Open Access Article
Open Access ArticleA FRET-based fluorescent and colorimetric probe for the specific detection of picric acid†
Ensheng
Zhang‡
 *ab,
Ping
Ju‡
*ab,
Ping
Ju‡
 ab,
Pu
Guo
a,
Xiufang
Hou
a,
Xueyan
Hou
a,
Haiming
Lv
a,
Ji-jiang
Wang
ab,
Pu
Guo
a,
Xiufang
Hou
a,
Xueyan
Hou
a,
Haiming
Lv
a,
Ji-jiang
Wang
 a and
Yuqi
Zhang
a and
Yuqi
Zhang
 *a
*a
aLaboratory of New Energy & New Function Materials, Shaanxi Key Laboratory of Chemical Reaction Engineering, College of Chemistry and Chemical Engineering, Yan'an University, Yan'an, Shaanxi 716000, P. R. China. E-mail: yqzhang@iccas.ac.cn; sdzes2006@163.com
bCollege of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong 273165, P. R. China
First published on 12th September 2018
Abstract
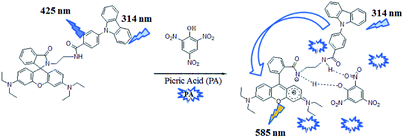
Picric acid (PA) as an environmental pollutant and high explosive, has recently received considerable attention. In this paper, a novel fluorescent and colorimetric chemo-probe (L) for the highly selective and sensitive detection of picric acid has been revealed. The probe was facilely constructed using rhodamine B, ethylenediamine and 4-(9H-carbazol-9-yl)benzoyl chloride. Significant fluorescence changes based on an intramolecular fluorescence resonance energy transfer (FRET) effect followed by a distinct color change from colorless to pink were observed after addition of picric acid to the probe solution. Selectivity measurements revealed that the as-synthesized probe exhibited high selectivity toward PA in the presence or absence of other analytes. The experimental titration results suggested that the as-synthesized probe is an effective tool for detection of PA with a nanomolar scale detection limit (820 nM) and could also serve as a “naked-eye” indicator for PA detection.
1. Introduction
Picric acid (PA), a typical water soluble polynitroaromatic compound, has been widely used in the manufacture of rocket fuels,1 fireworks,2 matches,3 dyes,4 medicines and pesticides.5 Its heavy use has caused severe environmental pollution6 and public health issues,7 such as headaches, weakness, anemia and liver injury. Another important concern regarding picric acid is its high explosive detonation properties and low safety coefficient, which might be used for terrorist attacks.7 Hence, there is an urgent need for convenient and specific analytical methods for the detection of picric acid. Especially, a high selectivity “naked-eye” probe is highly desirable.Currently, various universal analytical techniques and platforms such as high performance liquid chromatography (HPLC),8 Raman spectroscopy,9 ion-mobility spectroscopy (IMS)10 and electrochemical methods11etc., have been established for the detection of nitrogen containing aromatic (NCAs) explosives including picric acid. Although remarkable progress has been made for the above mentioned methods, some drawbacks such as high cost, less selectivity and lack of real-time monitoring limit their practical applications. Luckily, fluorescence and colorimetric sensors12,13 has emerged as a promising analysis technique in recent years and various novel materials have been designed and synthesized. For example, functional small organic molecules,14–23 metal or nonmetal nanoparticles,24,25 metal organic frameworks (MOFs),26–31etc. However, the fluorescence “turn-on” type and “naked-eye” sensors are still highly desirable for simple, sensitive, and selective detection of PA.
In this work, we report a fluorescent-colorimetric molecular probe L (Scheme 1) for the highly selective and sensitive detection of picric acid. The probe was facilely synthesized using rhodamine B, ethylenediamine and 4-(9H-carbazol-9-yl)benzoyl chloride. Distinct color change and rapid “turn-on” fluorescence were observed when treated L with picric acid, which revealed its potential application as a “naked-eye” and fluorescent indicator for picric acid. Furthermore, several advantages such as ready availability of the starting materials, efficient synthetic routes and nanomolar level (820 nM) detection limitation make this probe an useful chemosensor for PA detection.
2. Experimental sections
2.1 Instruments and reagents
1H NMR measurement were performed on Bruker AVII-400 MHz (1H NMR, 400 MHz) and OXFORD AS-500 500 MHz (1H NMR 500 MHz, 13C NMR 125 MHz) spectrometers using DMSO-d6, CD3Cl, and CD3OD as the solvents at 298 K. The chemical shifts were reported in parts per million (ppm). MS spectra were recorded on a Waters Micromass Quattro Micro API LC-MS in electrospray ionization mode and HRMS spectrum was measured on a LC-Q-TOF (ESI) apparatus. The FT-IR spectra were measured on a Shimadzu IR Affinity-1S FT-IR Spectrophotometer using the KBr pellet. Fluorescence spectra were recorded on an Agilent Cary Eclipse fluorescence spectrophotometer and the excitation wavelength is 314 nm (with excitation slit of 2.5 nm and emission slit of 5.0 nm) or 500 nm (with excitation slit of 2.5 nm and emission slit of 5.0 nm) at room temperature. Absorption spectra were monitored on a Shimadzu UV-2550 spectrophotometer.The 4-(9H-carbazol-9-yl)benzoyl chloride was synthesized from 4-(9H-carbazol-9-yl)benzoic acid and thionyl chloride (ESI, Part I†). The N-(rhodamine-B)lactam–ethylenediamine was synthesized according to the reported methods (ESI, Scheme S2†) and purified by recrystallization from anhydrous ethanol. The other chemical reagents were purchased from commercial suppliers and used without further purification.
The stock solutions of p-nitrophenol (p-NP), o-nitrodiphenol (o-NP), 4-methylphenol (4 MP), p-dihydroxybenzene (p-DHB), p-nitrobenzoic acid (p-NBA), 5-aminoisophthalic acid (5-AIPA), p-hydroxybenzoic acid (p-HBA), p-nitrophenylhydrazine (p-NPH), 2,4-dinitrophenylhydrazine (2,4-DNPH), phenylhydrazine (PH) and m-nitroaniline (m-NA), 2,4,6-trinitrotoluene (TNT, 4.4 mM in methanol) were prepared in anhydrous ethanol at the concentration of 10 mM. The concentration of the probe (L) stock solutions was 200 μM in anhydrous ethanol and diluted to 20 μM before used in the titration experiments.
Warning! Picric acid is highly explosive and should be handled carefully in small quantities.
2.2 Synthesis of the probe (L)
The purified N-(rhodamine-B)lactam–ethylenediamine (484 mg, 1.0 mmol) and triethylamine (2 mL) were dissolved in dry chloroform (5 mL) and then added to a three-necked flask. 4-(9H-carbazol-9-yl)benzoyl chloride (336 mg, 1.1 mmol) in dry chloroform (5 mL) was added dropwise to the flask over 1 h. The resulting mixture was stirred at room temperature under nitrogen atmosphere until completion of the reaction (monitored by TLC). The mixture was filtered and the filtrate was evaporated under reduced pressure. The crude product was purified by column chromatography using EtOAc–PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent. The probe (L) was obtained as white crystal in 85% yield. mp. 103 ∼ 105 °C, 1H NMR (500 MHz, CD3Cl) (T = 298 K) δ: ppm 8.55 (s, 1H, –CONHR), 8.17 (d, J = 8.55 Hz, 2H), 8.15 (d, J = 8.10 Hz, 2H), 7.96 (t, J = 4.30 Hz, 1H), 7.68 (d, J = 8.20 Hz, 2H), 7.47 (d, J = 7.50 Hz, 4H), 7.43 (t, J = 8.00 Hz, 2H), 7.31 (t, J = 7.45 Hz, 2H), 7.12 (t, J = 4.75 Hz, 1H), 6.49 (d, J = 8.85 Hz, 2H), 6.41 (d, J = 1.45 Hz, 2H), 6.29 (dd, J1 = 8.85 Hz, J2 = 1.55 Hz, 2H), 3.49 (2H, NCH2CH2N), 3.34 (q, J = 6.90 Hz, 8H), 3.25 (2H, NCH2CH2N), 1.18 (t, J = 6.90 Hz, 12H); 1H NMR (500 MHz, CD3OD) (T = 298 K) δ: ppm 8.19 (d, J = 7.65 Hz, 2H), 8.03 (d, J = 8.5 Hz, 2H), 7.95 (d, J = 6.70 Hz, 1H), 7.69 (d, J = 8.10 Hz, 2H), 7.55 (m, 2H), 7.43 (m, 4H), 7.32 (t, J = 7.15 Hz, 2H), 7.09 (d, J = 6.75 Hz, 1H), 6.47 (m, 4H), 6.39 (d, J = 8.80 Hz, 2H), 3.47 (t, J = 6.10 Hz, 2H, NCH2CH2N), 3.38 (q, J = 6.80 Hz, 8H), 3.31 (t, J = 5.95 Hz, 2H, NCH2CH2N), 1.18 (t, J = 6.75 Hz, 12H); 13C NMR (125 MHz, CD3OD) δ: ppm 169.8 (C
2) as eluent. The probe (L) was obtained as white crystal in 85% yield. mp. 103 ∼ 105 °C, 1H NMR (500 MHz, CD3Cl) (T = 298 K) δ: ppm 8.55 (s, 1H, –CONHR), 8.17 (d, J = 8.55 Hz, 2H), 8.15 (d, J = 8.10 Hz, 2H), 7.96 (t, J = 4.30 Hz, 1H), 7.68 (d, J = 8.20 Hz, 2H), 7.47 (d, J = 7.50 Hz, 4H), 7.43 (t, J = 8.00 Hz, 2H), 7.31 (t, J = 7.45 Hz, 2H), 7.12 (t, J = 4.75 Hz, 1H), 6.49 (d, J = 8.85 Hz, 2H), 6.41 (d, J = 1.45 Hz, 2H), 6.29 (dd, J1 = 8.85 Hz, J2 = 1.55 Hz, 2H), 3.49 (2H, NCH2CH2N), 3.34 (q, J = 6.90 Hz, 8H), 3.25 (2H, NCH2CH2N), 1.18 (t, J = 6.90 Hz, 12H); 1H NMR (500 MHz, CD3OD) (T = 298 K) δ: ppm 8.19 (d, J = 7.65 Hz, 2H), 8.03 (d, J = 8.5 Hz, 2H), 7.95 (d, J = 6.70 Hz, 1H), 7.69 (d, J = 8.10 Hz, 2H), 7.55 (m, 2H), 7.43 (m, 4H), 7.32 (t, J = 7.15 Hz, 2H), 7.09 (d, J = 6.75 Hz, 1H), 6.47 (m, 4H), 6.39 (d, J = 8.80 Hz, 2H), 3.47 (t, J = 6.10 Hz, 2H, NCH2CH2N), 3.38 (q, J = 6.80 Hz, 8H), 3.31 (t, J = 5.95 Hz, 2H, NCH2CH2N), 1.18 (t, J = 6.75 Hz, 12H); 13C NMR (125 MHz, CD3OD) δ: ppm 169.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 167.5 (C
O), 167.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 154.0, 153.4, 149.0, 140.6, 140.3, 132.8, 132.7, 130.3, 128.8, 128.1, 126.2, 125.8, 123.7, 123.6, 122.2, 120.1, 120.0, 119.9, 109.3, 108.3, 104.5, 97.7, 66.0 (spiro-atom), 44.0, 39.4, 39.3, 11.5; FT-IR (KBr) νmax/cm−1 3325 (NH), 3054, 2971, 2931, 1677 (spirolactam ring C
O), 154.0, 153.4, 149.0, 140.6, 140.3, 132.8, 132.7, 130.3, 128.8, 128.1, 126.2, 125.8, 123.7, 123.6, 122.2, 120.1, 120.0, 119.9, 109.3, 108.3, 104.5, 97.7, 66.0 (spiro-atom), 44.0, 39.4, 39.3, 11.5; FT-IR (KBr) νmax/cm−1 3325 (NH), 3054, 2971, 2931, 1677 (spirolactam ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1620 (C
O), 1620 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1488, 1445, 1225, 1121, 759; MS (ESI) calcd for C49H48N5O3 [M + H]+, found: 754.7; HRMS (ESI-TOF): m/z [M + H]+ calcd for C49H48N5O3: 754.3752; found: 754.3757.
O), 1488, 1445, 1225, 1121, 759; MS (ESI) calcd for C49H48N5O3 [M + H]+, found: 754.7; HRMS (ESI-TOF): m/z [M + H]+ calcd for C49H48N5O3: 754.3752; found: 754.3757.
3. Results and discussion
3.1 Solvent selection and response kinetics
In order to obtain a suitable and green reaction system, the fluorescence intensity of L in the presence or absence of PA was studied in different solvents (λex = 500 nm, λem = 586 nm, slits: 2.5 nm/5 nm). As shown in Fig. 1, no-fluorescence was detected in the absence of PA in all the tested solvents. However, remarkable turn-on fluorescence enhancement was observed after the addition of PA in methanol (MeOH) and ethanol (EtOH) solution which were more efficient hydrogen bond donors. Meanwhile, the fluorescence intensity was extremely weak in dimethyl formamide (DMF) and tetrahydrofuran (THF). Hence, ethanol was chosen as the solvent for the following fluorescent and colorimetric tests.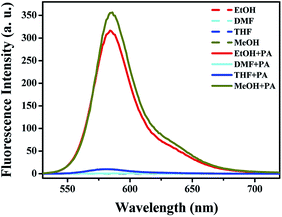 | ||
| Fig. 1 Fluorescence intensity of L (20 μM) in the presence (200 μM) or absence of PA (λex = 500 nm, λem = 586 nm, slits: 2.5 nm/5 nm) in different solvents. | ||
The fluorescence and UV-vis spectra of the probe solution (20 μM) were recorded every 5 min after addition of PA (200 μM). As depicted in Fig. S10,† a new and remarkable fluorescence enhancement at the wavelength of 586 nm could be observed after addition of PA for 5 min. With increasing the response time, the fluorescence intensity increased and reached the maximum after 80 min (Fig. 2a). Correspondingly, a new UV-vis absorption band centered at 560 nm appeared after addition of PA for 5 min (Fig. S11†) and the absorbance intensity reached the equilibrium at 80 min (Fig. 2b and S11†). Hence, all the fluorescence and UV-vis spectra were recorded at 80 min after addition of PA at room temperature. It is worth mentioning that, a recognizable color change of the probe solution from colorless (without PA) to pink (after reacted with PA) was observed together with the above mentioned process.
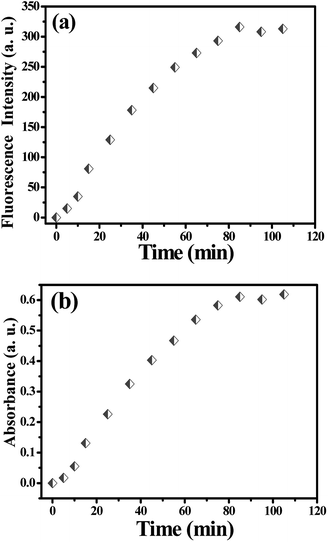 | ||
| Fig. 2 (a) The fluorescence intensity (λem = 586 nm) of the probe solution after addition of PA with time; (b) the absorbance (λ = 560 nm) of the probe solution after addition of PA with time. | ||
3.2 Selectivity of the probe L
Probe solutions (2 mL, 20 μM) were treated with various phenols (4-MP, p-DHB, p-HBA) and NCAs (PA, p-NP, o-NP, p-NBA, 5-AIPA, p-NPH, 2,4-DNPH, PH, m-NA and TNT) at the same concentration (200 μM) for the selectivity studies. The UV-Visible absorbance spectra were shown in Fig. 3a. Obviously, a new absorption band centered at about 560 nm occurred only when adding PA to the solution. As recorded in the photograph (Fig. 3b), the solution color changed from colorless to pink after the addition of PA. This could be attributed to the formation of the spiro-lactam ring-opening product, which implied that the as-synthesized molecular probe L might be served as a “naked-eyes” probe for PA.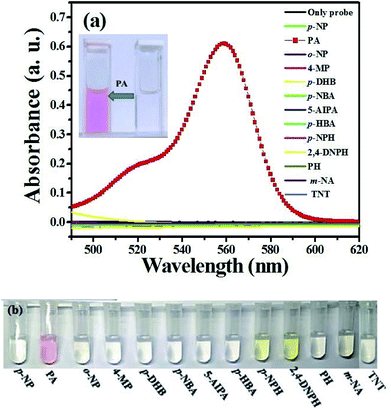 | ||
| Fig. 3 (a) Absorption spectra of L (20 μM) in the presence of various phenols (200 μM) or NCAs (200 μM); (b) corresponding photographs. | ||
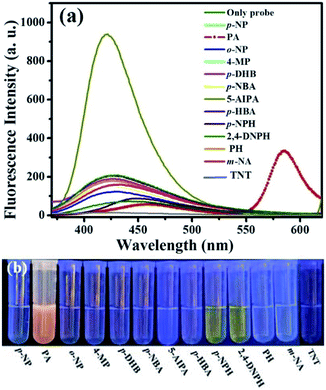
The selectivity of this probe was further evaluated by observing the fluorescence spectra of the probe solutions in the presence of various analytes. We chose 314 nm as the excitation wavelength because there was an absorbance band centred at 314 nm for the free probe solution, which could be attributed to the absorbance of 4-(9H-carbazol-9-yl)benzamide fluorophore (Fig. S12 in ESI†). For the original solution of probe L, a fluorescence emission at the wavelength of 420 nm occurred (Fig. 4a) based on the emission of the 4-(9H-carbazol-9-yl)benzamide fluorophore. Interestingly, after the addition of analytes including phenols and NCAs, this emission band changed irregularly, but only PA generated a remarkable new emission band centered at 586 nm. It can be seen that the fluorescence emission peak red-shifted from 420 nm to 460 nm and the intensity decreased in the presence of PA. Particularly, a strong fluorescence emission at 586 nm could be clearly observed, which resulted from the emission of the newly formed delocalized xanthane fluorophore (spiro-lactam ring-opening product). Fig. 4b displays the photograph of probe solutions with different analytes under 365 nm UV-light. A bright orange emission was observed after adding PA to the probe solution, which was obviously different from other solutions. For the compound 5-AIPA, the solution emitted bright blue fluorescence which might be due to the enhancement effect of 5-AIPA and probe (Fig. 4a).
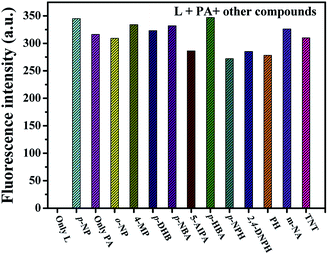
The interference measurements were carried out by recording the fluorescence emission intensity changes (λem = 586 nm) after adding 200 μM other analytes (phenols and other NCAs) to the mixture of L and PA (L 20 μM and PA 200 μM). As shown in Fig. 5, no remarkable fluorescence intensity change was observed for the mixture solution (probe 20 μM and PA 200 μM) before and after addition of interfering compounds (200 μM). These results demonstrated that the as-synthesized probe L is highly selective towards PA and its response cannot be interfered by other compounds in ethanol solution.
3.3 Sensitivity of probe L
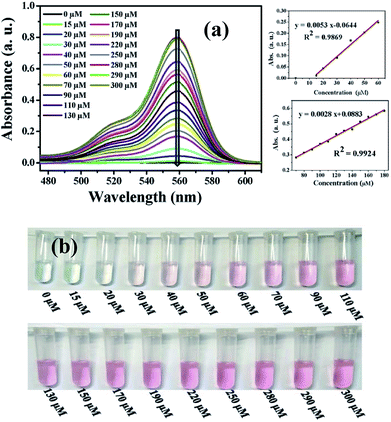
The sensitivity were assessed by observing the fluorescence and UV-visible spectra of probe solution (2 mL, 20 μM) with different concentration of PA (0–300 μM). In addition, all the fluorescence and UV-vis spectra were recorded at room temperature and the measurement system were transparent and homogeneous.Fig. 6a (left) shows that there is no absorption band at wavelength range from 500–600 nm in the absence of PA and the probe solution is colorless (Fig. 6b). However, a new absorption band centered at 560 nm appeared when upon addition of 15 μM PA. With increasing the concentration of PA from 15 μM to 280 μM, the absorbance intensity at 560 nm steadily increased and leveled off to a constant value at 280 μM, accompanying with a gradual color change from colorless to pink (Fig. 6b). Linear fitting curves of the absorption intensity at 560 nm versus the concentration of PA were obtained, and displayed in Fig. 6a (right). Obviously, the absorbance intensity displays two good linear correlations with the concentration of PA in the range of 15–60 μM (R2 = 0.9869) and 70–180 μM (R2 = 0.9924). The detection limit of L for PA calculated according to the eq. 3σ/k (ref. 32 and 33) was 820 nM, indicating the probe molecule is high sensitive to PA.
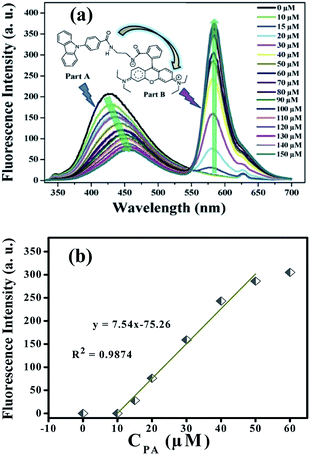
To further investigate the detection performance of the probe (L) towards PA, a series of fluorescence titration experiments were carried out. As shown in Fig. 7a, the fluorescence emission intensity at 420 nm gradually decreased and the emission peak shifted from 420 to 460 nm with the increasing of PA concentration. Most importantly, a new fluorescence emission band at 586 nm appeared after the addition of 15 μM PA, and the intensity increased gradually in this process. (Photograph of probe solutions with different concentration of PA under 365 nm UV light was exhibited in Fig. S13†). Linear fitting analysis (λex = 314 nm, λem = 586 nm) according to the fluorescence titration experiments was created and displayed in Fig. 7b. As shown in Fig. 7b, the emission intensity at 586 nm show a good linear relationship with R2 = 0.9874 over the range of 10 μM to 50 μM, which indicating good sensing properties for PA.
Interestingly, a fluorescence resonance energy transfer (FRET) phenomenon might occurred in the molecule of L. The emission at 420 nm can be attributed to 4-(9H-carbazol-9-yl)benzamide subunit (Part A of inset in Fig. 7a), which is a potential energy donor. After the addition of PA, the spiro-lactam ring opened and the rhodamine fluorophore (Part B of inset in Fig. 7a, the energy acceptor) formed simultaneously. A good overlap of the fluorescence spectrum of Part A (the energy donor unit) and the absorption spectrum of Part B (the energy acceptor unit) was observed (ESI, Part III Fig. S14†). In this case, the fluorescence emitted by Part A was absorbed by Part B, and used as the excitation light which induced the remarkable fluorescence at 586 nm. A series of control experiments were performed under the identical conditions using N-(rhodamine-B)lactam–ethylenediamine and PA in ethanol, which also proved the existence of the intramolecular FRET effect (ESI, Part III Fig. S15 and S16†). In addition, this intramolecular FRET effect was further confirmed by the fluorescence decays assay34–37 (ESI, Part III Fig. S17†)
3.4 Binding mode investigation
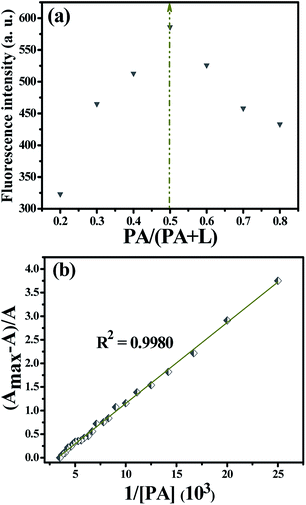
In order to reveal the stoichiometry between L and PA, mixture solutions of probe and PA with a total concentration of 200 μM (L + PA) were used in the preparation of the Job's plot of complex (L + PA) in ethanol38–40 and the results were displayed in Fig. 8a. Obviously, the plot revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for PA and L (Fig. 8a). This binding mode was also verified by the Benesi–Hildebrand plots,41,42 which was obtained according to the colorimetric titration experiments (ESI, Part IV†). The association constant (Ka) calculated according to the Benesi–Hildebrand plots (Fig. 8b) was 5834 L mol−1.
1 stoichiometry for PA and L (Fig. 8a). This binding mode was also verified by the Benesi–Hildebrand plots,41,42 which was obtained according to the colorimetric titration experiments (ESI, Part IV†). The association constant (Ka) calculated according to the Benesi–Hildebrand plots (Fig. 8b) was 5834 L mol−1.
3.5 Sensing mechanism
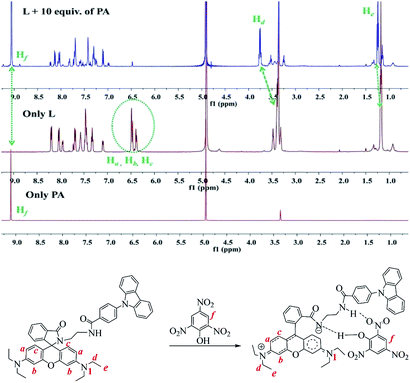
1H NMR spectroscopic titrations were performed in CD3OD to reveal the turn-on fluorescence mechanism and identify the possible binding position of PA (Fig. 9 and S19†). As depicted in Fig. 9, the peaks of Hd and He were clearly shifted downfield (0.36 ppm for Hd and 0.07 ppm for He), which indicated the positive electricity of N1 increased after the treatment with PA. Another remarkable change is the signals of Ha, Hb and Hc at 6.37–6.48 ppm, which almost disappeared in the presence of 1 equiv. of PA (shifted downfield from 6.37–6.48 ppm to 6.90–8.25 ppm, Fig. 9 and S19†). However, no significant change was observed with further increasing of PA from 2 equiv. to 10 equiv. revealing that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry was formed between L and PA (Fig. S19†). Furthermore, no obvious shift for Hf was observed, which suggested there was no significant π–π stacking or electron transfer between probe and PA.43,44
1 binding stoichiometry was formed between L and PA (Fig. S19†). Furthermore, no obvious shift for Hf was observed, which suggested there was no significant π–π stacking or electron transfer between probe and PA.43,44
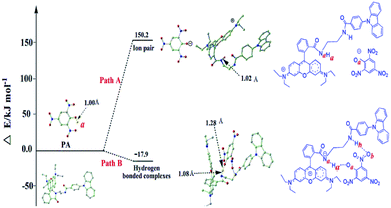
The detection mechanism of this fluorescence “OFF–ON” type and “naked-eye” chemo-sensor for PA was further studied through theoretical calculations method (DFT calculation)45. Optimized geometries of the probe and PA as well as the energy changes are displayed in Fig. 10 It is clear that energy will be released when the hydrogen bonded complex is formed (ΔE = −17.9 kJ mol−1, Fig. 10 Path B). However, if the hydrogen completely transfers from PA (Ha) to L (Na) and form the ion pair, it would be a significantly endothermic process (ΔE = +150.2 kJ mol−1, Fig. 10 Path A) which is not preferred.
The bond distances of Na–Ha and Oa–Ha are 1.08 Å and 1.28 Å, respectively, which indicates that there is a strong attraction between L and PA linked by Ha. Another hydrogen bond between Ob–Hh could also be observed as shown in Fig. 10 Path B.
With the above results in hand, a plausible sensing mechanism was proposed, as shown in Scheme 2. The spiro-lactam ring in probe L was opened after the addition of PA and hydrogen bonds formed between probe and PA. The formation of the delocalized xanthene motif would be response for the remarkable color changes (from colorless to pink) and fluorescence enhancement (intramolecular FRET phenomenon) of the sensor solution.
3.6 Practical application
In order to reveal the potential application of the as-synthesized probe, test strips were prepared. Filter papers were immersed in the probe stock solution and dried in air, then immersed into different concentration of PA solutions in water (50 μM, 100 μM, 1 mM, 10 mM). As shown in Fig. 11, the pink color as well as the fluorescence intensity of the test papers intensified with the increasing PA concentrations. It is worth mention that the naked eye detectable concentration is about 50 μM. | ||
| Fig. 11 Test strips for the detection of PA with the concentration from 50 μM to 10 mM under sunlight (a) and 365 nm UV light (b). | ||
4. Conclusion
In summary, a FRET based fluorescent and colorimetric chemo-probe for the specific detection of PA was revealed. The good selectivity and high sensitivity were demonstrated by fluorescence and colorimetric titration experiments, and an intermolecular FRET phenomenon was also observed and confirmed. The detection limit according to the colorimetric titration was found to be 820 nM, which indicating high detection sensitivity. Quantitative fluorescence and colorimetric titration revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry for PA and the probe. A plausible mechanism was postulated according to the binding mode studies, NMR analysis and the theoretical calculations. In addition, the notable features of this probe such as good selectivity and high sensitivity make it an useful indicator for PA detection.
1 stoichiometry for PA and the probe. A plausible mechanism was postulated according to the binding mode studies, NMR analysis and the theoretical calculations. In addition, the notable features of this probe such as good selectivity and high sensitivity make it an useful indicator for PA detection.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Natural Science Foundation of China (21663032), Natural Science Foundation of Shaanxi Province (2018JQ2079, 2018JQ2040), Scientific Research Program Funded by Shaanxi Provincial Education Department (17JK0869, 15JS121), Yan'an Science &Technology Innovation Team (No. 2015CHTD-04), Startup Foundation for Docotors and Youth Science Foundation of Yan'an University (YDBK2015-06, YDBY2016-001, YDQ2017-14), National Undergraduate Training Programs for Innovation and Entrepreneurship (201710719005).References
- J. Shen, J. Zhang, Y. Zuo, L. Wang, X. Sun, J. Li, W. Han and R. He, J. Hazard. Mater., 2009, 163, 1199–1206 CrossRef CAS.
- V. Bhalla, A. Gupta, M. Kumar, D. S. Rao and S. K. Prasad, ACS Appl. Mater. Interfaces, 2013, 5(3), 672–679 CrossRef CAS.
- V. Bhalla, S. Kaur, V. Vij and M. Kumar, Inorg. Chem., 2013, 52(9), 4860–4865 CrossRef CAS.
- E. H. Volwiler, Ind. Eng. Chem., 1926, 18(12), 1336–1337 CrossRef CAS.
- P. G. Thorne and T. F. Jenkins, Field Anal. Chem. Technol., 1997, 1(3), 165–170 CrossRef.
- Y. J. Tan, S. Z. Hu, G. L. Shen and R. Q. Yu, Anal. Chim. Acta, 2006, 570(2), 170–175 CrossRef PubMed.
- B. Roy, A. K. Bar, B. Gole and P. S. Mukherjee, J. Org. Chem., 2013, 78(3), 1306–1310 CrossRef CAS.
- C. Behrend and K. Heesche-Wagner, Appl. Environ. Microbiol., 1999, 65(4), 1372–1377 CAS.
- J. M. Sylvia, J. A. Janni, J. D. Klein and K. M. Spencer, Anal. Chem., 2000, 72(23), 5834–5840 CrossRef CAS.
- J. S. Caygill, F. Davis and S. P. Higson, Talanta, 2012, 88, 14–29 CrossRef CAS.
- R. Hodyss and J. L. Beauchamp, Anal. Chem., 2005, 77(11), 3607–3610 CrossRef CAS.
- Y. B. Ding, Y. Y. Tang, W. H. Zhu and Y. S. Xie, Chem. Soc. Rev., 2015, 44, 1101–1112 RSC.
- Y. B. Ding, W. H. Zhu and Y. S. Xie, Chem. Rev., 2017, 117(4), 2203–2256 CrossRef CAS.
- S. Sandhu, R. Kumar, P. Singh, A. Mahajan, M. Kaur and S. Kumar, ACS Appl. Mater. Interfaces, 2015, 7(19), 10491–10500 CrossRef CAS.
- K. Maiti, A. K. Mahapatra, A. Gangopadhyay, R. Maji, S. Mondal, S. S. Ali, S. Das, R. Sarkar, P. Datta and D. Mandal, ACS Omega, 2017, 2(4), 1583–1593 CrossRef CAS.
- B. Gogoi and N. Sen Sarma, ACS Appl. Mater. Interfaces, 2015, 7(21), 11195–11202 CrossRef CAS.
- C. Wu, J. L. Zhao, X. K. Jiang, X. L. Ni, X. Zeng, C. Redshaw and T. Yamato, Anal. Chim. Acta, 2016, 936, 216–221 CrossRef CAS.
- J. F. Xiong, J. X. Li, G. Z. Mo, J. P. Huo, J. Y. Liu, X. Y. Chen and Z. Y. Wang, J. Org. Chem., 2014, 79(23), 11619–11630 CrossRef CAS.
- G. Sathiyan and P. Sakthivel, RSC Adv., 2016, 6(108), 106705–106715 RSC.
- L. Cao, J. F. Xiong, Y. C. Wu, S. Ding, M. B. Li, F. Xie, Z. H. Ma and Z. Y. Wang, Chin. J. Org. Chem., 2016, 36, 2053–2074 CrossRef CAS.
- Y. C. Wu, S. H. Luo, L. Cao, K. Jiang, L. Y. Wang, J. C. Xie and Z. Y. Wang, Anal. Chim. Acta, 2017, 976, 74–83 CrossRef CAS PubMed.
- S. Nath, S. K. Pathak, B. Pradhan, R. K. Gupta, K. A. Reddy, G. Krishnamoorthy and A. S. Achalkumar, New J. Chem., 2018, 42(7), 5382–5394 RSC.
- Y. Gao, Y. Qi, K. Zhao, Q. Wen, J. Shen, L. Qiu and W. Mou, Sens. Actuators, B, 2018, 257, 553–560 CrossRef CAS.
- J. R. Zhang, Y. Y. Yue, H. Q. Luo and N. B. Li, Analyst, 2016, 141(3), 1091–1097 RSC.
- T. M. Geng, S. N. Ye, Y. Wang, H. Zhu, X. Wang and X. Liu, Talanta, 2017, 165, 282–288 CrossRef CAS.
- A. Buragohain, M. Yousufuddin, M. Sarma and S. Biswas, Cryst. Growth Des., 2016, 16(2), 842–851 CrossRef CAS.
- Y. H. Zhang, B. Li, H. P. Ma, L. M. Zhang and W. X. Zhang, J. Mater. Chem. C, 2017, 5(19), 4661–4669 RSC.
- H. Guo, Y. Zhang, Z. Zheng, H. Lin and Y. Zhang, Talanta, 2017, 170, 146–151 CrossRef CAS.
- J. Ye, L. Zhao, R. F. Bogale, Y. Gao, X. Wang, X. Qian and G. Ning, Chem.–Eur. J., 2015, 21(5), 2029–2037 CrossRef CAS.
- Y. Deng, N. Chen, Q. Li, X. Wu, X. Huang, Z. Lin and Y. Zhao, Cryst. Growth Des., 2017, 17(6), 3170–3177 CrossRef CAS.
- B. Joarder, A. V. Desai, P. Samanta, S. Mukherjee and S. K. Ghosh, Chem.–Eur. J., 2015, 21(3), 965–969 CrossRef CAS.
- J. Liu and Y. Lu, Angew. Chem., 2007, 119, 7731–7734 CrossRef.
- L. Peng, Q. Zhao, D. Wang, J. Zhai, P. Wang, S. Pang and T. Xie, Sens. Actuators, B, 2009, 136, 80–85 CrossRef CAS.
- K. E. Sapsford, L. Berti and I. L. Medintz, Angew. Chem., Int. Ed., 2006, 45, 4562–4588 CrossRef CAS.
- M. Tramier, M. Zahid, J. C. Mevel, M. J. Masse and M. Coppey-Moisan, Microsc. Res. Tech., 2006, 69(11), 933–939 CrossRef CAS.
- P. I. Bastiaens and A. Squire, Trends Cell Biol., 1999, 9(2), 48–52 CrossRef CAS.
- N. Andrews, M. C. Ramel, S. Kumar, Y. Alexandrov, D. J. Kelly, S. C. Warren, L. Kerry, N. Lockwood, A. Frolov, P. Frankel, L. Bugeon, J. McGinty, M. J. Dallman and P. M. W. French, J. Biophotonics, 2016, 9(4), 414–424 CrossRef CAS.
- Q. H. You, P. S. Chan, W. H. Chan, S. C. Hau, A. W. Lee, N. K. Mak and R. N. Wong, RSC Adv., 2012, 2(29), 11078–11083 RSC.
- L. Zhang, J. Fan and X. Peng, Spectrochim. Acta, Part A, 2009, 73(2), 398–402 CrossRef.
- A. Kumar and N. Ahmed, Ind. Eng. Chem. Res., 2017, 56(22), 6358–6368 CrossRef.
- M. Suresh, A. K. Mandal, S. Saha, E. Suresh, A. Mandoli, R. Di Liddo and A. Das, Org. Lett., 2010, 12(23), 5406–5409 CrossRef CAS.
- B. Chowdhury, S. Khata, R. Dutta, S. Chakraborty and P. Ghosh, Inorg. Chem., 2014, 53(15), 8061–8070 CrossRef CAS.
- A. Kumar, A. Pandith and H. S. Kim, Sens. Actuators, B, 2016, 231, 293–301 CrossRef CAS.
- D. C. Santra, M. K. Bera, P. K. Sukul and S. Malik, Chem.–Eur. J., 2016, 22(6), 2012–2019 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.1, Gaussian Inc, Wallingford CT, USA, 2009 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05468a |
| ‡ Ensheng Zhang and Ping Ju equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2018 |