One-pot controllable synthesis of carboxylic group functionalized hollow mesoporous silica nanospheres for efficient cisplatin delivery†
Z. Jomeh Farsangia,
A. Beitollahi*b,
B. D. Hattonc,
S. Sarkara,
M. R. Jaafarid,
M. Rezayata,
A. Amania and
F. Gheybia
aSchool of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Iran
bSchool of Metallurgy & Materials Eng., Iran University of Science and Technology, Iran. E-mail: beitolla@iust.ac.ir
cDepartment of Materials Science & Eng., University of Toronto, Canada
dDepartment of Pharmaceutical Sciences, Mashhad University of Medical Sciences, Iran
First published on 11th July 2016
Abstract
Biocompatible hollow porous materials, due to their high void volume and shell porosity, are favorable platforms for efficient drug delivery. In this study, carboxylic group functionalized hollow mesoporous silica nanospheres (HMSNs-COOH) were successfully synthesized in a simple and one-pot co-condensation method using polystyrene nanospheres as a hard template, and carboxyethylsilanetriol as a source of carboxyl groups and reaction catalyzer. The synthesized HMSNs-COOH were studied by characterizing their morphology, nanostructure, specific surface area, particle size distribution and chemical composition using FESEM, HRTEM, SAXRD, N2-sorption, DLS, FTIR and TGA techniques. The large specific surface area measured (973 m2 g−1) for the prepared HMSNs-COOH was found to increase the loading capacity of cisplatin to ∼48%, as an anti-neoplastic agent. Furthermore, release tests performed at three different pH values of 7.4, 6.5 and 5.5 showed a sustained and pH dependent release of the drug from the prepared HMSNs-COOH. The cytotoxicity of the formulated drug was also examined in c26 colon carcinoma cell lines using the MTT assay. We demonstrate that the drug loaded HMSNs-COOH samples have a lower toxicity than the free drug, due to controlled and sustained drug release.
1. Introduction
There is increasing interest in developing new effective treatments for cancer based on nano-therapeutic systems, to offer passive or active targeting compared to conventional chemo-therapy, which often causes toxicity to healthy cells.1,2 In passive targeting a nanocarrier can accumulate in the tumor tissue due to leaky blood vessels and poor lymphatic drainage due to the enhanced permeability and retention (EPR) effect.1 Hollow mesoporous silica nanospheres (HMSNs) are attractive for their potential clinical applications.3 They are considered to be appropriate drug delivery systems for cancer therapy due to their interesting properties, such as large specific surface area, low density, high pore volume, highly permeable porous shell, colloidal, chemical and mechanical stability, biodegradability and high biocompatibility.3,4 The hollow void volume of HMSNs can incorporate more drug molecules than conventional (sol–gel) SiO2; the pores connect the interior hollow space of HMSNs to the exterior environment. As a result, a multiple functionalization of the pore surfaces means the HMSNs can not only hold a drug cargo, but can also effectively control the drug release rate.5,6HMSNs have been synthesized by a number of colloidal methods, such as a template-mediated approach.7 Hard templating is a relatively simple and straightforward procedure for the synthesis of HMSNs using templates such as carbon spheres, SiO2 beads, polymer, or metal oxide nanoparticles.8,9 Furthermore, this method has the potential of independently controlling the shell thickness, hollow cavity size, pore size, overall particle morphology and size dispersity which can all be used to optimize both drug loading and release.10
Cisplatin [cis-dichlorodiammineplatinum(II)] is a potent cytotoxic agent, commonly used for treatment of different types of cancer including; ovarian, lung, testicular, cervical and some carcinomas.11 In reality, relatively high doses of cisplatin are necessary, which unavoidably often result in severe toxicity, obtained resistance, chronic neurotoxicity and acute nephrotoxicity, which can also cause kidney failure.12,13 In the past few decades, new drug delivery systems (DDS) have been under development, with the aim of minimizing the risk of overdose by enhancing the cisplatin accumulation in cancer cells. An interesting property of cisplatin is the formation of coordination complexes when the central platinum atom is linked to functional groups.14,15 Carboxylic groups are very suitable for this purpose as they can effectively bond to the cisplatin. Surprisingly, in addition they can be again exchanged through NAD(P)H-reductase, the high Cl− concentration or H2O in the cytosol. Furthermore, ionization of carboxylic groups provokes electrostatic repulsion between nanoparticles to avoid particle aggregation and increase dispersibility and stability in aqueous media.16,17
Mesoporous silica nanoparticles (MSNs) functionalized with carboxyl group scan be consistently synthesized by incorporating –CN groups into the structure, then oxidizing them to –COOH.18,19 This procedure requires extra care and can be effectively improved by introducing the pendant –COOH groups with a suitable water-soluble organosilane such as carboxyethylsilanetriol sodium (CES), co-condensed with tetraethoxysilane (TEOS). CES, however, is a strong base and accelerates the kinetics of hydrolysis and condensation of silica precursors, and can result in the formation of aggregated particles with irregular morphologies.20,21 For efficiently bonding of cisplatin, incorporation of a sufficient quantity of carboxylic groups is necessary. However, for effective internalization and cisplatin delivery into cancer cells, it is vital to moderate the particle size of COOH-modified MSNs at the nanometer scale.22
There have been few attempts at a one-pot method to both incorporate COOH groups in MSNs and control the particle size.23 Jinlou Gu et al. synthesized MSNs in one pot with CES for cisplatin loading, and the, drug loading capacity in, three different trials (with different amounts of COOH groups) was 8.7, and 11.3 wt%, respectively.24 Chia-Hui Lin et al. have reported a novel pH-sensitive release of cisplatin loaded in MSN in a multi-step functionalization reaction by the combination of a COOH group with a pH-sensitive hydrazone bond at the MSN surfaces.25 Arean et al., studied the effect of functional groups on three MCM-41 type mesoporous silica nanoparticles, and the amount of cisplatin adsorbed by the MCM-41, NH2-MCM-41 and COOH-MCM-41 samples, respectively, was 8.7(±0.7), 61.4(±4.6) and 14.7(±0.6) mg of cisplatin per gram of sample.11 Jinlou Gu et al. have grafted relatively high-density of COOH groups onto the pore surface of their mesoporous carrier in order to make a complex with platinum atoms.26
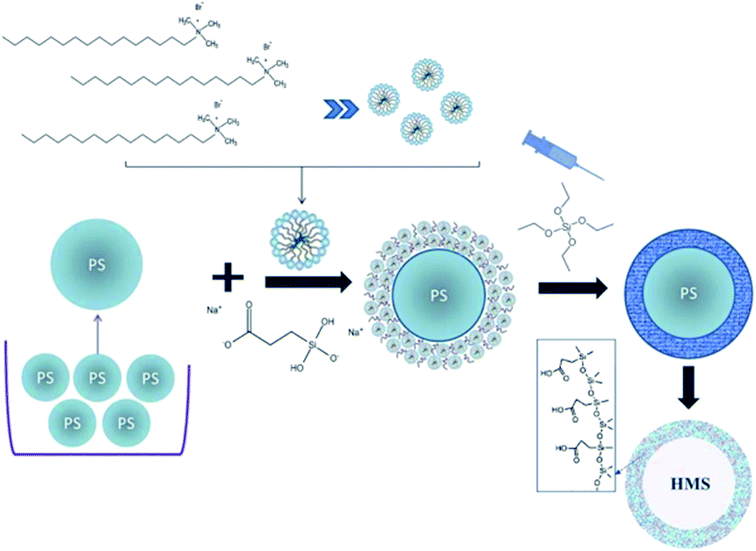
To the best of our knowledge, there is no report for making HMSNs in a one-pot method that incorporates a high ratio of COOH groups. Herein, we demonstrate a simple one-pot synthesis for well-ordered HMSN particles with a relatively high degree of COOH functionalization, based on a co-condensation of CES and TEOS templated by polystyrene (PS) spheres and cetyltrimethylammonium bromide (CTAB) for making the pores. Compared to conventional MSNs, the HMSNs-COOH synthesized in this work exhibit a much higher potential for drug loading and delivery. In addition, the presence of functional groups on the surface of the mesopores enables effective loading and controlled release. Therefore, the difficulties in drug loading and the problem of sudden drug release can be avoided. This nano-carrier is designed to use passive targeting and the EPR effect to become internalized in tumour tissue, due to the high permeability of leaky micro vessels associated with tumor angiogenesis.
2. Materials and methods
2.1. Chemicals and reagents
Analytically pure styrene (>99.0%) washed through an inhibitor remover column, 2,2′-azobis(2-methylpropionamidine)dihydrochloride (V-50, >97.0%), [2-(acryloyloxy)ethyl]trimethylammonium chloride (AETAC, 80 wt% in H2O), cetyltrimethylammonium bromide (CTAB), o-phenylenediamine (OPDA), dimethylformamide (DMF) and tetraethyl orthosilicate (TEOS, ≥99.0%) were purchased from Sigma Aldrich. Carboxyethylsilanetriol sodium salt (25% in water) (CES) was purchased from Gelest. Analytically pure tetrahydrfuran (THF) and ethanol were purchased from VWR. Ammonia solution (28%) was purchased from Merck. All other chemicals and reagents used were of analytical grade. Deionized water was generated with a Milli-Q integral pure and ultrapure water purification system.2.2. Synthesis of HMSNs-COOH
The PS latex suspensions were prepared through emulsion polymerization.27 Typically for making 120 nm PS, 0.25 g of AETAC (80 wt% in H2O) was dissolved in 100.00 g H2O and 10.00 g styrene was added to the solution in a 500 ml round-bottom flask. The mixture stirred at 1000 rpm for 30 min then was purged with nitrogen for 20 min and heated to 90 °C. Afterwards, 2 ml of an aqueous solution containing 0.25 g AIBN was added. The emulsion was kept at 90 °C for 12 h under nitrogen to allow the polymerization to complete.The HMSNs-COOH with the average size of 150 nm were prepared as follows: 0.50 g of CTAB was dissolved in a mixture of 50.00 g H2O, 20.00 g ethanol. Then 100 μl CES was added drop-wise to the CTAB mixture solution, and continually stirred until the solution became clear. Then 5 ml of PS 9 wt% was added drop-wise to the CTAB solution at room temperature under vigorous stirring, followed by sonicating for 15 min. The resulted milky mixture was then stirred for 30 min before the drop-wise injection of 2.0 g TEOS within 2 h. The mixture was kept at room temperature for 48 h before the mesoporous silica coated latex was collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The precipitate was then washed with ethanol three times before drying under vacuum at room temperature overnight. The CTAB surfactant and PS template were extracted simultaneously by refluxing the obtained mesoporous composite (0.5 g) at 70 °C with 100 ml THF and concentrated HCl (1 ml 37%) twice. The final product was obtained by centrifugation, and dried under vacuum overnight.
000 rpm. The precipitate was then washed with ethanol three times before drying under vacuum at room temperature overnight. The CTAB surfactant and PS template were extracted simultaneously by refluxing the obtained mesoporous composite (0.5 g) at 70 °C with 100 ml THF and concentrated HCl (1 ml 37%) twice. The final product was obtained by centrifugation, and dried under vacuum overnight.
As a control experiment the HMSNs were also synthesized by a modified method. Details of the synthesis procedure for non-functionalized HMSNs are given in the ESI† files.
2.3. Loading the cisplatin anticancer drug
Cisplatin (20.00 mg) was dissolved in 20 ml water–DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The as prepared HMSNs (40.00 mg) were added to the above cisplatin solution and stirred (dark) for 48 h at 37 °C. These suspensions were centrifuged at 10
1, v/v). The as prepared HMSNs (40.00 mg) were added to the above cisplatin solution and stirred (dark) for 48 h at 37 °C. These suspensions were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min. The cisplatin loaded HMSNs-COOH were washed with 20 ml of EtOH and DI water and dried in a vacuum oven overnight. The residual cisplatin content was measured in supernatant and washing supernatant using UV-Vis spectroscopy to determine the cisplatin loading.
000 rpm for 30 min. The cisplatin loaded HMSNs-COOH were washed with 20 ml of EtOH and DI water and dried in a vacuum oven overnight. The residual cisplatin content was measured in supernatant and washing supernatant using UV-Vis spectroscopy to determine the cisplatin loading.
2.4. In vitro release of cisplatin
To measure the in vitro release of the drug, the cisplatin-loaded HMSNs-COOH samples were immersed in PBS buffer at three different pH values of 5.5, 6.5 and 7.4. Three individual HMSNs samples were suspended in 10 ml of PBS buffer solution at these pH values. The supernatants were collected at fixed time intervals. The supernatant was measured by UV-Vis spectroscopy at the wavelength of 706 nm to determine the concentration of released cisplatin. The spectrophotometric measurements were recorded using a mixture of 1.40 mg ml−1 of OPDA solution, phosphate buffer pH 6.8, and DMF as a blank solution. Each sample was heated for 10 min at 100 °C before adding the DMF. The samples cooled before the UV-Vis measurements to determine the amount of residual cisplatin not loaded in the nanospheres. This measurement was done using a standard calibration curve, constructed by measuring the absorbance of cisplatin solutions at varying concentrations. The amount of cisplatin loaded in the nanospheres was then calculated by subtracting the unloaded amount from that initially added.2.5. In vitro cell viability assay
We used a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay to measure the in vitro cytotoxicity of HMSNs-COOH, cisplatin-loaded HMSNs-COOH, and free cisplatin against a c26 colon carcinoma cell line. The murine colon carcinoma cell line c26 was cultured in RPMI medium in 5% CO2 at 37 °C. Cells were harvested from sub-confluent cultures using trypsin, and were re-suspended in fresh complete medium. 4000 cells were seeded in 96-well plates for 24 h. Each plate also included untreated c26 cells as positive controls, and blank wells with medium free of cells. After overnight incubation of plates (37 °C, 5% CO2), the medium was removed carefully and exposed to the fresh medium (200 μl), containing up to 100 μl of serial concentration of drug formulations. After incubation at 37 °C, 5% CO2 for 48 h, the medium was removed carefully and replaced by 100 μl FCS free cell cultured medium containing 10 μl of MTT solution. The produced insoluble formazan by living cells was dissolved by adding 200 μl DMSO and its optical density (OD) was read with a multi-well scanning spectrophotometer (at 570 nm wavelength).2.6. Microstructural and physical characterization of HMSNs
Microstructural analysis was carried out using field emission scanning electron microscope (FE-SEM) with a SIGMA VP ZEISS instrument operated at 20 kV. High resolution transmission electron microscopy (HRTEM) was carried out with a HF3300 Hitachi TEM operated at 200 kV. We used an X-ray diffractometer Rigaku Mini Flex 600 to obtain small angle X-ray diffraction (XRD) patterns. Nitrogen adsorption–desorption isotherms were collected according to Brunauer–Emmett–Teller (BET) theory using a Belsorp pore analyzer at 77 K. The pore size distributions and pore volumes were extracted using the Barrett–Joyner–Halenda (BJH) model, and the total pore volumes were determined from the adsorbed amount at a relative pressure P/P0 of 0.99. Fourier transformed infrared (FTIR) spectra were collected at room temperature using Thermo Scientific Nicolet iS10 spectrophotometer. Thermogravimetric analysis (TGA) was performed by a TGA Q 50 instrument thermal analyzer from 100 to 800 °C with a heating rate of 10 K min−1. Cisplatin concentration measurements were performed by an Agilent Cary 60 UV-Vis spectrophotometer at 706 nm wavelength. The particle size was measured by Scatteroscope I nanoparticle size analyzer.3. Results and discussion
HMSNs-COOH were prepared by a hard templating method of PS spheres of well-defined size. This one pot co-condensation method involves CTAB as the mesostructure directing agent, CES as the source of COOH groups, and TEOS as the silica precursor. In comparison to grafting, the co-condensation method has several advantages. For instance, there is a uniform distribution of organic functional groups integrated into the shell walls, and a favorable loading of these groups without closing the shell nanopores. Also, there is the least possible number of synthesis steps. The cooperation between the CTAB and the CES can create an equal distribution of the COOH groups, and following the self-assembly of the surfactant micelles will make a uniform array groups into the pores and surface around the PS spheres.21 Finally, the PS and CTAB could be synchronously extracted by refluxing in a mixture of THF and HCl. One can expect that the synthesized HMSNs have a homogeneous distribution of COOH groups throughout the pore surfaces, in addition to the shell interior and exterior surfaces. Fig. 1 demonstrates the schematic diagram of the whole HMSNs-COOH synthesis procedure.Fig. 2 shows the FTIR spectra of PS, HMSNs-COOH before and after template extraction. The common spectral bands about 1105, 808, 960 cm−1 are due to asymmetric vibration, symmetric stretching vibration and stretching of Si–OH, respectively. The broad band around 3480 cm−1 is attributed to absorbed water and also silanol groups. The HMSNs-COOH spectrum, however, shows an additional peak at 1730 cm−1 which is related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of COOH, confirming the presence of terminal carboxylic groups. These spectra also confirmed the removal of the CTAB and PS after THF treatment as the C–H vibration bands at ∼2850 and 2930, the C–H deformation bands at ∼1470; also the characteristic band of C–H, C–C and C–aromatic group between 1500 and 1600 almost disappeared.26
O vibration of COOH, confirming the presence of terminal carboxylic groups. These spectra also confirmed the removal of the CTAB and PS after THF treatment as the C–H vibration bands at ∼2850 and 2930, the C–H deformation bands at ∼1470; also the characteristic band of C–H, C–C and C–aromatic group between 1500 and 1600 almost disappeared.26
The density of the COOH groups, integrally linked to the nanostructure surfaces, was estimated by calculating the corresponding weight loss in TGA. Fig. 3 shows the TGA result obtained for COOH-HMSNs after template removal. The plot shows the weight losses of the sample upon heating to 800 °C. The relatively small weight loss at temperatures up to 120 °C was related to physically adsorbed water and volatile solvent molecules. The weight loss between 120 °C and 200 °C was related to chemically combined water and silanol group dehydroxylation. As seen, the main weight loss related to degradation of chemically bonded COOH groups occurred between 400 °C and 500 °C.21 This significant weight loss of approximately 17% of the sample that corresponds to the decomposing of CH2CH2COOH confirms that the synthesized nanostructure has a relatively high surface density of COOH groups, to provide a desirable loading capacity for the cisplatin drug in the hollow cavity.
The amount of available COOH groups was also measured by titration experiment. Since it was difficult to precisely determine the titration endpoint the values were not as precise as TGA method (ESI†).
Small angle XRD was used to characterize the mesostructure of the synthesized HMSNs-COOH. The spectra revealed a relatively broad peak which confirms the presence of a likely worm-like and micropore structure (Fig. 4).
The spherical morphology and mono-dispersity of the as-prepared PS particles are clearly seen in FESEM (Fig. 5). The HMSNs-COOH are relatively uniform, spherical and monodisperse, with the particle size around 150 nm (Fig. 5). HRTEM imaging (Fig. 6) clearly shows uniform shells of about 15 nm thickness. Further, we can see the worm-like microporous structure in this shell region.
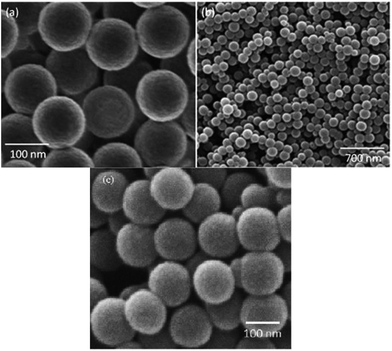 | ||
| Fig. 5 FESEM images of polystyrene nanospheres (a) and HMSNs-COOH sample with different magnification (b), (c). | ||
As shown in Fig. 7, the HMSNs-COOH exhibit type IV isotherms indicating the presence of well-defined mesopores and a specific surface area of 973 m2 g−1. This specific surface area belongs to the porosity of the shell, and does not include the internal surface area. The particles showed a high pore volume of 1.722 cm3 g−1. The average diameter of pore is 19.071 Å in regard to Barrett–Joyner–Halenda (BJH) curve and the poresizedistribution is quiet narrow.
Since cisplatin has a low molar absorptivity and no florescence absorption, a colorimetric based selective derivitization method has been used to determine the amount of cisplatin loading.28 By this method, carefully measurement of the amount of drug loading and release with a relatively high precision and accuracy in the 1–10 μg ml−1 range will be possible. Derivatization of cisplatin with O-phenylenediamine (OPDA) at pH = 6.8 and 90 °C resulted in a maximum absorption at 706 nm in the UV-Vis spectra.
The loading capacity was calculated using the following equation:
The amount of drug loading was relatively high and found to be almost 48 wt%. For comparison purposes, we also measured the amount of drug loading capacity in non-functionalized HMSNs as a control experiment and the cisplatin was loaded only up to 35 wt%, that is almost 27% lower than loading capacity of functionalized HMSNs. To examine the cisplatin release profile, three COOH-HMSN samples were loaded with cisplatin and then immersed in PBS buffers with pH values of 7.4, 6.5 and 5.5 at 37 °C. Fig. 8 shows the time dependent release behavior of the COOH-HMSNs sample after drug loading. The overall release patterns show a low, sustained and pH dependent pattern. A fast release, however, happened in the first 20 h, but it was slower in the remaining assay. After 4 days, 70, 56 and 40% of the total loaded drug was released in pH values of 5.5, 6.5 and 7.4, respectively. Clearly, it should be mentioned that at a lower pH promotes protonation of the COOH groups can accelerate the release profile.
The percentage of cytotoxicity was calculated according to following formulas:
| % viability = 100 − % cytotoxicity | (1) |
The cytotoxicity comparison of free cisplatin and cisplatin loaded HMSNs-COOH were evaluated for 24 h and 48 h respectively. These results are shown in Fig. 9 and 10. When the c26 colon carcinoma cells were treated with either HMSNs-COOH-cisplatin or free cisplatin the cytotoxicity was dose dependent. Although HMSNs-COOH-cisplatin exhibited a prolonged cytotoxicity effect on c26 cells over a longer period of 48 h. It can be seen that the drug loaded HMSNs-COOH have a lower toxicity than free drug. We suggest this effect is due to the controlled and sustained drug release, which provides enough time for the nanocarrier to accumulate in tumour tissue by EPR effect. Therefore, the cytotoxicity of the HMSNs-COOH was found to be relatively low and concentration dependent in the cell treated range of 0.3 to 2.5 μg ml−1. The half maximal inhibitory concentration values (IC50s) of cisplatin and cisplatin-HMSNs-COOH carrier against c26 was 0.64, 0.68 μg ml−1 over a period of 48 h drug treatment, respectively (Fig. 11).
 | ||
| Fig. 9 The cytotoxicity of HMSNs-COOH-cisplatin and free cisplatin to c26 colon carcinoma cells by MTT assay for 24 h. | ||
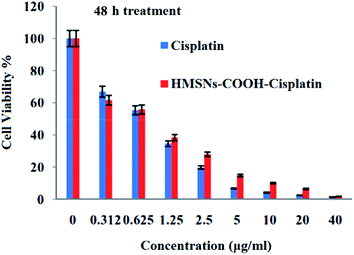 | ||
| Fig. 10 The cytotoxicity of HMSNs-COOH-cisplatin and free cisplatin to c26 colon carcinoma cells by MTT assay for 48 h. | ||
4. Conclusions
In this study we have reported a sustained and controlled drug delivery system based on HMSNs synthesized and functionalized in a one-pot procedure. The HMSNs-COOH exhibited relatively narrow size distribution, good morphological uniformity, and high dispersibility in aqueous media. The conjugation of the cisplatin–carboxylate complexes is designed for efficient linking to cisplatin and specifically releasing the drug within an endosomal medium. This drug delivery carrier synthesis and design procedure offers some features, for example; a one-pot synthesis method, a very low density carrier with high loading capacity (48 wt%) and loading efficiency (∼95%), a localize and sustained release of cisplatin (around 70% over 100 h), and an enhanced therapeutic efficacy by decreasing the necessary dose and side effects that arise from premature release. We suggest that this synthesis procedure has a highly controllable number of structural (PS sphere size, mesoporous template, shell thickness) and compositional (functional groups by co-assembly) parameters that can be fully optimized to precisely tailor the drug loading and release of a wide range of anticancer drugs.Acknowledgements
The authors would like to greatly acknowledge the invaluable support of Jane Howe and Cameron Stwarts for TEM and FESEM studies performed in the Ontario Centre for the Characterisation of Advanced Materials (OCCAM).References
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- M. E. Davis, Z. Chen and D. M. Shin, Nat. Rev. Drug Discovery, 2008, 7, 771–782 CrossRef CAS PubMed.
- Z. Feng, Y. Li, D. Niu, L. Li, W. Zhao, H. Chen, L. Li, J. Gao, M. Ruan and J. Shi, Chem. Commun., 2008, 2629–2631, 10.1039/B804594A.
- J. Shen, G. Song, M. An, X. Li, N. Wu, K. Ruan, J. Hu and R. Hu, Biomaterials, 2014, 316–326 CrossRef CAS PubMed.
- T. Wang, F. Chai, Q. Fu, L. Zhang, H. Liu, L. Li, Y. Liao, Z. Su, C. Wang, B. Duan and D. Ren, J. Mater. Chem., 2011, 21, 5299–5306 RSC.
- X. She, L. Chen, L. Velleman, C. Li, H. Zhu, C. He, T. Wang, S. Shigdar, W. Duan and L. Kong, J. Colloid Interface Sci., 2015, 445, 151–160 CrossRef CAS PubMed.
- K. An and T. Hyeon, Nano Today, 2009, 4, 359–373 CrossRef CAS.
- Y. Chen, H. Chen and J. Shi, Adv. Porous Mater., 2013, 1, 34–62 CrossRef CAS.
- Y. Jiao, J. Guo, S. Shen, B. Chang, Y. Zhang, X. Jiang and W. Yang, J. Mater. Chem., 2012, 22, 17636–17643 RSC.
- X. Fang, X. Zhao, W. Fang, C. Chen and N. Zheng, Nanoscale, 2013, 5, 2205–2218 RSC.
- C. O. Arean, M. J. Vesga, J. B. Parra and M. R. Delgado, Ceram. Int., 2013, 39, 7407–7414 CrossRef CAS.
- D. Roberts, P. J. O'Dwyer and S. W. Johnson, in Cancer Management in Man: Chemotherapy, Biological Therapy, Hyperthermia and Supporting Measures, ed. R. B. Minev, Springer, Netherlands, Dordrecht, 2011, vol. 8, pp. 145–164 Search PubMed.
- M. Kartalou and J. M. Essigmann, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2001, 478, 23–43 CrossRef CAS PubMed.
- S. Aryal, C. M. J. Hu and L. Zhang, ACS Nano, 2010, 4, 251–258 CrossRef CAS PubMed.
- Y. Min, C. Q. Mao, S. Chen, G. Ma, J. Wang and Y. Liu, Angew. Chem., Int. Ed., 2012, 51, 6742–6747 CrossRef CAS PubMed.
- C. Xu, B. Wang and S. Sun, J. Am. Chem. Soc., 2009, 131, 4216–4217 CrossRef CAS PubMed.
- K. J. Haxton and H. M. Burt, Dalton Trans., 2008, 5872–5875, 10.1039/B809949A.
- L. Han, O. Terasaki and S. Che, J. Mater. Chem., 2011, 21, 11033–11039 RSC.
- C.-m. Yang, B. Zibrowius and F. Schuth, Chem. Commun., 2003, 1772–1773, 10.1039/B304626E.
- C.-T. Tsai, Y.-C. Pan, C.-C. Ting, S. Vetrivel, A. S. T. Chiang, G. T. K. Fey and H.-M. Kao, Chem. Commun., 2009, 5018–5020, 10.1039/B909680A.
- L. Han, Y. Sakamoto, O. Terasaki, Y. Li and S. Che, J. Mater. Chem., 2007, 17, 1216–1221 RSC.
- Y.-S. Lin, C.-P. Tsai, H.-Y. Huang, C.-T. Kuo, Y. Hung, D.-M. Huang, Y.-C. Chen and C.-Y. Mou, Chem. Mater., 2005, 17, 4570–4573 CrossRef CAS.
- Y. Ma, L. Xing, H. Zheng and S. Che, Langmuir, 2010, 27, 517–520 CrossRef PubMed.
- J. Gu, J. Liu, Y. sheng Li, W. Zhao and J. Shi, Langmuir, 2012, 29, 403–410 CrossRef PubMed.
- C.-H. Lin, S.-H. Cheng, W.-N. Liao, P.-R. Wei, P.-J. Sung, C.-F. Weng and C.-H. Lee, Int. J. Pharm., 2012, 429, 138–147 CrossRef CAS PubMed.
- G. Jinlou, S. Shansha, L. Yongsheng, H. Qianjun, Z. Jiaying and S. Jianlin, J. Phys. Chem. Lett., 2010, 1, 3446–3450 CrossRef.
- G. Qi, Y. Wang, L. Estevez, A. K. Switzer, X. Duan, X. Yang and E. P. Giannelis, Chem. Mater., 2010, 22, 2693–2695 CrossRef CAS.
- M. Basotra, S. K. Singh and M. Gulati, ISRN Anal. Chem., 2013, 2013, 1–8 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10856c |
| This journal is © The Royal Society of Chemistry 2016 |