Assessing the reactivity of sodium alkyl-magnesiates towards quinoxaline: single electron transfer (SET) vs. nucleophilic alkylation processes†
Abstract
By exploring the reactivity of sodium butyl-magnesiate 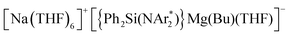 (1) supported by the bulky chelating silyl(bisamido) ligand {Ph2Si(NAr*)2}2− (Ar* = 2,6-iPr2-C6H3) towards Quinoxaline (Qx), the ability of this bimetallic system to effectively promote SET processes has been disclosed. Thus 1 executes the single-electron reduction of Qx affording complex
(1) supported by the bulky chelating silyl(bisamido) ligand {Ph2Si(NAr*)2}2− (Ar* = 2,6-iPr2-C6H3) towards Quinoxaline (Qx), the ability of this bimetallic system to effectively promote SET processes has been disclosed. Thus 1 executes the single-electron reduction of Qx affording complex 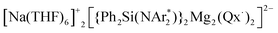 (2) whose structure in the solid state contains two quinaxolyl radical anions Qx˙ stabilised within a dimeric magnesiate framework. Combining multinuclear NMR and EPR measurements with DFT calculations, new insights into the constitution of 2 in solution and its magnetic behaviour have been gained. Further evidence on the SET reactivity of 1 was found when it was reacted with nitroxyl radical TEMPO which furnished contacted ion pair sodium magnesiate [(Ph2Si(NAr*)2)Mg(TEMPO−)Na(THF)3] (4) where both metals are connected by an alkoxide bridge, resulting from reduction of TEMPO. The role that the different ligands present in 1 can play in these new SET reactions has also been assessed. Using an amination approach, the Bu group in 1 can be replaced by the more basic amide TMP allowing the isolation of
(2) whose structure in the solid state contains two quinaxolyl radical anions Qx˙ stabilised within a dimeric magnesiate framework. Combining multinuclear NMR and EPR measurements with DFT calculations, new insights into the constitution of 2 in solution and its magnetic behaviour have been gained. Further evidence on the SET reactivity of 1 was found when it was reacted with nitroxyl radical TEMPO which furnished contacted ion pair sodium magnesiate [(Ph2Si(NAr*)2)Mg(TEMPO−)Na(THF)3] (4) where both metals are connected by an alkoxide bridge, resulting from reduction of TEMPO. The role that the different ligands present in 1 can play in these new SET reactions has also been assessed. Using an amination approach, the Bu group in 1 can be replaced by the more basic amide TMP allowing the isolation of 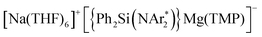 (3) which was characterised by multinuclear NMR and X-ray crystallography. 1H NMR monitoring of the reaction of 3 with Qx showed its conversion to 2, leaving the hydrogen atoms of the heterocycle untouched. Contrastingly, using sodium homoalkyl magnesiate [NaMg(CH2SiMe3)3] (5) led to the chemoselective C2 alkylation of this heterocycle, suggesting that the presence of the steric stabiliser {Ph2Si(NAr*)2}2− on the mixed-metal reagent is required in order to facilitate the Qx reduction.
(3) which was characterised by multinuclear NMR and X-ray crystallography. 1H NMR monitoring of the reaction of 3 with Qx showed its conversion to 2, leaving the hydrogen atoms of the heterocycle untouched. Contrastingly, using sodium homoalkyl magnesiate [NaMg(CH2SiMe3)3] (5) led to the chemoselective C2 alkylation of this heterocycle, suggesting that the presence of the steric stabiliser {Ph2Si(NAr*)2}2− on the mixed-metal reagent is required in order to facilitate the Qx reduction.

- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...