Zong Guan, Sascha Wiechmann, Martin Drafz, Eike Hübner and Andreas Schmidt
Org. Biomol. Chem., 2013,11, 3558-3567
DOI:
10.1039/C3OB40379C,
Paper
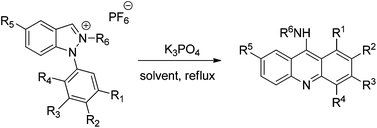
On deprotonation, 1-arylindazolium salts form 1-arylindazol-3-ylidenes which rearrange spontaneously via ring cleavage, ring closure and subsequent