.00 eV for 2.5CMC SDS and 3.5 eV for 2.5CMC CTAB. Triton X-100 at 2.5CMC increases the HOMO level, allowing the polarons and bipolarons to delocalize.2 CTAB and SDS provide higher band gap energies since they can obstruct the interaction of PSS with PEDOT, resulting in higher energy barriers between the HOMO and LUMO levels.
2 utilizing Triton X-100 at 2.5CMC, this condition provided a band gap energy of 1.80 eV, which is lower than that of 3.00 eV for 2.5CMC SDS and 3.5 eV for 2.5CMC CTAB. Triton X-100 at 2.5CMC increases the HOMO level, allowing the polarons and bipolarons to delocalize.2 CTAB and SDS provide higher band gap energies since they can obstruct the interaction of PSS with PEDOT, resulting in higher energy barriers between the HOMO and LUMO levels.
For the effect of Triton X-100 concentrations, a higher concentration provides a higher band gap energy above 2.5 CMC, as shown in Table 2, where too many Triton X-100 molecules can obstruct the PEDOT and PSS interaction and form the TX-PSS complex, but can be removed by methanol washing.54 The optimum Triton-X 100 concentration is 2.5CMC due to its lowest band gap energy (1.80 eV).
3.5 Morphology of PEDOT:PSS
The PEDOT:PSS morphology was investigated via FE-SEM. The effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios at the EDOT
PSS weight ratios at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on the PEDOT:PSS morphology is shown Fig. 5. The particle shapes are all spherical, as tabulated in Table 3. The PEDOT:PSS particle shapes were all spherical because PSS forms spherical micelles.56 It can be noted that the packing parameter, VH/lca0, is less than 1/3 (where, VH is the volume occupied by the hydrophobic groups in the micelle core, lc is the length of the hydrophobic group in the core, and a0 is the cross-sectional area occupied by the hydrophilic groups), indicating a spherical micelle.57 The particle sizes of PEDOT:PSS at the EDOT
1 on the PEDOT:PSS morphology is shown Fig. 5. The particle shapes are all spherical, as tabulated in Table 3. The PEDOT:PSS particle shapes were all spherical because PSS forms spherical micelles.56 It can be noted that the packing parameter, VH/lca0, is less than 1/3 (where, VH is the volume occupied by the hydrophobic groups in the micelle core, lc is the length of the hydrophobic group in the core, and a0 is the cross-sectional area occupied by the hydrophilic groups), indicating a spherical micelle.57 The particle sizes of PEDOT:PSS at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios of 1
PSS weight ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, and 1
11, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 are 33.20 ± 4.29, 16.57 ± 1.99, 16.26 ± 1.40, and 17.19 ± 2.05 nm, respectively, as tabulated in Table 3. The particle size of PEDOT:PSS decreased with an increase in PSS because PSS is an anionic surfactant,58 which forms PSS micelles at the CMC of 7 × 10−5 mol L−1. The EDOT
13 are 33.20 ± 4.29, 16.57 ± 1.99, 16.26 ± 1.40, and 17.19 ± 2.05 nm, respectively, as tabulated in Table 3. The particle size of PEDOT:PSS decreased with an increase in PSS because PSS is an anionic surfactant,58 which forms PSS micelles at the CMC of 7 × 10−5 mol L−1. The EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 is the condition of 4.75CMC PSS, and thus the average particle size of PEDOT:PSS became smaller. Above the EDOT
5 is the condition of 4.75CMC PSS, and thus the average particle size of PEDOT:PSS became smaller. Above the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the particle size of PEDOT:PSS is not significantly different.
5, the particle size of PEDOT:PSS is not significantly different.
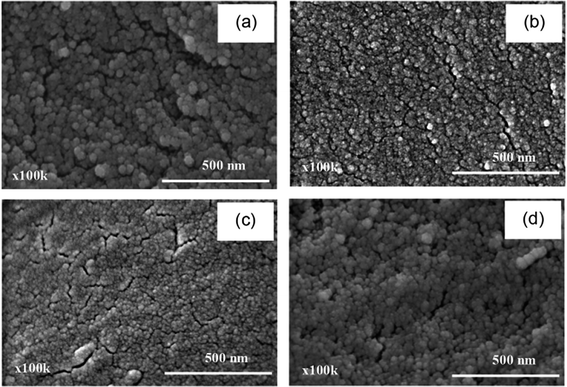 | ||
Fig. 5 PEDOT:PSS surface morphology at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and at various EDOT 1 and at various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios: EDOT PSS weight ratios: EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of (a) 1 PSS weight ratio of (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (b) 1 1, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (c) 1 5, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and (d) 1 11 and (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13. 13. | ||
Sample code EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS and EDOT PSS and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 Na2S2O8 |
Electrical conductivity (S cm−1) | Particle shape | Particle size |
|---|---|---|---|
| Effect of PSS (no surfactant) | |||
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and mole ratio 1 1 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
452.81 ± 31.59 | Spherical | 33.20 ± 4.29 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and mole ratio 1 3 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
366.55 ± 28.82 | Spherical | 32.88 ± 2.88 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and mole ratio 1 5 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
250.23 ± 85.13 | Spherical | 16.57 ± 1.99 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and mole ratio 1 7 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
477.17 ± 36.97 | Spherical | 16.60 ± 1.77 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and mole ratio 1 9 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
828.01 ± 81.77 | Spherical | 16.75 ± 2.81 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
999.74 ± 10.86 | Spherical | 16.26 ± 1.40 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and mole ratio 1 13 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
524.23 ± 42.29 | Spherical | 17.19 ± 2.05 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Effect of oxidant (no surfactant) | |||
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
999.74 ± 10.86 | Spherical | 16.26 ± 1.40 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
1048.78 ± 35.10 | Spherical | 17.28 ± 2.07 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1556.85 ± 46.84 | Spherical | 19.84 ± 2.36 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
564.71 ± 53.44 | Spherical | 21.82 ± 2.36 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
199.46 ± 37.51 | Spherical | 22.95 ± 2.20 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
55.61 ± 0.10 | Spherical | 23.97 ± 3.48 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Effect of surfactant | |||
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with CTAB (at CMC) 2 with CTAB (at CMC) |
12.43 ± 1.33 | Spherical | 24.98 ± 2.54 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with CTAB (at 2.5CMC) 2 with CTAB (at 2.5CMC) |
0.41 ± 0.06 | Spherical | 24.46 ± 2.35 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with CTAB (at 10CMC) 2 with CTAB (at 10CMC) |
29.82 ± 13.02 | Spherical | 20.91 ± 2.91 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with SDS (at CMC) 2 with SDS (at CMC) |
13.23 ± 4.45 | Spherical | 23.73 ± 3.49 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with SDS (at 2.5CMC) 2 with SDS (at 2.5CMC) |
25.04 ± 5.48 | Spherical | 44.01 ± 9.14 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with SDS (at 10CMC) 2 with SDS (at 10CMC) |
45.12 ± 6.02 | Spherical | 26.44 ± 7.16 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at CMC) 2 with Triton X-100 (at CMC) |
1289.43 ± 81.14 | Spherical | 21.37 ± 3.05 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 2.5CMC) 2 with Triton X-100 (at 2.5CMC) |
1879.49 ± 13.87 | Spherical | 56.77 ± 5.54 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 3.5CMC) 2 with Triton X-100 (at 3.5CMC) |
328.69 ± 35.90 | Spherical | 36.48 ± 4.30 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 5CMC) 2 with Triton X-100 (at 5CMC) |
298.92 ± 1.49 | Spherical | 33.95 ± 4.07 |
wt ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and mole ratio 1 11 and mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with Triton X-100 (at 10CMC) 2 with Triton X-100 (at 10CMC) |
238.87 ± 4.04 | Spherical | 30.28 ± 3.62 |
For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio at the EDOT
Na2S2O8 mole ratio at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, the particle shapes and sizes are tabulated Table 3. The particle sizes of PEDOT:PSS at the weight ratio of 1
11, the particle shapes and sizes are tabulated Table 3. The particle sizes of PEDOT:PSS at the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios of 1
Na2S2O8 mole ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, 1
1.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, 1
2.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 are 16.26 ± 1.40, 17.28 ± 2.07, 19.84 ± 2.36, 21.82 ± 2.36, 22.95 ± 2.20, and 23.97 ± 3.48 nm, respectively. The particle size of PEDOT:PSS increased monotonically with an increase in EDOT
4 are 16.26 ± 1.40, 17.28 ± 2.07, 19.84 ± 2.36, 21.82 ± 2.36, 22.95 ± 2.20, and 23.97 ± 3.48 nm, respectively. The particle size of PEDOT:PSS increased monotonically with an increase in EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio. This result is possibly derived from many successive steps: a larger oxidant amount yields a faster nucleation rate for EDOT radical ions; PEDOT chains are polymerized with a lower molecular weight; easier access into PSS micelles leads to a larger EDOT content in a given PSS micelle volume; and PEDOT chains aggregate to form a larger particle size. Additionally, the oxidant ions (Na+ and SO42−) can reduce the electro-repulsion among the SO3− groups of the PSS micelle, leading to an increase in the PSS micelle size, consistent with the effect of salt ions on SDS micelles, as reported by Kim et al., 2001.59 The result can be clearly observed in the FE-SEM images of PEDOT:PSS at the EDOT
Na2S2O8 mole ratio. This result is possibly derived from many successive steps: a larger oxidant amount yields a faster nucleation rate for EDOT radical ions; PEDOT chains are polymerized with a lower molecular weight; easier access into PSS micelles leads to a larger EDOT content in a given PSS micelle volume; and PEDOT chains aggregate to form a larger particle size. Additionally, the oxidant ions (Na+ and SO42−) can reduce the electro-repulsion among the SO3− groups of the PSS micelle, leading to an increase in the PSS micelle size, consistent with the effect of salt ions on SDS micelles, as reported by Kim et al., 2001.59 The result can be clearly observed in the FE-SEM images of PEDOT:PSS at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios of 1
Na2S2O8 mole ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as shown in Fig. 5(c) and 6(a), respectively.
2, as shown in Fig. 5(c) and 6(a), respectively.
The effect of surfactant type on the PEDOT:PSS morphology at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and the EDOT
11 and the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 using CTAB (cationic surfactant), SDS (anionic surfactant), and Triton X-100 (non-ionic surfactant) at 2.5CMC is shown in Fig. 6(b)–(d), respectively. The particle shapes and sizes of the synthesized PEDOT:PSS are tabulated in Table 3. The shapes of PEDOT:PSS in the three surfactant systems are spherical. The particle size of PEDOT:PSS without surfactant is smaller than that with the surfactants since the PSS micelles are likely disturbed by the surfactant molecules. Among the surfactants, PEDOT:PSS synthesized with CTAB at 2.5CMC showed the smallest particle size of 24.46 ± 2.35 nm since the electro-attractive force between the cationic CTAB surfactant and PSS− disturbs the formation of PSS micelles.53 For the anionic surfactant SDS at 2.5CMC, the particle size of PEDOT:PSS is 44.01 ± 9.14 nm, and SDS can repel and replace PSS−; thus, disturbing the formation of PSS micelles.37 For the non-ionic surfactant Triton X-100 at 2.5CMC, the particle size of PEDOT:PSS was the largest at 56.77 ± 5.54 nm since Triton X-100 interferes with the PSS micelle formation. Triton X-100 consists of a large ethoxy chain acting as a bulky polar head group, which can separate the SO3− groups of the PSS micelle; thus, reducing the electrostatic repulsive interaction, leading to an increase in the PSS micelle size.60
2 using CTAB (cationic surfactant), SDS (anionic surfactant), and Triton X-100 (non-ionic surfactant) at 2.5CMC is shown in Fig. 6(b)–(d), respectively. The particle shapes and sizes of the synthesized PEDOT:PSS are tabulated in Table 3. The shapes of PEDOT:PSS in the three surfactant systems are spherical. The particle size of PEDOT:PSS without surfactant is smaller than that with the surfactants since the PSS micelles are likely disturbed by the surfactant molecules. Among the surfactants, PEDOT:PSS synthesized with CTAB at 2.5CMC showed the smallest particle size of 24.46 ± 2.35 nm since the electro-attractive force between the cationic CTAB surfactant and PSS− disturbs the formation of PSS micelles.53 For the anionic surfactant SDS at 2.5CMC, the particle size of PEDOT:PSS is 44.01 ± 9.14 nm, and SDS can repel and replace PSS−; thus, disturbing the formation of PSS micelles.37 For the non-ionic surfactant Triton X-100 at 2.5CMC, the particle size of PEDOT:PSS was the largest at 56.77 ± 5.54 nm since Triton X-100 interferes with the PSS micelle formation. Triton X-100 consists of a large ethoxy chain acting as a bulky polar head group, which can separate the SO3− groups of the PSS micelle; thus, reducing the electrostatic repulsive interaction, leading to an increase in the PSS micelle size.60
The effect of surfactant concentration at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 on the PEDOT:PSS morphology is shown in Table 3. The shapes of PEDOT:PSS are still spherical. At the CMC, the surfactants form micelles, which are unstable and can disturb the interaction between PEDOT and PSS; thus, the particle sizes are larger than PEDOT:PSS without a surfactant.30 Above the CMC, surfactants are prone to interact with the PSS molecules, and thus the formation of PSS micelles is interrupted, leading to larger particle sizes.
2 on the PEDOT:PSS morphology is shown in Table 3. The shapes of PEDOT:PSS are still spherical. At the CMC, the surfactants form micelles, which are unstable and can disturb the interaction between PEDOT and PSS; thus, the particle sizes are larger than PEDOT:PSS without a surfactant.30 Above the CMC, surfactants are prone to interact with the PSS molecules, and thus the formation of PSS micelles is interrupted, leading to larger particle sizes.
3.6 Thermal stability
The thermal stability of PEDOT:PSS was investigated by TG-DTA under an N2 flow, as shown in Fig. 7, and the onset decomposition temperatures (Td,onset) of PEDOT:PSS with different surfactant systems were measured. The Td,onset of PEDOT:PSS at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 without surfactant was 499 °C and the char yield at 790 °C was 53.43%. The Td,onset of PEDOT:PSS synthesized under the same conditions using Triton X-100 at 2.5CMC was 492.97 °C and the char yield at 790 °C was 44.05%. For the CTAB system (2.5CMC), the Td,onset of PEDOT:PSS was 407.98 °C and the char yield at 790 °C was 51.62%. For the SDS system (2.5CMC), the Td,onset of PEDOT:PSS was 413.52 °C and the char yield at 790 °C was 50.52%. The PEDOT:PSS with the surfactants possessed lower Td,onset and % char yield values than that without surfactant. Thus, PEDOT:PSS with the surfactants possessed higher thermal degradation values because the surfactants disturbed the polymerization of PEDOT:PSS, resulting in a shorter conjugated polymer.61 For the PEDOT:PSS without surfactant and with Triton X-100, CTAB, and SDS, the thermograms show four decomposition stages: the first stage is the decomposition of water and moisture in the temperature range of 100–180 °C; the second stage is the decomposition of the side chains in the temperature range of 180–320 °C; the third stage exhibits the decomposition of PSS in the temperature range of 320–400 °C; and the last stage illustrates the decomposition of PEDOT (main chain) in the temperature range of 400–790 °C.23 For the effect of EDOT
2 without surfactant was 499 °C and the char yield at 790 °C was 53.43%. The Td,onset of PEDOT:PSS synthesized under the same conditions using Triton X-100 at 2.5CMC was 492.97 °C and the char yield at 790 °C was 44.05%. For the CTAB system (2.5CMC), the Td,onset of PEDOT:PSS was 407.98 °C and the char yield at 790 °C was 51.62%. For the SDS system (2.5CMC), the Td,onset of PEDOT:PSS was 413.52 °C and the char yield at 790 °C was 50.52%. The PEDOT:PSS with the surfactants possessed lower Td,onset and % char yield values than that without surfactant. Thus, PEDOT:PSS with the surfactants possessed higher thermal degradation values because the surfactants disturbed the polymerization of PEDOT:PSS, resulting in a shorter conjugated polymer.61 For the PEDOT:PSS without surfactant and with Triton X-100, CTAB, and SDS, the thermograms show four decomposition stages: the first stage is the decomposition of water and moisture in the temperature range of 100–180 °C; the second stage is the decomposition of the side chains in the temperature range of 180–320 °C; the third stage exhibits the decomposition of PSS in the temperature range of 320–400 °C; and the last stage illustrates the decomposition of PEDOT (main chain) in the temperature range of 400–790 °C.23 For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio at the EDOT
Na2S2O8 mole ratio at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, the TGA thermograms were nearly the same as the above results.
11, the TGA thermograms were nearly the same as the above results.
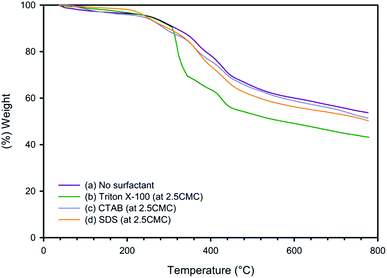 | ||
Fig. 7 TGA spectra of PEDOT:PSS at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2: (a) no surfactant, (b) Triton X-100 (2.5CMC), (c) CTAB (2.5CMC) and (d) SDS (2.5CMC). 2: (a) no surfactant, (b) Triton X-100 (2.5CMC), (c) CTAB (2.5CMC) and (d) SDS (2.5CMC). | ||
3.7 Electrical conductivity
The electrical conductivity of the PEDOT:PSS synthesized at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and at various EDOT
1 and at various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios is shown in Fig. 8. For EDOT
PSS weight ratios is shown in Fig. 8. For EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios between 1
PSS weight ratios between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the electrical conductivity of PEDOT:PSS decreased from 452.81 ± 31.59 to 250.23 ± 85.13 S cm−1 since the PSS amount is low and it may act as an insulator.20 At EDOT
5, the electrical conductivity of PEDOT:PSS decreased from 452.81 ± 31.59 to 250.23 ± 85.13 S cm−1 since the PSS amount is low and it may act as an insulator.20 At EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios between 1
PSS weight ratios between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, the electrical conductivity of PEDOT:PSS increased from 250.23 ± 85.13 to 999.74 ± 10.86 S cm−1 since PSS acts effectively as a dopant.13 The electrical conductivity increases due to an increase in the number of charge carriers.52 This finding is consistent with the increase in % PSS− and % SO42− from XPS and the decrease in the band gap energy from UV-Vis. However, at the EDOT
11, the electrical conductivity of PEDOT:PSS increased from 250.23 ± 85.13 to 999.74 ± 10.86 S cm−1 since PSS acts effectively as a dopant.13 The electrical conductivity increases due to an increase in the number of charge carriers.52 This finding is consistent with the increase in % PSS− and % SO42− from XPS and the decrease in the band gap energy from UV-Vis. However, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, the electrical conductivity of PEDOT:PSS decreased to 524.23 ± 42.29 S cm−1 due to over-doping.51 Hence, the EDOT
13, the electrical conductivity of PEDOT:PSS decreased to 524.23 ± 42.29 S cm−1 due to over-doping.51 Hence, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 is optimal owing to the highest electrical conductivity obtained (999.74 ± 10.86 S cm−1).
11 is optimal owing to the highest electrical conductivity obtained (999.74 ± 10.86 S cm−1).
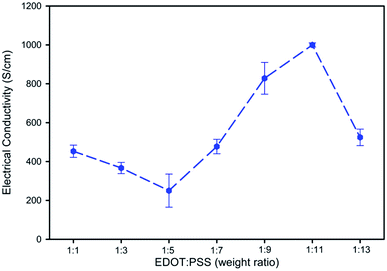 | ||
Fig. 8 Electrical conductivity of PEDOT:PSS at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and at various EDOT 1 and at various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratios. PSS weight ratios. | ||
The electrical conductivity of PEDOT:PSS using the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 at various EDOT
11 at various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios is shown in Fig. 9. As the EDOT
Na2S2O8 mole ratios is shown in Fig. 9. As the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio increased from 1
Na2S2O8 mole ratio increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the electrical conductivity of PEDOT:PSS increased from 999.74 ± 10.86 to 1556.85 ± 46.84 S cm−1 since SO42− from the oxidant may also act as a dopant12 by interacting with the oxidized PEDOT chain. This finding is consistent with the % SO42− increase from XPS, the decrease in band gap energy, and the low PSSNa amount. At EDOT
2, the electrical conductivity of PEDOT:PSS increased from 999.74 ± 10.86 to 1556.85 ± 46.84 S cm−1 since SO42− from the oxidant may also act as a dopant12 by interacting with the oxidized PEDOT chain. This finding is consistent with the % SO42− increase from XPS, the decrease in band gap energy, and the low PSSNa amount. At EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios above 1
Na2S2O8 mole ratios above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the electrical conductivity of PEDOT:PSS decreased from 1556.85 ± 46.84 S cm−1 to 55.61 ± 0.10 S cm−1 because Na+ ions from the oxidant can react with PSS− to form PSSNa, which reduces the amount of PSS available to dope PEDOT, leading to a decrease in electrical conductivity.42 The increase in PSSNa was identified at the EDOT
2, the electrical conductivity of PEDOT:PSS decreased from 1556.85 ± 46.84 S cm−1 to 55.61 ± 0.10 S cm−1 because Na+ ions from the oxidant can react with PSS− to form PSSNa, which reduces the amount of PSS available to dope PEDOT, leading to a decrease in electrical conductivity.42 The increase in PSSNa was identified at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios of 1
Na2S2O8 mole ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by XPS and XRD. Moreover, the decrease in electrical conductivity is related to the increase in the band gap energy. Thus, the EDOT
4 by XPS and XRD. Moreover, the decrease in electrical conductivity is related to the increase in the band gap energy. Thus, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is optimal to acquire the high PEDOT:PSS electrical conductivity of 1556.85 ± 46.84 S cm−1, as confirmed by the low band gap energy of 1.90 eV compared to other EDOT
2 is optimal to acquire the high PEDOT:PSS electrical conductivity of 1556.85 ± 46.84 S cm−1, as confirmed by the low band gap energy of 1.90 eV compared to other EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios.
Na2S2O8 mole ratios.
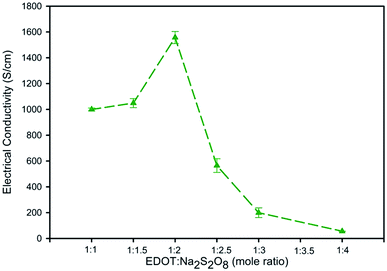 | ||
Fig. 9 Electrical conductivity of PEDOT:PSS at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and at various EDOT 11 and at various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios. Na2S2O8 mole ratios. | ||
The electrical conductivity of PEDOT:PSS synthesized using the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with various surfactant types and concentrations is shown in Fig. 10. The CTAB and SDS systems are not suitable to synthesize PEDOT:PSS since the electrical conductivity is 100 times lower than that of PEDOT:PSS without these two surfactants. CTAB can react with the PSS chain;53 whereas, SDS can replace PSS.37 Moreover, these two systems induce a decrease in the amount of dopant (% SO3− of PSS and % SO42− of Na2S2O8), as confirmed by XPS and the increase in band gap energy from 1.90 eV to 3.50 eV for CTAB and 1.90 eV to 3.00 eV for SDS. For Triton X-100, it can react with both PEDOT and the PSS chains, and can form a TX-PSS complex, which can be easily removed by methanol washing,54 resulting in the removal of PSS− acting as a dopant and PSSNa as a salt. This suggests that Triton X-100 reduces the PEDOT and PSS interaction as well as the PSSNa amount. From the XPS result, the decrease in the amount of PSSNa amount was clearly observed with an increase Triton X-100 concentration together with a decrease in the amount of dopant (% SO3− of PSS and % SO42− of Na2S2O8). However, using Triton X-100 at 2.5CMC provides a suitable condition for the removal of PSSNa since the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 with the lowest band gap energy of 1.80 eV were obtained, while the dopant amount remained high. A higher Triton X-100 concentration tended to remove the dopants, although PSSNa was preferentially eliminated.
2 with various surfactant types and concentrations is shown in Fig. 10. The CTAB and SDS systems are not suitable to synthesize PEDOT:PSS since the electrical conductivity is 100 times lower than that of PEDOT:PSS without these two surfactants. CTAB can react with the PSS chain;53 whereas, SDS can replace PSS.37 Moreover, these two systems induce a decrease in the amount of dopant (% SO3− of PSS and % SO42− of Na2S2O8), as confirmed by XPS and the increase in band gap energy from 1.90 eV to 3.50 eV for CTAB and 1.90 eV to 3.00 eV for SDS. For Triton X-100, it can react with both PEDOT and the PSS chains, and can form a TX-PSS complex, which can be easily removed by methanol washing,54 resulting in the removal of PSS− acting as a dopant and PSSNa as a salt. This suggests that Triton X-100 reduces the PEDOT and PSS interaction as well as the PSSNa amount. From the XPS result, the decrease in the amount of PSSNa amount was clearly observed with an increase Triton X-100 concentration together with a decrease in the amount of dopant (% SO3− of PSS and % SO42− of Na2S2O8). However, using Triton X-100 at 2.5CMC provides a suitable condition for the removal of PSSNa since the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 with the lowest band gap energy of 1.80 eV were obtained, while the dopant amount remained high. A higher Triton X-100 concentration tended to remove the dopants, although PSSNa was preferentially eliminated.
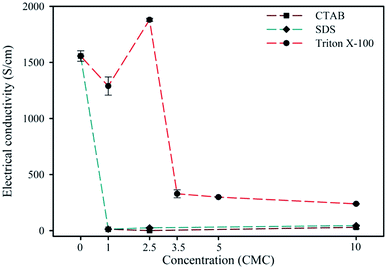 | ||
Fig. 10 Electrical conductivity of PEDOT:PSS at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1 PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT 11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1 Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 with various surfactant types and concentrations. 2 with various surfactant types and concentrations. | ||
To confirm the electrical conductivity of the synthesized PEDOT:PSS as measured by a custom-built 2-point probe, the electrical conductivity of graphite (particle size < 20 μm, Sigma Aldrich) and multi-walled carbon nanotubes (specific of diameter of 30–50 nm, >95 wt% purity, Alphanano Technology Co., Ltd.) was also measured by using the same equipment and the same sample thickness. The obtained electrical conductivity of graphite was 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 282.18 S cm−1 (ref. 62) and MWCNT was 1589.17 S cm−1,63 consistent with the data from previous work. It should be noted that the electrical conductivity of the synthesized PEDOT:PSS powder was in the same range as that of MWCNT.
282.18 S cm−1 (ref. 62) and MWCNT was 1589.17 S cm−1,63 consistent with the data from previous work. It should be noted that the electrical conductivity of the synthesized PEDOT:PSS powder was in the same range as that of MWCNT.
The related works of the synthesized PEDOT:PSS powder are tabulated in Table 4, where Qi and co-workers (1998) prepared PEDOT:PSS via chemical oxidative polymerization. They used EDOT and PSSNa as the reactants and Fe(NO3)3·9H2O as the oxidant. The reaction time was 2 h to obtain a dark blue solution. The electrical conductivity of PEDOT:PSS was 9.9 S cm−1.64 Lefebvre et al. synthesized PEDOT:PSS using a mixed solvent of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water at a ratio of 1
water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. EDOT and NaPSS were used as the reactants and Fe(NO3)3·9H2O and FeCl3 were used the oxidants. The electrical conductivity value at the EDOT
1. EDOT and NaPSS were used as the reactants and Fe(NO3)3·9H2O and FeCl3 were used the oxidants. The electrical conductivity value at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe(NO3)3·9H2O mole ratio of 1
Fe(NO3)3·9H2O mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was 2.50 S cm−1, while at the EDOT
5 was 2.50 S cm−1, while at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeCl3 mole ratio of 1
FeCl3 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 it was 0.006 S cm−1.24 Lefebvre et al. prepared PEDOT:PSS using Fe(NO3)3·9H2O as the oxidant and a mixture of acetonitrile
10 it was 0.006 S cm−1.24 Lefebvre et al. prepared PEDOT:PSS using Fe(NO3)3·9H2O as the oxidant and a mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water at a ratio of 1
water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the solvent. Using the EDOT
1 as the solvent. Using the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe(NO3)3·9H2O mole ratio of 1
Fe(NO3)3·9H2O mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the electrical conductivity was 1.50 S cm−1.21 Dai et al., 2008, synthesized PEDOT:PSS using Fe(NO3)3·9H2O as the oxidant and water as the solvent and the reaction time was 24 h at room temperature, giving an electrical conductivity value of 4.3 S cm−1.65 Wichiansee and co-worker prepared PEDOT:PSS by mixing EDOT, PSS, and Na2S2O8 in distilled water. Subsequently, Fe2(SO4)3 was added to the solution and it was stirred continuously for 24 h. The electrical conductivity was 27.50 S cm−1.22 Chanthanont and co-workers, 2013, synthesized PEDOT:PSS using an EDOT
10, the electrical conductivity was 1.50 S cm−1.21 Dai et al., 2008, synthesized PEDOT:PSS using Fe(NO3)3·9H2O as the oxidant and water as the solvent and the reaction time was 24 h at room temperature, giving an electrical conductivity value of 4.3 S cm−1.65 Wichiansee and co-worker prepared PEDOT:PSS by mixing EDOT, PSS, and Na2S2O8 in distilled water. Subsequently, Fe2(SO4)3 was added to the solution and it was stirred continuously for 24 h. The electrical conductivity was 27.50 S cm−1.22 Chanthanont and co-workers, 2013, synthesized PEDOT:PSS using an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS mole ratio of 1
PSS mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in distilled water. Na2S2O8 was used as the oxidant and Fe2(SO4)3 was added to the solution and stirred vigorously for 24 h. The electrical conductivity was 11.69 S cm−1.23
1 in distilled water. Na2S2O8 was used as the oxidant and Fe2(SO4)3 was added to the solution and stirred vigorously for 24 h. The electrical conductivity was 11.69 S cm−1.23
| Sample | Oxidant | Electrical conductivity (S cm−1) | Particle shape | Particle size (nm) | Reference |
|---|---|---|---|---|---|
| PEDOT:PSS | Fe(NO3)3·9H2O | 9.90 | — | — | Qi et al., 1998 |
| PEDOT:PSS | Fe(NO3)3·9H2O | 2.50 | — | — | Lefebvre et al., 1998 |
| FeCl3 | 0.006 | — | — | ||
| PEDOT:PSS | Fe(NO3)3·9H2O | 1.50 | — | — | Lefebvre et al., 1999 |
| PEDOT:PSS | Fe(NO3)3·9H2O | 4.30 | — | — | Dai et al., 2008 |
| PEDOT:PSS | Na2S2O8 | 27.50 | Irregular | 3000 | Wichiansee et al., 2008 |
| PEDOT:PSS | Na2S2O8 | 11.69 | Irregular | 3400 | Chanthanont et al., 2013 |
| PEDOT:PSS | Na2S2O8 | 1556.85 | Spherical | 19.84 | Present work |
| PEDOT:PSS with Triton X-100 at 2.5CMC | Na2S2O8 | 1879.49 | Spherical | 56.77 |
3.8 Interaction of PEDOT:PSS and dopants including PSSNa
For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio, at the EDOT
PSS weight ratio, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, PSS as the dopant reacts with PEDOT, as shown in Scheme 2.13 At an EDOT
1, PSS as the dopant reacts with PEDOT, as shown in Scheme 2.13 At an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio lower than 1
PSS weight ratio lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, as shown in Scheme 2(a), a small amount of PSS interacts with PEDOT, as confirmed by XPS. At the EDOT
11, as shown in Scheme 2(a), a small amount of PSS interacts with PEDOT, as confirmed by XPS. At the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, as shown in Scheme 2(b), more PSS chains are available to dope the PEDOT chains. However, at a EDOT
11, as shown in Scheme 2(b), more PSS chains are available to dope the PEDOT chains. However, at a EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio above 1
PSS weight ratio above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, as shown in Scheme 2(c), PSS also reacts with Na+ from Na2S2O8 to form PSSNa on PSS, as confirmed by XPS and XRD. The presence of PSSNa reduces the number of doping sites on PSS. Hence, the EDOT
11, as shown in Scheme 2(c), PSS also reacts with Na+ from Na2S2O8 to form PSSNa on PSS, as confirmed by XPS and XRD. The presence of PSSNa reduces the number of doping sites on PSS. Hence, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 is the optimal condition because of the highest electrical conductivity (999.74 ± 10.86 S cm−1) and lowest UV-Vis energy gap (2.67 eV). This shows that only a proper amount of PSS increases the number of charge carriers, resulting in an increase in the electrical conductivity.52
11 is the optimal condition because of the highest electrical conductivity (999.74 ± 10.86 S cm−1) and lowest UV-Vis energy gap (2.67 eV). This shows that only a proper amount of PSS increases the number of charge carriers, resulting in an increase in the electrical conductivity.52
The PEDOT:PSS synthesized at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and various EDOT
11 and various EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratios is demonstrated in Scheme 3. At an EDOT
Na2S2O8 mole ratios is demonstrated in Scheme 3. At an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio lower than 1
Na2S2O8 mole ratio lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as shown in Scheme 3(a), SO42− acts as an oxidant and dopant12 and it interacts with the PEDOT chains, but a small number of SO42− ions are available to interact with PEDOT to generate a small number of charge carriers, resulting in low electrical conductivity.52 In addition, Na+ from Na2S2O8 also reacts with PSS to form PSSNa, as verified by XPS and XRD. At the EDOT
2, as shown in Scheme 3(a), SO42− acts as an oxidant and dopant12 and it interacts with the PEDOT chains, but a small number of SO42− ions are available to interact with PEDOT to generate a small number of charge carriers, resulting in low electrical conductivity.52 In addition, Na+ from Na2S2O8 also reacts with PSS to form PSSNa, as verified by XPS and XRD. At the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as shown in Scheme 3(b), more SO42− ions are available to interact with PEDOT, as confirmed by XPS, while the PSSNa amount does not significantly increase. At an EDOT
2, as shown in Scheme 3(b), more SO42− ions are available to interact with PEDOT, as confirmed by XPS, while the PSSNa amount does not significantly increase. At an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio above 1
Na2S2O8 mole ratio above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, as shown in Scheme 3(c), more SO42− ions interact with PEDOT, but at the expense of more Na+ ions from Na2S2O8 reacting with PSS to form PSSNa, as confirmed by XPS and XRD. Therefore, the EDOT
2, as shown in Scheme 3(c), more SO42− ions interact with PEDOT, but at the expense of more Na+ ions from Na2S2O8 reacting with PSS to form PSSNa, as confirmed by XPS and XRD. Therefore, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is the optimal condition since it provides the highest electrical conductivity (1556.85 ± 46.84 S cm−1) and lowest energy gap of 1.90 eV.
2 is the optimal condition since it provides the highest electrical conductivity (1556.85 ± 46.84 S cm−1) and lowest energy gap of 1.90 eV.
At the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the interactions are shown in Scheme 4(a) without a surfactant and in Scheme 4(b) with Triton X-100. As shown in Scheme 4(b), Triton X-100 reacts with PSS and it reduces the PEDOT and PSS interaction and the PSSNa amount, which can be easily removed by methanol washing,54 as verified by XPS. Hence, using Triton X-100 at 2.5CMC yielded the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 with the lowest energy gap of 1.80 eV.
2, the interactions are shown in Scheme 4(a) without a surfactant and in Scheme 4(b) with Triton X-100. As shown in Scheme 4(b), Triton X-100 reacts with PSS and it reduces the PEDOT and PSS interaction and the PSSNa amount, which can be easily removed by methanol washing,54 as verified by XPS. Hence, using Triton X-100 at 2.5CMC yielded the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 with the lowest energy gap of 1.80 eV.
4. Conclusion
PEDOT:PSS was successfully synthesized via chemical oxidative polymerization with high electrical conductivity and a systematic route was used to investigate the effects of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio, EDOT
PSS weight ratio, EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, and surfactant type and concentration on its properties. The EDOT
Na2S2O8 mole ratio, and surfactant type and concentration on its properties. The EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 provided the highest PSS− dopant amount to interact with PEDOT. For the effect of EDOT
11 provided the highest PSS− dopant amount to interact with PEDOT. For the effect of EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio, the EDOT
Na2S2O8 mole ratio, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was the optimal condition since SO42− from the oxidant acting as a secondary dopant increased the number of charge carriers, resulting in the high electrical conductivity of 1556.85 ± 46.84 S cm−1 and low energy gap of 1.90 eV. In the case of the three surfactant systems, Triton X-100 (2.5CMC) was the suitable surfactant to synthesize PEDOT:PSS, at the EDOT
2 was the optimal condition since SO42− from the oxidant acting as a secondary dopant increased the number of charge carriers, resulting in the high electrical conductivity of 1556.85 ± 46.84 S cm−1 and low energy gap of 1.90 eV. In the case of the three surfactant systems, Triton X-100 (2.5CMC) was the suitable surfactant to synthesize PEDOT:PSS, at the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS weight ratio of 1
PSS weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 and EDOT
11 and EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2S2O8 mole ratio of 1
Na2S2O8 mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 because of the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 and the lowest energy gap of 1.80 eV. Triton X-100 molecules reduced the PEDOT and PSS interactions and the amount of PSSNa, which could be easily removed by methanol washing. The washing reduced the PSSNa amount on PSS, which then provided a higher number of doping sites on PSS. The particle shape of the PEDOT:PSS synthesized in all conditions were spherical. The particle size of PEDOT:PSS varied from 16.57 ± 1.99 to 56.77 ± 5.54 nm. The thermogravimetric analysis revealed that PEDOT:PSS with surfactants exhibited lower thermal stability than PEDOT:PSS without surfactant. The synthesis of PEDOT:PSS in this work provides guidance for the production and use of PEDOT:PSS particles in powder form with very high electrical conductivity, potentially suitable for many electronic applications such as drug delivery, thin film transistors, light emitting diodes, sensors, photovoltaics, and actuators.
2 because of the highest electrical conductivity of 1879.49 ± 13.87 S cm−1 and the lowest energy gap of 1.80 eV. Triton X-100 molecules reduced the PEDOT and PSS interactions and the amount of PSSNa, which could be easily removed by methanol washing. The washing reduced the PSSNa amount on PSS, which then provided a higher number of doping sites on PSS. The particle shape of the PEDOT:PSS synthesized in all conditions were spherical. The particle size of PEDOT:PSS varied from 16.57 ± 1.99 to 56.77 ± 5.54 nm. The thermogravimetric analysis revealed that PEDOT:PSS with surfactants exhibited lower thermal stability than PEDOT:PSS without surfactant. The synthesis of PEDOT:PSS in this work provides guidance for the production and use of PEDOT:PSS particles in powder form with very high electrical conductivity, potentially suitable for many electronic applications such as drug delivery, thin film transistors, light emitting diodes, sensors, photovoltaics, and actuators.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are graceful for the scholarship from the Thailand Research Fund through the Royal Golden Jubilee PhD Program (RGJ PHD/0108/2559), the funding from the Conductive and Electroactive Polymer Research Unit, the Thailand Research Fund (TRF), and the Royal Thai Government.References
- T. H. Le, Y. Kim and H. Yoon, Polymers, 2017, 9, 150 CrossRef.
- P. Kar, Doping in conjugated polymers, Scrivener Publishing LLC, Canada, 1st edn, 2013 Search PubMed.
- H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang and A. J. Heeger, J. Chem. Soc., Chem. Commun., 1997, 578–580 Search PubMed.
- N. Hall, Chem. Commun., 2003, 1–4 Search PubMed.
- W. S. Huang, B. D. Humphrey and A. G. MacDiarmid, J. Chem. Soc., Faraday Trans. 1, 1986, 82, 2385–2400 RSC.
- R. D. McCullough, R. D. Lowe, M. Jayaraman and D. L. Anderson, J. Org. Chem., 1993, 58, 904–912 CrossRef CAS.
- L. Dai, B. Winkler, L. Dong, L. Tong and W. H. A. Mau, Adv. Mater., 2001, 13, 915–925 CrossRef CAS.
- H. E. Katz, J. Mater. Chem., 1997, 7, 369–376 RSC.
- M. Hiraoka, P. Fiorini, J. O'Callaghan, I. Yamashita, C. V. Hoof and M. Op de Beeck, Sens. Actuators, A, 2012, 177, 23–29 CrossRef CAS.
- K. Yan, Z.-X. Liu, X. Li, J. Chen, H. Chen and C.-Z. Li, Org. Chem. Front., 2018, 5, 2845 RSC.
- D. Svirskis, J. Travas-Sejdic, A. Rodgers and S. Garg, J. Controlled Release, 2010, 146, 6–15 CrossRef CAS PubMed.
- N. Paradee and A. Sirivat, Polym. Int., 2013, 63, 106–113 CrossRef.
- L. Ouyang, C. Musumeci, M. J. Jafari, T. Ederth and O. Inganäs, ACS Appl. Mater. Interfaces, 2015, 7, 19764–19773 CrossRef CAS PubMed.
- A. Cho, S. Kim, S. Kim, W. Cho, C. Park, F. S. Kim and J. H. Kim, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 1530–1536 CrossRef CAS.
- K. Muro, M. Watanabe, T. Tamai, K. Yazawa and K. Matsukawa, RSC Adv., 2016, 6, 87147–87152 RSC.
- C. Liu, F. Jiang, M. Huang, R. Yue, B. Lu, J. Xu and G. Liu, J. Electron. Mater., 2011, 40, 648–651 CrossRef CAS.
- S. Lim, S. H. Park, T. K. An, H. S. Lee and S. H. Kim, RSC Adv., 2015, 6, 2004–2010 RSC.
- J. Ouyang, Q. Xu, C.-W. Chu, Y. Yang, G. Li and J. Shinar, Polymer, 2004, 45, 8443–8450 CrossRef CAS.
- J. Y. Oh, M. Shin, J. B. Lee, J. H. Ahn, H. K. Baik and U. Jeong, ACS Appl. Mater. Interfaces, 2014, 6, 6954–6961 CrossRef CAS PubMed.
- T. Horii, H. Hikawa, Y. Mochizuki and H. Okuzaki, Trans. Mater. Res. Soc. Jpn., 2012, 37, 515–518 CrossRef CAS.
- M. C. Lefebvre, Z. Qi and P. G. Pick up, J. Electrochem. Soc., 1999, 146, 2054–2058 CrossRef CAS.
- W. Wichiansee and A. Sirivat, Materials Science and Engineering C., 2008, 29, 78–84 CrossRef.
- P. Chanthanont and A. Sirivat, Polym. Adv. Technol., 2013, 32, 21367 Search PubMed.
- M. Lefebvre, Z. Qi, D. Rana and P. G. Pickup, J. Electrochem. Soc., 1999, 146, 2054–2058 CrossRef CAS.
- S. Khan and A. K. Narula, Eur. Polym. J., 2016, 81, 161–172 CrossRef CAS.
- Polypyrrole Stability and Coatings for Radar Absorbing Materials, 2004, http://www.dtic.mil/dtic/tr/fulltext/u2/a436251.pdf.
- Z. Fufang, Z. Baogai, S. Zhuoran, H. Yuanming and P. Chanxu, Key Eng. Mater., 2009, 407–408, 573–576 Search PubMed.
- N. Sangiorgi, L. Aversa, R. Tatti, R. Verucchi and A. Sanson, Opt. Mater., 2016, 64, 18–27 CrossRef.
- T. Permpool, A. Sirivat and D. Aussawasathien, Polym. Int., 2014, 63, 2076–2083 CrossRef CAS.
- K. Phasuksom and A. Sirivat, Synth. Met., 2016, 219, 142–153 CrossRef CAS.
- C. Liang and H.-W. Su, Ind. Eng. Chem. Res., 2009, 48, 5558–5562 CrossRef CAS.
- C. Coletta, Z. Cui, A. Dazzi, J. M. Guigner, S. Neron, J.-L. Marignier and S. Remita, Radiat. Phys. Chem., 2016, 126, 21–31 CrossRef CAS.
- N. D. Koromilas, G. C. Lainioti, E. K. Oikonomou, G. Bokias and J. K. Kallitsis, Eur. Polym. J., 2014, 54, 39–51 CrossRef CAS.
- E. G. Langford, K. D. Shaughnessy, T. C. Devore, D. Lawrence and C. Constantin, MRS Adv., 2016, 1, 465–469 CrossRef CAS.
- C. Sriprachuabwong, C. Karuwan, A. Wisitsorrat, D. Phokharatkul, T. Lomas, P. Sritongkham and A. Tuantranont, J. Mater. Chem., 2012, 22, 5478–5485 RSC.
- C. Lin, B. Fan, J. X. Zhang, X. Yang and H. Zhang, Desalin. Water Treat., 2015, 57, 21627–21733 CrossRef.
- C. Yeon, G. Kim, J. W. Lim and S. J. Yun, RSC Adv., 2017, 7, 5888–5897 RSC.
- K. Sukchol, S. Thongyai, P. Praserthdam and G. A. Sotzing, Synth. Met., 2013, 179, 10–17 CrossRef CAS.
- T.-R. Chou, S.-H. Chen, Y.-T. Chiang, T.-T. Chang, C.-W. Lin and C.-Y. Chao, Org. Electron., 2017, 48, 223–229 CrossRef CAS.
- T. G. Kim, S. R. Ha, H. Choi, K. Uh, U. Kundapur, S. Park, C. W. Lee, S. Lee, J. Kim and J.-M. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 19231–19237 CrossRef CAS PubMed.
- C. Yeon, S. J. Yun, J. Kim and J. W. Lim, Adv. Electron. Mater., 2015, 1, 1500121 CrossRef.
- Y. Xia and J. Ouyang, Macromolecules, 2009, 42, 4141–4147 CrossRef CAS.
- G. Zotti, S. Zecchin, G. Schiavon, F. Louwet, L. Groenendaal, X. Crispin, W. Osikowicz, W. Salaneck and M. Fahhman, Macromolecules, 2003, 36, 3337–3344 CrossRef CAS.
- M. Chen, K. Shafer-Peltier, S. J. Randtke and E. Peltier, Chem. Eng. J., 2018, 344, 155–164 CrossRef CAS.
- H. Park, S. H. Lee, F. S. Kim, H. H. Choi, I. W. Cheong and J. H. Kim, J. Mater. Chem. A, 2014, 2, 6532–6539 RSC.
- L. Zhang, H. Deng, S. Liu, Q. Zhang, F. Chen and Q. Fu, RSC Adv., 2015, 5, 105592–105599 RSC.
- A. B. Volynsky, A. Y. Stakheev, N. S. Telegina, V. G. Senin, L. M. Kustov and R. Wennirch, Spectrochim. Acta, Part B, 2001, 56, 1387–1396 CrossRef.
- J. Zhao, S. Xu, K. Tschulik, R. G. Compton, M. Wei, D. O'Hare, D. G. Evans and X. Duan, Adv. Funct. Mater., 2015, 25, 2745–2753 CrossRef CAS.
- S. Fabiano, S. Braun, X. Liu, E. Weverberghs, P. Gerbaux, M. Fahlman, M. Berggren and X. Crispin, Adv. Mater., 2014, 26, 6000–6006 CrossRef CAS PubMed.
- L. Qie, W. Chen, X. Xiong, C. Hu, F. Zou, P. Hu and Y. Huang, Adv. Sci., 2015, 2, 1500195 Search PubMed.
- P. Tehrani, A. Kanciurzewska, X. Crispin, N. D. Robinson, M. Fahlman and M. Berggren, Solid State Ionics, 2007, 177, 3521–3527 CrossRef CAS.
- T.-C. Tsai, H.-C. Chang, C.-H. Chen and W.-T. Whang, Org. Electron., 2011, 12, 2159–2164 CrossRef CAS.
- B. Fan, X. Mei and J. Ouyang, Macromolecules, 2008, 41, 5971–5973 CrossRef CAS.
- S.-S. Yoon and D.-Y. Khang, J. Phys. Chem. C, 2016, 120, 29525–29532 CrossRef CAS.
- D. C. Sun and D. S. Sun, Mater. Chem. Phys., 2009, 118, 288–292 CrossRef CAS.
- S. Maruthamuthu, J. Chandrasekaran, D. Manoharan, S. N. Karthick and H. J. Kim, J. Appl. Polym. Sci., 2016, 133, 43772 CrossRef.
- D. Lensen, D. M. Vriezema and J. C. M. van Hest, Macromol. Biosci., 2008, 8, 991–1005 CrossRef CAS PubMed.
- S. Arunsawad, K. Srikulkit and S. Limpanart, J. Met., Mater. Miner., 2014, 24, 29–34 CAS.
- B.-J. Kim, S.-G. Oh, M.-G. Han and S.-S. Im, Synth. Met., 2000, 122, 297–304 CrossRef.
- H. Zhang and P. L. Dubin, J. Colloid Interface Sci., 1996, 186, 264–270 CrossRef.
- E. Eren, G. Celik, A. Uygun, J. Tabačiarová and M. Omastová, Synth. Met., 2012, 162, 1451–1458 CrossRef CAS.
- D. Wang, S. Karato and Z. Jiang, Geophys. Res. Lett., 2013, 40, 2028–2032 CrossRef CAS.
- T. Tungkavet, N. Seetapan, D. Pattavarakorn and A. Sirivat, Mater. Sci. Eng., C, 2014, 46, 281–289 CrossRef PubMed.
- Z. Qi and P. G. Pick up, Chem. Commun., 1998, 2299–2300 RSC.
- T. Dai, X. Jiang, S. Hua, X. Wang and Y. Lu, Chem. Commun., 2008, 4279–4281 RSC.
| This journal is © The Royal Society of Chemistry 2019 |