A chiral aluminum solvating agent (CASA) for 1H NMR chiral analysis of alcohols at low temperature†
Min-Seob
Seo
,
Sumin
Jang
and
Hyunwoo
Kim
 *
*
Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Korea. E-mail: hwkim@kaist.edu
First published on 6th March 2018
Abstract
A chiral aluminum solvating agent (CASA) was demonstrated to be a general and efficient reagent for 1H NMR chiral analysis of alcohols. The sodium salt of the CASA (CASA-Na) showed a complete baseline peak separation of the hydroxyl group for various chiral alcohols including primary, secondary, and tertiary alcohols with alkyl and aryl substituents in CD3CN. Due to the weak intermolecular interaction, 1H NMR measurement at low temperature (−40 to 10 °C) was required.
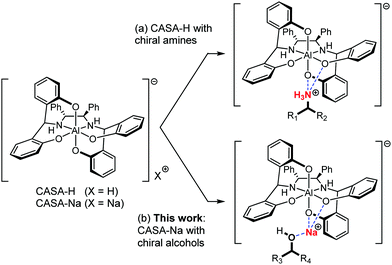
Chiral analysis such as determination of the absolute configuration and the relative ratio of enantiomers is indispensable in the asymmetric synthesis of chiral compounds.1,2 Quantitative chiral analysis has largely relied on chromatographic techniques using high-performance liquid chromatography (HPLC) or gas chromatography (GC) with a column coated or immobilized with chiral stationary phases.3 In addition, NMR spectroscopy can be a complementary analytical technique for chiral analysis because it is the most conveniently and widely used method for the structural determination of compounds. However, for the NMR chiral analysis, chiral analytes should be converted to diastereomeric mixtures by covalent bond formation or non-covalent interactions with chiral reagents,1 or by self-association with achiral reagents4 in order to generate anisochronous chemical shifts. Among these NMR chiral analyses, the use of chiral solvating agents (CSAs) using non-covalent interactions is operationally simple and convenient because the sample can be directly analyzed by mixing with a CSA. The reported CSAs include unsaturated metal complexes,5 Brønsted–Lowry acids/bases,6 hydrogen bonding reagents,7 supramolecular receptors,8 and others.9 However, analytes are mainly limited to amines or carboxylic acids because they can form relatively strong charged hydrogen bonding. Although several efficient CSAs have been reported, it is still quite challenging to use CSAs for other polar compounds. Indeed, for example, chiral alcohols are common structural motifs synthesized by many stereoselective reactions, but only a few cases of chiral analysis using CSAs have been reported and moreover, they tend to exhibit insufficient peak separation and narrow analyte scope.10,11 We recently introduced negatively charged octahedral aluminum complexes as an efficient CSA for charged compounds including amines (Fig. 1a).12 Here, we demonstrate that our chiral aluminum complexes can be generally applicable for chiral solvation of alcohols (Fig. 1b).
A chiral aluminum solvating agent (CASA), a negatively charged octahedral ate complex, was readily prepared by a stereoselective assembly of the N2O4 ligand with AlCl3. We demonstrated that the protonated form, CASA-H, can be used for chiral solvation of amines and the sodium form, CASA-Na, can be used for chiral solvation of carboxylic acids. The CASA is one of the most efficient CSAs efficiently working with almost all types of charged compounds including amines and carboxylic acids in polar and nonpolar solvents.13 In order to test the chiral solvation ability of the CASA for chiral alcohols, we initially compared the chiral solvation of an alcohol, 2-phenylethanol (1), and a structurally related carboxylic acid, 2-phenylpropionic acid (2), with CASA-Na.
When the (R,R)-form of CASA-Na was used in CD3CN, rac-2-phenylpropionic acid (2) showed a sufficient baseline peak separation of ΔΔδ14 = 0.047 ppm, but rac-1-phenylethanol (1) showed only a small peak separation of ΔΔδ = 0.013 ppm (Fig. 2). Because of the structural similarity of 1 and 2, we proposed that the binding strength of carboxylic acid and alcohol functionality to the CASA may be related to the chiral solvation ability. Indeed, the binding constants between (R,R)-CASA-Na and chiral alcohols (R)-1 and (S)-1 were measured to be 0.348 and 0.290 M−1, while those between (R,R)-CASA-Na and chiral acids (R)-2 and (S)-2 were measured to be 28.2 and 25.9 M−1, respectively (Fig. 2, ESI†). The binding constants for alcohol 1 are about 80–90 times weaker than those for carboxylic acid 2. Interestingly, (R,R)-CASA-Na binds 1.1–1.2 times stronger with the (R)-enantiomer than with the (S)-enantiomer. Such a weak intermolecular interaction between a receptor and an alcohol analyte is the reason why efficient CSAs for chiral alcohol have not been developed so far.
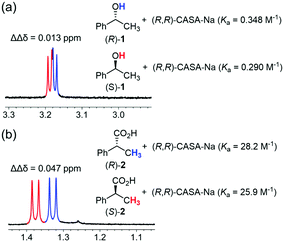 | ||
| Fig. 2 Partial 1H NMR spectra and measured binding constants for chiral solvation of (a) 1-phenylethanol (1) and (b) 2-phenylpropionic acid (2) with (R,R)-CASA-Na at 25 °C in CD3CN. | ||
We then attempted to improve the peak separation ability of the CASA for chiral alcohols. Changing solvents or counter cations was not successful. In a series of analyte tests, we found that 1-phenylbutanol (3) showed a peak separation (ΔΔδ = 0.020 ppm) at 25 °C in CD3CN. Because 1-phenylbutanol is an extended structure of 1-phenylethanol by substituting a methyl group with an n-propyl group, we proposed that the increase of hydrophobic interaction would improve the peak separation of diastereomeric mixtures of chiral alcohols and CASA-Na. Indeed, when rac-1-phenyldodecanol (4) was used, a better peak separation (ΔΔδ = 0.028 ppm) was observed (Fig. 3a). Thus, increasing hydrophobicity is a method to achieve a baseline peak separation for chiral solvation of alcohols with CASA-Na. In addition, we wanted to develop a more general method applicable to most chiral alcohol analytes. As a reduction in temperature can shift the equilibrium towards the formation of the complex, we lowered the temperature to increase the enantiodifferentiation. To our delight, an increase of peak separation of rac-1-phenylethanol (1) was observed with CASA-Na and a sufficient baseline peak separation was obtained at −10 °C (ΔΔδ = 0.032 ppm) and −20 °C (ΔΔδ = 0.046 ppm) (Fig. 3b). Because the temperature control is a simple operation in modern NMR spectroscopy, the chiral solvation with CASA-Na can be conveniently used for most chiral alcohols.
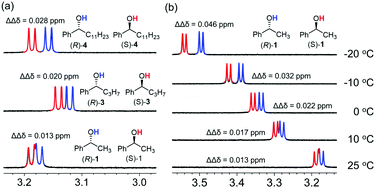 | ||
| Fig. 3 The effect of (a) hydrophobicity and (b) temperature for chiral solvation of hydroxyl groups of alcohols with CASA-Na in CD3CN. | ||
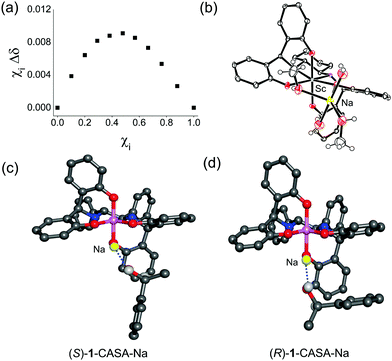
In order to understand the origin of chiral solvation of alcohols with a CASA, we first obtained a Job plot to find the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct formed between CASA-Na and 1-phenyldodecanol (4) (Fig. 4a). All our efforts to obtain an X-ray structure of such a 1
1 adduct formed between CASA-Na and 1-phenyldodecanol (4) (Fig. 4a). All our efforts to obtain an X-ray structure of such a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct with CASA-Na and alcohol analyte failed, but we obtained the X-ray structure of the Sc(III) complex from methanolic solution (Fig. 4b). In the crystal structure of the Sc complex, two MeOH and two H2O molecules coordinate to the Na+ ion in the unit cell.13 Thus, the Job plot and the crystal structure provided us insight to propose a 1
1 adduct with CASA-Na and alcohol analyte failed, but we obtained the X-ray structure of the Sc(III) complex from methanolic solution (Fig. 4b). In the crystal structure of the Sc complex, two MeOH and two H2O molecules coordinate to the Na+ ion in the unit cell.13 Thus, the Job plot and the crystal structure provided us insight to propose a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model between CASA-Na and 1-phenylethanol (1). We then calculated the energy minimum structures of (R,R)-CASA-Na/(R)-1 and (R,R)-CASA-Na/(S)-1 (Fig. 4c and d). The DFT computation showed that both complexes have comparable energy with a free energy difference of 0.04 kcal mol−1. In both optimized structures, chiral alcohols (R)-1 and (S)-1 bind to the sodium cation with a geometry where the proton at the chiral carbon center is placed toward the CASA in order to minimize the steric repulsion. Thus, the methyl group of (S)-1 is placed toward the phenol groups of the CASA, whereas that of (R)-1 is placed away from the phenol groups. On the basis of the proposed 1-CASA complexes, the methyl group of (S)-1 is expected to be more shielded than that of (R)-1. Indeed the calculated methyl peaks (1.71 and 1.15 ppm for (R)-1 and (S)-1, respectively) relatively agree with the measured methyl peaks (1.41 and 1.40 ppm for (R)-1 and (S)-1, respectively). Moreover, the calculated chemical shifts for the proton of the OH groups were found to be 1.49 and 1.59 ppm for (R)-1 and (S)-1, respectively, which also agreed with the observed 1H NMR spectra.
1 binding model between CASA-Na and 1-phenylethanol (1). We then calculated the energy minimum structures of (R,R)-CASA-Na/(R)-1 and (R,R)-CASA-Na/(S)-1 (Fig. 4c and d). The DFT computation showed that both complexes have comparable energy with a free energy difference of 0.04 kcal mol−1. In both optimized structures, chiral alcohols (R)-1 and (S)-1 bind to the sodium cation with a geometry where the proton at the chiral carbon center is placed toward the CASA in order to minimize the steric repulsion. Thus, the methyl group of (S)-1 is placed toward the phenol groups of the CASA, whereas that of (R)-1 is placed away from the phenol groups. On the basis of the proposed 1-CASA complexes, the methyl group of (S)-1 is expected to be more shielded than that of (R)-1. Indeed the calculated methyl peaks (1.71 and 1.15 ppm for (R)-1 and (S)-1, respectively) relatively agree with the measured methyl peaks (1.41 and 1.40 ppm for (R)-1 and (S)-1, respectively). Moreover, the calculated chemical shifts for the proton of the OH groups were found to be 1.49 and 1.59 ppm for (R)-1 and (S)-1, respectively, which also agreed with the observed 1H NMR spectra.
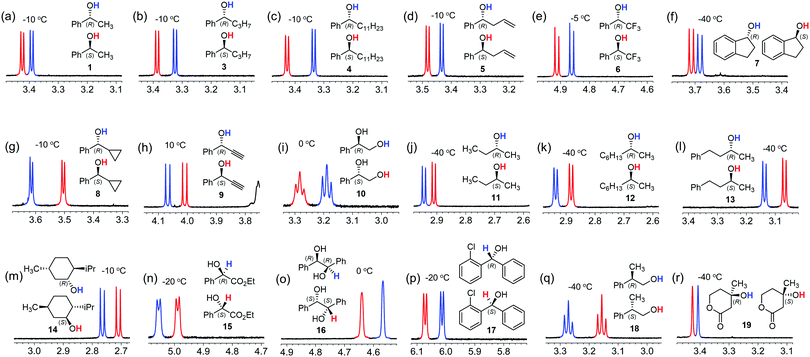
The utility of the CASA for chiral solvation of alcohols was demonstrated with various chiral alcohols 1 and 3–19 as shown in Fig. 5. To our delight, we could achieve baseline peak separation for all of the chiral alcohols tested. In many cases, the proton peak of the hydroxy group was well-separated in the 1H NMR spectra in the presence of CASA-Na. The recorded temperature ranged from −40 °C to 10 °C based on the substituents of the chiral alcohols related to hydrophobicity. First, we successfully differentiated secondary alcohols with one phenyl substituent at the chiral carbon center. All OH peaks from (R)-phenyl-substituted alcohols 1 and 3–7 with linear alkyl, allyl, CF3, and cyclic alkyl groups are located at more upfield positions than those from (S)-alcohols (Fig. 5a–f). However, the relative peak pattern was inverted with substituents of cyclopropyl and alkynyl groups (Fig. 5g and h). Thus, it is still primitive to generally apply chiral solvation with a CASA to predict the absolute chirality of chiral alcohols. In addition, CASA-Na was also effective for alkyl substituted secondary alcohols 11–14. In cases of chiral secondary alcohols with alkyl substituents, a low temperature of −40 °C was generally required to achieve baseline peak separation and all OH peaks of (R)-alcohols appeared in more downfield positions than those of (S)-alcohols. Finally, other chiral alcohols including diol 16, diaryl-substituted secondary alcohol 17, primary alcohol 18, and tertiary alcohol 19 were well resolved in the 1H NMR spectra with CASA-Na in CD3CN. Thus, the chiral solvation with CASA-Na can be a general method for chiral solvation of chiral alcohols.
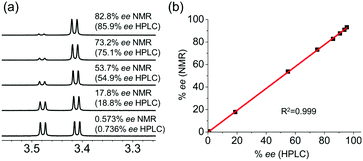
We next investigated the quantitative chiral analysis of a chiral alcohol. As shown in Fig. 6a, 1-phenylbutanol 3 with various % ee was analyzed with CASA-Na. The chemical shifts of the resolved peaks were almost constant, implying that Ka values are rather close.15 Accordingly, reliable integration of major and minor peaks can be achieved. In order to ensure a fair comparison, we measured % ee with both NMR and HPLC, and both measurements showed an excellent linear relationship (Fig. 6b). Accordingly, 1H NMR chiral analysis of alcohols with CASA-Na can be a complementary analytical tool together with classical chromatographic methods. In addition, when compared with several reported CSAs,11 CASA-Na showed better peak separation of the hydroxyl groups in CD3CN (ESI†).
In summary, we have demonstrated a general and efficient chiral solvation of chiral alcohols with a sodium salt of negatively charged octahedral Al complex, CASA-Na. Baseline peak separation of hydroxyl groups has been achieved at low temperature by simple addition of CASA-Na. We have shown that low temperature measurement is necessary due to the small binding constants between CASA-Na and chiral alcohols and that the hydrophobicity of chiral alcohols enhances the peak resolution. The origin of chiral discrimination of chiral alcohols has been proposed by DFT computation together with 1H NMR and X-ray crystallographic data. Because the CASA provides well resolved and sharp signals of various chiral alcohols including aryl- and alkyl-substituted primary, secondary, and tertiary alcohols, it can be a general method for the determination of the % ee and absolute configuration of alcohols. As we have successfully extended the analyte scope of the CASA, we anticipate that the CASA will be used as a universal chiral solvating agent for amines, carboxylic acids, and alcohols.
The authors are grateful for the financial support provided by Samsung Science and Technology Foundation (SSTF-BA1601-10). The authors thank Dr Alan J. Lough (University of Toronto) for his X-ray analysis.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For reviews on chiral analysis by NMR: (a) T. J. Wenzel, Tetrahedron: Asymmetry, 2017, 28, 1212 CrossRef CAS; (b) T. J. Wenzel and C. D. Chisholm, Chirality, 2011, 23, 190 CrossRef CAS PubMed; (c) J. Lacour and D. Moraleda, Chem. Commun., 2009, 7073 RSC; (d) T. J. Wenzel, Discrimination of Chiral Compounds Using NMR Spectroscopy, Wiley-VCH, Weinheim, 2007 Search PubMed; (e) J. M. Seco, E. Quiñoá and R. Riguera, Chem. Rev., 2004, 104, 17 CrossRef CAS; (f) H. Schneider, F. Hacket and V. Rudiger, Chem. Rev., 1998, 98, 1755 CrossRef CAS PubMed; (g) D. Parker, Chem. Rev., 1991, 91, 1441 CrossRef CAS.
- For reviews on chiral analysis using optical sensing: (a) L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840 CrossRef CAS PubMed; (b) Z. Chen, Q. Wang, X. Wu, Z. Li and Y.-B. Jiang, Chem. Rev., 2015, 44, 4249 RSC; (c) D. Leung, S. O. Kang and E. V. Anslyn, Chem. Soc. Rev., 2012, 41, 448 RSC; (d) E. B. Bauer, Chem. Soc. Rev., 2012, 41, 3153 RSC; (e) G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2007, 108, 1 CrossRef PubMed.
- (a) S. M. Han, Biomed. Chromatogr., 1997, 11, 259 CrossRef CAS PubMed; (b) V. Schurig and H.-P. Nowotny, Angew. Chem., Int. Ed. Engl., 1990, 29, 939 CrossRef.
- (a) R. García-Rodríguez, S. Hanf, A. D. Bond and D. S. Wright, Chem. Commun., 2017, 53, 1225 RSC; (b) J. Labuta, J. P. Hill, S. Ishihara, L. Hanykova and K. Ariga, Acc. Chem. Res., 2015, 48, 521 CrossRef CAS PubMed; (c) J. Labuta, S. Ishihara, T. Sikorsky, Z. Futera, A. Shundo, L. Hanykova, J. V. Burda, K. Ariga and J. P. Hill, Nat. Commun., 2013, 4, 1 Search PubMed.
- For recent examples, see: (a) L.-P. Li and B.-H. Ye, Inorg. Chem., 2017, 56, 10717 CrossRef CAS PubMed; (b) Y. Zhao and T. M. Swager, J. Am. Chem. Soc., 2015, 139, 3321 Search PubMed; (c) F. Freire, E. Quiñoá and R. Riguera, Chem. Commun., 2008, 4147 RSC.
- For recent examples, see: (a) C.-X. Liu, L. Zheng, L. Zhu, H.-P. Xiao, X. Li and J. Jiang, Org. Biomol. Chem., 2017, 15, 4314 RSC; (b) R. Gupta, R. G. Gonnade and A. V. Bedekar, J. Org. Chem., 2016, 81, 7384 CrossRef CAS PubMed; (c) A. Lakshmipriya, S. R. Chaudhari and N. Suryaprakash, New J. Chem., 2016, 40, 8118 RSC; (d) H. Huang, G. Bian, H. Zong, Y. Wang, S. Yang, H. Yue, L. Song and H. Fan, Org. Lett., 2016, 18, 2524 CrossRef CAS PubMed.
- For recent examples, see: (a) Y. Li, G.-H. Yang, C. Q. He, X. Li, K. N. Houk and J.-P. Cheng, Org. Lett., 2017, 15, 4314 Search PubMed; (b) E. Monteagudo, P. March, A. Alvarez-Larena and A. Virgili, ChemistrySelect, 2017, 2, 7362 CrossRef CAS; (c) G. Bian, H. Fan, H. Huang, S. Yang, H. Zong, L. Song and G. Yang, Org. Lett., 2015, 17, 1369 CrossRef CAS PubMed; (d) G. Bian, H. Fan, S. Yang, H. Yue, H. Huang, H. Zong and L. Song, J. Org. Chem., 2013, 78, 9137 CrossRef CAS PubMed.
- For recent examples, see: (a) S. Ito, M. Okuno and M. Asami, Org. Biomol. Chem., 2018, 16, 213 RSC; (b) N. Jain, A. N. Khanvilkar, S. Sahoo and A. V. Bedekar, Tetrahedron, 2018, 74, 68 CrossRef CAS; (c) S. K. Mishra and N. Suryaprakash, Tetrahedron: Asymmetry, 2017, 28, 1220 CrossRef CAS; (d) A. Lakshmipriya, G. Sumana and N. Suryaprakash, Tetrahedron: Asymmetry, 2017, 28, 1290 CrossRef CAS; (e) B. E. Dalvano and T. J. Wenzel, Tetrahedron: Asymmetry, 2017, 28, 1061 CrossRef CAS; (f) C. Lv, L. Feng, H. Zhao, G. Wang, P. Stavropoulos and L. Ai, Org. Biomol. Chem., 2017, 15, 1642 RSC; (g) L. Fang, C. Lv, G. Wang, L. Feng, P. Stavropoulos, G. Gao, L. Ai and J. Zhang, Org. Chem. Front., 2016, 3, 1716 RSC; (h) S. Ito, K. Ikeda and M. Asami, Chem. Lett., 2016, 45, 1379 CrossRef CAS; (i) T. Ema, D. Tanida and T. Sakai, J. Am. Chem. Soc., 2007, 129, 10591 CrossRef CAS PubMed; (j) A. E. Lovely and T. J. Wenzel, J. Org. Chem., 2006, 71, 9178 CrossRef CAS PubMed.
- L. Yang, T. Wenzel, R. T. Williamson, M. Christensen, W. Schafer and C. J. Welch, ACS Cent. Sci., 2016, 2, 332 CrossRef CAS PubMed.
- (a) G. Bian, S. Yang, H. Huang, H. Zong, L. Song, H. Fan and X. Sun, Chem. Sci., 2016, 7, 932 RSC; (b) L. S. Moon, M. Pal, Y. Kasetti, P. V. Bharatam and R. S. Jolly, J. Org. Chem., 2010, 75, 5487 CrossRef CAS PubMed; (c) L. S. Moon, R. S. Jolly, Y. Kasetti and P. V. Bharatam, Chem. Commun., 2009, 1067 RSC.
- (a) G. Bian, S. Yang, H. Huang, H. Zong and L. Song, Sens. Actuators, B, 2016, 231, 129 CrossRef CAS; (b) I. Pal, S. R. Chaudhari and N. R. Suryaprakash, Magn. Reson. Chem., 2015, 53, 142 CrossRef CAS PubMed; (c) I. Pal, S. R. Chaudhari and N. Suryaprakash, New J. Chem., 2014, 38, 4908 RSC; (d) C. Wolf, A. M. Cook and J. E. Dannatt, Tetrahedron: Asymmetry, 2014, 25, 163 CrossRef CAS.
- M.-S. Seo and H. Kim, J. Am. Chem. Soc., 2015, 137, 14190 CrossRef CAS PubMed.
- CCDC 1816058 contain the supplementary crystallographic data for this paper†.
- ΔΔδ = |ΔδR − Δδs|, ΔδR = |δR
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) with
with![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CSA − δR|, and ΔδS = |δS
CSA − δR|, and ΔδS = |δS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) with
with![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CSA − δS|.
CSA − δS|. - K. D. Klika, Tetrahedron: Asymmetry, 2009, 20, 1099 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic and calculation data, and crystallographic details. CCDC 1816058. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc00574e |
| This journal is © The Royal Society of Chemistry 2018 |