Open Access Article
Open Access ArticleSuccessive diastereoselective C(sp3)–H arylation and Suzuki coupling toward enantioenriched polyaryl unnatural amino acid motifs†
Shefali Banga and
Srinivasarao Arulananda Babu *
*
Department of Chemical Sciences Indian Institute of Science Education and Research (IISER) Mohali Knowledge City, Sector 81, SAS Nagar, Mohali, Manauli P.O., Punjab 140306, India. E-mail: sababu@iisermohali.ac.in
First published on 25th June 2025
Abstract
This paper reports the preliminary efforts in constructing (teraryl-, quateraryl- and hexaryl-based) polyaryl unnatural amino acid motifs. At first, chemo- and diastereoselective Pd(II)-catalyzed bidentate directing group-aided arylation of prochiral β-C(sp3)–H bonds of carboxamides of amino acids with 4-bromo-4′-iodo-1,1′-biphenyl was performed. This process generated amino acid motifs possessing the 4-bromobiphenyl unit. Subsequently, the Suzuki–Miyaura coupling reaction with the 4-bromobiphenyl unit present in amino acid motifs has led to the assembling of a library of teraryl-, quateraryl-, and hexaaryl-based polyaryl unnatural amino acid motifs. Emission spectra of representative teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs are recorded, and some are found to be fluorescent. In the literature, various teraryl- and quateraryl-based molecules have been reported as medicinally relevant compounds. Consequently, there is scope for synthesizing novel and functionalized teraryl-, quateraryl-, and hexaaryl-based molecules to aid future investigations into the biological activities of such scaffolds. Thus, this work on the construction of teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs via successive sp3 C–H arylation and Suzuki coupling would be a valuable effort toward strengthening the library of polyaryl-based unnatural amino acid scaffolds.
Introduction
Oligoaryls or π-extended biaryls (e.g., teraryls, quateraryls, hexaaryls, etc) have gained significant attention in materials, organic synthesis, and drug discovery/medicinal chemistry.1,2 Of particular interest, various naturally occurring and synthetically derived teraryls are known to exhibit potential bio-activities and medicinal properties (Fig. 1).2–4 Several teraryls, quateraryls, and hexaaryls are vital motifs in developing various functional organic materials and additionally, various terphenyls are known to exhibit fluorescence properties.5On the other hand, the secondary structures of protein were imagined as templates in the design of drug-like small molecules that may act as proteomimetics.6,7 It is reported that various types of biaryl, terphenyl-inspired templates, polycyclic ether, benzodiazepinedione, and indane motifs were envisioned to act as drug-like non-peptidyl α-helix mimetics, which may disrupt protein–protein interactions (Fig. 1).6 Along these lines, various non-peptidyl teraryl-based α-helix mimetics (e.g., 1i, and 1f, Fig. 1), were tested as potent Bcl-XL antagonists7b,c and acted to disrupt the p53/HDM2 interaction.7d
In general, the transition metal-catalyzed cross-coupling method has been used as a viable route for obtaining biaryl and oligoaryl-based compounds.8–11 Along these lines, the synthesis of bio-active and non-peptidyl teraryl-based α-helix mimetics (e.g., 1i,7c 1f,7a,g 1e,3a 2j,4a,b) and pyridine-based teraryl/quateraryl α-helix mimetics7e,f,h have been accomplished using the sequential cross-coupling reactions (Scheme 1).
 | ||
| Scheme 1 Representative cross-coupling approaches toward the synthesis of bio-active polyaryl-based unnatural amino acid motifs. | ||
Unnatural amino acid derivatives (viz., noncanonical or non-proteinogenic amino acids) have proven to be vital and privileged molecules in various research fields, including organic synthesis, chemical biology, and drug discovery.12 Diverse unnatural amino acid molecules have been used as starting materials for synthesizing natural products, drug molecules, and peptides, etc. Furthermore, a wide range of L- and D-unnatural amino acids are used as organocatalysts, ligands, tools, or probes to study and understand the functions of macromolecules and biomolecules.12
In recent years, the C–H functionalization of sp3 C–H bonds has facilitated the regio- or site-selective installation of a wide range of functional groups in aliphatic chains.13 Especially, the Pd(II)-catalyzed, bidentate directing group-aided C–H functionalization14,15 of the prochiral or diastereotopic sp3 C–H bonds has enabled the installation of a wide range of functional groups in the backbone of carboxamides of α-amino acids.16–18
Given the importance of teraryls and quateraryls as inhibitors of medicinally relevant protein–protein and protein–nucleic acid interactions.6,7 There is scope for synthesizing new teraryls and quateraryls. In this work, we explored the construction of teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs via the successive sp3 C–H arylation and Suzuki coupling. We envisaged that this approach would be a valuable effort and a contribution towards strengthening the library of polyaryl-based unnatural amino acid motifs.
With regard to polyaryl-based unnatural amino acid motifs, while the synthesis of biaryl-based unnatural amino acid molecules via the traditional cross-coupling reaction has been well documented (Scheme 1).18a,19 However, apart from the Suzuki coupling-based synthesis of arginine-based tripeptide 2j encompassing a terphenyl unit (Scheme 1),4a,b to the best of our knowledge, the synthesis of teraryl-, quateraryl, and hexaaryl-based unnatural amino acid analogues is rarely explored via the C–H functionalization route. Recently, we reported the synthesis of biaryl-based unnatural amino acid molecules via the sp3 C–H arylation method.18a As a part of the extension of our previous report, this work aimed to generate teraryl-, quateraryl, and hexaaryl-based unnatural amino acid motifs (Scheme 2), which may be useful in developing amino acid-based proteomimetics.
 | ||
| Scheme 2 Successive diastereoselective C(sp3)–H arylation and Suzuki coupling toward (teraryl, quateraryl, hexaaryl-based) polyaryl unnatural amino acid motifs. | ||
Results and discussion
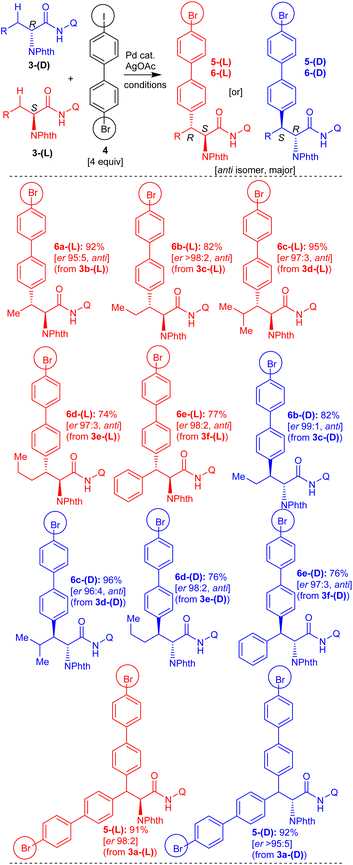
To commence with the conceived teraryl and quaternary unnatural amino acid targets, initially, we planned to assemble various amino acid derivatives possessing a 4-bromobiphenyl moiety, which then can be subjected to the Suzuki coupling reaction. Based on previous experience,18a,20 we prepared carboxamide of 2-aminobutyric acid 3b-(DL) linked with the bidentate directing group 8-aminoquinoline for performing the Pd(II)-catalyzed arylation of the prochiral β-C(sp3)–H bond of 2-aminobutyric acid 3b-(DL) with 4-bromo-4′-iodobiphenyl (4). Carboxamide 3b-(DL) was treated with 4-bromo-4′-iodobiphenyl (4) under the standard C–H arylation conditions16–18 involving Pd(OAc)2 catalyst, AgOAc (iodide ion scavenging additive) in toluene at 110 °C for 48 h (Scheme 3). This reaction afforded the expected 2-aminobutyric acid derivative 6a-(DL), possessing a 4-bromobiphenyl moiety in 90% yield as the major diastereomer (having the anti-stereochemistry).Similarly, carboxamides of norvaline 3c-(DL), leucine 3d-(DL), norleucine 3e-(DL), phenylalanine 3f-(DL) and 2-aminooctanoic acid 3g-(DL) possessing 8-aminoquinoline directing group were assembled.18,20 Then, the carboxamides 3c-g-(DL) were subjected to the β-C(sp3)–H arylation with 4-bromo-4′-iodobiphenyl (4) in the presence of Pd(OAc)2 and AgOAc in toluene at 110 °C for 48 h (Scheme 3). These reactions afforded the corresponding norvaline 6b-(DL), leucine 6c-(DL), norleucine 6d-(DL), phenylalanine 6e-(DL), and 2-aminooctanoic acid 6f-(DL) possessing a 4-bromobiphenyl moiety in 73–97% yields (major diastereomers having the anti-stereochemistry).
Next, we assembled enantioenriched carboxamides of L-amino acids, such as L-2-aminobutyric acid 3b-(L), L-norvaline 3c-(L), L-leucine 3d-(L), L-norleucine 3e-(L), and L-phenylalanine 3f-(L), possessing an 8-aminoquinoline directing group. Subsequently, enantioenriched carboxamides of L-amino acids 3b-f-(L) were subjected to the Pd(OAc)2-catalyzed β-C(sp3)–H arylation with 4-bromo-4′-iodobiphenyl (4) in toluene at 110 °C for 48 h (Scheme 4). These reactions afforded the corresponding enantioenriched 2-aminobutyric acid 6a-(L), norvaline 6b-(L), leucine 6c-(L), norleucine 6d-(L) and phenylalanine 6e-(L) possessing 4-bromobiphenyl moiety in 74–95% yields (major diastereomers, anti-stereochemistry) with good enantiopurity.
Along this line, we then assembled enantioenriched carboxamides of D-amino acids such as D-norvaline 3c-(D), D-leucine 3d-(D), D-norleucine 3e-(D), and D-phenylalanine 3f-(D) possessing an 8-aminoquinoline directing group. Subsequently, enantiopure carboxamides of D-amino acids 3c-f-(D) were subjected to the Pd(OAc)2-catalyzed β-C(sp3)–H arylation with 4-bromo-4′-iodobiphenyl (4) (Scheme 4). These reactions afforded the corresponding enantioenriched norvaline 6b-(D), leucine 6c-(D), norleucine 6d-(D), and phenylalanine 6e-(D) possessing a 4-bromobiphenyl moiety in 76–96% yields (major diastereomers, anti-stereochemistry) with good enantiopurity.
Additionally, we assembled racemic and enantiopure carboxamides of alanine, such as DL-alanine 3a-(DL), D-alanine 3a-(D), and L-alanine 3a-(L) possessing an 8-aminoquinoline directing group.18a Carboxamide 3a-(DL) was treated with 4-bromo-4′-iodobiphenyl (4) under the standard β-C(sp3)–H conditions involving Pd(OAc)2 and AgOAc in toluene at 110 °C for 48 h (Scheme 3). This reaction afforded the alanine derivative 5-(DL), possessing two 4-bromobiphenyl moieties via double β-C(sp3)–H arylation of 3a-(DL). Along this line, enantiopure L- or D-alanine carboxamides 3a-(L) or 3a-(D) were subjected to the β-C(sp3)–H arylation with 4-bromo-4′-iodobiphenyl (4) in the presence of Pd(OAc)2 and AgOAc in toluene at 110 °C for 48 h (Scheme 4). These reactions afforded the corresponding enantioenriched alanine derivatives 5-(L) and 5-(D) possessing two 4-bromobiphenyl moieties via double β-C(sp3)–H arylation of respective carboxamides 3a-(L) and 3a-(D).
Initially, we tried to perform the Suzuki cross-coupling reaction with the 4-bromobiphenyl moiety present in the amino acid derivative having 8-aminoquinoline and phthalimide protecting groups (see ESI†). Unfortunately, we could not achieve the expected Suzuki cross-coupling and the synthesis of the π-extended biaryl, such as teraryl- or quateraryl-based unnatural amino acid motifs having 8-aminoquinoline and phthalimide protecting groups. The presence of 8-aminoquinoline and phthalimide protecting groups presumably interfered with the Suzuki cross-coupling reaction conditions, and thereby, the expected Suzuki cross-coupling with the 4-bromobiphenyl moiety present in the amino acid derivative did not occur.
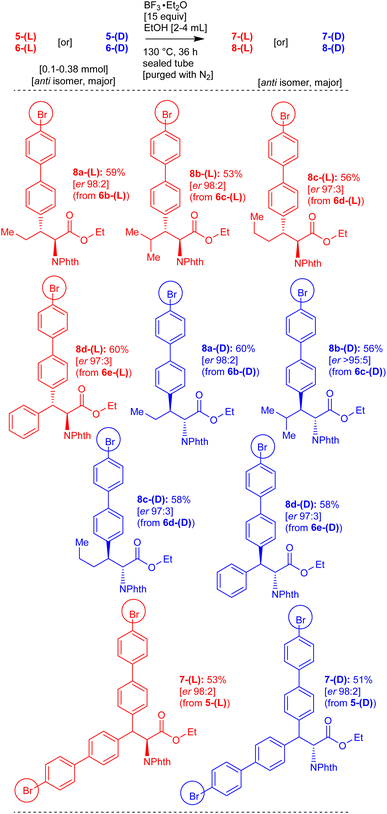
Therefore, we attempted the removal of the directing group (8-aminoquinoline) and phthalimide protecting groups from the amino acid derivatives 5-(DL), 6-(DL), 5-(D), 6-(D), 5-(L) and 6-(L) which were obtained via the Pd(II)-catalyzed β-C(sp3)–H arylation reactions. Based our previous experience,18 we performed the BF3·Et2O-mediated direct amide to ester conversion reactions in the substrates 5-(DL), 6-(DL), 5-(D), 6-(D), 5-(L) and 6-(L). Accordingly, carboxamide of norvaline 6b-(DL), having 8-aminoquinoline and phthalimide protecting group, was treated with ethanol in the presence of BF3·Et2O at 130 °C for 36 h. This reaction enabled the removal of the 8-aminoquinoline directing group and afforded the corresponding norvaline ethyl ester derivative 8a-(DL) in 61% yield (Scheme 5). Similarly, the substrates leucine 6c-(DL), norleucine 6d-(DL), and phenylalanine 6e-(DL), having the 8-aminoquinoline directing group, were treated with ethanol/methanol in the presence of BF3·Et2O at 130 °C for 36 h. These reactions afforded the corresponding products including leucine 8b-(DL), norleucine 8c-(DL), phenylalanine 8d-(DL) and phenylalanine 8e-(DL) ester derivatives (Scheme 5).
Subsequently, enantioenriched L-carboxamide substrates including norvaline 6b-(L), leucine 6c-(L), norleucine 6d-(L), phenylalanine 6e-(L) and D-carboxamide substrates including norvaline 6b-(D), leucine 6c-(D), norleucine 6d-(D), phenylalanine 6e-(D) were treated with EtOH in the presence of BF3·Et2O to remove the 8-aminoquinoline group. These reaction afforded the corresponding enantioenriched products including norvaline 8a-(L), leucine 8b-(L), norleucine 8c-(L), phenylalanine 8d-(L) and norvaline 8a-(D), leucine 8b-(D), norleucine 8c-(D), phenylalanine 8d-(D) ester derivatives (Scheme 6). Additionally, the carboxamides of alanine 5-(DL), enantioenriched alanine 5-(D), and alanine 5-(L) were treated with EtOH in the presence of BF3·Et2O to remove the 8-aminoquinoline group. These reactions afforded the corresponding products, including alanine 7-(DL), enantioenriched alanine 7-(D) and alanine 7-(L) ester derivatives (Schemes 5 and 6).
Before performing the Suzuki coupling reaction using the amino acid derivatives 7-(DL), 8-(DL), 7-(D), 8-(D), 7-(L) and 8-(L) possessing the phthalimide group, we decided to deprotect the phthalimide group also. Accordingly, we attempted the deprotection of phthalimide group from the amino acid ester derivatives 7-(DL), 8-(DL), 7-(D), 8-(D), 7-(L) and 8-(L) to obtain the amino acid ester derivatives 9-(DL), 10-(DL), 9-(D), 10-(D), 9-(L) and 10-(L) possessing the free amino group and 4-bromobiphenyl moiety (Schemes 7 and 8).
 | ||
| Scheme 7 Deprotection of the phthalimide group in substrates 7-(DL), and 8-(DL), affording ester derivatives of 9-(DL), and 10-(DL), having free amino group. | ||
 | ||
| Scheme 8 Deprotection of the phthalimide group in substrates 7-(D), 8-(D), 7-(L) and 8-(L) affording ester derivatives of 9-(D), 10-(D), 9-(L) and 10-(L) having free amino group. | ||
Based on our previous works, we treated norvaline ethyl ester derivative 8a-(DL) with 1,2-ethylenediamine in t-BuOH at rt, which enabled the phthalimide deprotection. The norvaline ethyl ester derivative 10a-(DL), having the free amino group, was obtained in 81% yield (Scheme 7). Similarly, ester derivatives of leucine 8b-(DL), norleucine 8c-(DL), phenylalanine 8d-(DL), and phenylalanine 8e-(DL) were treated with 1,2-ethylenediamine in t-BuOH at rt. These reactions afforded the corresponding ethyl ester derivatives of leucine 10b-(DL), norleucine 10c-(DL), phenylalanine 10d-(DL), and phenylalanine 10e-(DL) possessing the free amino group.
Subsequently, enantioenriched ester derivatives of norvaline 8a-(L), leucine 8b-(L), norleucine 8c-(L), phenylalanine 8d-(L), and norvaline 8a-(D), leucine 8b-(D), norleucine 8c-(D) and phenylalanine 8d-(D) were treated with 1,2-ethylenediamine in t-BuOH at rt. These reactions afforded the corresponding enantioenriched ester derivatives of norvaline 10a-(L), leucine 10b-(L), norleucine 10c-(L), phenylalanine 10d-(L) and norvaline 10a-(D), leucine 10b-(D), norleucine 10c-(D) and phenylalanine 10d-(D) having the free amino group (Scheme 8). Additionally, alanine ester derivatives 7-(DL), enantioenriched alanine 7-(D), and alanine 7-(L) were treated with 1,2-ethylenediamine in t-BuOH at rt. These reactions afforded the corresponding alanine ester derivatives 9-(DL), enantioenriched alanine 9-(D), and alanine 9-(L) possessing the free amino group (Schemes 7 and 8).
Having obtained amino acid ester derivatives 9-(DL), 10-(DL), 9-(D), 10-(D), 9-(L) and 10-(L) possessing free amino group and 4-bromobiphenyl moiety, we then commenced the synthesis of teraryl- and quateraryl amino acid motifs via the Suzuki coupling. Firstly, we attempted the Suzuki coupling reaction on norvaline 10a-(DL) containing a 4-bromobiphenyl moiety with (4-methoxyphenyl)boronic acid in the presence of Pd(PPh3)4 (5 mol%) and K2CO3 in toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL) at 110 °C for 24 h. This reaction afforded the targeted teraryl-based norvaline 12a-(DL) in 84% yield (Scheme 9). Then, the Pd-catalyzed Suzuki coupling reaction was performed using enantioenriched norvalines 10a-(D) and 10a-(L) containing a 4-bromobiphenyl moiety with (4-methoxyphenyl)boronic acid. These reactions afforded the targeted enantioenriched teraryl-based norvalines 12a-(D) and 12a-(L) in 78–83% yields (anti isomers) with good enantiopurity (Scheme 10). Next, we performed the Pd-catalyzed Suzuki coupling reaction on leucine 10b-(DL), norleucine 10c-(DL), and phenylalanine 10d-(DL) containing a 4-bromobiphenyl moiety with (4-methoxyphenyl)boronic acid. These reactions afforded the corresponding targeted teraryl-based leucine 13a-(DL), norleucine 14a-(DL), and phenylalanine 15a-(DL) in 67–92% yields (anti isomers) (Scheme 9).
1 mL) at 110 °C for 24 h. This reaction afforded the targeted teraryl-based norvaline 12a-(DL) in 84% yield (Scheme 9). Then, the Pd-catalyzed Suzuki coupling reaction was performed using enantioenriched norvalines 10a-(D) and 10a-(L) containing a 4-bromobiphenyl moiety with (4-methoxyphenyl)boronic acid. These reactions afforded the targeted enantioenriched teraryl-based norvalines 12a-(D) and 12a-(L) in 78–83% yields (anti isomers) with good enantiopurity (Scheme 10). Next, we performed the Pd-catalyzed Suzuki coupling reaction on leucine 10b-(DL), norleucine 10c-(DL), and phenylalanine 10d-(DL) containing a 4-bromobiphenyl moiety with (4-methoxyphenyl)boronic acid. These reactions afforded the corresponding targeted teraryl-based leucine 13a-(DL), norleucine 14a-(DL), and phenylalanine 15a-(DL) in 67–92% yields (anti isomers) (Scheme 9).
 | ||
| Scheme 10 Construction of terphenyl-based amino acid derivatives 12-(D), 13-(D), 14-(D), 15-(D), 12-(L), 13-(L), 14-(L), and 15-(L) via the Pd(II)-catalyzed Suzuki coupling reaction. | ||
Additionally, we carried out the Pd-catalyzed Suzuki coupling reaction on leucine 10b-(DL) or norleucine 10c-(DL) or phenylalanine 10d-(DL) containing a 4-bromobiphenyl moiety using different arylboronic acids. These attempts afforded the corresponding targeted teraryl-based leucines 13b-(DL), 13c-(DL), norleucines 14b-(DL), 14c-(DL), and phenylalanines 15b-(DL), 15c-(DL) in 57–82% yields (Scheme 9). While the Suzuki coupling reactions were successful in amino acid ester derivatives 10-(DL), having a free amino group. To prepare the orthogonally protected teraryl amino acid motifs, the free amino group in norvaline 10a-(DL) or norleucine 10c-(DL) or phenylalanine 10d-(DL) was protected as an N-Boc group. Then, we carried out the Pd-catalyzed Suzuki coupling reaction on N-Boc norvaline 10a-(DL) or N-Boc norleucine 10c-(DL), or N-Boc phenylalanine 10d-(DL) containing a 4-bromobiphenyl moiety with different arylboronic acids. These reactions afforded the corresponding targeted teraryl-based N-Boc norvaline 12b-(DL) or N-Boc norleucine 14d-(DL) and N-Boc phenylalanine 29-(DL) (Scheme 9).
Next, we carried out the Pd-catalyzed Suzuki coupling reaction on enantioenriched L-amino acid substrates, leucine 10b-(L), norleucine 10c-(L), and phenylalanine 10d-(L) containing a 4-bromobiphenyl moiety with (4-methoxyphenyl) boronic acid. These reactions afforded the corresponding targeted enantioenriched teraryl-based leucine 13a-(L), norleucine 14a-(L), and phenylalanine 15a-(L) (anti isomers) with good enantiopurity (Scheme 10). Subsequently, we carried out the Pd-catalyzed Suzuki coupling reaction on enantioenriched D-amino acid substrates, including leucine 10b-(D), norleucine 10c-(D), and phenylalanine 10d-(D) containing a 4-bromobiphenyl moiety with (4-methoxyphenyl)boronic acid. These reactions afforded the corresponding targeted enantioenriched teraryl-based leucine 13a-(D), norleucine 14a-(D), and phenylalanine 15a-(D) (anti-isomers) with good enantiopurity (Scheme 10).
Having synthesized teraryl-based α-amino acids (Schemes 9 and 10), next to extend the substrate scope and generality of this protocol, we performed the Suzuki coupling on the long chain unnatural amino acid derivatives 16a, 16b containing the 4-bromobiaryl moiety (Scheme 11).18a We heated 11-aminoundecanoic acid ester substrate 16a with boronic acid 11a in the presence of Pd(PPh3)4 and K3PO4 in DMF at 110 °C for 24 h. This reaction afforded teraryl-based 11-aminoundecanoic acid motif 18a in 48% yield (Scheme 11). Similarly, teraryl-based 7-aminoheptanoic acid motif 18b was obtained from 16b. Next, we attempted the Heck coupling reaction on 16b with ethyl acrylate 17a in the presence of Pd(OAc)2, P(o-tolyl)3, and Et3N in MeCN at 85 °C for 17 h. This reaction afforded the olefin-unit appended, biaryl-based 7-aminoheptanoic acid ester substrate 18c in 60% yield (Scheme 11). We continued to attempt the Sonogashira cross-coupling reaction on the 4-bromobiphenyl moiety in substrate 16b and 4-phenylalanine derivative 10d-(DL) containing the 4-bromobiphenyl moiety with phenylacetylene 17b. Accordingly, treatment of 16b or 10d-(DL) in the presence of Pd(PPh3)2Cl2, CuI and Et3N in DMF for 110 °C for 17 h afforded the corresponding terphenyl-based compounds 7-aminoheptanoic acid motif 19a and phenylalanine motif 19b-(DL) possessing an alkyne unit (Scheme 11).
After having constructed a library of racemic and enantioenriched teraryl-based amino acid motifs, we shifted our attention towards the synthesis of quarteraryl-based amino acid scaffolds. Firstly, we attempted the Suzuki coupling reaction on norvaline 10a-(DL) containing a 4-bromobiphenyl moiety with [1,1′-biphenyl]-4-ylboronic acid in the presence of Pd(PPh3)4, (5 mol%) and K2CO3 in toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL) at 110 °C for 24 h. This reaction afforded the targeted quateraryl-based norvaline 26a-(DL) in 71% yield (Scheme 12). Along this line,18a the Pd-catalyzed Suzuki coupling reaction on norvaline 10aa-(DL) containing a 4-bromobiphenyl moiety (and 8-aminoquinoline directing group) with (6-methoxynaphthalen-2-yl)boronic acid afforded the targeted quateraryl-based norvaline 26b-(DL) in 69% yield.
1 mL) at 110 °C for 24 h. This reaction afforded the targeted quateraryl-based norvaline 26a-(DL) in 71% yield (Scheme 12). Along this line,18a the Pd-catalyzed Suzuki coupling reaction on norvaline 10aa-(DL) containing a 4-bromobiphenyl moiety (and 8-aminoquinoline directing group) with (6-methoxynaphthalen-2-yl)boronic acid afforded the targeted quateraryl-based norvaline 26b-(DL) in 69% yield.
 | ||
Scheme 12 Construction of quarteraryl-based amino acid derivatives 26–30 via the Pd(II)-catalyzed Suzuki coupling reaction. Conditions: (a) Pd(PPh3)4 (5 mol%), K2CO3 (3 equiv.), toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL), 110 °C, 24 h, sealed tube (purged with N2). (b) Compounds 28a-(DL) and 30 are obtained from 8c-(DL) and 16b using reaction conditions, Pd(OAc)2 (10 mol%), P(o-tolyl)3 (25 mol%), Et3N (0.2 mL), MeCN (3 mL), 85 °C, 17 h, sealed tube (purged with N2). (c) Substrate 26b-(DL) was obtained from 10aa-(DL).18a 1 mL), 110 °C, 24 h, sealed tube (purged with N2). (b) Compounds 28a-(DL) and 30 are obtained from 8c-(DL) and 16b using reaction conditions, Pd(OAc)2 (10 mol%), P(o-tolyl)3 (25 mol%), Et3N (0.2 mL), MeCN (3 mL), 85 °C, 17 h, sealed tube (purged with N2). (c) Substrate 26b-(DL) was obtained from 10aa-(DL).18a | ||
Next, we conducted the Pd-catalyzed Suzuki coupling reaction on leucine 10b-(DL) with [1,1′-biphenyl]-4-ylboronic acid or [1,1′-biphenyl]-3-ylboronic acid. These reactions afforded the corresponding targeted quateraryl-based leucine 27a-(DL) and leucine 27b-(DL) in 65–78% yields. Then, the Pd-catalyzed Suzuki coupling reaction on enantioenriched leucine 10b-(D) with [1,1′-biphenyl]-4-ylboronic acid afforded the targeted quateraryl-based enantioenriched leucine 27a-(D) in 74% yield with good enantiopurity.
Subsequently, we carried out the Pd-catalyzed Suzuki coupling reaction on N-Phth norleucine 8c-(DL) with [1,1′-biphenyl]-4-ylboronic acid, which afforded the targeted quateraryl-based N-Phth norleucine 28a-(DL) in 47% yield. Along this line, the Pd-catalyzed Suzuki coupling reaction on norleucine 10c-(DL) with [1,1′-biphenyl]-4-ylboronic acid gave the targeted quateraryl-based norleucine 28b-(DL) in 78% yield. Furthermore, the Pd-catalyzed Suzuki coupling reaction on enantioenriched norleucine 10c-(D) or 10c-(L) with [1,1′-biphenyl]-4-ylboronic acid gave the corresponding targeted enantioenriched quateraryl-based norleucine 28b-(D) and norleucine 28b-(L) in 74–78% yields with good enantiopurity (Scheme 12). Additionally, the Pd-catalyzed Suzuki reaction of 7-aminooctanoic acid ester substrate 16b with [1,1′-biphenyl]-4-ylboronic acid gave the teraryl-based 7-aminooctanoic acid motif 30 in 37% yield (Scheme 12).
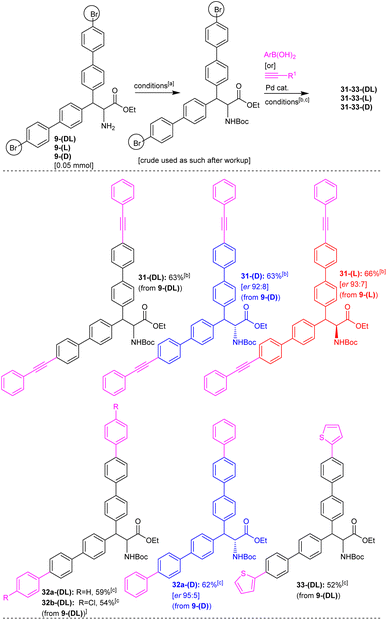
Having obtained a series of teraryl- and quateraryl-based amino acid derivatives, we shifted our attention toward the synthesis of hexaaryl-based amino acids (Scheme 13). Towards this end, we attempted the Sonogashira reaction on the 4-bromobiphenyl moiety present in alanine ethyl ester derivatives 9-(DL), 9-(D), and 9-(L). At first, the free amino group in alanine substrates 9-(DL), 9-(D) and 9-(L) was protected as N-Boc and then, we subjected the N-Boc 9-(DL), 9-(D) and 9-(L) to the Pd-catalyzed Sonogashira reaction conditions. The corresponding N-Boc protected alanine 9a-(DL), having two 4-bromobiphenyl moieties, was heated with phenylacetylene 17b in the presence of Pd(PPh3)2Cl2, CuI, Et3N in DMF at 110 °C for 17 h. This reaction afforded the dialkyne-unit incorporated, hexaaryl-based alanine ester derivative 31-(DL) in 63% yield (Scheme 13). Along this line, the Pd-catalyzed Sonogashira reactions of corresponding N-Boc protected, enantioenriched alanines 9-(D) and 9-(L) having two 4-bromobiphenyl moieties with phenylacetylene 17b were carried out. These reactions afforded the dialkyne-unit incorporated, enantioenriched hexaaryl-based alanine derivatives 31-(D) and 31-(L) in 63–66% yields with good enantiopurity (Scheme 13). Similarly, the corresponding N-Boc protected alanine 9a-(DL), having two 4-bromobiphenyl moieties, was subjected to the Pd-catalyzed Suzuki coupling reaction with phenylboronic acid or (4-chlorophenyl)boronic acid or thiophen-2-ylboronic acid. These reactions afforded the corresponding hexaaryl-based alanine ester derivatives 32a-(DL), 32b-(DL), and 33-(DL) in 52–59% yields (Scheme 13). Additionally, the N-Boc protected enantioenriched alanine 9a-(D), having two 4-bromobiphenyl moieties, was subjected to the Pd-catalyzed Suzuki coupling reactions with phenylboronic acid. This reaction gave the targeted enantioenriched hexaaryl-based alanine ester derivative 32a-(D) in 62% yield with good enantiopurity (Scheme 13).
After performing the cross-coupling reactions on amino acids and the synthesis of teraryl or tetraaryl, or hexaaryl-based amino acids, to expand the synthetic utility and scope of this work, we intended to perform the Miyaura borylation reaction on the 4-bromobiphenyl moiety present in amino acid motifs (Scheme 14). This approach would enable the synthesis of various biaryl-based amino acid motifs possessing boronate ester units, which may be used as coupling partners in the cross-coupling reactions. At the outset, carboxamide of 2-aminobutyric acid containing a 4-bromobiphenyl moiety 6a-(DL) was subjected to the standard Miyaura borylation conditions involving B2Pin2 (2 equiv.) in the presence of Pd(dppf)2Cl2 and KOAc in 1,4-dioxane at 80 °C for 36 h. This reaction afforded the biaryl-based 2-aminobutyric acid motif possessing boronate ester unit 21a-(DL) in 82% yield (Scheme 14). Along this line, the enantioenriched carboxamide of 2-aminobutyric acid containing a 4-bromobiphenyl moiety 6a-(L) was subjected to the Pd-catalyzed reaction with B2Pin2. This reaction afforded the enantioenriched biaryl-based 2-aminobutyric acid motif possessing boronate ester unit 21a-(L) in 85% yield with good enantiopurity (Scheme 14). The structure and anti-stereochemistry of the compound 21a-(L) were confirmed by the X-ray structure analysis (Fig. 2).21
 | ||
Scheme 14 Construction of biaryl-based amino acid motifs possessing boronate ester unit 21–24 and construction of quateraryl-based amino acid motif 25-(DL). (a) Substrate 10d,e-(DL) were first treated with (Boc)2O (1.5 equiv.), (CH3)2CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (0.5 H2O (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 mL), DCM (2 mL), rt, 12 h, and the corresponding crude compound of N-Boc compound obtained after workup was used in the next step. (b) Product 22 was obtained from substrate 16c.18a 9 mL), DCM (2 mL), rt, 12 h, and the corresponding crude compound of N-Boc compound obtained after workup was used in the next step. (b) Product 22 was obtained from substrate 16c.18a | ||
Similarly, carboxamides of norvaline 6b-(DL), leucine 6c-(DL), and norleucine 6d-(DL) containing a 4-bromobiphenyl moiety were subjected to the Pd-catalyzed reaction with B2Pin2. The corresponding norvaline 21b-(DL), leucine 21c-(DL), and norleucine 21d-(DL) compounds possessing boronate ester units were obtained in 78–89% yields (Scheme 14). Additionally, carboxamide of 2-aminooctanoic acid 6f-(DL) and 11-aminoundecanoic acid 16c containing a 4-bromobiphenyl moiety,18a was subjected to the Pd-catalyzed reaction with B2Pin2. The corresponding 2-aminooctanoic acid 21e-(DL), and 11-aminoundecanoic acid 22 compounds possessing boronate ester unit were obtained in 78–88% yields. Finally, phenylalanines 23-(DL) and 24-(DL) possessing boronate ester unit were obtained from their corresponding N-Boc phenylalanines 10d-(DL) and 10e-(DL) containing the 4-bromobiphenyl moiety. Furthermore, to show the utility, the synthesized N-Boc phenylalanine 24-(DL) possessing boronate ester unit was subjected to the Pd-catalyzed Suzuki coupling reaction with N-Boc phenylalanine 10d-(DL) containing the 4-bromobiphenyl moiety. This reaction afforded the quateraryl-based compound 25-(DL) appended with two phenylalanine units in 67% (Scheme 14).
Having prepared a variety of teraryl-, quarteraryl-, and hexaaryl amino acid scaffolds, we wished to expand the synthetic utility of these compounds by assembling representative examples of teraryl-based peptides (Scheme 15). Initially, we subjected teraryl-based norvaline 12a-(DL) possessing a free amino group to the standard peptide coupling with N-Boc glycine. This reaction afforded the corresponding teraryl-based dipeptide norvaline-Gly 34a-(DL) in 78% yield (Scheme 15). Similarly, the teraryl-based tripeptide norvaline-Gly-Gly 34b-(DL) was prepared by treating 12a-(DL) with N-Boc-Gly-Gly-OH under standard peptide coupling conditions (Scheme 15). Furthermore, enantioenriched teraryl-based norvalines 12a-(D) or 12a-(L) possessing free amino groups were subjected to peptide coupling with N-Boc-Gly-Gly-OH. These reactions afforded the corresponding enantioenriched teraryl-based tripeptides, norvaline-Gly-Gly 34b-(D) and 34b-(L) in 81–84% yields (Scheme 15).
 | ||
| Scheme 15 Synthetic transformations. Construction of representative examples of teraryl-based peptides. Reaction conditions: (a) EDC-HCl (1.1 equiv.), HOBt (1.1 equiv.), 0 °C to rt, 24 h. | ||
We have performed the HPLC analysis of the substrates used and polyaryl-based α-amino acid motifs synthesized in this work (see the ESI†). The HPLC analysis patterns of the racemic unnatural amino acid starting materials 3a-f-(DL) were determined. Subsequently, enantiopurity of starting material substrates,18 such as N-phthaloyl 8-aminoquinoline carboxamides of alanine 3a-(D) (er 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), alanine 3a-(L) (er 97
3), alanine 3a-(L) (er 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 2-aminobutyric acid 3b-(L) (er 95
3), 2-aminobutyric acid 3b-(L) (er 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), norvaline 3c-(D) (er 98
5), norvaline 3c-(D) (er 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), norvaline 3c-(L) (er 98
2), norvaline 3c-(L) (er 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), leucine 3d-(D) (er 98
2), leucine 3d-(D) (er 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), leucine 3d-(L) (er 98
2), leucine 3d-(L) (er 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), norleucine 3e-(D) (er 96
2), norleucine 3e-(D) (er 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), norleucine 3e-(L) (er 96
4), norleucine 3e-(L) (er 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), phenylalanine 3f-(D) (er 96
4), phenylalanine 3f-(D) (er 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), phenylalanine 3f-(L) (er 97
4), phenylalanine 3f-(L) (er 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were ascertained from HPLC analysis.
3) were ascertained from HPLC analysis.
Next, the HPLC analysis patterns of the racemic 4-bromobiphenyl-based unnatural amino acid motifs including alanine 5-(DL), 2-aminobutyric acid 6a-(DL), norvaline 6b-(DL), leucine 6c-(DL), norleucine 6d-(DL) and phenylalanine 6e-(DL) were ascertained. Then, the HPLC analysis patterns of the enantioenriched 4-bromobiphenyl-based unnatural amino acid motifs such as alanine 5-(D), alanine 5-(L), 2-aminobutyric acid 6a-(L), norvaline 6b-(D), norvaline 6b-(L), leucine 6c-(D), leucine 6c-(L), norleucine 6d-(D), norleucine 6d-(L), and phenylalanine 6e-(D) phenylalanine 6e-(L) were ascertained.
Along this line, the HPLC patterns of 8-aminoquinoline DG-free 4-bromobiphenyl-based amino acid esters such as alanine 7-(DL), norvaline 8a-(DL), leucine 8b-(DL), norleucine 8c-(DL), and phenylalanine 8d-(DL) were obtained. Then, the HPLC analysis of their corresponding enantioenriched 8-aminoquinoline directing group-free 4-bromobiphenyl-based amino acid esters such as alanine 7-(D), alanine 7-(L), norvaline 8a-(D), norvaline 8a-(L), leucine 8b-(D), leucine 8b-(L), norleucine 8c-(D), norleucine 8c-(L), phenylalanine 8d-(D) and phenylalanine 8d-(L) was obtained.
Similarly, the HPLC patterns of 8-aminoquinoline directing group-free and phthalimide group-deprotected 4-bromobiphenyl-based amino acid derivatives such as alanine 9-(DL), norvaline 10a-(DL), leucine 10b-(DL), norleucine 10c-(DL), and phenylalanine 10d-(DL) have been obtained. Subsequently, the HPLC patterns of their corresponding enantioenriched derivatives such as alanine 9-(D), alanine 9-(L), norvaline 10a-(D), norvaline 10a-(L), leucine 10b-(D), leucine 10b-(L), norleucine 10c-(D), norleucine 10c-(L), phenylalanine 10d-(D) and phenylalanine 10d-(L) were also ascertained.
We next established the HPLC analysis patterns of racemic polyaryl-based products such as norvaline 12a-(DL), leucine 13a-(DL), norleucine 14a-(DL), phenylalanine 15a-(DL), 2-aminobutyric acid 21a-(DL), leucine 27a-(DL), norleucine 28b-(DL), alanine 31-(DL) and alanine 32a-(DL). Subsequently, we obtained the HPLC analysis of corresponding enantioenriched polyaryl-based products such as norvaline 12a-(D), norvaline 12a-(L), leucine 13a-(D), leucine 13a-(L), norleucine 14a-(D), norleucine 14a-(L), phenylalanine 15a-(D), phenylalanine 15a-(L), 2-aminobutyric acid 21a-(L), leucine 27a-(D), norleucine 28b-(D), norleucine 28b-(L), alanine 31-(D), alanine 31-(L) and alanine 32a-(D). Thereafter, the HPLC analysis pattern of tripeptide 34b-(DL) and enantioenriched tripeptides 34b-(D) and 34b-(L) were ascertained.
The structures of all the products obtained in this work were established by their respective NMR spectra and HRMS data. In addition to this, the structure and anti-stereochemistry of a representative biaryl-based 2-aminobutyric acid motif possessing boronate ester unit 21a-(L) was unambiguously ascertained by the single-crystal X-ray structure analysis (Fig. 2). This indirectly indicated Pd(II)-catalyzed 8-aminoquinoline bidentate directing group-aided arylation of prochiral β–C(sp3)–H bonds of carboxamides of amino acids with 4-bromo-4′-iodo-1,1′-biphenyl is a diastereoselective reaction.14d,16,18 This process afforded the corresponding amino acid motifs possessing 4-bromobiphenyl as the major diastereomer having the anti-stereochemistry (Schemes 3 and 4). This observation is in concurrence with the reported works and the mechanism of the Pd(II)-catalyzed 8-aminoquinoline bidentate directing group-aided arylation of prochiral β–C(sp3)–H bonds carboxamides is well documented.15–18
In concurrence with the mechanism proposed in the literature,14d,15-18 we divulge that the coordination of the 8-aminoquinoline directing group in the substrate 3f-(L) to the Pd(II) metal center is followed by concerted metalation deprotonation (CMP), affording the five-membered Pd(II) species 35b. Oxidative addition of 35b with an aryl iodide then forms the Pd(IV) species 35c, which experiences reductive elimination to afford the new C–C bond in intermediate 35d. Halide abstraction by a halide ion scavenger (e.g., Ag(I) salt) followed by proteolysis of 35d generated the β–C–H arylated product 6e-(L) and regenerates the active Pd(II) species in the catalytic cycle (Scheme 16). The formation of an anti-isomer as the major compound from the arylation of the prochiral C(sp3)–H bond of amino acid can be corroborated with the participation of possible conformations 35ba or 35bb of the palladacycle intermediate (generated after the β-C–H activation of the corresponding substrate 3f-(L)). This observation is defended with the Pd(II)-catalyzed 8-aminoquinoline-aided deuteration experiments performed by Daugulis's group.17m Daugulis detected17m a 64% and less than 10% of deuterium incorporation at the 3S and 3R positions in the product 36, respectively (Scheme 16). Since the protonation likely transpires with retention of configuration, it is anticipated that 35b has an anti-arrangement of the N-Phth and phenyl groups in the conformation 35bb or Pd and N-Phth groups in the conformation 35ba.14d Thus, it was envisioned that the diastereoselectivity of the arylation of substrate 3f-(L) is established at the palladation step.17m
 | ||
| Scheme 16 Proposed mechanism for the diastereoselective C–H functionalization.14d,15-18 | ||
Literature reports revealed that the π-extended aryl systems, including teraryl-based motifs, have been found to exhibit fluorescent properties and have been used as analytical probes.5h–l Preliminary efforts were made to ascertain the UV-Vis absorption spectra (λmax (absorption)) of representative teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs synthesized in this work (Fig. 3, Charts A to F). Further, we have conducted a preliminary examination of fluorescence emission of representative teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs obtained via the successive sp3 C–H arylation and Suzuki coupling method (Fig. 3, Charts G to I). It was noted that teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs exhibit fluorescence. Further, a screening of the fluorescence emission of teraryl-based unnatural amino acid motif 13c-(DL) and teraryl-based peptide 34a-(DL) under different concentrations was conducted. Increasing the concentration of the solution of teraryl-based unnatural amino acid/peptide motif did not show any quenching of fluorescence emission. There were no intermolecular interactions, and it was noted that the fluorescence emission increased when the concentration of the solution 13c-(DL) or 34a-(DL) was increased. The potential of fluorescence properties is yet to be investigated and will be reported in the context of future work.
Conclusions
In summary, this work reports the preliminary efforts in generating racemic and enantioenriched teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs. The goal was accomplished via the chemo-, diastereoselective Pd(II)-catalyzed bidentate directing group-aided arylation of prochiral β–C(sp3)–H bonds of carboxamides of amino acids with 4-bromo-4′-iodo-1,1′-biphenyl. As this process generated amino acids possessing the 4-bromobiphenyl units, subsequently, the Suzuki–Miyaura coupling reaction with the 4-bromobiphenyl unit present in amino acids has led to the assembling of teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs. Racemic and enantioenriched polyaryl-based unnatural amino acids comprising norvaline, leucine, norleucine, phenylalanine, 2-aminobutyric acid, 2-aminooctanoic acid, and alanine were synthesized. Pd-catalyzed C–H arylation and Suzuki or Sonogashira, or Heck coupling reactions were used as key steps to accomplish the synthesis of polyaryl-based unnatural amino acid derivatives. We also performed the Miyaura borylation reaction on the 4-bromobiphenyl moiety present in the biaryl-based amino acid motifs, and this process has led to the construction of biaryl-based amino acid motifs possessing a boronate ester unit. The substrate scope and generality of the protocol and synthesis of representative examples of teraryl-based peptides were shown. The yields of the C–H arylation, Suzuki coupling reactions affording the polyaryl-based unnatural amino acid derivatives are reasonably good. Accordingly, we believe that this process may be a scalable and practically useful route. It was noted that the representative teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid derivatives synthesized in this work are fluorescent. In the literature, various non-peptidyl teraryl- and quateraryl-based α-helix mimetics have been reported as inhibitors of medicinally relevant protein–protein and protein-nucleic acid interactions. Thus, this work on the construction of teraryl-, quateraryl-, and hexaaryl-based unnatural amino acid motifs via the successive sp3 C–H arylation and Suzuki coupling is a contribution towards strengthening the proteomimetic design and library of oligoaryl-based unnatural amino acids. Further works on establishing the photophysical properties and the application of this method for targeting unnatural amino acid-based α-helix mimetics will be carried out in the future.Experimental section
General information
The reagents used are commercially available and used without purification. The TLC analyses were performed on silica gel 60 F254 pre-coated plates or preparative alumina TLC plates and visualized by observation under irradiation with a UV lamp or iodine vapor. Column chromatography separation of crude reaction mixtures/samples was conducted on silica gel (100–200 mesh). 1H NMR and 13C{1H} NMR spectra were recorded on 400 and ∼101 MHz spectrometers, respectively (with TMS as an internal standard). The HRMS analysis data were obtained from the QTOF mass analyser using the electrospray ionization (ESI) method. The IR spectra were recorded either as neat samples/thin films or by using KBr for preparing pellets for solid samples, or in a solvent. The required anhydrous solvents were prepared under standard solvent drying procedures and reactions were conducted under a nitrogen atmosphere or in ambient air in an RB flask or the sealed tube as mentioned in the respective Schemes/Tables. Organic layers obtained from the work-up procedure were dried using anhydrous Na2SO4. Isolated yields of products were reported, and yields have not been optimized. The column chromatographic purification of the crude mixture of all the reactions comprising diastereomer formation gave only the major isomer, and we did not obtain the minor isomer in characterizable/detectable amounts from the fractions collected in the column purification process. In the case of reactions involving enantioenriched carboxamides, there is a minor gain or loss of er when compared to the starting substrates, and at this stage, we feel this may be due to handling/sampling error. The starting material amino acid carboxamides used for the construction of racemic and enantioenriched amino acid derivatives possessing a 4-bromobiphenyl moiety in the Pd(II)-catalyzed sp3 C–H arylation are known compounds and prepared using the standard synthetic procedures18General procedure for the synthesis of 4-bromobiaryl-based amino acid derivatives via the Pd(II)-catalyzed, 8-aminoquinoline-aided C–H arylation of amino acid carboxamides
A mixture of an appropriate amino acid carboxamide18a (0.25–4.6 mmol, 1 equiv.), 4-bromo-4′-iodobiphenyl (4, 4 equiv.), Pd(OAc)2 (10 mol%) and AgOAc (2.5 equiv.) in anhydrous toluene (2–10 mL) was heated at 110 °C for 48 h under a nitrogen atm. After the reaction period, the reaction mixture was concentrated under reduced pressure, and the crude reaction mixture was purified by column chromatography on neutral alumina or silica gel (eluent = EtOAc:hexane) to afford the corresponding bromobiaryl-based amino acid derivatives 5/6 (see the corresponding scheme for specific entry).General procedure for the removal of the 8-aminoquinoline directing group and synthesis of amino acid ester derivatives
A mixture of an appropriate 8-aminoquinoline-based carboxamide (0.10–0.38 mmol, 1 equiv.), BF3·Et2O (15 equiv.), and anhydrous EtOH (3–4 mL) in a screw-capped sealed tube containing a magnetic bead was stirred, and the tube was heated at 130 °C for 36 h. Then, the reaction mixture was allowed to attain the rt and concentrated under reduced pressure to afford the corresponding crude reaction mixture. The crude reaction mixture was then purified by column chromatography to afford the corresponding amino acid ester derivatives 7/8 (see the corresponding Scheme for specific entry).General procedure for the deprotection of phthalimide group and synthesis of Phth-free amino acid derivatives
To an appropriate Phth-protected amino acid derivative (0.2–0.35 mmol, 1 equiv.) in t-BuOH (1–3 mL), ethane-1,2-diamine (10 equiv.) was added. The reaction mixture was stirred at rt for 24 h, and then, the solvent was removed under reduced pressure. The resultant reaction mixture was diluted with EtOAc (5–7 mL) and washed with water. The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure. The resulting crude reaction mixture was then purified by column chromatography to afford the corresponding Phth-free amino acid derivatives 9/10 (see the corresponding scheme for specific entry).General procedure for the Pd-catalyzed Suzuki–Miyaura cross-coupling reaction of N-Boc protected or free amino group-containing amino acid ester derivative
To a mixture of an appropriate free amino group or N-Boc protected amino acid derivative possessing 4-bromobiaryl moiety (0.054–0.2 mmol, 1 equiv.), arylboronic acid (1.5–3 equiv.), Pd(PPh3)4 (5–10 mol%), K2CO3 (3–5 equiv.) in toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL) was heated at 110 °C for 24 h in a sealed tube (filled with N2). After the reaction period was over, the crude reaction mixture was concentrated under vacuum and purified by column chromatography on silica gel (EtOAc
1 mL) was heated at 110 °C for 24 h in a sealed tube (filled with N2). After the reaction period was over, the crude reaction mixture was concentrated under vacuum and purified by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane) to afford the corresponding Suzuki–Miyaura cross-coupling products (see the corresponding Schemes for specific entry).
hexane) to afford the corresponding Suzuki–Miyaura cross-coupling products (see the corresponding Schemes for specific entry).
General procedure for the Pd(II)-catalyzed Suzuki–Miyaura and Heck cross-coupling reaction of phthalimide-protected amino acid ester derivative
A solution of phthalimide-protected amino acid derivative possessing 4-bromobiaryl moiety (0.09 mmol, 1 equiv.), arylboronic acid (1.5 equiv.), or ethyl acrylate (1.5 equiv.), Pd(OAc)2 (10 mol%), P(o-tolyl)3 (40 mol%), Et3N (0.2 mL) and CH3CN (2 mL) were taken in a sealed tube under a nitrogen atm and the tube was then submerged in a silicon oil bath preheated at 85 °C. After 17 h, the reaction mixture was cooled down to rt and the solvent was removed under reduced pressure to provide the crude reaction mixture. Purification of the crude reaction mixture by column chromatography on silica gel (EtOAc/hexane) afforded the corresponding products (see the corresponding Schemes for specific entry).General procedure for the Sonogashira cross-coupling reaction
A solution of the appropriate amino acid derivative possessing 4-bromobiaryl moiety (0.044–0.09 mmol, 1 equiv.), phenylacetylene (1.5–3 equiv.), Et3N (0.12–0.25 mL), CuI (3–5 mol%), Pd(PPh3)2Cl2 (5–10 mol%) and dry DMF (1–2 mL) was taken in a sealed tube under nitrogen atmosphere. The reaction tube was dipped in a silicon-containing oil bath preheated at 110 °C. After 17 h the reaction mixture was cooled down to rt and the reaction mixture was cooled down to rt and extracted with EtOAc (5–7 mL). The organic layer was washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by column chromatography on silica gel (EtOAc/hexanes) to afford the cross-coupled products (see the corresponding Scheme for specific entry).General procedure Pd-catalysed Miyaura borylation on 4-bromobiphenyl-based biaryl amino acid derivatives
A solution of an appropriate 4-bromobiphenyl-based biaryl amino acid derivative (0.1–0.25 mmol, 1 equiv.) was heated with B2Pin2 (2 equiv.) in Pd(dppf)2Cl2 (5 mol%), KOAc (3 equiv.), 1,4-dioxane (1–3 mL) at 80 °C for 36 h in a sealed tube purged with N2 atmosphere. After the reaction time was over, the reaction mixture was concentrated and purified by column chromatography on silica gel (EtOAc/hexanes as eluent) to afford the corresponding biaryl amino acid derivative possessing boronate ester moiety (see the corresponding Scheme for specific entry).3,3-Bis(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)propanamide (5-(DL))
For the data see ref. 18a the HPLC of the compound 5-(DL) was determined using the Daicel Chiralpak IC column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 53.19 min, tL = 63.38 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 53.19 min, tL = 63.38 min.
(R)-3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)propanamide (5-(D))
The compound 5-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a light yellow solid (209 mg, 92%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 3025, 1714, 769 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.22 (s, 1H), 8.72–8.68 (m, 1H), 8.59 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 8.06 (dd, J1 = 8.3, J2 = 1.5 Hz, 1H), 7.79 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.75 (d, J = 8.3 Hz, 2H), 7.63 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.55–7.40 (m, 12H), 7.34–7.27 (m, 5H), 6.11 (d, J = 12.3 Hz, 1H), 5.82 (d, J = 12.4 Hz, 1H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 167.9, 165.4, 148.0, 140.1, 139.9, 139.2, 139.1, 138.8, 138.3, 138.2, 135.9, 134.1, 133.8, 131.7, 131.6, 131.3, 128.6, 128.4, 128.3, 128.2, 127.7, 127.6, 127.1, 127.0, 123.4, 121.9, 121.4, 121.4, 116.8, 58.5, 49.6. HRMS (ESI): m/z [M + H]+ calcd for C44H30Br2N3O3: 806.0654 found, 806.0653. [α]25 D = −40.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = >95
70) as a light yellow solid (209 mg, 92%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 3025, 1714, 769 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.22 (s, 1H), 8.72–8.68 (m, 1H), 8.59 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 8.06 (dd, J1 = 8.3, J2 = 1.5 Hz, 1H), 7.79 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.75 (d, J = 8.3 Hz, 2H), 7.63 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.55–7.40 (m, 12H), 7.34–7.27 (m, 5H), 6.11 (d, J = 12.3 Hz, 1H), 5.82 (d, J = 12.4 Hz, 1H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 167.9, 165.4, 148.0, 140.1, 139.9, 139.2, 139.1, 138.8, 138.3, 138.2, 135.9, 134.1, 133.8, 131.7, 131.6, 131.3, 128.6, 128.4, 128.3, 128.2, 127.7, 127.6, 127.1, 127.0, 123.4, 121.9, 121.4, 121.4, 116.8, 58.5, 49.6. HRMS (ESI): m/z [M + H]+ calcd for C44H30Br2N3O3: 806.0654 found, 806.0653. [α]25 D = −40.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 5-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (80
5) of the compound 5-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 54.08 min, tL = 63.34 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 54.08 min, tL = 63.34 min.
(S)-3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)propanamide (5-(L))
The compound 5-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a light yellow solid (206 mg, 91%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 169–171 °C; IR (DCM): 3026, 1713, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.18 (s, 1H), 8.68–8.63 (m, 1H), 8.54 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 8.00 (dd, J1 = 8.3, J2 = 1.6 Hz, 1H), 7.74 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.71 (d, J = 8.3 Hz, 2H), 7.57 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.50–7.35 (m, 12H), 7.28–7.22 (m, 5H), 6.07 (d, J = 12.4 Hz, 1H), 5.77 (d, J = 12.4 Hz, 1H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 167.9, 165.4, 148.0, 140.0, 140.0, 139.2, 139.1, 138.8, 138.3, 138.2, 135.9, 134.1, 133.8, 131.7, 131.6, 131.3, 128.6, 128.4, 128.3, 128.2, 127.7, 127.6, 127.1, 127.0, 123.4, 121.9, 121.4, 121.4, 116.8, 58.5, 49.6. HRMS (ESI): m/z [M + H]+ calcd for C44H30Br2N3O3: 806.0654 found, 806.0649. [α]25 D = +37.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
70) as a light yellow solid (206 mg, 91%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 169–171 °C; IR (DCM): 3026, 1713, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.18 (s, 1H), 8.68–8.63 (m, 1H), 8.54 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 8.00 (dd, J1 = 8.3, J2 = 1.6 Hz, 1H), 7.74 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.71 (d, J = 8.3 Hz, 2H), 7.57 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.50–7.35 (m, 12H), 7.28–7.22 (m, 5H), 6.07 (d, J = 12.4 Hz, 1H), 5.77 (d, J = 12.4 Hz, 1H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 167.9, 165.4, 148.0, 140.0, 140.0, 139.2, 139.1, 138.8, 138.3, 138.2, 135.9, 134.1, 133.8, 131.7, 131.6, 131.3, 128.6, 128.4, 128.3, 128.2, 127.7, 127.6, 127.1, 127.0, 123.4, 121.9, 121.4, 121.4, 116.8, 58.5, 49.6. HRMS (ESI): m/z [M + H]+ calcd for C44H30Br2N3O3: 806.0654 found, 806.0649. [α]25 D = +37.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 5-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (80
2) of the compound 5-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 53.75 min, tL = 64.97 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 53.75 min, tL = 64.97 min.
(2S*,3R*)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)butanamide (6a-(DL))
The compound 6a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (149 mg, 90%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 192–194 °C; IR (DCM): 2971, 1716, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.59 (dd, J1 = 6.4, J2 = 2.6 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.5 Hz, 1H), 8.03 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.77 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.58 (d, J = 8.2 Hz, 2H), 7.52–7.50 (m, 4H), 7.41–7.40 (m, 2H), 7.33–7.26 (m, 3H), 5.33 (d, J = 11.6 Hz, 1H), 4.45–4.37 (m, 1H), 1.35 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 147.9, 142.2, 139.6, 138.8, 138.3, 135.9, 134.3, 133.9, 131.7, 131.7, 128.5, 128.4, 127.6, 127.6, 127.1, 123.7, 121.8, 121.4, 116.7, 61.4, 38.5, 20.3. HRMS (ESI): m/z [M + H]+ calcd for C33H25BrN3O3: 590.1079 found, 590.1083. The HPLC of the compound 6a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
70) as a colorless solid (149 mg, 90%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 192–194 °C; IR (DCM): 2971, 1716, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.59 (dd, J1 = 6.4, J2 = 2.6 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.5 Hz, 1H), 8.03 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.77 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.58 (d, J = 8.2 Hz, 2H), 7.52–7.50 (m, 4H), 7.41–7.40 (m, 2H), 7.33–7.26 (m, 3H), 5.33 (d, J = 11.6 Hz, 1H), 4.45–4.37 (m, 1H), 1.35 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 147.9, 142.2, 139.6, 138.8, 138.3, 135.9, 134.3, 133.9, 131.7, 131.7, 128.5, 128.4, 127.6, 127.6, 127.1, 123.7, 121.8, 121.4, 116.7, 61.4, 38.5, 20.3. HRMS (ESI): m/z [M + H]+ calcd for C33H25BrN3O3: 590.1079 found, 590.1083. The HPLC of the compound 6a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 33.32 min, tD = 38.36 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 33.32 min, tD = 38.36 min.
(2S,3R)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)butanamide (6a-(L))
The compound 6a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a semi-solid (152 mg, 92%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 191–193 °C; IR (DCM): 2972, 1716, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.94 (s, 1H), 8.59 (dd, J1 = 5.9, J2 = 3.1 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.5 Hz, 1H), 8.02 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.95 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.75 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58 (d, J = 8.2 Hz, 2H), 7.49 (d, J = 8.1 Hz, 4H), 7.40–7.39 (m, 2H), 7.32–7.5 (m, 3H), 5.33 (d, J = 11.5 Hz, 1H), 4.45–4.37 (m, 1H), 1.35 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 165.7, 147.8, 142.1, 139.3, 138.6, 138.1, 135.8, 134.2, 133.7, 131.6, 131.5, 128.3, 128.2, 127.4, 127.4, 126.9, 123.5, 121.7, 121.3, 121.3, 116.5, 61.2, 38.3, 20.2. HRMS (ESI): m/z [M + H]+ calcd for C33H25BrN3O3: 590.1079 found, 590.1080. [α]25 D = +38.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
70) as a semi-solid (152 mg, 92%, 0.28 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 191–193 °C; IR (DCM): 2972, 1716, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.94 (s, 1H), 8.59 (dd, J1 = 5.9, J2 = 3.1 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.5 Hz, 1H), 8.02 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.95 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.75 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58 (d, J = 8.2 Hz, 2H), 7.49 (d, J = 8.1 Hz, 4H), 7.40–7.39 (m, 2H), 7.32–7.5 (m, 3H), 5.33 (d, J = 11.5 Hz, 1H), 4.45–4.37 (m, 1H), 1.35 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 165.7, 147.8, 142.1, 139.3, 138.6, 138.1, 135.8, 134.2, 133.7, 131.6, 131.5, 128.3, 128.2, 127.4, 127.4, 126.9, 123.5, 121.7, 121.3, 121.3, 116.5, 61.2, 38.3, 20.2. HRMS (ESI): m/z [M + H]+ calcd for C33H25BrN3O3: 590.1079 found, 590.1080. [α]25 D = +38.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 6a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
5) of the compound 6a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 35.88 min, tD = 42.23 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 35.88 min, tD = 42.23 min.
(2S*,3R*)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)pentanamide (6b-(DL))
For the data see ref. 18a The HPLC of the compound 6b-(DL) was determined using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 14.93 min, tL = 31.69 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 14.93 min, tL = 31.69 min.
(2R,3S)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)pentanamide (6b-(D))
The compound 6b-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a light yellow solid (129 mg, 82%, 0.26 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 181–183 °C; IR (DCM): 2967, 1713, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.50–8.48 (m, 2H), 7.97 (dd, J1 = 8.2, J2 = 1.2 Hz, 1H), 7.86 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.70 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.48–7.42 (m, 6H), 7.35–7.22 (m, 5H), 5.30 (d, J = 11.7 Hz, 1H), 4.09 (td, J1 = 11.4, J2 = 3.4 Hz, 1H), 1.76–1.51 (m, 2H), 0.67 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 165.7, 147.8, 139.6, 139.2, 138.4, 138.0, 135.7, 134.1, 133.7, 131.5, 131.4, 129.0, 128.2, 127.4, 127.2, 126.8, 123.4, 121.6, 121.2, 121.1, 116.4, 60.7, 45.1, 25.9, 11.0; HRMS (ESI): m/z [M + Na]+ calcd for C34H26BrN3NaO3: 626.1055 found, 626.1063. [α]25 D = −39.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er 99
70) as a light yellow solid (129 mg, 82%, 0.26 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 181–183 °C; IR (DCM): 2967, 1713, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.50–8.48 (m, 2H), 7.97 (dd, J1 = 8.2, J2 = 1.2 Hz, 1H), 7.86 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.70 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.48–7.42 (m, 6H), 7.35–7.22 (m, 5H), 5.30 (d, J = 11.7 Hz, 1H), 4.09 (td, J1 = 11.4, J2 = 3.4 Hz, 1H), 1.76–1.51 (m, 2H), 0.67 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 165.7, 147.8, 139.6, 139.2, 138.4, 138.0, 135.7, 134.1, 133.7, 131.5, 131.4, 129.0, 128.2, 127.4, 127.2, 126.8, 123.4, 121.6, 121.2, 121.1, 116.4, 60.7, 45.1, 25.9, 11.0; HRMS (ESI): m/z [M + Na]+ calcd for C34H26BrN3NaO3: 626.1055 found, 626.1063. [α]25 D = −39.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the compound 6b-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50
1) of the compound 6b-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 14.95 min, tL = 31.83 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 14.95 min, tL = 31.83 min.
(2S,3R)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)pentanamide (6b-(L))
The compound 6b-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a light yellow solid (129 mg, 82%, 0.26 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 182–184 °C; IR (DCM): 2967, 1714, 769 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.94 (s, 1H), 8.58–8.55 (m, 2H), 8.04 (d, J = 8.2 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.77 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.59–7.49 (m, 6H), 7.42–7.29 (m, 5H), 5.37 (d, J = 11.7 Hz, 1H), 4.17 (td, J1 = 11.4, J2 = 3.6 Hz, 1H), 1.83–1.57 (m, 2H), 0.74 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.2, 165.8, 147.9, 139.7, 139.4, 138.6, 138.2, 135.9, 134.2, 133.9, 131.6, 131.6, 129.2, 128.4, 127.5, 127.3, 127.0, 123.6, 121.7, 121.3, 121.3, 116.6, 60.8, 45.2, 26.1, 11.6; HRMS (ESI): m/z [M + H]+ calcd for C34H26BrN3NaO3: 626.1055 found, 626.1059. [α]25 D = +42.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = >98
70) as a light yellow solid (129 mg, 82%, 0.26 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 182–184 °C; IR (DCM): 2967, 1714, 769 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.94 (s, 1H), 8.58–8.55 (m, 2H), 8.04 (d, J = 8.2 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.77 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.59–7.49 (m, 6H), 7.42–7.29 (m, 5H), 5.37 (d, J = 11.7 Hz, 1H), 4.17 (td, J1 = 11.4, J2 = 3.6 Hz, 1H), 1.83–1.57 (m, 2H), 0.74 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.2, 165.8, 147.9, 139.7, 139.4, 138.6, 138.2, 135.9, 134.2, 133.9, 131.6, 131.6, 129.2, 128.4, 127.5, 127.3, 127.0, 123.6, 121.7, 121.3, 121.3, 116.6, 60.8, 45.2, 26.1, 11.6; HRMS (ESI): m/z [M + H]+ calcd for C34H26BrN3NaO3: 626.1055 found, 626.1059. [α]25 D = +42.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 6b-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50
2) of the compound 6b-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 15.03 min, tL = 31.52 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 15.03 min, tL = 31.52 min.
(2S*,3R*)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-4-methyl-N-(quinolin-8-yl)pentanamide (6c-(DL))
For the data see ref. 18a the HPLC of the compound 6c-(DL) was determined using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 11.68 min, tL = 17.68 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 11.68 min, tL = 17.68 min.
(2R,3S)3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-4-methyl-N-(quinolin-8-yl)pentanamide (6c-(D))
The compound 6c-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (148 mg, 96%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 233–235 °C; IR (DCM): 2962, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.11 (s, 1H), 8.60–8.56 (m, 2H), 8.04 (dd, J1 = 8.3, J2 = 1.6 Hz, 1H), 7.96 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.76 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.57–7.53 (m, 6H), 7.44–7.32 (m, 5H), 5.72 (d, J = 12.4 Hz, 1H), 4.35 (dd, J1 = 12.4, J2 = 3.4 Hz, 1H), 2.10–2.03 (m, 1H), 0.90 (d, J = 6.8 Hz, 3H), 0.85 (d, J = 6.8 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 166.1, 147.9, 139.5, 138.7, 138.4, 136.2, 135.9, 134.3, 134.1, 131.8, 131.8, 130.6, 128.5, 127.6, 127.0, 126.8, 123.7, 121.8, 121.4, 121.4, 116.8, 57.9, 48.1, 29.0, 21.5, 16.4; HRMS (ESI): m/z [M + Na]+ calcd for C35H28BrN3NaO3: 640.1212 found, 640.1208. [α]25 D = −46.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96
70) as a colorless solid (148 mg, 96%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 233–235 °C; IR (DCM): 2962, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.11 (s, 1H), 8.60–8.56 (m, 2H), 8.04 (dd, J1 = 8.3, J2 = 1.6 Hz, 1H), 7.96 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.76 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.57–7.53 (m, 6H), 7.44–7.32 (m, 5H), 5.72 (d, J = 12.4 Hz, 1H), 4.35 (dd, J1 = 12.4, J2 = 3.4 Hz, 1H), 2.10–2.03 (m, 1H), 0.90 (d, J = 6.8 Hz, 3H), 0.85 (d, J = 6.8 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 166.1, 147.9, 139.5, 138.7, 138.4, 136.2, 135.9, 134.3, 134.1, 131.8, 131.8, 130.6, 128.5, 127.6, 127.0, 126.8, 123.7, 121.8, 121.4, 121.4, 116.8, 57.9, 48.1, 29.0, 21.5, 16.4; HRMS (ESI): m/z [M + Na]+ calcd for C35H28BrN3NaO3: 640.1212 found, 640.1208. [α]25 D = −46.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of the compound 6c-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50
4) of the compound 6c-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 11.25 min, tL = 17.25 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 11.25 min, tL = 17.25 min.
(2S,3R)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-4-methyl-N-(quinolin-8-yl)pentanamide (6c-(L))
The compound 6c-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (147 mg, 95%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 234–236 °C; IR (DCM): 2962, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.09 (s, 1H), 8.57–8.53 (m, 2H), 8.02 (dd, J1 = 8.3, J2 = 1.5 Hz, 1H), 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.76 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.55–7.51 (m, 6H), 7.41–7.29 (m, 5H), 5.70 (d, J = 12.4 Hz, 1H), 4.32 (dd, J1 = 12.4, J2 = 3.5 Hz, 1H), 2.07–2.00 (m, 1H), 0.87 (d, J = 6.9 Hz, 3H), 0.82 (d, J = 6.8 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 166.1, 147.9, 139.5, 138.6, 138.4, 136.2, 135.9, 134.3, 134.1, 131.8, 131.8, 130.5, 128.5, 127.6, 127.0, 126.8, 123.7, 121.8, 121.4, 121.4, 116.8, 57.9, 48.1, 29.0, 21.5, 16.3; HRMS (ESI): m/z [M + H]+ calcd for C35H29BrN3O3: 618.1392 found, 618.1404. [α]25 D = +49.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
70) as a colorless solid (147 mg, 95%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 234–236 °C; IR (DCM): 2962, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.09 (s, 1H), 8.57–8.53 (m, 2H), 8.02 (dd, J1 = 8.3, J2 = 1.5 Hz, 1H), 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.76 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.55–7.51 (m, 6H), 7.41–7.29 (m, 5H), 5.70 (d, J = 12.4 Hz, 1H), 4.32 (dd, J1 = 12.4, J2 = 3.5 Hz, 1H), 2.07–2.00 (m, 1H), 0.87 (d, J = 6.9 Hz, 3H), 0.82 (d, J = 6.8 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 166.1, 147.9, 139.5, 138.6, 138.4, 136.2, 135.9, 134.3, 134.1, 131.8, 131.8, 130.5, 128.5, 127.6, 127.0, 126.8, 123.7, 121.8, 121.4, 121.4, 116.8, 57.9, 48.1, 29.0, 21.5, 16.3; HRMS (ESI): m/z [M + H]+ calcd for C35H29BrN3O3: 618.1392 found, 618.1404. [α]25 D = +49.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 6c-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50
3) of the compound 6c-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 11.28 min, tL = 17.16 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 11.28 min, tL = 17.16 min.
(2S*,3R*)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)hexanamide (6d-(DL))
For the data see ref. 18a the HPLC of the compound 6d-(DL) was determined using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 13.38 min, tL = 28.17 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 13.38 min, tL = 28.17 min.
(2R,3S)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)hexanamide (6d-(D))
The compound 6d-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (117 mg, 76%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 187–189 °C; IR (DCM): 2959, 1714, 747 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.90 (s, 1H), 8.57–8.55 (m, 2H), 8.04–8.02 (m, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.77 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.54 (d, J = 8.1 Hz, 2H), 7.50–7.47 (m, 4H), 7.41–7.36 (m, 2H), 7.31–7.26 (m, 3H), 5.34 (d, J = 11.6 Hz, 1H), 4.30–4.24 (m, 1H), 1.68–1.62 (m, 2H), 1.16–1.09 (m, 2H), 0.80 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 165.8, 147.8, 140.0, 139.2, 138.4, 138.0, 135.7, 134.1, 133.7, 131.5, 131.4, 129.0, 128.2, 127.4, 127.2, 126.8, 123.5, 121.6, 121.2, 121.2, 116.5, 61.0, 43.4, 35.0, 19.6, 13.7. HRMS (ESI): m/z [M + H]+ calcd for C35H29BrN3O3: 618.1392 found, 618.1389. [α]25 D = −52.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
70) as a colorless solid (117 mg, 76%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 187–189 °C; IR (DCM): 2959, 1714, 747 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.90 (s, 1H), 8.57–8.55 (m, 2H), 8.04–8.02 (m, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.77 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.54 (d, J = 8.1 Hz, 2H), 7.50–7.47 (m, 4H), 7.41–7.36 (m, 2H), 7.31–7.26 (m, 3H), 5.34 (d, J = 11.6 Hz, 1H), 4.30–4.24 (m, 1H), 1.68–1.62 (m, 2H), 1.16–1.09 (m, 2H), 0.80 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 165.8, 147.8, 140.0, 139.2, 138.4, 138.0, 135.7, 134.1, 133.7, 131.5, 131.4, 129.0, 128.2, 127.4, 127.2, 126.8, 123.5, 121.6, 121.2, 121.2, 116.5, 61.0, 43.4, 35.0, 19.6, 13.7. HRMS (ESI): m/z [M + H]+ calcd for C35H29BrN3O3: 618.1392 found, 618.1389. [α]25 D = −52.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 6d-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50
2) of the compound 6d-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 14.39 min, tL = 29.52 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 14.39 min, tL = 29.52 min.
(2S,3R)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)hexanamide (6d-(L))
The compound 6d-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (115 mg, 74%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 185–187 °C; IR (DCM): 2958, 1714, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.98 (s, 1H), 8.62–8.56 (m, 2H), 8.01–7.99 (m, 1H), 7.93 (dd, J1 = 5.2, J2 = 3.1 Hz, 2H), 7.74 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58 (d, J = 8.2 Hz, 2H), 7.51–7.47 (m, 4H), 7.38–7.37 (m, 2H), 7.31–7.26 (m, 3H), 5.41 (d, J = 11.6 Hz, 1H), 4.37–4.30 (m, 1H), 1.72–1.67 (m, 2H), 1.21–1.11 (m, 2H), 0.83 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.2, 165.8, 147.9, 140.0, 139.4, 138.5, 138.1, 135.8, 134.2, 133.8, 131.6, 131.5, 129.0, 128.3, 127.5, 127.3, 126.9, 123.6, 121.7, 121.3, 121.2, 116.6, 61.1, 43.5, 35.0, 19.7, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C35H28BrN3NaO3: 640.1212 found, 640.1203. [α]25 D = +49.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
70) as a colorless solid (115 mg, 74%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 185–187 °C; IR (DCM): 2958, 1714, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.98 (s, 1H), 8.62–8.56 (m, 2H), 8.01–7.99 (m, 1H), 7.93 (dd, J1 = 5.2, J2 = 3.1 Hz, 2H), 7.74 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58 (d, J = 8.2 Hz, 2H), 7.51–7.47 (m, 4H), 7.38–7.37 (m, 2H), 7.31–7.26 (m, 3H), 5.41 (d, J = 11.6 Hz, 1H), 4.37–4.30 (m, 1H), 1.72–1.67 (m, 2H), 1.21–1.11 (m, 2H), 0.83 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.2, 165.8, 147.9, 140.0, 139.4, 138.5, 138.1, 135.8, 134.2, 133.8, 131.6, 131.5, 129.0, 128.3, 127.5, 127.3, 126.9, 123.6, 121.7, 121.3, 121.2, 116.6, 61.1, 43.5, 35.0, 19.7, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C35H28BrN3NaO3: 640.1212 found, 640.1203. [α]25 D = +49.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 6d-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50
3) of the compound 6d-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 13.90 min, tL = 29.31 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 13.90 min, tL = 29.31 min.
(2S*,3R*)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-3-phenyl-N-(quinolin-8-yl)propanamide (6e-(DL))
For the data see ref. 18a The HPLC of the compound 6e-(DL) was determined using the Daicel Chiralpak IC column, hexane/i-PrOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 19.54 min, tL = 28.87 min.
60), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 19.54 min, tL = 28.87 min.
(2R,3S)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-3-phenyl-N-(quinolin-8-yl)propanamide (6e-(D))
The compound 6e-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (2270 mg, 76%, 4.6 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 233–235 °C; IR (DCM): 2924, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.08 (s, 1H), 8.57 (dd, J1 = 6.2, J2 = 2.7 Hz, 1H), 8.49 (dd, J1 = 4.2, J2 = 1.4 Hz, 1H), 7.99 (dd, J1 = 8.2, J2 = 1.5 Hz, 1H), 7.69 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.61 (d, J = 8.2 Hz, 2H), 7.57 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.50–7.32 (m, 8H), 7.26–7.19 (m, 3H), 7.11 (t, J = 7.6 Hz, 2H), 6.98 (t, J = 7.4 Hz, 1H), 5.94 (d, J = 12.3 Hz, 1H), 5.61 (d, J = 12.3 Hz, 1H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 167.8, 165.5, 148.0, 140.6, 140.2, 139.4, 138.8, 138.4, 136.0, 134.0, 134.0, 131.8, 131.4, 128.7, 128.6, 128.5, 127.8, 127.7, 127.1, 127.0, 123.4, 121.9, 121.5, 121.4, 116.9, 58.5, 50.0. HRMS (ESI): m/z [M + H]+ calcd for C38H27BrN3O3: 652.1236 found, 652.1234. [α]25 D = −52.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
70) as a colorless solid (2270 mg, 76%, 4.6 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 233–235 °C; IR (DCM): 2924, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.08 (s, 1H), 8.57 (dd, J1 = 6.2, J2 = 2.7 Hz, 1H), 8.49 (dd, J1 = 4.2, J2 = 1.4 Hz, 1H), 7.99 (dd, J1 = 8.2, J2 = 1.5 Hz, 1H), 7.69 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.61 (d, J = 8.2 Hz, 2H), 7.57 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.50–7.32 (m, 8H), 7.26–7.19 (m, 3H), 7.11 (t, J = 7.6 Hz, 2H), 6.98 (t, J = 7.4 Hz, 1H), 5.94 (d, J = 12.3 Hz, 1H), 5.61 (d, J = 12.3 Hz, 1H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 167.8, 165.5, 148.0, 140.6, 140.2, 139.4, 138.8, 138.4, 136.0, 134.0, 134.0, 131.8, 131.4, 128.7, 128.6, 128.5, 127.8, 127.7, 127.1, 127.0, 123.4, 121.9, 121.5, 121.4, 116.9, 58.5, 50.0. HRMS (ESI): m/z [M + H]+ calcd for C38H27BrN3O3: 652.1236 found, 652.1234. [α]25 D = −52.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 6e-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (40
3) of the compound 6e-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 19.76 min, tL = 29.84 min.
60), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 19.76 min, tL = 29.84 min.
(2S,3R)-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-3-phenyl-N-(quinolin-8-yl)propanamide (6e-(L))
For the data see ref. 18a the enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 6e-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (40
2) of the compound 6e-(L) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 19.86 min, tL = 29.24 min.
60), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 19.86 min, tL = 29.24 min.
Ethyl 3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoate (7-(DL))
For the data see ref. 18a the HPLC of the compound 7-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 31.48 min, tD = 38.88 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 31.48 min, tD = 38.88 min.
(R)-ethyl 3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoate (7-(D))
The compound 7-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (109 mg, 51%, 0.3 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 147–149 °C; IR (DCM): 2925, 1718, 757 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.75 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.64 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 7.9 Hz, 4H), 7.43 (t, J = 8.2 Hz, 4H), 7.37–7.30 (m, 4H), 7.24 (d, J = 9.0 Hz, 2H), 5.79 (d, J = 11.9 Hz, 1H), 5.37 (d, J = 11.9 Hz, 1H), 4.09–4.03 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 167.4, 141.0, 139.8, 139.5, 139.2, 138.5, 138.3, 134.1, 131.8, 131.7, 131.2, 128.5, 128.4, 128.3, 128.2, 127.2, 127.0, 123.4, 121.4, 121.4, 61.8, 54.9, 50.0, 13.7. HRMS (ESI): m/z [M + H]+ calcd for C37H28Br2NO4: 708.0385 found, 708.0400. [α]25 D = −102.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a colorless solid (109 mg, 51%, 0.3 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 147–149 °C; IR (DCM): 2925, 1718, 757 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.75 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.64 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 7.9 Hz, 4H), 7.43 (t, J = 8.2 Hz, 4H), 7.37–7.30 (m, 4H), 7.24 (d, J = 9.0 Hz, 2H), 5.79 (d, J = 11.9 Hz, 1H), 5.37 (d, J = 11.9 Hz, 1H), 4.09–4.03 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 167.4, 141.0, 139.8, 139.5, 139.2, 138.5, 138.3, 134.1, 131.8, 131.7, 131.2, 128.5, 128.4, 128.3, 128.2, 127.2, 127.0, 123.4, 121.4, 121.4, 61.8, 54.9, 50.0, 13.7. HRMS (ESI): m/z [M + H]+ calcd for C37H28Br2NO4: 708.0385 found, 708.0400. [α]25 D = −102.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 7-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80
2) of the compound 7-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 32.10 min, tD = 38.46 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 32.10 min, tD = 38.46 min.
(S)-Ethyl 3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)propanoate (7-(L))
The compound 7-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (112 mg, 53%, 0.3 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 146–148 °C; IR (DCM): 2925, 1714, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.75 (dd, J1 = 5.3, J2 = 3.1 Hz, 2H), 7.64 (dd, J1 = 5.3, J2 = 3.0 Hz, 2H), 7.60 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 7.9 Hz, 4H), 7.43 (t, J = 8.1 Hz, 4H), 7.37–7.30 (m, 4H), 7.24 (d, J = 8.9 Hz, 2H), 5.79 (d, J = 12.0 Hz, 1H), 5.37 (d, J = 12.0 Hz, 1H), 4.09–4.03 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 167.4, 141.1, 139.9, 139.6, 139.2, 138.5, 138.4, 134.1, 131.8, 131.7, 131.3, 128.5, 128.4, 128.3, 128.2, 127.3, 127.0, 123.5, 121.5, 121.4, 61.8, 54.9, 50.0, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C37H27Br2NNaO4: 730.0205 found, 730.0193. [α]25 D = +100.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a semi-solid (112 mg, 53%, 0.3 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 146–148 °C; IR (DCM): 2925, 1714, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.75 (dd, J1 = 5.3, J2 = 3.1 Hz, 2H), 7.64 (dd, J1 = 5.3, J2 = 3.0 Hz, 2H), 7.60 (d, J = 8.2 Hz, 2H), 7.53 (d, J = 7.9 Hz, 4H), 7.43 (t, J = 8.1 Hz, 4H), 7.37–7.30 (m, 4H), 7.24 (d, J = 8.9 Hz, 2H), 5.79 (d, J = 12.0 Hz, 1H), 5.37 (d, J = 12.0 Hz, 1H), 4.09–4.03 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 167.4, 141.1, 139.9, 139.6, 139.2, 138.5, 138.4, 134.1, 131.8, 131.7, 131.3, 128.5, 128.4, 128.3, 128.2, 127.3, 127.0, 123.5, 121.5, 121.4, 61.8, 54.9, 50.0, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C37H27Br2NNaO4: 730.0205 found, 730.0193. [α]25 D = +100.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 7-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80
2) of the compound 7-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 31.95 min, tD = 40.50 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 31.95 min, tD = 40.50 min.
(2S*,3R*)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)pentanoate (8a-(DL))
For the data see ref. 18a The HPLC of the compound 8a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 15.00 min, tL = 17.36 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 15.00 min, tL = 17.36 min.
(2R,3S)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)pentanoate (8a-(D))
The compound 8a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (91 mg, 60%, 0.3 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 130–132 °C; IR (DCM): 2932, 1718, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.79 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.57–7.55 (m, 4H), 7.49 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 5.17 (d, J = 10.4 Hz, 1H), 4.09–3.98 (m, 2H), 3.79 (td, J1 = 11.1, J2 = 3.8 Hz, 1H), 1.71–1.52 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H), 0.68 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 167.6, 141.1, 139.7, 138.2, 134.3, 131.7, 131.5, 129.0, 128.5, 126.7, 123.6, 121.2, 61.4, 56.9, 45.8, 25.3, 13.7, 11.3. HRMS (ESI): m/z [M + Na]+ calcd for C27H24BrNNaO4: 528.0786 found, 528.0786. [α]25 D = −30.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a colorless solid (91 mg, 60%, 0.3 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 130–132 °C; IR (DCM): 2932, 1718, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.79 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.57–7.55 (m, 4H), 7.49 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 5.17 (d, J = 10.4 Hz, 1H), 4.09–3.98 (m, 2H), 3.79 (td, J1 = 11.1, J2 = 3.8 Hz, 1H), 1.71–1.52 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H), 0.68 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 167.6, 141.1, 139.7, 138.2, 134.3, 131.7, 131.5, 129.0, 128.5, 126.7, 123.6, 121.2, 61.4, 56.9, 45.8, 25.3, 13.7, 11.3. HRMS (ESI): m/z [M + Na]+ calcd for C27H24BrNNaO4: 528.0786 found, 528.0786. [α]25 D = −30.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 8a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
2) of the compound 8a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 16.00 min, tL = 18.76 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 16.00 min, tL = 18.76 min.
(2S,3R)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)pentanoate (8a-(L))
The compound 8a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (90 mg, 59%, 0.3 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 131–133 °C; IR (DCM): 2931, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.78 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.56–7.53 (m, 4H), 7.47 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.1 Hz, 2H), 5.14 (d, J = 10.4 Hz, 1H), 4.07–3.94 (m, 2H), 3.76 (td, J1 = 11.1, J2 = 3.8 Hz, 1H), 1.69–1.48 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.67 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 167.7, 141.1, 139.7, 138.2, 134.3, 131.8, 131.6, 129.1, 128.5, 126.8, 123.6, 121.3, 61.4, 56.9, 45.8, 25.4, 13.7, 11.4. HRMS (ESI): m/z [M + Na]+ calcd for C27H24BrNNaO4: 528.0786 found, 528.0779. [α]25 D = +33.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
80) as a colorless solid (90 mg, 59%, 0.3 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 131–133 °C; IR (DCM): 2931, 1715, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.78 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.56–7.53 (m, 4H), 7.47 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.1 Hz, 2H), 5.14 (d, J = 10.4 Hz, 1H), 4.07–3.94 (m, 2H), 3.76 (td, J1 = 11.1, J2 = 3.8 Hz, 1H), 1.69–1.48 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.67 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 167.7, 141.1, 139.7, 138.2, 134.3, 131.8, 131.6, 129.1, 128.5, 126.8, 123.6, 121.3, 61.4, 56.9, 45.8, 25.4, 13.7, 11.4. HRMS (ESI): m/z [M + Na]+ calcd for C27H24BrNNaO4: 528.0786 found, 528.0779. [α]25 D = +33.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 8a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
2) of the compound 8a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 16.41 min, tL = 18.80 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 16.41 min, tL = 18.80 min.
(2S*,3R*)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-4-methylpentanoate (8b-(DL))
The compound 8b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (28 mg, 54%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 150–152 °C; IR (DCM): 2926, 1714, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.78 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.56–7.47 (m, 6H), 7.38 (d, J = 8.2 Hz, 2H), 5.42 (d, J = 11.7 Hz, 1H), 4.00–3.88 (m, 3H), 1.95–1.87 (m, 1H), 0.91 (t, J = 7.1 Hz, 3H), 0.80 (d, J = 2.8 Hz, 3H), 0.79 (d, J = 2.8 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 139.7, 138.1, 137.7, 134.3, 131.8, 131.6, 130.2, 128.5, 126.1, 123.6, 121.3, 61.4, 54.4, 48.7, 28.6, 21.5, 16.7, 13.6. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0942. The HPLC of the compound 8b-(DL) was determined using the Daicel Chiralpak AD column, hexane/i-PrOH (70
80) as a colorless solid (28 mg, 54%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 150–152 °C; IR (DCM): 2926, 1714, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.78 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.56–7.47 (m, 6H), 7.38 (d, J = 8.2 Hz, 2H), 5.42 (d, J = 11.7 Hz, 1H), 4.00–3.88 (m, 3H), 1.95–1.87 (m, 1H), 0.91 (t, J = 7.1 Hz, 3H), 0.80 (d, J = 2.8 Hz, 3H), 0.79 (d, J = 2.8 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 139.7, 138.1, 137.7, 134.3, 131.8, 131.6, 130.2, 128.5, 126.1, 123.6, 121.3, 61.4, 54.4, 48.7, 28.6, 21.5, 16.7, 13.6. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0942. The HPLC of the compound 8b-(DL) was determined using the Daicel Chiralpak AD column, hexane/i-PrOH (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.63 min, tD = 15.62 min.
30), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.63 min, tD = 15.62 min.
(2R, 3S)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-4-methylpentanoate (8b-(D))
The compound 8b-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (29 mg, 56%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 153–155 °C; IR (DCM): 2927, 1715, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.94 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.80 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58–7.49 (m, 6H), 7.40 (d, J = 8.2 Hz, 2H), 5.44 (d, J = 11.7 Hz, 1H), 3.99–3.93 (m, 3H), 1.97–1.90 (m, 1H), 0.93 (t, J = 7.1 Hz, 3H), 0.82 (d, J = 3.0 Hz, 3H), 0.80 (d, J = 3.0 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 139.7, 138.1, 137.7, 134.3, 131.8, 131.7, 130.2, 128.5, 126.1, 123.6, 121.3, 61.4, 54.5, 48.7, 28.6, 21.5, 16.7, 13.6. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0939. [α]25 D = −20.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = >95
80) as a colorless solid (29 mg, 56%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 153–155 °C; IR (DCM): 2927, 1715, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.94 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.80 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58–7.49 (m, 6H), 7.40 (d, J = 8.2 Hz, 2H), 5.44 (d, J = 11.7 Hz, 1H), 3.99–3.93 (m, 3H), 1.97–1.90 (m, 1H), 0.93 (t, J = 7.1 Hz, 3H), 0.82 (d, J = 3.0 Hz, 3H), 0.80 (d, J = 3.0 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 139.7, 138.1, 137.7, 134.3, 131.8, 131.7, 130.2, 128.5, 126.1, 123.6, 121.3, 61.4, 54.5, 48.7, 28.6, 21.5, 16.7, 13.6. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0939. [α]25 D = −20.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 8b-(D) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (70
5) of the compound 8b-(D) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.36 min, tD = 15.56 min.
30), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.36 min, tD = 15.56 min.
(2S,3R)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-4-methylpentanoate (8b-(L))
The compound 8b-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (28 mg, 53%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 151–153 °C; IR (DCM): 2926, 1714, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.84 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.70 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.48–7.39 (m, 6H), 7.30 (d, J = 8.1 Hz, 2H), 5.34 (d, J = 11.7 Hz, 1H), 3.88–3.83 (m, 3H), 1.87–1.79 (m, 1H), 0.83 (t, J = 7.1 Hz, 3H), 0.72 (d, J = 2.7 Hz, 3H), 0.70 (d, J = 2.6 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 139.7, 138.1, 137.7, 134.3, 131.8, 131.7, 130.2, 128.5, 126.1, 123.6, 121.3, 61.4, 54.5, 48.7, 28.6, 21.5, 16.8, 13.6. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0941. [α]25 D = +18.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
80) as a colorless solid (28 mg, 53%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 151–153 °C; IR (DCM): 2926, 1714, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.84 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.70 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.48–7.39 (m, 6H), 7.30 (d, J = 8.1 Hz, 2H), 5.34 (d, J = 11.7 Hz, 1H), 3.88–3.83 (m, 3H), 1.87–1.79 (m, 1H), 0.83 (t, J = 7.1 Hz, 3H), 0.72 (d, J = 2.7 Hz, 3H), 0.70 (d, J = 2.6 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 139.7, 138.1, 137.7, 134.3, 131.8, 131.7, 130.2, 128.5, 126.1, 123.6, 121.3, 61.4, 54.5, 48.7, 28.6, 21.5, 16.8, 13.6. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0941. [α]25 D = +18.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 8b-(L) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (70
2) of the compound 8b-(L) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.68 min, tD = 15.75 min.
30), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.68 min, tD = 15.75 min.
(2S*,3R*)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)hexanoate (8c-(DL))
For the data see ref. 18a the HPLC of the compound 8c-(DL) was determined using the Daicel Chiralpak AD column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 16.81 min, tD = 21.55 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 16.81 min, tD = 21.55 min.
(2R,3S)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)hexanoate (8c-(D))
The compound 8c-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (93 mg, 58%, 0.31 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 131–133 °C; IR (DCM): 2932, 1716, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.78 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.56–7.41 (m, 8H), 5.11 (d, J = 10.4 Hz, 1H), 4.06–3.93 (m, 2H), 3.87 (td, J1 = 11.0, J2 = 4.2 Hz, 1H), 1.67–1.43 (m, 2H), 1.08–1.02 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 167.7, 141.4, 139.7, 138.2, 134.3, 131.8, 131.6, 129.0, 128.5, 126.8, 123.7, 121.3, 61.5, 57.1, 44.0, 34.4, 19.9, 13.8, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0947.[α]25 D = −45.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
80) as a colorless solid (93 mg, 58%, 0.31 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 131–133 °C; IR (DCM): 2932, 1716, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.78 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.56–7.41 (m, 8H), 5.11 (d, J = 10.4 Hz, 1H), 4.06–3.93 (m, 2H), 3.87 (td, J1 = 11.0, J2 = 4.2 Hz, 1H), 1.67–1.43 (m, 2H), 1.08–1.02 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 167.7, 141.4, 139.7, 138.2, 134.3, 131.8, 131.6, 129.0, 128.5, 126.8, 123.7, 121.3, 61.5, 57.1, 44.0, 34.4, 19.9, 13.8, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0947.[α]25 D = −45.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 8c-(D) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (90
3) of the compound 8c-(D) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 17.14 min, tD = 22.03 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 17.14 min, tD = 22.03 min.
(2S,3R)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)hexanoate (8c-(L))
The compound 8c-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (90 mg, 56%, 0.31 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 133–135 °C; IR (DCM): 2932, 1716, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.79 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.56–7.41 (m, 8H), 5.11 (d, J = 10.4 Hz, 1H), 4.06–3.93 (m, 2H), 3.87 (td, J1 = 11.0, J2 = 4.2 Hz, 1H), 1.64–1.43 (m, 2H), 1.09–1.04 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 167.7, 141.4, 139.7, 138.2, 134.3, 131.8, 131.6, 129.0, 128.5, 126.8, 123.7, 121.3, 61.5, 57.1, 44.0, 34.4, 19.9, 13.8, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0937. [α]25 D = +42.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
80) as a colorless solid (90 mg, 56%, 0.31 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 133–135 °C; IR (DCM): 2932, 1716, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.92 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.79 (dd, J1 = 5.5, J2 = 3.0 Hz, 2H), 7.56–7.41 (m, 8H), 5.11 (d, J = 10.4 Hz, 1H), 4.06–3.93 (m, 2H), 3.87 (td, J1 = 11.0, J2 = 4.2 Hz, 1H), 1.64–1.43 (m, 2H), 1.09–1.04 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.4, 167.7, 141.4, 139.7, 138.2, 134.3, 131.8, 131.6, 129.0, 128.5, 126.8, 123.7, 121.3, 61.5, 57.1, 44.0, 34.4, 19.9, 13.8, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C28H26BrNNaO4: 542.0943 found, 542.0937. [α]25 D = +42.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 8c-(L) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (90
3) of the compound 8c-(L) was determined by HPLC using the Daicel Chiralpak AD column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 16.91 min, tD = 21.92 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 16.91 min, tD = 21.92 min.
(2S*,3R*)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-3-phenylpropanoate (8d-(DL))
For the data see ref. 18a the HPLC of the compound 8d-(DL) was determined using the Daicel Chiralcel IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 9.37 min, tL = 11.48 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 9.37 min, tL = 11.48 min.
(2R,3S)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-3-phenylpropanoate (8d-(D))
The compound 8d-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (123 mg, 58%, 0.38 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 2930, 1715, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.64 (dd, J1 = 5.5, J2 = 3.1 Hz, 2H), 7.58–7.50 (m, 6H), 7.42 (d, J = 8.4 Hz, 2H), 7.29–7.26 (m, 2H), 7.11 (t, J = 7.7 Hz, 2H), 7.00 (t, J = 7.4 Hz, 1H), 5.76 (d, J = 13.5 Hz, 1H), 5.31 (d, J = 11.8 Hz, 1H), 4.10–4.02 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 167.2, 141.1, 140.2, 139.4, 138.2, 134.0, 131.7, 131.1, 128.5, 128.4, 128.1, 127.8, 127.0, 126.9, 123.2, 121.3, 61.6, 54.8, 50.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C31H24BrNNaO4: 576.0786 found, 576.0789. [α]25 D = −38.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
80) as a colorless solid (123 mg, 58%, 0.38 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 2930, 1715, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.64 (dd, J1 = 5.5, J2 = 3.1 Hz, 2H), 7.58–7.50 (m, 6H), 7.42 (d, J = 8.4 Hz, 2H), 7.29–7.26 (m, 2H), 7.11 (t, J = 7.7 Hz, 2H), 7.00 (t, J = 7.4 Hz, 1H), 5.76 (d, J = 13.5 Hz, 1H), 5.31 (d, J = 11.8 Hz, 1H), 4.10–4.02 (m, 2H), 1.02 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.1, 167.2, 141.1, 140.2, 139.4, 138.2, 134.0, 131.7, 131.1, 128.5, 128.4, 128.1, 127.8, 127.0, 126.9, 123.2, 121.3, 61.6, 54.8, 50.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C31H24BrNNaO4: 576.0786 found, 576.0789. [α]25 D = −38.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 8d-(D) was determined by HPLC using the Daicel Chiralcel IC column, hexane/i-PrOH (50
3) of the compound 8d-(D) was determined by HPLC using the Daicel Chiralcel IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 10.46 min, tL = 13.00 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 10.46 min, tL = 13.00 min.
(2S,3R)-ethyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-3-phenylpropanoate (8d-(L))
For the data see ref. 18a the enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 8d-(L) was determined by HPLC using the Daicel Chiralcel IC column, hexane/i-PrOH (50
3) of the compound 8d-(L) was determined by HPLC using the Daicel Chiralcel IC column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 10.93 min, tL = 13.16 min.
50), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 10.93 min, tL = 13.16 min.
(2S*,3R*)-methyl 3-(4′-bromo-[1,1′-biphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)-3-phenylpropanoate (8e-(DL))
The compound 8e-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless semi-solid (123 mg, 60%, 0.38 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2925, 1715, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74 (dd, J1 = 5.5, J2 = 3.1 Hz, 2H), 7.65 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.58–7.51 (m, 6H), 7.42 (d, J = 8.5 Hz, 2H), 7.27–7.26 (m, 2H), 7.11 (t, J = 7.7 Hz, 2H), 7.00 (t, J = 7.4 Hz, 1H), 5.78 (d, J = 11.9 Hz, 1H), 5.30 (d, J = 11.8 Hz, 1H), 3.61 (s, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.6, 167.2, 141.1, 140.1, 139.6, 138.4, 134.1, 131.8, 131.2, 128.6, 128.2, 127.9, 127.2, 127.0, 123.4, 121.4, 54.6, 52.7, 50.3. HRMS (ESI): m/z [M + Na]+ calcd for C30H22BrNNaO4: 562.0630 found, 562.0615.
80) as a colorless semi-solid (123 mg, 60%, 0.38 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2925, 1715, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74 (dd, J1 = 5.5, J2 = 3.1 Hz, 2H), 7.65 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.58–7.51 (m, 6H), 7.42 (d, J = 8.5 Hz, 2H), 7.27–7.26 (m, 2H), 7.11 (t, J = 7.7 Hz, 2H), 7.00 (t, J = 7.4 Hz, 1H), 5.78 (d, J = 11.9 Hz, 1H), 5.30 (d, J = 11.8 Hz, 1H), 3.61 (s, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.6, 167.2, 141.1, 140.1, 139.6, 138.4, 134.1, 131.8, 131.2, 128.6, 128.2, 127.9, 127.2, 127.0, 123.4, 121.4, 54.6, 52.7, 50.3. HRMS (ESI): m/z [M + Na]+ calcd for C30H22BrNNaO4: 562.0630 found, 562.0615.
Ethyl 2-amino-3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)propanoate (9-(DL))
The compound 9-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (90 mg, 78%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2926, 1730, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.53–7.23 (m, 16H), 4.32 (d, J = 8.8 Hz, 1H), 4.26 (d, J = 8.8 Hz, 1H), 3.99 (q, J = 7.1 Hz, 2H), 1.01 (t, J = 7.1 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.7, 139.8, 139.4, 139.4, 138.6, 138.4, 131.8, 129.1, 128.7, 128.5, 128.4, 127.2, 126.9, 121.4, 121.4, 60.8, 58.6, 55.6, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H26Br2NO2: 578.0330 found, 578.0344. The HPLC of the compound 9-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
50) as a semi-solid (90 mg, 78%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2926, 1730, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.53–7.23 (m, 16H), 4.32 (d, J = 8.8 Hz, 1H), 4.26 (d, J = 8.8 Hz, 1H), 3.99 (q, J = 7.1 Hz, 2H), 1.01 (t, J = 7.1 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.7, 139.8, 139.4, 139.4, 138.6, 138.4, 131.8, 129.1, 128.7, 128.5, 128.4, 127.2, 126.9, 121.4, 121.4, 60.8, 58.6, 55.6, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H26Br2NO2: 578.0330 found, 578.0344. The HPLC of the compound 9-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 17.23 min, tL = 22.43 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 17.23 min, tL = 22.43 min.
(R)-ethyl 2-amino-3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)propanoate (9-(D))
The compound 9-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (94 mg, 81%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2928, 1732, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.54–7.38 (m, 16H), 4.32 (d, J = 8.7 Hz, 1H), 4.27 (d, J = 8.8 Hz, 1H), 4.01 (q, J = 7.1 Hz, 2H), 1.02 (t, J = 7.2 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.7, 139.9, 139.5, 139.4, 138.7, 138.4, 131.8, 129.2, 128.7, 128.5, 128.5, 127.3, 127.0, 121.5, 121.5, 60.9, 58.6, 55.7, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H26Br2NO2: 578.0330 found, 578.0334. [α]25 D = −40.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
50) as a semi-solid (94 mg, 81%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2928, 1732, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.54–7.38 (m, 16H), 4.32 (d, J = 8.7 Hz, 1H), 4.27 (d, J = 8.8 Hz, 1H), 4.01 (q, J = 7.1 Hz, 2H), 1.02 (t, J = 7.2 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.7, 139.9, 139.5, 139.4, 138.7, 138.4, 131.8, 129.2, 128.7, 128.5, 128.5, 127.3, 127.0, 121.5, 121.5, 60.9, 58.6, 55.7, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H26Br2NO2: 578.0330 found, 578.0334. [α]25 D = −40.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 9-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
5) of the compound 9-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 18.37 min, tL = 22.53 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 18.37 min, tL = 22.53 min.
(S)-ethyl 2-amino-3,3-bis(4′-bromo-[1,1′-biphenyl]-4-yl)propanoate (9-(L))
The compound 9-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (91 mg, 79%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1730, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.58–7.42 (m, 16H), 4.35 (d, J = 8.9 Hz, 1H), 4.30 (d, J = 8.8 Hz, 1H), 4.04 (q, J = 7.1 Hz, 2H), 1.05 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.7, 139.9, 139.5, 139.5, 138.7, 138.5, 131.8, 129.2, 128.8, 128.5, 128.5, 127.3, 127.0, 121.5, 121.5, 60.9, 58.6, 55.7, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H26Br2NO2: 578.0330 found, 578.0329. [α]25 D = +45.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
50) as a semi-solid (91 mg, 79%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1730, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.58–7.42 (m, 16H), 4.35 (d, J = 8.9 Hz, 1H), 4.30 (d, J = 8.8 Hz, 1H), 4.04 (q, J = 7.1 Hz, 2H), 1.05 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.7, 139.9, 139.5, 139.5, 138.7, 138.5, 131.8, 129.2, 128.8, 128.5, 128.5, 127.3, 127.0, 121.5, 121.5, 60.9, 58.6, 55.7, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H26Br2NO2: 578.0330 found, 578.0329. [α]25 D = +45.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 9-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
5) of the compound 9-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 18.96 min, tL = 22.92 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 18.96 min, tL = 22.92 min.
(2S*,3R*)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)pentanoate (10a-(DL))
The compound 10a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (85 mg, 81%, 0.28 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1719, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.56–7.43 (m, 6H), 7.29–7.26 (m, 2H), 4.09–3.98 (m, 2H), 3.59 (d, J = 6.3 Hz, 1H), 2.89–2.83 (m, 1H), 1.98–1.73 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.6, 139.8, 138.4, 131.8, 129.1, 128.5, 126.8, 121.4, 60.7, 59.9, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C19H23BrNO2: 376.0912 found, 376.0903. The HPLC of the compound 10a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
50) as a semi-solid (85 mg, 81%, 0.28 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1719, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.56–7.43 (m, 6H), 7.29–7.26 (m, 2H), 4.09–3.98 (m, 2H), 3.59 (d, J = 6.3 Hz, 1H), 2.89–2.83 (m, 1H), 1.98–1.73 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.6, 139.8, 138.4, 131.8, 129.1, 128.5, 126.8, 121.4, 60.7, 59.9, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C19H23BrNO2: 376.0912 found, 376.0903. The HPLC of the compound 10a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 6.66 min, tD = 8.14 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 6.66 min, tD = 8.14 min.
(2R,3S)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)pentanoate (10a-(D))
The compound 10a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (86 mg, 82%, 0.28 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1719, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.49–7.36 (m, 6H), 7.22–7.19 (m, 2H), 3.99–3.94 (m, 2H), 3.53 (d, J = 6.2 Hz, 1H), 2.82–2.77 (m, 1H), 1.89–1.70 (m, 2H), 1.03 (t, J = 7.1 Hz, 3H), 0.76 (t, J = 7.4 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.5, 139.8, 138.4, 131.8, 129.1, 128.5, 126.7, 121.3, 60.7, 59.9, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C19H23BrNO2: 376.0912 found, 376.0919. [α]25 D = −13.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a semi-solid (86 mg, 82%, 0.28 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1719, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.49–7.36 (m, 6H), 7.22–7.19 (m, 2H), 3.99–3.94 (m, 2H), 3.53 (d, J = 6.2 Hz, 1H), 2.82–2.77 (m, 1H), 1.89–1.70 (m, 2H), 1.03 (t, J = 7.1 Hz, 3H), 0.76 (t, J = 7.4 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.5, 139.8, 138.4, 131.8, 129.1, 128.5, 126.7, 121.3, 60.7, 59.9, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C19H23BrNO2: 376.0912 found, 376.0919. [α]25 D = −13.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 10a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
2) of the compound 10a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 6.70 min, tD = 7.79 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 6.70 min, tD = 7.79 min.
(2S,3R)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)pentanoate (10a-(L))
The compound 10a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (84 mg, 80%, 0.28 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1719, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.42 (m, 6H), 7.28–7.26 (m, 2H), 4.09–3.97 (m, 2H), 3.59 (d, J = 6.3 Hz, 1H), 2.88–2.83 (m, 1H), 1.96–1.74 (m, 2H), 1.09 (t, J = 7.2 Hz, 3H), 0.82 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.7, 140.5, 139.7, 138.3, 131.7, 129.0, 128.4, 126.7, 121.3, 60.6, 59.8, 51.7, 23.1, 13.9, 12.1. HRMS (ESI): m/z [M + H]+ calcd for C19H23BrNO2: 376.0912 found, 376.0923. [α]25 D = +10.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a semi-solid (84 mg, 80%, 0.28 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2927, 1719, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.42 (m, 6H), 7.28–7.26 (m, 2H), 4.09–3.97 (m, 2H), 3.59 (d, J = 6.3 Hz, 1H), 2.88–2.83 (m, 1H), 1.96–1.74 (m, 2H), 1.09 (t, J = 7.2 Hz, 3H), 0.82 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.7, 140.5, 139.7, 138.3, 131.7, 129.0, 128.4, 126.7, 121.3, 60.6, 59.8, 51.7, 23.1, 13.9, 12.1. HRMS (ESI): m/z [M + H]+ calcd for C19H23BrNO2: 376.0912 found, 376.0923. [α]25 D = +10.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 10a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90
2) of the compound 10a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 7.07 min, tD = 8.13 min.
10), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 7.07 min, tD = 8.13 min.
(2S*,3R*)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-4-methylpentanoate (10b-(DL))
The compound 10b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (100 mg, 80%, 0.32 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2924, 1732, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.43 (m, 6H), 7.20 (d, J = 8.2 Hz, 2H), 4.12–3.99 (m, 2H), 3.84 (d, J = 6.6 Hz, 1H), 2.72–2.68 (m, 1H), 2.45–2.37 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.6 Hz, 3H), 0.79 (d, J = 6.7 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 139.7, 139.2, 138.2, 131.7, 129.8, 128.4, 126.3, 121.3, 60.5, 57.1, 56.5, 28.1, 21.4, 20.0, 13.9. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1062. The HPLC of the compound 10b-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
50) as a semi-solid (100 mg, 80%, 0.32 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2924, 1732, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.43 (m, 6H), 7.20 (d, J = 8.2 Hz, 2H), 4.12–3.99 (m, 2H), 3.84 (d, J = 6.6 Hz, 1H), 2.72–2.68 (m, 1H), 2.45–2.37 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.6 Hz, 3H), 0.79 (d, J = 6.7 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 139.7, 139.2, 138.2, 131.7, 129.8, 128.4, 126.3, 121.3, 60.5, 57.1, 56.5, 28.1, 21.4, 20.0, 13.9. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1062. The HPLC of the compound 10b-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 8.61 min, tD = 11.40 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 8.61 min, tD = 11.40 min.
(2R,3S)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-4-methylpentanoate (10b-(D))
The compound 10b-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (102 mg, 82%, 0.32 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2924, 1732, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.43 (m, 6H), 7.20 (d, J = 8.2 Hz, 2H), 4.11–4.00 (m, 2H), 3.86 (d, J = 6.5 Hz, 1H), 2.72–2.69 (m, 1H), 2.45–2.37 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.6 Hz, 3H), 0.79 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.0, 139.7, 139.2, 138.3, 131.8, 129.9, 128.5, 126.4, 121.3, 60.7, 57.1, 56.5, 28.2, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1065. [α]25 D = −38.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a semi-solid (102 mg, 82%, 0.32 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2924, 1732, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.43 (m, 6H), 7.20 (d, J = 8.2 Hz, 2H), 4.11–4.00 (m, 2H), 3.86 (d, J = 6.5 Hz, 1H), 2.72–2.69 (m, 1H), 2.45–2.37 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.05 (d, J = 6.6 Hz, 3H), 0.79 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.0, 139.7, 139.2, 138.3, 131.8, 129.9, 128.5, 126.4, 121.3, 60.7, 57.1, 56.5, 28.2, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1065. [α]25 D = −38.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 10b-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
2) of the compound 10b-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 9.85 min, tD = 12.71 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 9.85 min, tD = 12.71 min.
(2S,3R)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-4-methylpentanoate (10b-(L))
The compound 10b-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (104 mg, 83%, 0.32 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2924, 1732, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.43 (m, 6H), 7.20 (d, J = 8.2 Hz, 2H), 4.11–4.01 (m, 2H), 3.85 (d, J = 6.6 Hz, 1H), 2.72–2.68 (m, 1H), 2.45–2.37 (m, 1H), 1.12 (t, J = 7.2 Hz, 3H), 1.04 (d, J = 6.6 Hz, 3H), 0.79 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 139.7, 139.2, 138.2, 131.8, 129.8, 128.5, 126.3, 121.3, 60.6, 57.1, 56.5, 28.2, 21.4, 20.0, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1060. [α]25 D = +40.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 90
50) as a semi-solid (104 mg, 83%, 0.32 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2924, 1732, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.43 (m, 6H), 7.20 (d, J = 8.2 Hz, 2H), 4.11–4.01 (m, 2H), 3.85 (d, J = 6.6 Hz, 1H), 2.72–2.68 (m, 1H), 2.45–2.37 (m, 1H), 1.12 (t, J = 7.2 Hz, 3H), 1.04 (d, J = 6.6 Hz, 3H), 0.79 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 139.7, 139.2, 138.2, 131.8, 129.8, 128.5, 126.3, 121.3, 60.6, 57.1, 56.5, 28.2, 21.4, 20.0, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1060. [α]25 D = +40.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10)* of the compound 10b-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
10)* of the compound 10b-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 9.76 min, tD = 12.70 min. (*Ascertained using best peak integration. The peaks could not be clearly resolved).
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 9.76 min, tD = 12.70 min. (*Ascertained using best peak integration. The peaks could not be clearly resolved).
(2R*,3S*)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-4-ylhexanoate (10c-(DL))
The compound 10c-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (100 mg, 74%, 0.35 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1729, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.42 (m, 6H), 7.29–7.23 (m, 2H), 4.09–3.98 (m, 2H), 3.57 (d, J = 6.3 Hz, 1H), 3.00–2.94 (m, 1H), 1.82–1.76 (m, 2H), 1.30–1.14 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.9, 139.7, 138.3, 131.8, 129.0, 128.5, 126.7, 121.3, 60.6, 60.0, 49.5, 32.2, 20.5, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1058. The HPLC of the compound 10c-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
50) as a semi-solid (100 mg, 74%, 0.35 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1729, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.42 (m, 6H), 7.29–7.23 (m, 2H), 4.09–3.98 (m, 2H), 3.57 (d, J = 6.3 Hz, 1H), 3.00–2.94 (m, 1H), 1.82–1.76 (m, 2H), 1.30–1.14 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.9, 139.7, 138.3, 131.8, 129.0, 128.5, 126.7, 121.3, 60.6, 60.0, 49.5, 32.2, 20.5, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1058. The HPLC of the compound 10c-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 12.53 min, tD = 14.10 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 12.53 min, tD = 14.10 min.
(2R,3S)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-4-ylhexanoate (10c-(D))
The compound 10c-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (102 mg, 75%, 0.35 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1729, 751 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.56–7.42 (m, 6H), 7.29–7.26 (m, 2H), 4.09–4.00 (m, 2H), 3.62 (d, J = 6.2 Hz, 1H), 3.03–2.98 (m, 1H), 1.83–1.78 (m, 2H), 1.33–1.14 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.88 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.7, 140.8, 139.8, 138.4, 131.8, 129.0, 128.5, 126.7, 121.4, 60.7, 60.1, 49.5, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1065. [α]25 D = −25.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
50) as a semi-solid (102 mg, 75%, 0.35 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1729, 751 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.56–7.42 (m, 6H), 7.29–7.26 (m, 2H), 4.09–4.00 (m, 2H), 3.62 (d, J = 6.2 Hz, 1H), 3.03–2.98 (m, 1H), 1.83–1.78 (m, 2H), 1.33–1.14 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.88 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.7, 140.8, 139.8, 138.4, 131.8, 129.0, 128.5, 126.7, 121.4, 60.7, 60.1, 49.5, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1065. [α]25 D = −25.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 10c-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
3) of the compound 10c-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 12.57 min, tD = 14.10 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 12.57 min, tD = 14.10 min.
(2S,3R)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-4-ylhexanoate (10c-(L))
The compound 10c-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (105 mg, 77%, 0.35 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1729, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.42 (m, 6H), 7.29–7.23 (m, 2H), 4.10–3.97 (m, 2H), 3.58 (d, J = 6.2 Hz, 1H), 3.00–2.94 (m, 1H), 1.82–1.77 (m, 2H), 1.34–1.14 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.88 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.9, 139.8, 138.3, 131.8, 129.0, 128.5, 126.7, 121.3, 60.6, 60.1, 49.5, 32.2, 20.5, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1060. [α]25 D = +26.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a semi-solid (105 mg, 77%, 0.35 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1729, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.55–7.42 (m, 6H), 7.29–7.23 (m, 2H), 4.10–3.97 (m, 2H), 3.58 (d, J = 6.2 Hz, 1H), 3.00–2.94 (m, 1H), 1.82–1.77 (m, 2H), 1.34–1.14 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.88 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 140.9, 139.8, 138.3, 131.8, 129.0, 128.5, 126.7, 121.3, 60.6, 60.1, 49.5, 32.2, 20.5, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C20H25BrNO2: 390.1069 found, 390.1060. [α]25 D = +26.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 10c-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
2) of the compound 10c-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 12.57 min, tD = 14.69 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 12.57 min, tD = 14.69 min.
(2S*,3R*)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-3-phenylpropanoate (10d-(DL))
The compound 10d-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (90 mg, 85%, 0.25 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1732, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.52–7.23 (m, 13H), 4.27 (d, J = 8.8 Hz, 1H), 4.23 (d, J = 8.8 Hz, 1H), 3.99 (q, J = 7.1 Hz, 2H), 1.00 (t, J = 7.1 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C NMR (101 MHz, CDCl3): δC 174.2, 140.9, 140.2, 139.5, 138.2, 131.7, 128.7, 128.7, 128.6, 128.4, 127.0, 126.8, 121.3, 60.7, 58.6, 56.1, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C23H23BrNO2: 424.0912 found, 424.0923. The HPLC of the compound 10d-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
50) as a semi-solid (90 mg, 85%, 0.25 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2930, 1732, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.52–7.23 (m, 13H), 4.27 (d, J = 8.8 Hz, 1H), 4.23 (d, J = 8.8 Hz, 1H), 3.99 (q, J = 7.1 Hz, 2H), 1.00 (t, J = 7.1 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C NMR (101 MHz, CDCl3): δC 174.2, 140.9, 140.2, 139.5, 138.2, 131.7, 128.7, 128.7, 128.6, 128.4, 127.0, 126.8, 121.3, 60.7, 58.6, 56.1, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C23H23BrNO2: 424.0912 found, 424.0923. The HPLC of the compound 10d-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 0.5 mL min−1, UV detection at 254 nm, tL = 36.19 min, tD = 37.94 min.
05), flow rate 0.5 mL min−1, UV detection at 254 nm, tL = 36.19 min, tD = 37.94 min.
(2R,3S)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-3-phenylpropanoate (10d-(D))
The compound 10d-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (92 mg, 87%, 0.25 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2929, 1731, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.54–7.24 (m, 13H), 4.27 (d, J = 9.0 Hz, 1H), 4.24 (d, J = 8.9 Hz, 1H), 4.00 (q, J = 7.1 Hz, 2H), 1.01 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C NMR (101 MHz, CDCl3): δC 174.3, 140.9, 140.3, 139.6, 138.4, 131.8, 128.8, 128.8, 128.7, 128.5, 127.1, 126.9, 121.4, 60.8, 58.7, 56.2, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C23H23BrNO2: 424.0912 found, 424.0920. [α]25 D = −26.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96
50) as a semi-solid (92 mg, 87%, 0.25 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2929, 1731, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.54–7.24 (m, 13H), 4.27 (d, J = 9.0 Hz, 1H), 4.24 (d, J = 8.9 Hz, 1H), 4.00 (q, J = 7.1 Hz, 2H), 1.01 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C NMR (101 MHz, CDCl3): δC 174.3, 140.9, 140.3, 139.6, 138.4, 131.8, 128.8, 128.8, 128.7, 128.5, 127.1, 126.9, 121.4, 60.8, 58.7, 56.2, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C23H23BrNO2: 424.0912 found, 424.0920. [α]25 D = −26.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of the compound 10d-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
4) of the compound 10d-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 0.5 mL min−1, UV detection at 254 nm, tL = 34.80 min, tD = 36.65 min.
05), flow rate 0.5 mL min−1, UV detection at 254 nm, tL = 34.80 min, tD = 36.65 min.
(2S,3R)-ethyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-3-phenylpropanoate (10d-(L))
The compound 10d-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a semi-solid (92 mg, 87%, 0.25 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2920, 1732, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.54–7.23 (m, 13H), 4.27 (d, J = 8.8 Hz, 1H), 4.24 (d, J = 8.8 Hz, 1H), 4.00 (q, J = 8.9 Hz, 2H), 1.01 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C NMR (101 MHz, CDCl3): δC 174.3, 141.0, 140.3, 139.6, 138.4, 131.8, 128.8, 128.8, 128.7, 128.5, 127.1, 126.9, 121.4, 60.8, 58.7, 56.2, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C23H23BrNO2: 424.0912 found, 424.0922. [α]25 D = +28.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
50) as a semi-solid (92 mg, 87%, 0.25 mmol scale); Rf (50% EtOAc/hexane) 0.5; IR (DCM): 2920, 1732, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.54–7.23 (m, 13H), 4.27 (d, J = 8.8 Hz, 1H), 4.24 (d, J = 8.8 Hz, 1H), 4.00 (q, J = 8.9 Hz, 2H), 1.01 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C NMR (101 MHz, CDCl3): δC 174.3, 141.0, 140.3, 139.6, 138.4, 131.8, 128.8, 128.8, 128.7, 128.5, 127.1, 126.9, 121.4, 60.8, 58.7, 56.2, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C23H23BrNO2: 424.0912 found, 424.0922. [α]25 D = +28.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 10d-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
5) of the compound 10d-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 0.5 mL min−1, UV detection at 254 nm, tL = 35.41 min, tD = 37.19 min.
05), flow rate 0.5 mL min−1, UV detection at 254 nm, tL = 35.41 min, tD = 37.19 min.
(2S*,3R*)-methyl 2-amino-3-(4′-bromo-[1,1′-biphenyl]-4-yl)-3-phenylpropanoate (10e-(DL))
The compound 10e-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless semi-solid (69 mg, 84%, 0.2 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2925, 1734, 765 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.50–7.20 (m, 13H), 4.30 (d, J = 8.5 Hz, 1H), 4.24 (d, J = 8.5 Hz, 1H), 3.52 (s, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.6, 140.8, 140.0, 139.3, 138.0, 131.6, 128.6, 128.6, 128.5, 128.3, 126.9, 126.7, 121.3, 58.5, 55.6, 51.7. HRMS (ESI): m/z [M + H]+ calcd for C22H21BrNO2: 410.0756 found, 410.0771.
80) as a colorless semi-solid (69 mg, 84%, 0.2 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2925, 1734, 765 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.50–7.20 (m, 13H), 4.30 (d, J = 8.5 Hz, 1H), 4.24 (d, J = 8.5 Hz, 1H), 3.52 (s, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.6, 140.8, 140.0, 139.3, 138.0, 131.6, 128.6, 128.6, 128.5, 128.3, 126.9, 126.7, 121.3, 58.5, 55.6, 51.7. HRMS (ESI): m/z [M + H]+ calcd for C22H21BrNO2: 410.0756 found, 410.0771.
(2S*,3R*)-ethyl 2-amino-3-(4′′-methoxy-[1,1':4′,1′′-terphenyl]-4-yl)pentanoate (12a-(DL))
The compound 12a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (68 mg, 84%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2934, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.57 (m, 8H), 7.30–7.26 (m, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.06–4.01 (m, 2H), 3.86 (s, 3H), 3.61 (d, J = 6.3 Hz, 1H), 2.89–2.84 (m, 1H), 1.97–1.76 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.84 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.8, 159.2, 140.1, 139.6, 139.2, 133.2, 129.0, 128.0, 127.2, 127.0, 126.8, 114.2, 60.6, 60.0, 55.3, 51.8, 23.3, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C26H30NO3: 404.2226 found, 404.2239. The HPLC of the compound 12a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (80
50) as a colorless solid (68 mg, 84%, 0.2 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2934, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.57 (m, 8H), 7.30–7.26 (m, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.06–4.01 (m, 2H), 3.86 (s, 3H), 3.61 (d, J = 6.3 Hz, 1H), 2.89–2.84 (m, 1H), 1.97–1.76 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.84 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.8, 159.2, 140.1, 139.6, 139.2, 133.2, 129.0, 128.0, 127.2, 127.0, 126.8, 114.2, 60.6, 60.0, 55.3, 51.8, 23.3, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C26H30NO3: 404.2226 found, 404.2239. The HPLC of the compound 12a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 291.7 nm, tL = 12.12 min, tD = 14.08 min.
20), flow rate 1.0 mL min−1, UV detection at 291.7 nm, tL = 12.12 min, tD = 14.08 min.
(2R,3S)-ethyl 2-amino-3-(4′′-methoxy-[1,1':4′,1′′-terphenyl]-4-yl)pentanoate (12a-(D))
The compound 12a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (18 mg, 83%, 0.054 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 165–167 °C; IR (DCM): 2934, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.29–7.25 (m, 2H), 7.00 (d, J = 8.6 Hz, 2H), 4.07–4.00 (m, 2H), 3.85 (s, 3H), 3.61 (d, J = 6.4 Hz, 1H), 2.89–2.84 (m, 1H), 1.97–1.76 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.7, 159.1, 140.0, 139.5, 139.1, 139.1, 133.1, 129.0, 128.0, 127.2, 127.0, 126.8, 114.2, 60.7, 59.9, 55.3, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C26H30NO3: 404.2226 found, 404.2239. [α]25 D = −22.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
50) as a colorless solid (18 mg, 83%, 0.054 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 165–167 °C; IR (DCM): 2934, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.29–7.25 (m, 2H), 7.00 (d, J = 8.6 Hz, 2H), 4.07–4.00 (m, 2H), 3.85 (s, 3H), 3.61 (d, J = 6.4 Hz, 1H), 2.89–2.84 (m, 1H), 1.97–1.76 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.7, 159.1, 140.0, 139.5, 139.1, 139.1, 133.1, 129.0, 128.0, 127.2, 127.0, 126.8, 114.2, 60.7, 59.9, 55.3, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C26H30NO3: 404.2226 found, 404.2239. [α]25 D = −22.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 12a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80
5) of the compound 12a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 291.7 nm, tL = 12.15 min, tD = 14.17 min.
20), flow rate 1.0 mL min−1, UV detection at 291.7 nm, tL = 12.15 min, tD = 14.17 min.
(2S,3R)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)pentanoate (12a-(L))
The compound 12a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (17 mg, 78%, 0.054 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2934, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.57 (m, 8H), 7.30–7.26 (m, 2H), 7.00 (d, J = 8.6 Hz, 2H), 4.06–4.01 (m, 2H), 3.86 (s, 3H), 3.61 (d, J = 6.3 Hz, 1H), 2.89–2.84 (m, 1H), 1.98–1.76 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 159.1, 140.0, 139.5, 139.1, 139.1, 133.1, 129.0, 128.0, 127.2, 127.0, 126.8, 114.2, 60.7, 59.9, 55.3, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C26H30NO3: 404.2226 found, 404.2229. [α]25 D = +19.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
50) as a colorless solid (17 mg, 78%, 0.054 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2934, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.57 (m, 8H), 7.30–7.26 (m, 2H), 7.00 (d, J = 8.6 Hz, 2H), 4.06–4.01 (m, 2H), 3.86 (s, 3H), 3.61 (d, J = 6.3 Hz, 1H), 2.89–2.84 (m, 1H), 1.98–1.76 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.8, 159.1, 140.0, 139.5, 139.1, 139.1, 133.1, 129.0, 128.0, 127.2, 127.0, 126.8, 114.2, 60.7, 59.9, 55.3, 51.7, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C26H30NO3: 404.2226 found, 404.2229. [α]25 D = +19.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 12a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80
5) of the compound 12a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 291.7 nm, tL = 12.05 min, tD = 13.77 min.
20), flow rate 1.0 mL min−1, UV detection at 291.7 nm, tL = 12.05 min, tD = 13.77 min.
(2S*,3R*)-ethyl 2-((tert-butoxycarbonyl)amino)-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)pentanoate (12b-(DL))
The compound 12b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (22 mg, 62%, 0.07 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 201–203 °C; IR (DCM): 2933, 1742, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.55 (m, 8H), 7.23 (d, J = 7.9 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 5.08 (d, J = 9.0 Hz, 1H), 4.51 (t, J = 8.2 Hz, 1H), 4.00 (q, J = 6.6 Hz, 2H), 3.86 (s, 3H), 2.90–2.84 (m, 1H), 1.96–1.77 (m, 2H), 1.45 (s, 9H), 1.04 (t, J = 6.9 Hz, 3H), 0.85 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.8, 159.2, 155.2, 139.6, 139.4, 139.1, 138.7, 133.2, 129.0, 128.0, 127.3, 127.0, 126.8, 114.2, 79.9, 61.0, 58.3, 55.3, 51.0, 28.3, 24.3, 13.9, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C31H37NNaO5: 526.2569 found, 526.2582.
80) as a colorless solid (22 mg, 62%, 0.07 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 201–203 °C; IR (DCM): 2933, 1742, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.55 (m, 8H), 7.23 (d, J = 7.9 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 5.08 (d, J = 9.0 Hz, 1H), 4.51 (t, J = 8.2 Hz, 1H), 4.00 (q, J = 6.6 Hz, 2H), 3.86 (s, 3H), 2.90–2.84 (m, 1H), 1.96–1.77 (m, 2H), 1.45 (s, 9H), 1.04 (t, J = 6.9 Hz, 3H), 0.85 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.8, 159.2, 155.2, 139.6, 139.4, 139.1, 138.7, 133.2, 129.0, 128.0, 127.3, 127.0, 126.8, 114.2, 79.9, 61.0, 58.3, 55.3, 51.0, 28.3, 24.3, 13.9, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C31H37NNaO5: 526.2569 found, 526.2582.
(2S*,3R*)-ethyl 2-amino-3-(4′′-methoxy-[1,1':4′,1′′-terphenyl]-4-yl)-4-methylpentanoate (13a-(DL))
The compound 13a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (14 mg, 67%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 129–131 °C; IR (DCM): 2924, 1729, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.53 (m, 8H), 7.21 (d, J = 8.2 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.11–4.02 (m, 2H), 3.88 (d, J = 6.5 Hz, 1H), 3.86 (s, 3H), 2.72 (t, J = 7.8 Hz, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 159.2, 139.5, 139.1, 138.6, 133.2, 129.8, 128.0, 127.2, 127.0, 126.4, 114.2, 60.7, 57.2, 56.6, 55.3, 28.2, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2398. The HPLC of the compound 13a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (80
50) as a colorless solid (14 mg, 67%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 129–131 °C; IR (DCM): 2924, 1729, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.53 (m, 8H), 7.21 (d, J = 8.2 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.11–4.02 (m, 2H), 3.88 (d, J = 6.5 Hz, 1H), 3.86 (s, 3H), 2.72 (t, J = 7.8 Hz, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 159.2, 139.5, 139.1, 138.6, 133.2, 129.8, 128.0, 127.2, 127.0, 126.4, 114.2, 60.7, 57.2, 56.6, 55.3, 28.2, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2398. The HPLC of the compound 13a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 9.18 min, tD = 12.48 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 9.18 min, tD = 12.48 min.
(2R,3S)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)-4-methylpentanoate (13a-(D))
The compound 13a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (15 mg, 72%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 130–132 °C; IR (DCM): 2925, 1729, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.53 (m, 8H), 7.21 (d, J = 8.2 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.12–4.01 (m, 2H), 3.88 (d, J = 6.6 Hz, 1H), 3.86 (s, 3H), 2.72 (t, J = 7.6 Hz, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.0, 159.2, 139.5, 139.1, 138.6, 133.2, 129.8, 128.0, 127.2, 127.0, 126.4, 114.2, 60.7, 57.1, 56.5, 55.3, 28.3, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2381. [α]25 D = −16.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
50) as a colorless solid (15 mg, 72%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 130–132 °C; IR (DCM): 2925, 1729, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.53 (m, 8H), 7.21 (d, J = 8.2 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.12–4.01 (m, 2H), 3.88 (d, J = 6.6 Hz, 1H), 3.86 (s, 3H), 2.72 (t, J = 7.6 Hz, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.0, 159.2, 139.5, 139.1, 138.6, 133.2, 129.8, 128.0, 127.2, 127.0, 126.4, 114.2, 60.7, 57.1, 56.5, 55.3, 28.3, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2381. [α]25 D = −16.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 13a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80
5) of the compound 13a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.87 min, tD = 14.33 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.87 min, tD = 14.33 min.
(2S,3R)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)-4-methylpentanoate (13a-(L))
The compound 13a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (14 mg, 67%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 128–130 °C; IR (DCM): 2925, 1729, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.69–7.49 (m, 8H), 7.22 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 4.11–4.03 (m, 2H), 3.91 (d, J = 6.4 Hz, 1H), 3.86 (s, 3H), 2.73 (t, J = 7.7 Hz, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.07 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.6 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.0, 159.2, 139.5, 139.1, 138.6, 133.2, 129.8, 128.0, 127.2, 127.0, 126.4, 114.2, 60.7, 57.2, 56.6, 55.3, 28.3, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2384. [α]25 D = +18.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96
50) as a colorless solid (14 mg, 67%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 128–130 °C; IR (DCM): 2925, 1729, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.69–7.49 (m, 8H), 7.22 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 4.11–4.03 (m, 2H), 3.91 (d, J = 6.4 Hz, 1H), 3.86 (s, 3H), 2.73 (t, J = 7.7 Hz, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.07 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.6 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.0, 159.2, 139.5, 139.1, 138.6, 133.2, 129.8, 128.0, 127.2, 127.0, 126.4, 114.2, 60.7, 57.2, 56.6, 55.3, 28.3, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2384. [α]25 D = +18.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of the compound 13a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80
4) of the compound 13a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.53 min, tD = 14.41 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 10.53 min, tD = 14.41 min.
(2S*,3R*)-ethyl 2-amino-3-(4′′-chloro-[1,1′:4′,1′′-terphenyl]-4-yl)-4-methylpentanoate (13b-(DL))
The compound 13b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (12 mg, 57%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 135–137 °C; IR (DCM): 2927, 1728, 749 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74–7.37 (m, 10H), 7.22 (d, J = 8.2 Hz, 2H), 4.15–4.02 (m, 2H), 3.87 (d, J = 6.5 Hz, 1H), 2.73–2.69 (m, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 140.1, 139.1, 138.9, 138.8, 138.7, 133.4, 129.8, 128.9, 128.2, 127.4, 127.3, 126.5, 60.7, 57.2, 56.6, 28.3, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + Na]+ calcd for C26H28ClNNaO2: 444.1706 found, 444.1704.
50) as a colorless solid (12 mg, 57%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 135–137 °C; IR (DCM): 2927, 1728, 749 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74–7.37 (m, 10H), 7.22 (d, J = 8.2 Hz, 2H), 4.15–4.02 (m, 2H), 3.87 (d, J = 6.5 Hz, 1H), 2.73–2.69 (m, 1H), 2.46–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 140.1, 139.1, 138.9, 138.8, 138.7, 133.4, 129.8, 128.9, 128.2, 127.4, 127.3, 126.5, 60.7, 57.2, 56.6, 28.3, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + Na]+ calcd for C26H28ClNNaO2: 444.1706 found, 444.1704.
(2S*,3R*)-ethyl 2-amino-3-(4′′-fluoro-[1,1′:4′,1′′-terphenyl]-4-yl)-4-methylpentanoate (13c-(DL))
The compound 13c-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (16 mg, 80%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 153–155 °C; IR (DCM): 2928, 1728, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.67–7.53 (m, 8H), 7.26–7.12 (m, 4H), 4.11–4.02 (m, 2H), 3.87 (d, J = 6.5 Hz, 1H), 2.72 (t, J = 7.6 Hz, 1H), 2.47–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 162.5 (d, JC-F = 247.3 Hz), 139.7, 138.9 (d, JC-F = 5.2 Hz), 136.8 (d, JC-F = 3.4 Hz), 129.8, 128.6, 128.5, 127.3, 127.3, 126.5, 115.8 (d, JC-F = 21.4 Hz), 60.7, 57.2, 56.6, 28.2, 21.5, 20.1, 14.0. 19F{1H} NMR (∼376 MHz, CDCl3): δF −115.64. HRMS (ESI): m/z [M + H]+ calcd for C26H29FNO2: 406.2182 found, 406.2189.
50) as a colorless solid (16 mg, 80%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 153–155 °C; IR (DCM): 2928, 1728, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.67–7.53 (m, 8H), 7.26–7.12 (m, 4H), 4.11–4.02 (m, 2H), 3.87 (d, J = 6.5 Hz, 1H), 2.72 (t, J = 7.6 Hz, 1H), 2.47–2.38 (m, 1H), 1.13 (t, J = 7.2 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 162.5 (d, JC-F = 247.3 Hz), 139.7, 138.9 (d, JC-F = 5.2 Hz), 136.8 (d, JC-F = 3.4 Hz), 129.8, 128.6, 128.5, 127.3, 127.3, 126.5, 115.8 (d, JC-F = 21.4 Hz), 60.7, 57.2, 56.6, 28.2, 21.5, 20.1, 14.0. 19F{1H} NMR (∼376 MHz, CDCl3): δF −115.64. HRMS (ESI): m/z [M + H]+ calcd for C26H29FNO2: 406.2182 found, 406.2189.
(2S*,3R*)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)hexanoate (14a-(DL))
The compound 14a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (15 mg, 72%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2933, 1724, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.29 (d, J = 8.2 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.08–4.00 (m, 2H), 3.86 (s, 3H), 3.59 (d, J = 6.3 Hz, 1H), 2.98 (dd, J1 = 14.5, J2 = 7.0 Hz, 1H), 1.81 (q, J = 7.8 Hz, 2H), 1.33–1.16 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 159.2, 140.3, 139.6, 139.2, 133.2, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 60.7, 60.2, 55.3, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2379. The HPLC of the compound 14a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
50) as a colorless solid (15 mg, 72%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2933, 1724, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.29 (d, J = 8.2 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 4.08–4.00 (m, 2H), 3.86 (s, 3H), 3.59 (d, J = 6.3 Hz, 1H), 2.98 (dd, J1 = 14.5, J2 = 7.0 Hz, 1H), 1.81 (q, J = 7.8 Hz, 2H), 1.33–1.16 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 159.2, 140.3, 139.6, 139.2, 133.2, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 60.7, 60.2, 55.3, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2379. The HPLC of the compound 14a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 24.43 min, tD = 34.81 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 24.43 min, tD = 34.81 min.
(2R,3S)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)hexanoate (14a-(D))
The compound 14a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (14 mg, 67%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 163–165 °C; IR (DCM): 2933, 1724, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.29 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 4.08–4.00 (m, 2H), 3.86 (s, 3H), 3.59 (d, J = 6.3 Hz, 1H), 2.98 (dd, J1 = 14.6, J2 = 7.0 Hz, 1H), 1.81 (q, J = 8.0 Hz, 2H), 1.33–1.16 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 159.2, 140.4, 139.6, 139.1, 139.1, 133.2, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 60.6, 60.2, 55.3, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2378. [α]25 D = −17.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96
50) as a colorless solid (14 mg, 67%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 163–165 °C; IR (DCM): 2933, 1724, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.29 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 4.08–4.00 (m, 2H), 3.86 (s, 3H), 3.59 (d, J = 6.3 Hz, 1H), 2.98 (dd, J1 = 14.6, J2 = 7.0 Hz, 1H), 1.81 (q, J = 8.0 Hz, 2H), 1.33–1.16 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 159.2, 140.4, 139.6, 139.1, 139.1, 133.2, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 60.6, 60.2, 55.3, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2378. [α]25 D = −17.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of the compound 14a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
4) of the compound 14a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 25.01 min, tD = 34.20 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 25.01 min, tD = 34.20 min.
(2S,3R)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)hexanoate (14a-(L))
The compound 14a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (15 mg, 72%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 161–163 °C; IR (DCM): 2933, 1724, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.56 (m, 8H), 7.29 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 4.08–4.00 (m, 2H), 3.86 (s, 3H), 3.59 (d, J = 6.3 Hz, 1H), 2.97 (dd, J1 = 14.5, J2 = 6.8 Hz, 1H), 1.81 (q, J = 7.9 Hz, 2H), 1.31–1.14 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 159.2, 140.4, 139.6, 139.1, 139.1, 133.2, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 60.6, 60.2, 55.3, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2390. [α]25 D = +14.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
50) as a colorless solid (15 mg, 72%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 161–163 °C; IR (DCM): 2933, 1724, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.56 (m, 8H), 7.29 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 4.08–4.00 (m, 2H), 3.86 (s, 3H), 3.59 (d, J = 6.3 Hz, 1H), 2.97 (dd, J1 = 14.5, J2 = 6.8 Hz, 1H), 1.81 (q, J = 7.9 Hz, 2H), 1.31–1.14 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.89 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 159.2, 140.4, 139.6, 139.1, 139.1, 133.2, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 60.6, 60.2, 55.3, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C27H32NO3: 418.2382 found, 418.2390. [α]25 D = +14.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 14a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95
3) of the compound 14a-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 22.11 min, tD = 31.86 min.
05), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 22.11 min, tD = 31.86 min.
(2S*,3R*)-ethyl 2-amino-3-(4′′-chloro-[1,1′:4′,1′′-terphenyl]-4-yl)hexanoate (14b-(DL))
The compound 14b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (12 mg, 57%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 133–135 °C; IR (DCM): 2930, 1729, 756 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.79–7.55 (m, 8H), 7.43–7.41 (m, 2H), 7.31–7.25 (m, 2H), 4.10–3.97 (m, 2H), 3.64 (d, J = 6.1 Hz, 1H), 3.03–2.98 (m, 1H), 1.84–1.73 (m, 2H), 1.31–1.15 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.2, 140.0, 139.1, 138.7, 135.0, 133.4, 128.9, 128.9, 128.2, 127.7, 127.4, 127.3, 127.0, 60.9, 59.9, 49.1, 32.0, 20.5, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C26H29ClNO2: 422.1887 found, 422.1885.
50) as a colorless solid (12 mg, 57%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 133–135 °C; IR (DCM): 2930, 1729, 756 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.79–7.55 (m, 8H), 7.43–7.41 (m, 2H), 7.31–7.25 (m, 2H), 4.10–3.97 (m, 2H), 3.64 (d, J = 6.1 Hz, 1H), 3.03–2.98 (m, 1H), 1.84–1.73 (m, 2H), 1.31–1.15 (m, 2H), 1.10 (t, J = 7.2 Hz, 3H), 0.88 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.2, 140.0, 139.1, 138.7, 135.0, 133.4, 128.9, 128.9, 128.2, 127.7, 127.4, 127.3, 127.0, 60.9, 59.9, 49.1, 32.0, 20.5, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C26H29ClNO2: 422.1887 found, 422.1885.
(2S*,3R*)-ethyl 2-amino-3-(4′′-fluoro-[1,1′:4′,1′′-terphenyl]-4-yl)hexanoate (14c-(DL))
The compound 14c-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (13 mg, 64%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2928, 1727, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.67–7.56 (m, 8H), 7.31–7.26 (m, 2H), 7.16–7.12 (m, 2H), 4.10–3.98 (m, 2H), 3.59 (d, J = 6.3 Hz, 1H), 2.98 (dd, J1 = 14.9, J2 = 6.6 Hz, 1H), 1.81 (q, J1 = 15.1, J2 = 7.9 Hz, 2H), 1.33–1.16 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 162.5 (d, JC–F = 247.3 Hz), 140.6, 139.8, 138.9 (d, JC–F = 2.1 Hz), 136.8 (d, JC–F = 3.0 Hz), 129.0, 128.6, 128.5, 127.3, 127.3, 126.8, 115.7 (d, JC–F = 21.5 Hz), 60.6, 60.2, 49.6, 32.3, 20.6, 14.0. 19F{1H} NMR (∼376 MHz, CDCl3): δF −115.65. HRMS (ESI): m/z [M + H]+ calcd for C26H29FNO2: 406.2182 found, 406.2175.
50) as a colorless solid (13 mg, 64%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2928, 1727, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.67–7.56 (m, 8H), 7.31–7.26 (m, 2H), 7.16–7.12 (m, 2H), 4.10–3.98 (m, 2H), 3.59 (d, J = 6.3 Hz, 1H), 2.98 (dd, J1 = 14.9, J2 = 6.6 Hz, 1H), 1.81 (q, J1 = 15.1, J2 = 7.9 Hz, 2H), 1.33–1.16 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 162.5 (d, JC–F = 247.3 Hz), 140.6, 139.8, 138.9 (d, JC–F = 2.1 Hz), 136.8 (d, JC–F = 3.0 Hz), 129.0, 128.6, 128.5, 127.3, 127.3, 126.8, 115.7 (d, JC–F = 21.5 Hz), 60.6, 60.2, 49.6, 32.3, 20.6, 14.0. 19F{1H} NMR (∼376 MHz, CDCl3): δF −115.65. HRMS (ESI): m/z [M + H]+ calcd for C26H29FNO2: 406.2182 found, 406.2175.
(2S*,3R*)-ethyl 2-((tert-butoxycarbonyl)amino)-3-(3′′-nitro-[1,1′:4′,1′′-terphenyl]-4-yl)hexanoate (14d-(DL))
The compound 14d-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (22 mg, 59%, 0.07 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 156–158 °C; IR (DCM): 2929, 1708, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.51–8.50 (m, 1H), 8.23–8.20 (m, 1H), 7.98–7.96 (m, 1H), 7.71 (s, 4H), 7.65–7.57 (m, 3H), 7.27–7.25 (m, 2H), 5.10 (d, J = 9.3 Hz, 1H), 4.53–4.49 (m, 1H), 4.09–3.99 (m, 2H), 3.01 (dd, J1 = 14.9, J2 = 7.2 Hz, 1H), 1.81 (q, J = 8.8 Hz, 2H), 1.45 (s, 9H), 1.29–1.17 (m, 2H), 1.07 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.8, 155.2, 148.8, 142.3, 141.1, 139.6, 138.8, 137.3, 132.8, 129.8, 129.0, 127.7, 127.5, 126.9, 122.0, 121.8, 79.9, 61.0, 58.4, 48.8, 33.2, 28.3, 20.6, 13.9, 13.9. HRMS (ESI): m/z [M + Na]+ calcd for C31H36N2NaO6: 555.2471 found, 555.2471.
80) as a colorless solid (22 mg, 59%, 0.07 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 156–158 °C; IR (DCM): 2929, 1708, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 8.51–8.50 (m, 1H), 8.23–8.20 (m, 1H), 7.98–7.96 (m, 1H), 7.71 (s, 4H), 7.65–7.57 (m, 3H), 7.27–7.25 (m, 2H), 5.10 (d, J = 9.3 Hz, 1H), 4.53–4.49 (m, 1H), 4.09–3.99 (m, 2H), 3.01 (dd, J1 = 14.9, J2 = 7.2 Hz, 1H), 1.81 (q, J = 8.8 Hz, 2H), 1.45 (s, 9H), 1.29–1.17 (m, 2H), 1.07 (t, J = 7.1 Hz, 3H), 0.89 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.8, 155.2, 148.8, 142.3, 141.1, 139.6, 138.8, 137.3, 132.8, 129.8, 129.0, 127.7, 127.5, 126.9, 122.0, 121.8, 79.9, 61.0, 58.4, 48.8, 33.2, 28.3, 20.6, 13.9, 13.9. HRMS (ESI): m/z [M + Na]+ calcd for C31H36N2NaO6: 555.2471 found, 555.2471.
(2S*,3R*)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)-3-phenylpropanoate (15a-(DL))
The compound 15a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (25 mg, 92%, 0.06 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 186–188 °C; IR (DCM): 2924, 1731, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.60–7.52 (m, 8H), 7.40–7.25 (m, 7H), 6.99 (d, J = 8.5 Hz, 2H), 4.26 (s, 2H), 3.98 (q, J = 6.8 Hz, 2H), 3.85 (s, 3H), 0.99 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.4, 159.2, 140.4, 140.4, 139.7, 139.2, 139.0, 133.2, 128.8, 128.7, 128.0, 127.3, 127.2, 127.1, 127.0, 126.9, 114.3, 60.8, 58.8, 56.3, 55.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C30H30NO3: 452.2226 found, 452.2238. The HPLC of the compound 15a-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (85
50) as a colorless solid (25 mg, 92%, 0.06 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 186–188 °C; IR (DCM): 2924, 1731, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.60–7.52 (m, 8H), 7.40–7.25 (m, 7H), 6.99 (d, J = 8.5 Hz, 2H), 4.26 (s, 2H), 3.98 (q, J = 6.8 Hz, 2H), 3.85 (s, 3H), 0.99 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.4, 159.2, 140.4, 140.4, 139.7, 139.2, 139.0, 133.2, 128.8, 128.7, 128.0, 127.3, 127.2, 127.1, 127.0, 126.9, 114.3, 60.8, 58.8, 56.3, 55.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C30H30NO3: 452.2226 found, 452.2238. The HPLC of the compound 15a-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 24.25 min, tL = 26.13 min.
15), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 24.25 min, tL = 26.13 min.
(2R,3S)-ethyl 2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)-3-phenylpropanoate (15a-(D))
The compound 15a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (24 mg, 89%, 0.06 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 185–187 °C; IR (DCM): 2923, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.64–7.55 (m, 8H), 7.44–7.28 (m, 7H), 7.02 (d, J = 8.7 Hz, 2H), 4.30 (s, 2H), 4.02 (q, J = 7.1 Hz, 2H), 3.88 (s, 3H), 1.03 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.4, 159.2, 140.4, 140.4, 139.7, 139.2, 139.0, 133.1, 128.8, 128.7, 128.0, 127.3, 127.2, 127.1, 127.0, 126.9, 114.2, 60.8, 58.8, 56.3, 55.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C30H30NO3: 452.2226 found, 452.2227. [α]25 D = −36.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98
50) as a colorless solid (24 mg, 89%, 0.06 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 185–187 °C; IR (DCM): 2923, 1730, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.64–7.55 (m, 8H), 7.44–7.28 (m, 7H), 7.02 (d, J = 8.7 Hz, 2H), 4.30 (s, 2H), 4.02 (q, J = 7.1 Hz, 2H), 3.88 (s, 3H), 1.03 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.4, 159.2, 140.4, 140.4, 139.7, 139.2, 139.0, 133.1, 128.8, 128.7, 128.0, 127.3, 127.2, 127.1, 127.0, 126.9, 114.2, 60.8, 58.8, 56.3, 55.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C30H30NO3: 452.2226 found, 452.2227. [α]25 D = −36.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 15a-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (85
2) of the compound 15a-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 24.55 min, tL = 26.76 min.
15), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 24.55 min, tL = 26.76 min.
Ethyl (2S,3R)-2-amino-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)-3-phenylpropanoate (15a-(L))
The compound 15a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (25 mg, 92%, 0.06 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 184–186 °C; IR (DCM): 2925, 1730, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.57–7.53 (m, 8H), 7.41–7.25 (m, 7H), 6.99 (d, J = 8.6 Hz, 2H), 4.26 (s, 2H), 4.00 (q, J = 7.1 Hz, 2H), 3.85 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.4, 159.2, 140.4, 139.7, 139.2, 139.0, 133.2, 128.8, 128.7, 128.7, 128.0, 127.3, 127.2, 127.1, 127.0, 126.9, 114.3, 60.8, 58.8, 56.3, 55.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C30H30NO3: 452.2226 found, 452.2220. [α]25 D = +33.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 99
50) as a colorless solid (25 mg, 92%, 0.06 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 184–186 °C; IR (DCM): 2925, 1730, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.57–7.53 (m, 8H), 7.41–7.25 (m, 7H), 6.99 (d, J = 8.6 Hz, 2H), 4.26 (s, 2H), 4.00 (q, J = 7.1 Hz, 2H), 3.85 (s, 3H), 1.00 (t, J = 7.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.4, 159.2, 140.4, 139.7, 139.2, 139.0, 133.2, 128.8, 128.7, 128.7, 128.0, 127.3, 127.2, 127.1, 127.0, 126.9, 114.3, 60.8, 58.8, 56.3, 55.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C30H30NO3: 452.2226 found, 452.2220. [α]25 D = +33.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the compound 15a-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (85
1) of the compound 15a-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 24.68 min, tL = 26.01 min.
15), flow rate 1.0 mL min−1, UV detection at 254 nm, tD = 24.68 min, tL = 26.01 min.
(2S*,3R*)-ethyl 3-([1,1′:4′,1′′-terphenyl]-4-yl)-2-amino-3-phenylpropanoate (15b-(DL))
The compound 15b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (45 mg, 71%, 0.15 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 140–142 °C; IR (DCM): 2928, 1731, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.66–7.33 (m, 18H), 4.29–4.25 (m, 2H), 4.00 (q, J = 7.0 Hz, 2H), 1.01 (t, J = 7.2 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.4, 140.6, 140.6, 140.4, 140.1, 139.6, 139.1, 128.8, 128.8, 128.7, 127.5, 127.3, 127.3, 127.1, 127.0, 60.8, 58.8, 56.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H28NO2: 422.2120 found, 422.2135.
50) as a colorless solid (45 mg, 71%, 0.15 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 140–142 °C; IR (DCM): 2928, 1731, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.66–7.33 (m, 18H), 4.29–4.25 (m, 2H), 4.00 (q, J = 7.0 Hz, 2H), 1.01 (t, J = 7.2 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼126 MHz, CDCl3): δC 174.4, 140.6, 140.6, 140.4, 140.1, 139.6, 139.1, 128.8, 128.8, 128.7, 127.5, 127.3, 127.3, 127.1, 127.0, 60.8, 58.8, 56.3, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H28NO2: 422.2120 found, 422.2135.
(2S*,3R*)-ethyl 2-amino-3-(4′′-chloro-[1,1′:4′,1′′-terphenyl]-4-yl)-3-phenylpropanoate (15c-(DL))
The compound 15c-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (60 mg, 82%, 0.16 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 140–142 °C; IR (DCM): 2929, 1731, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.68–7.53 (m, 8H), 7.43–7.35 (m, 8H), 7.29–7.22 (m, 1H), 4.29–4.24 (m, 2H), 4.00 (q, J = 7.1 Hz, 2H), 1.01 (t, J = 7.2 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.6, 140.3, 139.9, 139.0, 138.8, 138.7, 133.4, 128.9, 128.8, 128.7, 128.6, 128.2, 127.3, 127.2, 127.1, 126.9, 60.8, 58.7, 56.2, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H27ClNO2: 456.1730 found, 456.1735.
50) as a colorless solid (60 mg, 82%, 0.16 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 140–142 °C; IR (DCM): 2929, 1731, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.68–7.53 (m, 8H), 7.43–7.35 (m, 8H), 7.29–7.22 (m, 1H), 4.29–4.24 (m, 2H), 4.00 (q, J = 7.1 Hz, 2H), 1.01 (t, J = 7.2 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.6, 140.3, 139.9, 139.0, 138.8, 138.7, 133.4, 128.9, 128.8, 128.7, 128.6, 128.2, 127.3, 127.2, 127.1, 126.9, 60.8, 58.7, 56.2, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C29H27ClNO2: 456.1730 found, 456.1735.
Ethyl 11-(1,3-dioxoisoindolin-2-yl)-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)undecanoate (18a)
The compound 18a was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (15 mg, 48%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 115–117 °C; IR (DCM): 2929, 1710, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.82 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.69 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.65–7.55 (m, 8H), 7.25 (d, J = 6.4 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 3.65 (t, J = 7.4 Hz, 2H), 3.16–3.08 (m, 1H), 2.67–2.55 (m, 2H), 1.69–1.59 (m, 4H), 1.33–1.24 (m, 10H), 1.14 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.5, 168.4, 159.2, 143.3, 139.4, 139.2, 138.7, 133.8, 133.2, 132.2, 128.0, 127.9, 127.2, 126.9, 126.9, 123.1, 114.2, 60.2, 55.4, 41.9, 41.8, 38.0, 36.2, 29.4, 29.2, 29.1, 28.5, 27.3, 26.8, 14.1. HRMS (ESI): m/z [M + H]+ calcd for C40H44NO5: 618.3219 found, 618.3221.
80) as a colorless solid (15 mg, 48%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 115–117 °C; IR (DCM): 2929, 1710, 772 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.82 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.69 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.65–7.55 (m, 8H), 7.25 (d, J = 6.4 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 3.65 (t, J = 7.4 Hz, 2H), 3.16–3.08 (m, 1H), 2.67–2.55 (m, 2H), 1.69–1.59 (m, 4H), 1.33–1.24 (m, 10H), 1.14 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.5, 168.4, 159.2, 143.3, 139.4, 139.2, 138.7, 133.8, 133.2, 132.2, 128.0, 127.9, 127.2, 126.9, 126.9, 123.1, 114.2, 60.2, 55.4, 41.9, 41.8, 38.0, 36.2, 29.4, 29.2, 29.1, 28.5, 27.3, 26.8, 14.1. HRMS (ESI): m/z [M + H]+ calcd for C40H44NO5: 618.3219 found, 618.3221.
Ethyl 7-(1,3-dioxoisoindolin-2-yl)-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)heptanoate (18b)
The compound 18b was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (25 mg, 50%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2928, 1708, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.80 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.66 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.67–7.50 (m, 8H), 7.23 (d, J = 8.2 Hz, 2H), 7.01–6.99 (m, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 3.62 (t, J = 7.3 Hz, 2H), 3.17–3.10 (m, 1H), 2.68–2.56 (m, 2H), 1.78–1.58 (m, 4H), 1.27–1.21 (m, 2H), 1.14 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.3, 168.4, 159.1, 142.7, 139.4, 139.1, 138.8, 133.8, 133.2, 132.0, 128.0, 127.9, 127.2, 126.9, 123.1, 114.2, 60.3, 55.3, 41.7, 41.6, 37.7, 35.5, 28.3, 24.5, 14.1. HRMS (ESI): m/z [M + Na]+ calcd for C36H35NNaO5: 584.2413 found, 584.2432.
80) as a colorless solid (25 mg, 50%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2928, 1708, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.80 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.66 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.67–7.50 (m, 8H), 7.23 (d, J = 8.2 Hz, 2H), 7.01–6.99 (m, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.86 (s, 3H), 3.62 (t, J = 7.3 Hz, 2H), 3.17–3.10 (m, 1H), 2.68–2.56 (m, 2H), 1.78–1.58 (m, 4H), 1.27–1.21 (m, 2H), 1.14 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.3, 168.4, 159.1, 142.7, 139.4, 139.1, 138.8, 133.8, 133.2, 132.0, 128.0, 127.9, 127.2, 126.9, 123.1, 114.2, 60.3, 55.3, 41.7, 41.6, 37.7, 35.5, 28.3, 24.5, 14.1. HRMS (ESI): m/z [M + Na]+ calcd for C36H35NNaO5: 584.2413 found, 584.2432.
(E)-ethyl 7-(1,3-dioxoisoindolin-2-yl)-3-(4′-(3-ethoxy-3-oxoprop-1-en-1-yl)-[1,1′-biphenyl]-4-yl)heptanoate (18c)
The compound 18c was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless semi-solid (30 mg, 60%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2931, 1707, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.83 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.74 (d, J = 16.0 Hz, 1H), 7.68 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.62–7.58 (m, 4H), 7.51 (d, J = 8.2 Hz, 2H), 7.26 (d, J = 8.2 Hz, 2H), 6.50 (d, J = 16.0 Hz, 1H), 4.30 (q, J = 7.1 Hz, 2H), 4.06 (q, J = 7.1 Hz, 2H), 3.63 (t, J = 7.3 Hz, 2H), 3.19–3.11 (m, 1H), 2.70–2.58 (m, 2H), 1.78–1.58 (m, 4H), 1.38 (t, J = 7.1 Hz, 3H), 1.31–1.22 (m, 2H), 1.16 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 168.3, 167.1, 144.1, 143.5, 142.7, 138.2, 133.8, 133.2, 132.1, 128.5, 128.0, 127.3, 127.0, 123.1, 117.9, 60.5, 60.3, 41.6, 37.7, 35.5, 28.3, 24.5, 14.3, 14.1. HRMS (ESI): m/z [M + Na]+ calcd for C34H35NNaO6: 576.2362 found, 576.2380.
80) as a colorless semi-solid (30 mg, 60%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2931, 1707, 770 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.83 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.74 (d, J = 16.0 Hz, 1H), 7.68 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.62–7.58 (m, 4H), 7.51 (d, J = 8.2 Hz, 2H), 7.26 (d, J = 8.2 Hz, 2H), 6.50 (d, J = 16.0 Hz, 1H), 4.30 (q, J = 7.1 Hz, 2H), 4.06 (q, J = 7.1 Hz, 2H), 3.63 (t, J = 7.3 Hz, 2H), 3.19–3.11 (m, 1H), 2.70–2.58 (m, 2H), 1.78–1.58 (m, 4H), 1.38 (t, J = 7.1 Hz, 3H), 1.31–1.22 (m, 2H), 1.16 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 168.3, 167.1, 144.1, 143.5, 142.7, 138.2, 133.8, 133.2, 132.1, 128.5, 128.0, 127.3, 127.0, 123.1, 117.9, 60.5, 60.3, 41.6, 37.7, 35.5, 28.3, 24.5, 14.3, 14.1. HRMS (ESI): m/z [M + Na]+ calcd for C34H35NNaO6: 576.2362 found, 576.2380.
Ethyl 7-(1,3-dioxoisoindolin-2-yl)-3-(4′-(phenylethynyl)-[1,1′-biphenyl]-4-yl)heptanoate (19a)
The compound 19a was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (38 mg, 76%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 90–92 °C; IR (DCM): 2933, 1716, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.80 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.66 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59–7.47 (m, 8H), 7.36–7.35 (m, 3H), 7.26–7.22 (m, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.61 (t, J = 7.2 Hz, 2H), 3.17–3.09 (m, 1H), 2.67–2.55 (m, 2H), 1.75–1.58 (m, 4H), 1.29–1.13 (m, 2H), 1.15 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 168.4, 143.2, 140.6, 138.3, 133.8, 132.0, 131.9, 131.6, 128.3, 128.2, 127.9, 126.9, 126.8, 123.3, 123.1, 121.9, 90.0, 89.3, 60.3, 41.6, 41.6, 37.7, 35.5, 28.2, 24.4, 14.1. HRMS (ESI): m/z [M + H]+ calcd for C37H34NO4: 556.2488 found, 556.2512.
80) as a colorless solid (38 mg, 76%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 90–92 °C; IR (DCM): 2933, 1716, 771 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.80 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.66 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59–7.47 (m, 8H), 7.36–7.35 (m, 3H), 7.26–7.22 (m, 2H), 4.04 (q, J = 7.1 Hz, 2H), 3.61 (t, J = 7.2 Hz, 2H), 3.17–3.09 (m, 1H), 2.67–2.55 (m, 2H), 1.75–1.58 (m, 4H), 1.29–1.13 (m, 2H), 1.15 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 168.4, 143.2, 140.6, 138.3, 133.8, 132.0, 131.9, 131.6, 128.3, 128.2, 127.9, 126.9, 126.8, 123.3, 123.1, 121.9, 90.0, 89.3, 60.3, 41.6, 41.6, 37.7, 35.5, 28.2, 24.4, 14.1. HRMS (ESI): m/z [M + H]+ calcd for C37H34NO4: 556.2488 found, 556.2512.
(2S*,3R*)-ethyl 2-amino-3-phenyl-3-(4′-(phenylethynyl)-[1,1′-biphenyl]-4-yl)propanoate (19b-(DL))
The compound 19b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (15 mg, 67%, 0.05 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2924, 1731, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.58–7.50 (m, 9H), 7.41–7.39 (m, 2H), 7.36–7.34 (m, 7H), 4.28–4.23 (m, 2H), 4.00 (q, J = 7.2 Hz, 2H), 1.01 (t, J = 7.2 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.9, 140.4, 140.3, 138.7, 132.0, 131.6, 128.8, 128.7, 128.7, 128.3, 128.3, 127.1, 127.0, 126.8, 123.2, 122.1, 90.1, 89.2, 60.9, 58.8, 56.2, 13.9. HRMS (ESI): m/z [M + H]+ calcd for C31H28NO2: 446.2120 found, 446.2132.
70) as a colorless solid (15 mg, 67%, 0.05 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2924, 1731, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.58–7.50 (m, 9H), 7.41–7.39 (m, 2H), 7.36–7.34 (m, 7H), 4.28–4.23 (m, 2H), 4.00 (q, J = 7.2 Hz, 2H), 1.01 (t, J = 7.2 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.3, 140.9, 140.4, 140.3, 138.7, 132.0, 131.6, 128.8, 128.7, 128.7, 128.3, 128.3, 127.1, 127.0, 126.8, 123.2, 122.1, 90.1, 89.2, 60.9, 58.8, 56.2, 13.9. HRMS (ESI): m/z [M + H]+ calcd for C31H28NO2: 446.2120 found, 446.2132.
(2S*,3R*)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)butanamide (21a-(DL))
The compound 21a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a brown colored semi-solid (52 mg, 82%, 0.1 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 86–88 °C; IR (DCM): 2928, 1718, 758 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.89 (s, 1H), 8.60 (dd, J1 = 5.6, J2 = 3.3 Hz, 1H), 8.47 (dd, J1 = 4.0, J2 = 1.2 Hz, 1H), 8.02 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.84 (d, J = 7.8 Hz, 2H), 7.76 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59 (s, 4H), 7.49 (d, J = 7.9 Hz, 2H), 7.40–7.39 (m, 2H), 7.28–7.24 (m, 1H), 5.36 (d, J = 11.6 Hz, 1H), 4.44–4.36 (m, 1H), 1.43-1.36-1.35 (m, 15H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 148.0, 143.3, 142.1, 139.9, 138.3, 135.9, 135.2, 134.3, 133.9, 131.7, 128.3, 127.9, 127.6, 127.0, 126.2, 123.7, 121.8, 121.4, 116.7, 83.8, 61.3, 38.6, 24.8, 20.6. HRMS (ESI): m/z [M + H]+ calcd for C39H37BN3O5: 638.2826 found, 638.2834. The HPLC of the compound 21a-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (40
70) as a brown colored semi-solid (52 mg, 82%, 0.1 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 86–88 °C; IR (DCM): 2928, 1718, 758 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.89 (s, 1H), 8.60 (dd, J1 = 5.6, J2 = 3.3 Hz, 1H), 8.47 (dd, J1 = 4.0, J2 = 1.2 Hz, 1H), 8.02 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.84 (d, J = 7.8 Hz, 2H), 7.76 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59 (s, 4H), 7.49 (d, J = 7.9 Hz, 2H), 7.40–7.39 (m, 2H), 7.28–7.24 (m, 1H), 5.36 (d, J = 11.6 Hz, 1H), 4.44–4.36 (m, 1H), 1.43-1.36-1.35 (m, 15H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 148.0, 143.3, 142.1, 139.9, 138.3, 135.9, 135.2, 134.3, 133.9, 131.7, 128.3, 127.9, 127.6, 127.0, 126.2, 123.7, 121.8, 121.4, 116.7, 83.8, 61.3, 38.6, 24.8, 20.6. HRMS (ESI): m/z [M + H]+ calcd for C39H37BN3O5: 638.2826 found, 638.2834. The HPLC of the compound 21a-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 14.56 min, tD = 19.86 min.
60), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 14.56 min, tD = 19.86 min.
(2S,3R)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)butanamide (21a-(L))
The compound 21a-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a brown colored semi-solid (54 mg, 85%, 0.1 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 85–87 °C; IR (DCM): 2928, 1718, 757 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.59 (dd, J1 = 5.8, J2 = 3.1 Hz, 1H), 8.47 (dd, J1 = 4.0, J2 = 1.3 Hz, 1H), 8.02 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.84 (d, J = 7.8 Hz, 2H), 7.77 (dd, J1 = 5.3, J2 = 3.1 Hz, 2H), 7.58 (s, 4H), 7.48 (d, J = 7.8 Hz, 2H), 7.41–7.39 (m, 2H), 7.28–7.25 (m, 1H), 5.36 (d, J = 11.5 Hz, 1H), 4.43–4.35 (m, 1H), 1.36–1.35 (m, 15H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 148.0, 143.3, 142.2, 139.9, 138.3, 135.9, 135.2, 134.3, 134.0, 131.8, 128.3, 128.0, 127.6, 127.1, 126.3, 123.7, 121.8, 121.4, 116.7, 83.8, 61.3, 38.6, 24.9, 24.9, 20.6. HRMS (ESI): m/z [M + H]+ calcd for C39H37BN3O5: 638.2826 found, 638.2827. [α]25 D = +28.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96
70) as a brown colored semi-solid (54 mg, 85%, 0.1 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 85–87 °C; IR (DCM): 2928, 1718, 757 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.59 (dd, J1 = 5.8, J2 = 3.1 Hz, 1H), 8.47 (dd, J1 = 4.0, J2 = 1.3 Hz, 1H), 8.02 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.94 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.84 (d, J = 7.8 Hz, 2H), 7.77 (dd, J1 = 5.3, J2 = 3.1 Hz, 2H), 7.58 (s, 4H), 7.48 (d, J = 7.8 Hz, 2H), 7.41–7.39 (m, 2H), 7.28–7.25 (m, 1H), 5.36 (d, J = 11.5 Hz, 1H), 4.43–4.35 (m, 1H), 1.36–1.35 (m, 15H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 148.0, 143.3, 142.2, 139.9, 138.3, 135.9, 135.2, 134.3, 134.0, 131.8, 128.3, 128.0, 127.6, 127.1, 126.3, 123.7, 121.8, 121.4, 116.7, 83.8, 61.3, 38.6, 24.9, 24.9, 20.6. HRMS (ESI): m/z [M + H]+ calcd for C39H37BN3O5: 638.2826 found, 638.2827. [α]25 D = +28.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of the compound 21a-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (40
4) of the compound 21a-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 13.00 min, tD = 17.73 min.
60), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 13.00 min, tD = 17.73 min.
(2S*,3R*)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)pentanamide (21b-(DL))
The compound 21b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (145 mg, 89%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 120–122 °C; IR (DCM): 2931, 1715, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.59–8.57 (m, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.5 Hz, 1H), 7.98 (d, J = 8.3 Hz, 1H), 7.91 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.85 (d, J = 7.9 Hz, 2H), 7.72 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.61–7.55 (m, 4H), 7.49 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 4.1 Hz, 2H), 7.24 (dd, J1 = 8.2, J2 = 4.2 Hz, 1H), 5.42 (d, J = 11.6 Hz, 1H), 4.18 (td, J1 = 11.3, J2 = 3.2 Hz, 1H), 1.83–1.62 (m, 2H), 1.35 (s, 12H), 0.75 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 147.9, 143.2, 139.7, 139.6, 138.2, 135.8, 135.1, 134.2, 133.9, 131.7, 129.1, 127.7, 127.5, 127.0, 126.1, 123.6, 121.7, 121.3, 116.6, 83.7, 60.8, 45.3, 26.3, 24.8, 11.2. HRMS (ESI): m/z [M + H]+ calcd for C40H39BN3O5: 652.2983 found, 652.2993.
70) as a colorless solid (145 mg, 89%, 0.25 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 120–122 °C; IR (DCM): 2931, 1715, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.93 (s, 1H), 8.59–8.57 (m, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.5 Hz, 1H), 7.98 (d, J = 8.3 Hz, 1H), 7.91 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.85 (d, J = 7.9 Hz, 2H), 7.72 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.61–7.55 (m, 4H), 7.49 (d, J = 8.0 Hz, 2H), 7.36 (d, J = 4.1 Hz, 2H), 7.24 (dd, J1 = 8.2, J2 = 4.2 Hz, 1H), 5.42 (d, J = 11.6 Hz, 1H), 4.18 (td, J1 = 11.3, J2 = 3.2 Hz, 1H), 1.83–1.62 (m, 2H), 1.35 (s, 12H), 0.75 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 165.9, 147.9, 143.2, 139.7, 139.6, 138.2, 135.8, 135.1, 134.2, 133.9, 131.7, 129.1, 127.7, 127.5, 127.0, 126.1, 123.6, 121.7, 121.3, 116.6, 83.7, 60.8, 45.3, 26.3, 24.8, 11.2. HRMS (ESI): m/z [M + H]+ calcd for C40H39BN3O5: 652.2983 found, 652.2993.
(2S*,3R*)-2-(1,3-dioxoisoindolin-2-yl)-4-methyl-N-(quinolin-8-yl)-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)pentanamide (21c-(DL))
The compound 21c-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (52 mg, 78%, 0.1 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 140–142 °C; IR (DCM): 2927, 1716, 756 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.04 (s, 1H), 8.56–8.52 (m, 2H), 8.01–7.85 (m, 5H), 7.77–7.44 (s, 2H), 7.65–7.56 (m, 6H), 7.36–7.26 (m, 3H), 5.71 (d, J = 12.2 Hz, 1H), 4.31 (d, J = 12.0 Hz, 1H), 2.09–2.02 (m, 1H), 1.36 (s, 12H), 0.88 (d, J = 6.1 Hz, 3H), 0.82 (d, J = 6.0 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 166.2, 147.9, 143.3, 139.8, 138.4, 136.1, 135.8, 135.2, 134.2, 134.2, 131.9, 130.5, 128.7, 127.6, 127.2, 127.0, 126.2, 123.7, 121.7, 121.4, 116.8, 83.8, 57.9, 48.3, 29.2, 24.9, 21.5, 16.3. HRMS (ESI): m/z [M + H]+ calcd for C41H41BN3O5: 666.3139 found, 666.3145.
70) as a colorless solid (52 mg, 78%, 0.1 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 140–142 °C; IR (DCM): 2927, 1716, 756 cm−1; 1H NMR (400 MHz, CDCl3): δH 10.04 (s, 1H), 8.56–8.52 (m, 2H), 8.01–7.85 (m, 5H), 7.77–7.44 (s, 2H), 7.65–7.56 (m, 6H), 7.36–7.26 (m, 3H), 5.71 (d, J = 12.2 Hz, 1H), 4.31 (d, J = 12.0 Hz, 1H), 2.09–2.02 (m, 1H), 1.36 (s, 12H), 0.88 (d, J = 6.1 Hz, 3H), 0.82 (d, J = 6.0 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 166.2, 147.9, 143.3, 139.8, 138.4, 136.1, 135.8, 135.2, 134.2, 134.2, 131.9, 130.5, 128.7, 127.6, 127.2, 127.0, 126.2, 123.7, 121.7, 121.4, 116.8, 83.8, 57.9, 48.3, 29.2, 24.9, 21.5, 16.3. HRMS (ESI): m/z [M + H]+ calcd for C41H41BN3O5: 666.3139 found, 666.3145.
(2S*,3R*)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)hexanamide (21d-DL)
The compound 21d-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a colorless solid (115 mg, 86%, 0.2 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 123–125 °C; IR (DCM): 2928, 1716, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.58 (dd, J1 = 5.6, J2 = 3.4 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 7.99 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.84 (d, J = 8.0 Hz, 2H), 7.74 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59–7.54 (m, 4H), 7.47 (d, J = 8.1 Hz, 2H), 7.39–7.34 (m, 2H), 7.25 (dd, J1 = 8.5, J2 = 4.0 Hz, 1H), 5.39 (d, J = 11.7 Hz, 1H), 4.32–4.24 (m, 1H), 1.71–1.62 (m, 2H), 1.36 (s, 12H), 1.20–1.06 (m, 2H), 0.80 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 166.0, 147.9, 143.2, 140.0, 139.7, 138.2, 135.8, 135.1, 134.2, 133.9, 131.7, 129.0, 127.7, 127.5, 126.9, 126.1, 123.6, 121.7, 121.3, 116.6, 83.7, 61.0, 43.6, 35.3, 24.8, 19.7, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C41H41BN3O5: 666.3139 found, 666.3140.
70) as a colorless solid (115 mg, 86%, 0.2 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 123–125 °C; IR (DCM): 2928, 1716, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.88 (s, 1H), 8.58 (dd, J1 = 5.6, J2 = 3.4 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 7.99 (dd, J1 = 8.3, J2 = 1.4 Hz, 1H), 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.84 (d, J = 8.0 Hz, 2H), 7.74 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.59–7.54 (m, 4H), 7.47 (d, J = 8.1 Hz, 2H), 7.39–7.34 (m, 2H), 7.25 (dd, J1 = 8.5, J2 = 4.0 Hz, 1H), 5.39 (d, J = 11.7 Hz, 1H), 4.32–4.24 (m, 1H), 1.71–1.62 (m, 2H), 1.36 (s, 12H), 1.20–1.06 (m, 2H), 0.80 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 166.0, 147.9, 143.2, 140.0, 139.7, 138.2, 135.8, 135.1, 134.2, 133.9, 131.7, 129.0, 127.7, 127.5, 126.9, 126.1, 123.6, 121.7, 121.3, 116.6, 83.7, 61.0, 43.6, 35.3, 24.8, 19.7, 13.8. HRMS (ESI): m/z [M + H]+ calcd for C41H41BN3O5: 666.3139 found, 666.3140.
(2S*,3R*)-2-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)octanamide (21e-(DL))
The compound 21e-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a brown colored semi-solid (92 mg, 88%, 0.15 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 184–186 °C; IR (DCM): 2928, 1715, 751 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.57 (dd, J1 = 6.9, J2 = 3.4 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 8.00 (dd, J1 = 8.3, J2 = 1.6 Hz, 1H), 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.84 (d, J = 8.1 Hz, 2H), 7.75 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58–7.54 (m, 4H), 7.48 (d, J = 8.1 Hz, 2H), 7.38–7.37 (m, 2H), 7.27–7.23 (m, 1H), 5.38 (d, J = 11.7 Hz, 1H), 4.29–4.22 (m, 1H), 1.71–1.65 (m, 2H), 1.36 (s, 12H), 1.23–1.04 (m, 6H), 0.75 (t, J = 6.5 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 166.0, 147.9, 143.2, 140.0, 139.7, 138.2, 135.8, 135.1, 134.2, 133.9, 131.7, 129.0, 127.7, 127.5, 127.0, 126.1, 123.6, 121.7, 121.3, 116.6, 83.7, 61.1, 43.8, 33.1, 31.5, 26.2, 24.8, 22.4, 13.9. HRMS (ESI): m/z [M + H]+ calcd for C43H45BN3O5: 694.3452 found, 694.3461.
70) as a brown colored semi-solid (92 mg, 88%, 0.15 mmol scale); Rf (30% EtOAc/hexane) 0.5; mp: 184–186 °C; IR (DCM): 2928, 1715, 751 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.87 (s, 1H), 8.57 (dd, J1 = 6.9, J2 = 3.4 Hz, 1H), 8.51 (dd, J1 = 4.2, J2 = 1.6 Hz, 1H), 8.00 (dd, J1 = 8.3, J2 = 1.6 Hz, 1H), 7.93 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.84 (d, J = 8.1 Hz, 2H), 7.75 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.58–7.54 (m, 4H), 7.48 (d, J = 8.1 Hz, 2H), 7.38–7.37 (m, 2H), 7.27–7.23 (m, 1H), 5.38 (d, J = 11.7 Hz, 1H), 4.29–4.22 (m, 1H), 1.71–1.65 (m, 2H), 1.36 (s, 12H), 1.23–1.04 (m, 6H), 0.75 (t, J = 6.5 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.3, 166.0, 147.9, 143.2, 140.0, 139.7, 138.2, 135.8, 135.1, 134.2, 133.9, 131.7, 129.0, 127.7, 127.5, 127.0, 126.1, 123.6, 121.7, 121.3, 116.6, 83.7, 61.1, 43.8, 33.1, 31.5, 26.2, 24.8, 22.4, 13.9. HRMS (ESI): m/z [M + H]+ calcd for C43H45BN3O5: 694.3452 found, 694.3461.
11-(1,3-dioxoisoindolin-2-yl)-N-(quinolin-8-yl)-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)undecanamide (22)
The compound 22 was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 30
hexanes = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a brown colored semi-solid (115 mg, 78%, 0.20 mmol scale); Rf (30% EtOAc/hexane) 0.5; IR (DCM): 2928, 1711, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.67 (s, 1H), 8.73 (d, J = 7.4 Hz, 1H), 8.68 (dd, J1 = 4.2, J2 = 1.4 Hz, 1H), 8.07 (d, J = 8.2 Hz, 1H), 7.82 (d, J = 8.0 Hz, 2H), 7.79 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.65 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.55–7.34 (m, 9H), 3.63 (t, J = 7.4 Hz, 2H), 3.37–3.30 (m, 1H), 2.88–2.85 (m, 2H), 1.86–1.60 (m, 4H), 1.35 (s, 12H), 1.25–1.17 (m, 10H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 170.2, 168.3, 147.9, 143.8, 143.5, 138.8, 138.1, 136.1, 135.1, 134.3, 133.7, 132.0, 128.5, 127.9, 127.7, 127.2, 126.8, 126.1, 123.0, 121.4, 121.3, 116.3, 83.7, 45.7, 42.2, 37.9, 36.1, 29.3, 29.2, 29.0, 28.4, 27.3, 26.7, 24.8. HRMS (ESI): m/z [M + H]+ calcd for C46H51BN3O5: 736.3922 found, 736.3926.
70) as a brown colored semi-solid (115 mg, 78%, 0.20 mmol scale); Rf (30% EtOAc/hexane) 0.5; IR (DCM): 2928, 1711, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 9.67 (s, 1H), 8.73 (d, J = 7.4 Hz, 1H), 8.68 (dd, J1 = 4.2, J2 = 1.4 Hz, 1H), 8.07 (d, J = 8.2 Hz, 1H), 7.82 (d, J = 8.0 Hz, 2H), 7.79 (dd, J1 = 5.4, J2 = 3.1 Hz, 2H), 7.65 (dd, J1 = 5.4, J2 = 3.0 Hz, 2H), 7.55–7.34 (m, 9H), 3.63 (t, J = 7.4 Hz, 2H), 3.37–3.30 (m, 1H), 2.88–2.85 (m, 2H), 1.86–1.60 (m, 4H), 1.35 (s, 12H), 1.25–1.17 (m, 10H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 170.2, 168.3, 147.9, 143.8, 143.5, 138.8, 138.1, 136.1, 135.1, 134.3, 133.7, 132.0, 128.5, 127.9, 127.7, 127.2, 126.8, 126.1, 123.0, 121.4, 121.3, 116.3, 83.7, 45.7, 42.2, 37.9, 36.1, 29.3, 29.2, 29.0, 28.4, 27.3, 26.7, 24.8. HRMS (ESI): m/z [M + H]+ calcd for C46H51BN3O5: 736.3922 found, 736.3926.
(2S*,3R*)-methyl 2-((tert-butoxycarbonyl)amino)-3-phenyl-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)propanoate (23-(DL))
The compound 23-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless semi-solid (48 mg, 86%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2927, 1712, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.86 (d, J = 7.9 Hz, 2H), 7.57–7.52 (m, 4H), 7.35–7.22 (m, 7H), 5.12 (t, J = 8.4 Hz, 1H), 4.87 (d, J = 8.8 Hz, 1H), 4.44 (d, J = 8.2 Hz, 1H), 3.54 (s, 3H), 1.37 (s, 9H), 1.35 (s, 12H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.6, 155.2, 143.2, 139.6, 139.6, 139.5, 135.2, 129.0, 128.8, 128.7, 128.6, 128.3, 127.2, 126.2, 83.8, 80.2, 56.7, 53.3, 52.1, 28.2, 24.8. HRMS (ESI): m/z [M + Na]+ calcd for C33H40BNNaO6: 580.2846 found, 580.2831.
80) as a colorless semi-solid (48 mg, 86%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; IR (DCM): 2927, 1712, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.86 (d, J = 7.9 Hz, 2H), 7.57–7.52 (m, 4H), 7.35–7.22 (m, 7H), 5.12 (t, J = 8.4 Hz, 1H), 4.87 (d, J = 8.8 Hz, 1H), 4.44 (d, J = 8.2 Hz, 1H), 3.54 (s, 3H), 1.37 (s, 9H), 1.35 (s, 12H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.6, 155.2, 143.2, 139.6, 139.6, 139.5, 135.2, 129.0, 128.8, 128.7, 128.6, 128.3, 127.2, 126.2, 83.8, 80.2, 56.7, 53.3, 52.1, 28.2, 24.8. HRMS (ESI): m/z [M + Na]+ calcd for C33H40BNNaO6: 580.2846 found, 580.2831.
(2S*,3R*)-ethyl 2-((tert-butoxycarbonyl)amino)-3-phenyl-3-(4′-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-[1,1′-biphenyl]-4-yl)propanoate (24-(DL))
The compound 24-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (50 mg, 87%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 2926, 1715, 758 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.88 (d, J = 8.2 Hz, 2H), 7.59–7.54 (m, 4H), 7.39–7.25 (m, 7H), 5.12 (t, J = 9.0 Hz, 1H), 4.91 (d, J = 9.0 Hz, 1H), 4.42 (d, J = 8.8 Hz, 1H), 4.04–3.96 (m, 2H), 1.39 (s, 9H), 1.38 (s, 12H), 0.99 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 143.3, 139.7, 139.7, 139.6, 135.2, 128.8, 128.7, 128.6, 127.2, 126.2, 83.8, 80.1, 61.2, 56.8, 53.6, 28.2, 24.8, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C34H42BNNaO6: 594.3003 found, 594.3008.
80) as a colorless solid (50 mg, 87%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 2926, 1715, 758 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.88 (d, J = 8.2 Hz, 2H), 7.59–7.54 (m, 4H), 7.39–7.25 (m, 7H), 5.12 (t, J = 9.0 Hz, 1H), 4.91 (d, J = 9.0 Hz, 1H), 4.42 (d, J = 8.8 Hz, 1H), 4.04–3.96 (m, 2H), 1.39 (s, 9H), 1.38 (s, 12H), 0.99 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 143.3, 139.7, 139.7, 139.6, 135.2, 128.8, 128.7, 128.6, 127.2, 126.2, 83.8, 80.1, 61.2, 56.8, 53.6, 28.2, 24.8, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C34H42BNNaO6: 594.3003 found, 594.3008.
Diethyl 3,3′-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4,4′′′-diyl)(2S*,2′S*,3R*,3′R*)-bis(2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate) (25-(DL))
The compound 25-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (30 mg, 67%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2926, 1710, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.70 (m, 4H), 7.67–7.65 (m, 4H), 7.60–7.57 (m, 4H), 7.41–7.33 (m, 12H), 7.30–7.26 (m, 2H), 5.13 (t, J = 8.9 Hz, 2H), 4.92 (d, J = 9.1 Hz, 2H), 4.43 (d, J = 8.8 Hz, 2H), 4.06–3.98 (m, 4H), 1.40 (br. s, 18H), 1.01 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 139.6, 139.5, 139.3, 128.9, 128.7, 128.6, 127.3, 127.2, 127.0, 80.1, 61.2, 56.8, 53.6, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C56H60N2NaO8: 911.4247 found, 911.4243.
80) as a colorless solid (30 mg, 67%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2926, 1710, 752 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.70 (m, 4H), 7.67–7.65 (m, 4H), 7.60–7.57 (m, 4H), 7.41–7.33 (m, 12H), 7.30–7.26 (m, 2H), 5.13 (t, J = 8.9 Hz, 2H), 4.92 (d, J = 9.1 Hz, 2H), 4.43 (d, J = 8.8 Hz, 2H), 4.06–3.98 (m, 4H), 1.40 (br. s, 18H), 1.01 (t, J = 7.1 Hz, 6H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 139.6, 139.5, 139.3, 128.9, 128.7, 128.6, 127.3, 127.2, 127.0, 80.1, 61.2, 56.8, 53.6, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C56H60N2NaO8: 911.4247 found, 911.4243.
Ethyl (2S*,3R*)-3-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4-yl)-2-aminopentanoate (26a-(DL))
The compound 26a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (32 mg, 71%, 0.1 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 130–132 °C; IR (DCM): 2928, 1732, 760 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74–7.65 (m, 10H), 7.59 (d, J = 8.2 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H), 7.31–7.26 (m, 2H), 4.07–4.01 (m, 2H), 3.61 (d, J = 6.4 Hz, 1H), 2.90–2.85 (m, 1H), 1.98–1.77 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.6, 140.2, 140.1, 139.9, 139.5, 139.4, 139.0, 129.0, 128.8, 127.5, 127.3, 127.0, 126.8, 60.7, 60.0, 51.8, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C31H32NO2: 450.2433 found, 450.2426.
50) as a colorless solid (32 mg, 71%, 0.1 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 130–132 °C; IR (DCM): 2928, 1732, 760 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74–7.65 (m, 10H), 7.59 (d, J = 8.2 Hz, 2H), 7.47 (t, J = 7.8 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H), 7.31–7.26 (m, 2H), 4.07–4.01 (m, 2H), 3.61 (d, J = 6.4 Hz, 1H), 2.90–2.85 (m, 1H), 1.98–1.77 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.6, 140.2, 140.1, 139.9, 139.5, 139.4, 139.0, 129.0, 128.8, 127.5, 127.3, 127.0, 126.8, 60.7, 60.0, 51.8, 23.2, 14.0, 12.2. HRMS (ESI): m/z [M + H]+ calcd for C31H32NO2: 450.2433 found, 450.2426.
(2S*,3R*)-2-amino-3-(4′-(6-methoxynaphthalen-2-yl)-[1,1′-biphenyl]-4-yl)-N-(quinolin-8-yl)pentanamide (26b-(DL))
The compound 26b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (19 mg, 69%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 165–167 °C; IR (DCM): 2927, 1525, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 11.37 (s, 1H), 8.89 (dd, J1 = 7.2, J2 = 1.8 Hz, 1H), 8.85 (dd, J1 = 4.1, J2 = 1.6 Hz, 1H), 8.14 (dd, J1 = 8.2, J2 = 1.6 Hz, 1H), 8.08–8.02 (m, 1H), 7.83–7.75 (m, 5H), 7.67–7.51 (m, 6H), 7.44–7.41 (m, 2H), 7.20–7.17 (m, 2H), 3.94 (s, 3H), 3.80 (d, J = 4.0 Hz, 1H), 3.54–3.49 (m, 1H), 2.04–1.71 (m, 3H), 0.89 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.6, 157.8, 148.5, 140.7, 139.9, 139.4, 139.1, 139.0, 136.2, 135.7, 134.3, 133.8, 129.7, 129.2, 128.9, 128.0, 127.5, 127.3, 127.3, 127.2, 126.1, 125.8, 125.4, 121.8, 121.5, 119.2, 116.4, 105.5, 62.2, 55.3, 49.9, 20.3, 12.4. HRMS (ESI): m/z [M + H]+ calcd for C37H34N3O2: 552.2651 found, 552.2656.
80) as a colorless solid (19 mg, 69%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 165–167 °C; IR (DCM): 2927, 1525, 753 cm−1; 1H NMR (400 MHz, CDCl3): δH 11.37 (s, 1H), 8.89 (dd, J1 = 7.2, J2 = 1.8 Hz, 1H), 8.85 (dd, J1 = 4.1, J2 = 1.6 Hz, 1H), 8.14 (dd, J1 = 8.2, J2 = 1.6 Hz, 1H), 8.08–8.02 (m, 1H), 7.83–7.75 (m, 5H), 7.67–7.51 (m, 6H), 7.44–7.41 (m, 2H), 7.20–7.17 (m, 2H), 3.94 (s, 3H), 3.80 (d, J = 4.0 Hz, 1H), 3.54–3.49 (m, 1H), 2.04–1.71 (m, 3H), 0.89 (t, J = 7.3 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.6, 157.8, 148.5, 140.7, 139.9, 139.4, 139.1, 139.0, 136.2, 135.7, 134.3, 133.8, 129.7, 129.2, 128.9, 128.0, 127.5, 127.3, 127.3, 127.2, 126.1, 125.8, 125.4, 121.8, 121.5, 119.2, 116.4, 105.5, 62.2, 55.3, 49.9, 20.3, 12.4. HRMS (ESI): m/z [M + H]+ calcd for C37H34N3O2: 552.2651 found, 552.2656.
(2S*,3R*)-ethyl 3-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4-yl)-2-amino-4-methylpentanoate (27a-(DL))
The compound 27a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (16 mg, 78%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 230–232 °C; IR (DCM): 2929, 1727, 760 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74–7.68 (m, 10H), 7.60 (d, J = 7.3 Hz, 2H), 7.50 (t, J = 7.0 Hz, 2H), 7.40 (t, J = 6.8 Hz, 1H), 7.27 (d, J = 7.5 Hz, 2H), 4.16–4.06 (m, 2H), 3.93 (d, J = 5.9 Hz, 1H), 2.77 (t, J = 6.8 Hz, 1H), 2.51–2.42 (m, 1H), 1.17 (t, J = 7.0 Hz, 3H), 1.11 (d, J = 6.0 Hz, 3H), 0.86 (d, J = 6.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 140.6, 140.2, 140.1, 139.8, 139.5, 139.4, 139.0, 138.8, 129.8, 128.8, 127.5, 127.3, 127.0, 126.5, 60.7, 57.2, 56.6, 28.3, 21.5, 20.1, 14.0.HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2591. The HPLC of the compound 27a-(DL) was determined using the Daicel Chiralpak IC column, hexane/i-PrOH (80
50) as a colorless solid (16 mg, 78%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 230–232 °C; IR (DCM): 2929, 1727, 760 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.74–7.68 (m, 10H), 7.60 (d, J = 7.3 Hz, 2H), 7.50 (t, J = 7.0 Hz, 2H), 7.40 (t, J = 6.8 Hz, 1H), 7.27 (d, J = 7.5 Hz, 2H), 4.16–4.06 (m, 2H), 3.93 (d, J = 5.9 Hz, 1H), 2.77 (t, J = 6.8 Hz, 1H), 2.51–2.42 (m, 1H), 1.17 (t, J = 7.0 Hz, 3H), 1.11 (d, J = 6.0 Hz, 3H), 0.86 (d, J = 6.1 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 140.6, 140.2, 140.1, 139.8, 139.5, 139.4, 139.0, 138.8, 129.8, 128.8, 127.5, 127.3, 127.0, 126.5, 60.7, 57.2, 56.6, 28.3, 21.5, 20.1, 14.0.HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2591. The HPLC of the compound 27a-(DL) was determined using the Daicel Chiralpak IC column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 326.3 nm, tL = 16.31 min, tD = 19.30 min.
20), flow rate 1.0 mL min−1, UV detection at 326.3 nm, tL = 16.31 min, tD = 19.30 min.
(2R,3S)-ethyl 3-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4-yl)-2-amino-4-methylpentanoate (27a-(D))
The compound 27a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (15 mg, 74%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 231–233 °C; IR (DCM): 2929, 1728, 760 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.73–7.64 (m, 10H), 7.56 (d, J = 8.1 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.36 (t, J = 7.3 Hz, 1H), 7.25–7.21 (m, 2H), 4.13–4.00 (m, 2H), 3.88 (d, J = 6.6 Hz, 1H), 2.72 (t, J = 7.8 Hz, 1H), 2.47–2.38 (m, 1H), 1.13 (t, J = 7.1 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.82 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 140.6, 140.1, 139.8, 139.5, 139.4, 138.9, 138.8, 129.8, 128.8, 127.5, 127.3, 127.0, 126.4, 60.6, 57.2, 56.5, 28.2, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2587. [α]25 D = −21.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96
50) as a colorless solid (15 mg, 74%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 231–233 °C; IR (DCM): 2929, 1728, 760 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.73–7.64 (m, 10H), 7.56 (d, J = 8.1 Hz, 2H), 7.46 (t, J = 7.8 Hz, 2H), 7.36 (t, J = 7.3 Hz, 1H), 7.25–7.21 (m, 2H), 4.13–4.00 (m, 2H), 3.88 (d, J = 6.6 Hz, 1H), 2.72 (t, J = 7.8 Hz, 1H), 2.47–2.38 (m, 1H), 1.13 (t, J = 7.1 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.82 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 175.1, 140.6, 140.1, 139.8, 139.5, 139.4, 138.9, 138.8, 129.8, 128.8, 127.5, 127.3, 127.0, 126.4, 60.6, 57.2, 56.5, 28.2, 21.5, 20.1, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2587. [α]25 D = −21.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of the compound 27a-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (80
4) of the compound 27a-(D) was determined by HPLC using the Daicel Chiralpak IC column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 326.3 nm, tL = 14.74 min, tD = 18.49 min.
20), flow rate 1.0 mL min−1, UV detection at 326.3 nm, tL = 14.74 min, tD = 18.49 min.
(2S*,3R*)-ethyl 3-([1,1′:3′,1'':4′′,1′′′-quaterphenyl]-4′′′-yl)-2-amino-4-methylpentanoate (27b-(DL))
The compound 27b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (15 mg, 65%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 88–90 °C; IR (DCM): 2927, 1730, 758 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.85 (s, 1H), 7.73–7.36 (m, 14H), 7.25–7.21 (m, 2H), 4.13–4.01 (m, 2H), 3.88 (d, J = 6.5 Hz, 1H), 2.72 (t, J = 7.7 Hz, 1H), 2.47–2.38 (m, 1H), 1.13 (t, J = 7.1 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.82 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.4, 141.8, 141.2, 141.2, 139.9, 139.8, 139.1, 138.5, 129.8, 129.2, 128.8, 127.5, 127.4, 127.3, 127.3, 126.6, 126.2, 126.0, 125.9, 60.8, 56.9, 56.4, 28.3, 21.5, 20.2, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2603.
50) as a colorless solid (15 mg, 65%, 0.05 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 88–90 °C; IR (DCM): 2927, 1730, 758 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.85 (s, 1H), 7.73–7.36 (m, 14H), 7.25–7.21 (m, 2H), 4.13–4.01 (m, 2H), 3.88 (d, J = 6.5 Hz, 1H), 2.72 (t, J = 7.7 Hz, 1H), 2.47–2.38 (m, 1H), 1.13 (t, J = 7.1 Hz, 3H), 1.06 (d, J = 6.6 Hz, 3H), 0.82 (d, J = 6.7 Hz, 3H). (The NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.4, 141.8, 141.2, 141.2, 139.9, 139.8, 139.1, 138.5, 129.8, 129.2, 128.8, 127.5, 127.4, 127.3, 127.3, 126.6, 126.2, 126.0, 125.9, 60.8, 56.9, 56.4, 28.3, 21.5, 20.2, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2603.
(2S*,3R*)-ethyl 3-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4-yl)-2-(1,3-dioxoisoindolin-2-yl)hexanoate (28a-(DL))
The compound 28a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (25 mg, 47%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 214–216 °C; IR (DCM): 2926, 1714, 769 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.94–7.92 (m, 2H), 7.79–7.78 (m, 2H), 7.75–7.62 (m, 12H), 7.49–7.43 (m, 4H), 7.37 (t, J = 7.5 Hz, 1H), 5.13 (d, J = 10.3 Hz, 1H), 4.07–3.86 (m, 2H), 1.59–1.50 (m, 2H), 3.92–3.86 (m, 1H), 1.13–1.05 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.77 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 141.0, 140.7, 140.1, 139.8, 139.6, 139.4, 138.9, 134.3, 131.7, 129.0, 128.8, 127.5, 127.4, 127.3, 127.3, 127.0, 126.9, 123.7, 61.5, 57.3, 44.0, 34.5, 19.9, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C40H35NNaO4: 616.2464 found, 616.2438.
80) as a colorless solid (25 mg, 47%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 214–216 °C; IR (DCM): 2926, 1714, 769 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.94–7.92 (m, 2H), 7.79–7.78 (m, 2H), 7.75–7.62 (m, 12H), 7.49–7.43 (m, 4H), 7.37 (t, J = 7.5 Hz, 1H), 5.13 (d, J = 10.3 Hz, 1H), 4.07–3.86 (m, 2H), 1.59–1.50 (m, 2H), 3.92–3.86 (m, 1H), 1.13–1.05 (m, 2H), 1.00 (t, J = 7.1 Hz, 3H), 0.77 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 168.5, 167.8, 141.0, 140.7, 140.1, 139.8, 139.6, 139.4, 138.9, 134.3, 131.7, 129.0, 128.8, 127.5, 127.4, 127.3, 127.3, 127.0, 126.9, 123.7, 61.5, 57.3, 44.0, 34.5, 19.9, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C40H35NNaO4: 616.2464 found, 616.2438.
(2S*,3R*)-ethyl 3-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4-yl)-2-aminohexanoate (28b-(DL))
The compound 28b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (16 mg, 78%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 240–242 °C; IR (DCM): 2928, 1730, 748 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.65 (m, 11H), 7.59 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.4 Hz, 2H), 7.37 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 7.9 Hz, 1H), 4.08–4.00 (m, 2H), 3.60 (d, J = 6.2 Hz, 1H), 3.01–2.96 (m, 1H), 1.84–1.79 (m, 2H), 1.33–1.26 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.90 (t, J = 7.2 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.7, 140.6, 140.2, 139.9, 139.6, 139.4, 139.1, 129.0, 128.8, 127.5, 127.3, 127.0, 126.9, 60.7, 60.2, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2589. The HPLC of the compound 28b-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (50
50) as a colorless solid (16 mg, 78%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 240–242 °C; IR (DCM): 2928, 1730, 748 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.65 (m, 11H), 7.59 (d, J = 7.8 Hz, 2H), 7.47 (t, J = 7.4 Hz, 2H), 7.37 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 7.9 Hz, 1H), 4.08–4.00 (m, 2H), 3.60 (d, J = 6.2 Hz, 1H), 3.01–2.96 (m, 1H), 1.84–1.79 (m, 2H), 1.33–1.26 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.90 (t, J = 7.2 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.7, 140.6, 140.2, 139.9, 139.6, 139.4, 139.1, 129.0, 128.8, 127.5, 127.3, 127.0, 126.9, 60.7, 60.2, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2589. The HPLC of the compound 28b-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 291.9 nm, tL = 6.94 min, tD = 8.53 min.
50), flow rate 1.0 mL min−1, UV detection at 291.9 nm, tL = 6.94 min, tD = 8.53 min.
(2R,3S)-ethyl 3-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4-yl)-2-aminohexanoate (28b-(D))
The compound 28b-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (15 mg, 74%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 242–244 °C; IR (DCM): 2929, 1730, 747 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.65 (m, 11H), 7.59 (d, J = 8.0 Hz, 2H), 7.47 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 4.09–4.01 (m, 2H), 3.60 (d, J = 6.2 Hz, 1H), 3.01–2.96 (m, 1H), 1.82 (q, J = 7.8 Hz, 2H), 1.33–1.25 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.90 (t, J = 7.3 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.7, 140.5, 140.2, 139.9, 139.6, 139.4, 139.1, 129.0, 128.8, 127.5, 127.3, 127.0, 126.9, 60.7, 60.2, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2599. [α]25 D = −48.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96
50) as a colorless solid (15 mg, 74%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 242–244 °C; IR (DCM): 2929, 1730, 747 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.65 (m, 11H), 7.59 (d, J = 8.0 Hz, 2H), 7.47 (t, J = 7.6 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 4.09–4.01 (m, 2H), 3.60 (d, J = 6.2 Hz, 1H), 3.01–2.96 (m, 1H), 1.82 (q, J = 7.8 Hz, 2H), 1.33–1.25 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.90 (t, J = 7.3 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.7, 140.5, 140.2, 139.9, 139.6, 139.4, 139.1, 129.0, 128.8, 127.5, 127.3, 127.0, 126.9, 60.7, 60.2, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2599. [α]25 D = −48.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of the compound 28b-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (50
4) of the compound 28b-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 291.9 nm, tL = 6.87 min, tD = 8.39 min.
50), flow rate 1.0 mL min−1, UV detection at 291.9 nm, tL = 6.87 min, tD = 8.39 min.
(2S,3R)-ethyl 3-([1,1′:4′,1'':4′′,1′′′-quaterphenyl]-4-yl)-2-aminohexanoate (SB-1912, 28b-(L))
The compound 28b-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 50
hexanes = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) as a colorless solid (16 mg, 78%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 241–243 °C; IR (DCM): 2931, 1731, 745 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.65 (m, 11H), 7.59 (d, J = 8.1 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.37 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 4.09–4.01 (m, 2H), 3.61 (d, J = 6.3 Hz, 1H), 3.02–2.96 (m, 1H), 1.82 (q, J = 7.8 Hz, 2H), 1.33–1.25 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.90 (t, J = 7.3 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.6, 140.5, 140.1, 139.9, 139.5, 139.4, 139.0, 129.0, 128.8, 127.5, 127.3, 127.0, 126.9, 60.7, 60.2, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2601. [α]25 D = +50.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
50) as a colorless solid (16 mg, 78%, 0.044 mmol scale); Rf (50% EtOAc/hexane) 0.5; mp: 241–243 °C; IR (DCM): 2931, 1731, 745 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.72–7.65 (m, 11H), 7.59 (d, J = 8.1 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.37 (t, J = 7.2 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 4.09–4.01 (m, 2H), 3.61 (d, J = 6.3 Hz, 1H), 3.02–2.96 (m, 1H), 1.82 (q, J = 7.8 Hz, 2H), 1.33–1.25 (m, 2H), 1.11 (t, J = 7.1 Hz, 3H), 0.90 (t, J = 7.3 Hz, 3H). (the NH2 signal could not be clearly assigned in the proton NMR spectrum as it may be merged with the residual water peak). 13C{1H} NMR (∼101 MHz, CDCl3): δC 174.9, 140.6, 140.5, 140.1, 139.9, 139.5, 139.4, 139.0, 129.0, 128.8, 127.5, 127.3, 127.0, 126.9, 60.7, 60.2, 49.6, 32.3, 20.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd for C32H34NO2: 464.2590 found, 464.2601. [α]25 D = +50.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 28b-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (50
5) of the compound 28b-(L) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 291.9 nm, tL = 6.89 min, tD = 8.02 min.
50), flow rate 1.0 mL min−1, UV detection at 291.9 nm, tL = 6.89 min, tD = 8.02 min.
(2S*,3R*)-ethyl 3-(4′-(benzo[d][1,3]dioxol-5-yl)-[1,1′-biphenyl]-4-yl)-2-((tert-butoxycarbonyl)amino)-3-phenylpropanoate (29-(DL))
The compound 29-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (40 mg, 71%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 200–202 °C; IR (DCM): 2927, 1739, 754 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.60–7.53 (m, 6H), 7.37–7.30 (m, 6H), 7.27–7.23 (m, 1H), 7.10–7.08 (m, 2H), 6.90–6.88 (m, 1H), 6.00 (s, 2H), 5.10 (t, J = 8.9 Hz, 1H), 4.90 (d, J = 9.0 Hz, 1H), 4.40 (d, J = 8.7 Hz, 1H), 4.03–3.95 (m, 2H), 1.37 (s, 9H), 0.97 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 148.1, 147.1, 139.8, 139.6, 139.4, 139.3, 139.2, 135.0, 128.8, 128.7, 128.6, 127.2, 127.2, 127.0, 120.5, 108.6, 107.5, 101.1, 80.1, 61.2, 56.8, 53.6, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C35H35NNaO6: 588.2362 found, 588.2364.
80) as a colorless solid (40 mg, 71%, 0.1 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 200–202 °C; IR (DCM): 2927, 1739, 754 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.60–7.53 (m, 6H), 7.37–7.30 (m, 6H), 7.27–7.23 (m, 1H), 7.10–7.08 (m, 2H), 6.90–6.88 (m, 1H), 6.00 (s, 2H), 5.10 (t, J = 8.9 Hz, 1H), 4.90 (d, J = 9.0 Hz, 1H), 4.40 (d, J = 8.7 Hz, 1H), 4.03–3.95 (m, 2H), 1.37 (s, 9H), 0.97 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 148.1, 147.1, 139.8, 139.6, 139.4, 139.3, 139.2, 135.0, 128.8, 128.7, 128.6, 127.2, 127.2, 127.0, 120.5, 108.6, 107.5, 101.1, 80.1, 61.2, 56.8, 53.6, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C35H35NNaO6: 588.2362 found, 588.2364.
Ethyl 3-([1,1′:4′,1′′:4′′,1′′′-quaterphenyl]-4-yl)-7-(1,3-dioxoisoindolin-2-yl)heptanoate (30)
The compound 30 was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (20 mg, 37%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2930, 1708, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.81 (dd, J1 = 5.3, J2 = 3.1 Hz, 2H), 7.75–7.63 (m, 12H), 7.53 (d, J = 8.0 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 7.26–7.24 (m, 2H), 4.05 (q, J = 7.1 Hz, 2H), 3.62 (t, J = 7.2 Hz, 2H), 3.18–3.10 (m, 1H), 2.68–2.57 (m, 2H), 1.77–1.61 (m, 4H), 1.31–1.22 (m, 2H), 1.15 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.3, 168.4, 142.9, 140.7, 140.1, 139.9, 139.6, 139.3, 138.7, 133.8, 132.1, 128.8, 127.9, 127.5, 127.4, 127.3, 127.3, 127.0, 127.0, 123.1, 60.3, 41.7, 41.7, 37.7, 35.6, 28.3, 24.5, 14.1. HRMS (ESI): m/z [M + Na]+ calcd for C41H37NNaO4: 630.2620 found, 630.2607.
80) as a colorless solid (20 mg, 37%, 0.09 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 160–162 °C; IR (DCM): 2930, 1708, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.81 (dd, J1 = 5.3, J2 = 3.1 Hz, 2H), 7.75–7.63 (m, 12H), 7.53 (d, J = 8.0 Hz, 2H), 7.47 (t, J = 7.7 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 7.26–7.24 (m, 2H), 4.05 (q, J = 7.1 Hz, 2H), 3.62 (t, J = 7.2 Hz, 2H), 3.18–3.10 (m, 1H), 2.68–2.57 (m, 2H), 1.77–1.61 (m, 4H), 1.31–1.22 (m, 2H), 1.15 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.3, 168.4, 142.9, 140.7, 140.1, 139.9, 139.6, 139.3, 138.7, 133.8, 132.1, 128.8, 127.9, 127.5, 127.4, 127.3, 127.3, 127.0, 127.0, 123.1, 60.3, 41.7, 41.7, 37.7, 35.6, 28.3, 24.5, 14.1. HRMS (ESI): m/z [M + Na]+ calcd for C41H37NNaO4: 630.2620 found, 630.2607.
Ethyl 2-((tert-butoxycarbonyl)amino)-3,3-bis(4′-(phenylethynyl)-[1,1′-biphenyl]-4-yl)propanoate (31-(DL))
The compound 31-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (20 mg, 63%, 0.044 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 109–111 °C; IR (DCM): 2924, 1713, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.53–7.40 (m, 16H), 7.36–7.27 (m, 10H), 5.10–5.04 (m, 1H), 4.87–4.84 (m, 1H), 4.39–4.37 (m, 1H), 3.99–3.92 (m, 2H), 1.31 (s, 9H), 0.93 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.0, 155.2, 140.3, 140.2, 139.7, 139.4, 139.1, 132.0, 131.8, 131.6, 129.1, 128.9, 128.5, 128.3, 128.2, 127.2, 127.1, 127.0, 126.8, 123.2, 122.2, 122.2, 90.1, 89.2, 80.2, 61.2, 56.7, 53.3, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C50H43NNaO4: 744.3090 found, 744.3063. The HPLC of the compound 31-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (50
80) as a colorless solid (20 mg, 63%, 0.044 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 109–111 °C; IR (DCM): 2924, 1713, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.53–7.40 (m, 16H), 7.36–7.27 (m, 10H), 5.10–5.04 (m, 1H), 4.87–4.84 (m, 1H), 4.39–4.37 (m, 1H), 3.99–3.92 (m, 2H), 1.31 (s, 9H), 0.93 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.0, 155.2, 140.3, 140.2, 139.7, 139.4, 139.1, 132.0, 131.8, 131.6, 129.1, 128.9, 128.5, 128.3, 128.2, 127.2, 127.1, 127.0, 126.8, 123.2, 122.2, 122.2, 90.1, 89.2, 80.2, 61.2, 56.7, 53.3, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C50H43NNaO4: 744.3090 found, 744.3063. The HPLC of the compound 31-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 300 nm, tL = 14.93 min, tD = 28.67 min.
50), flow rate 1.0 mL min−1, UV detection at 300 nm, tL = 14.93 min, tD = 28.67 min.
Ethyl (R)-2-((tert-butoxycarbonyl)amino)-3,3-bis(4′-(phenylethynyl)-[1,1′-biphenyl]-4-yl)propanoate (31-(D))
The compound 31-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (20 mg, 63%, 0.044 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 108–110 °C; IR (DCM): 2925, 1715, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.51–7.39 (m, 16H), 7.33–7.25 (m, 10H), 5.09–5.01 (m, 1H), 4.87–4.84 (m, 1H), 4.38–4.36 (m, 1H), 3.97–3.89 (m, 2H), 1.30 (s, 9H), 0.92 (t, J = 6.9 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.0, 155.2, 140.3, 140.2, 139.6, 139.4, 139.1, 132.0, 131.8, 131.6, 129.1, 128.9, 128.5, 128.3, 128.3, 128.2, 127.3, 127.1, 126.8, 123.2, 122.2, 122.2, 90.1, 89.2, 80.2, 61.2, 56.7, 53.3, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C50H43NNaO4: 744.3090 found, 744.3087. [α]25 D = −39.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 92
80) as a colorless solid (20 mg, 63%, 0.044 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 108–110 °C; IR (DCM): 2925, 1715, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.51–7.39 (m, 16H), 7.33–7.25 (m, 10H), 5.09–5.01 (m, 1H), 4.87–4.84 (m, 1H), 4.38–4.36 (m, 1H), 3.97–3.89 (m, 2H), 1.30 (s, 9H), 0.92 (t, J = 6.9 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.0, 155.2, 140.3, 140.2, 139.6, 139.4, 139.1, 132.0, 131.8, 131.6, 129.1, 128.9, 128.5, 128.3, 128.3, 128.2, 127.3, 127.1, 126.8, 123.2, 122.2, 122.2, 90.1, 89.2, 80.2, 61.2, 56.7, 53.3, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C50H43NNaO4: 744.3090 found, 744.3087. [α]25 D = −39.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) of the compound 31-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (50
8) of the compound 31-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 300 nm, tL = 14.74 min, tD = 27.92 min.
50), flow rate 1.0 mL min−1, UV detection at 300 nm, tL = 14.74 min, tD = 27.92 min.
Ethyl (S)-2-((tert-butoxycarbonyl)amino)-3,3-bis(4′-(phenylethynyl)-[1,1′-biphenyl]-4-yl)propanoate (31-(L))
The compound 31-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (21 mg, 66%, 0.044 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 107–109 °C; IR (DCM): 2925, 1714, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.52–7.40 (m, 16H), 7.35–7.27 (m, 10H), 5.09–5.02 (m, 1H), 4.86–4.84 (m, 1H), 4.40–4.34 (m, 1H), 3.98–3.91 (m, 2H), 1.30 (s, 9H), 0.93 (t, J = 6.6 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.0, 155.2, 140.3, 140.2, 139.5, 139.3, 139.2, 132.0, 132.0, 131.9, 131.6, 129.1, 129.1, 128.9, 128.9, 128.6, 128.5, 128.3, 128.3, 127.3, 127.2, 127.1, 127.0, 126.8, 123.2, 122.3, 90.2, 89.2, 80.2, 61.3, 56.7, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C50H43NNaO4: 744.3090 found, 744.3089. [α]25 D = +42.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 93
80) as a colorless solid (21 mg, 66%, 0.044 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 107–109 °C; IR (DCM): 2925, 1714, 755 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.52–7.40 (m, 16H), 7.35–7.27 (m, 10H), 5.09–5.02 (m, 1H), 4.86–4.84 (m, 1H), 4.40–4.34 (m, 1H), 3.98–3.91 (m, 2H), 1.30 (s, 9H), 0.93 (t, J = 6.6 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.0, 155.2, 140.3, 140.2, 139.5, 139.3, 139.2, 132.0, 132.0, 131.9, 131.6, 129.1, 129.1, 128.9, 128.9, 128.6, 128.5, 128.3, 128.3, 127.3, 127.2, 127.1, 127.0, 126.8, 123.2, 122.3, 90.2, 89.2, 80.2, 61.3, 56.7, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C50H43NNaO4: 744.3090 found, 744.3089. [α]25 D = +42.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) of the compound 31-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (50
7) of the compound 31-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 300 nm, tL = 14.89 min, tD = 28.52 min.
50), flow rate 1.0 mL min−1, UV detection at 300 nm, tL = 14.89 min, tD = 28.52 min.
Ethyl 3,3-di([1,1′:4′,1′′-terphenyl]-4-yl)-2-((tert-butoxycarbonyl)amino)propanoate (32a-(DL))
The compound 32a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (20 mg, 59%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 175–177 °C; IR (DCM): 2926, 1712, 761 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.71–7.37 (m, 26H), 5.18 (t, J = 9.0 Hz, 1H), 4.97 (d, J = 9.0 Hz, 1H), 4.48 (d, J = 8.7 Hz, 1H), 4.08–4.00 (m, 2H), 1.41 (s, 9H), 1.03 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.3, 140.6, 140.6, 140.2, 139.5, 139.5, 139.4, 138.8, 129.1, 128.9, 128.8, 127.5, 127.3, 127.1, 127.0, 80.2, 61.2, 56.8, 53.4, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C46H43NNaO4: 696.3090 found, 696.3088. The HPLC of the compound 32a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (50
80) as a colorless solid (20 mg, 59%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 175–177 °C; IR (DCM): 2926, 1712, 761 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.71–7.37 (m, 26H), 5.18 (t, J = 9.0 Hz, 1H), 4.97 (d, J = 9.0 Hz, 1H), 4.48 (d, J = 8.7 Hz, 1H), 4.08–4.00 (m, 2H), 1.41 (s, 9H), 1.03 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.3, 140.6, 140.6, 140.2, 139.5, 139.5, 139.4, 138.8, 129.1, 128.9, 128.8, 127.5, 127.3, 127.1, 127.0, 80.2, 61.2, 56.8, 53.4, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C46H43NNaO4: 696.3090 found, 696.3088. The HPLC of the compound 32a-(DL) was determined using the Daicel Chiralcel ODH column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 296 nm, tD = 8.66 min, tL = 11.89 min.
50), flow rate 1.0 mL min−1, UV detection at 296 nm, tD = 8.66 min, tL = 11.89 min.
(R)-ethyl 3,3-di([1,1′:4′,1′′-terphenyl]-4-yl)-2-((tert-butoxycarbonyl)amino)propanoate (32a-(D))
The compound 32a-(D) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (21 mg, 62%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 176–178 °C; IR (DCM): 2926, 1712, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.68–7.33 (m, 26H), 5.16 (t, J = 8.9 Hz, 1H), 4.96 (d, J = 9.1 Hz, 1H), 4.46 (d, J = 8.8 Hz, 1H), 4.06–3.97 (m, 2H), 1.38 (s, 9H), 0.99 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 140.6, 140.6, 140.1, 139.5, 139.4, 139.4, 138.8, 129.0, 128.9, 128.8, 127.5, 127.3, 127.1, 127.0, 80.2, 61.2, 56.8, 53.4, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C46H43NNaO4: 696.3090 found, 696.3092. [α]25 D = −25.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95
80) as a colorless solid (21 mg, 62%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 176–178 °C; IR (DCM): 2926, 1712, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.68–7.33 (m, 26H), 5.16 (t, J = 8.9 Hz, 1H), 4.96 (d, J = 9.1 Hz, 1H), 4.46 (d, J = 8.8 Hz, 1H), 4.06–3.97 (m, 2H), 1.38 (s, 9H), 0.99 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 140.6, 140.6, 140.1, 139.5, 139.4, 139.4, 138.8, 129.0, 128.9, 128.8, 127.5, 127.3, 127.1, 127.0, 80.2, 61.2, 56.8, 53.4, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C46H43NNaO4: 696.3090 found, 696.3092. [α]25 D = −25.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) of the compound 32a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (50
5) of the compound 32a-(D) was determined by HPLC using the Daicel Chiralcel ODH column, hexane/i-PrOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), flow rate 1.0 mL min−1, UV detection at 296 nm, tD = 7.98 min, tL = 11.00 min.
50), flow rate 1.0 mL min−1, UV detection at 296 nm, tD = 7.98 min, tL = 11.00 min.
Ethyl 2-((tert-butoxycarbonyl)amino)-3,3-bis(4′′-chloro-[1,1′:4′,1′′-terphenyl]-4-yl)propanoate (32b-(DL))
The compound 32b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (20 mg, 54%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 230–232 °C; IR (DCM): 2925, 1712, 756 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.41 (m, 24H), 5.20–5.16 (m, 1H), 4.97 (d, J = 8.1 Hz, 1H), 4.48 (d, J = 8.6 Hz, 1H), 4.10–3.99 (m, 2H), 1.41 (s, 9H), 1.03 (d, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.1, 155.2, 139.9, 139.8, 139.5, 139.5, 139.4, 139.3, 139.1, 138.9, 133.5, 131.9, 129.2, 129.1, 129.0, 128.9, 128.5, 128.2, 127.4, 127.4, 127.3, 127.2, 127.1, 127.0, 80.2, 61.2, 56.8, 53.5, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C46H41Cl2NNaO4: 764.2310 found, 764.2323.
80) as a colorless solid (20 mg, 54%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 230–232 °C; IR (DCM): 2925, 1712, 756 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.65–7.41 (m, 24H), 5.20–5.16 (m, 1H), 4.97 (d, J = 8.1 Hz, 1H), 4.48 (d, J = 8.6 Hz, 1H), 4.10–3.99 (m, 2H), 1.41 (s, 9H), 1.03 (d, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.1, 155.2, 139.9, 139.8, 139.5, 139.5, 139.4, 139.3, 139.1, 138.9, 133.5, 131.9, 129.2, 129.1, 129.0, 128.9, 128.5, 128.2, 127.4, 127.4, 127.3, 127.2, 127.1, 127.0, 80.2, 61.2, 56.8, 53.5, 28.2, 13.8. HRMS (ESI): m/z [M + Na]+ calcd for C46H41Cl2NNaO4: 764.2310 found, 764.2323.
Ethyl 2-((tert-butoxycarbonyl)amino)-3,3-bis(4′-(thiophen-2-yl)-[1,1′-biphenyl]-4-yl)propanoate (33-(DL))
The compound 33-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 20
hexanes = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) as a colorless solid (18 mg, 52%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 166–168 °C; IR (DCM): 2925, 1711, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.69–7.08 (m, 22H), 5.13 (t, J = 8.8 Hz, 1H), 4.93 (d, J = 9.0 Hz, 1H), 4.43 (d, J = 8.7 Hz, 1H), 4.04–3.96 (m, 2H), 1.37 (s, 9H), 0.98 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 143.9, 140.6, 140.6, 140.1, 140.0, 140.0, 139.5, 139.3, 138.7, 129.1, 129.0, 128.9, 128.8, 128.8, 128.1, 127.4, 127.3, 127.3, 127.3, 127.2, 127.0, 126.3, 124.9, 123.1, 80.1, 61.2, 56.8, 53.4, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C42H39NNaO4S2: 708.2218 found, 708.2212.
80) as a colorless solid (18 mg, 52%, 0.05 mmol scale); Rf (20% EtOAc/hexane) 0.5; mp: 166–168 °C; IR (DCM): 2925, 1711, 750 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.69–7.08 (m, 22H), 5.13 (t, J = 8.8 Hz, 1H), 4.93 (d, J = 9.0 Hz, 1H), 4.43 (d, J = 8.7 Hz, 1H), 4.04–3.96 (m, 2H), 1.37 (s, 9H), 0.98 (t, J = 7.1 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 172.2, 155.2, 143.9, 140.6, 140.6, 140.1, 140.0, 140.0, 139.5, 139.3, 138.7, 129.1, 129.0, 128.9, 128.8, 128.8, 128.1, 127.4, 127.3, 127.3, 127.3, 127.2, 127.0, 126.3, 124.9, 123.1, 80.1, 61.2, 56.8, 53.4, 28.2, 13.7. HRMS (ESI): m/z [M + Na]+ calcd for C42H39NNaO4S2: 708.2218 found, 708.2212.
(2S*,3R*)-ethyl 2-(2-((tert-butoxycarbonyl)amino)acetamido)-3-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)pentanoate (34a-(DL))
The compound 34a-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 80
hexanes = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as a colorless solid (22 mg, 78%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 190–192 °C; IR (DCM): 2932, 1660, 812 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.55 (m, 8H), 7.22 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 6.71 (d, J = 8.6 Hz, 1H), 5.20 (br. s, 1H), 4.83 (t, J = 7.9 Hz, 1H), 3.98 (q, J = 7.0 Hz, 2H), 3.90–3.76 (m, 5H), 2.92–2.86 (m, 1H), 1.97–1.78 (m, 2H), 1.46 (s, 9H), 1.03 (t, J = 7.0 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.2, 169.1, 159.2, 139.7, 139.6, 139.0, 138.3, 133.1, 128.9, 128.0, 127.2, 127.0, 126.9, 114.2, 80.3, 61.2, 56.8, 55.3, 50.8, 44.4, 28.2, 24.4, 13.8, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C33H40N2NaO6: 583.2784 found, 583.2789.
20) as a colorless solid (22 mg, 78%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 190–192 °C; IR (DCM): 2932, 1660, 812 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.55 (m, 8H), 7.22 (d, J = 8.1 Hz, 2H), 6.99 (d, J = 8.7 Hz, 2H), 6.71 (d, J = 8.6 Hz, 1H), 5.20 (br. s, 1H), 4.83 (t, J = 7.9 Hz, 1H), 3.98 (q, J = 7.0 Hz, 2H), 3.90–3.76 (m, 5H), 2.92–2.86 (m, 1H), 1.97–1.78 (m, 2H), 1.46 (s, 9H), 1.03 (t, J = 7.0 Hz, 3H), 0.84 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.2, 169.1, 159.2, 139.7, 139.6, 139.0, 138.3, 133.1, 128.9, 128.0, 127.2, 127.0, 126.9, 114.2, 80.3, 61.2, 56.8, 55.3, 50.8, 44.4, 28.2, 24.4, 13.8, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C33H40N2NaO6: 583.2784 found, 583.2789.
Ethyl (S*)-12-((R*)-1-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)propyl)-2,2-dimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazatridecan-13-oate (34b-(DL))
The compound 34b-(DL) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 80
hexanes = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as a colorless solid (25 mg, 81%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 2932, 1655, 813 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.26–6.65 (m, 6H), 5.17 (s, 1H), 4.81 (t, J = 8.0 Hz, 1H), 4.05–3.94 (m, 4H), 3.86 (br. s, 5H), 2.96–2.90 (m, 1H), 1.96–1.70 (m, 2H), 1.47 (s, 9H), 1.04 (t, J = 7.1 Hz, 3H), 0.84 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.1, 170.0, 168.2, 159.2, 156.1, 139.7, 139.6, 138.9, 138.2, 133.1, 129.0, 128.0, 127.2, 127.0, 126.9, 114.3, 80.6, 61.3, 57.1, 55.4, 50.5, 44.3, 43.0, 28.3, 24.4, 13.8, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C35H43N3NaO7: 640.2999 found, 640.2984. The HPLC of the compound 34b-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (80
20) as a colorless solid (25 mg, 81%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 168–170 °C; IR (DCM): 2932, 1655, 813 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.63–7.56 (m, 8H), 7.26–6.65 (m, 6H), 5.17 (s, 1H), 4.81 (t, J = 8.0 Hz, 1H), 4.05–3.94 (m, 4H), 3.86 (br. s, 5H), 2.96–2.90 (m, 1H), 1.96–1.70 (m, 2H), 1.47 (s, 9H), 1.04 (t, J = 7.1 Hz, 3H), 0.84 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.1, 170.0, 168.2, 159.2, 156.1, 139.7, 139.6, 138.9, 138.2, 133.1, 129.0, 128.0, 127.2, 127.0, 126.9, 114.3, 80.6, 61.3, 57.1, 55.4, 50.5, 44.3, 43.0, 28.3, 24.4, 13.8, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C35H43N3NaO7: 640.2999 found, 640.2984. The HPLC of the compound 34b-(DL) was determined using the Daicel Chiralpak IA column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 15.30 min, tD = 17.91 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 15.30 min, tD = 17.91 min.
Ethyl (R)-12-((S)-1-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)propyl)-2,2-dimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazatridecan-13-oate (34b-(D))
The compound 34b-(D) was obtained after purification by column chromatography on silica gel (EtOAc:hexanes = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as a colorless solid (25 mg, 81%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2932, 1655, 813 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.67–7.58 (m, 8H), 7.28–7.00 (m, 6H), 5.41 (s, 1H), 4.82 (t, J = 8.2 Hz, 1H), 4.11–3.96 (m, 4H), 3.88 (br. s, 5H), 2.97–2.91 (m, 1H), 2.21–1.79 (m, 2H), 1.48 (s, 9H), 1.03 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.1, 170.0, 168.2, 159.2, 156.1, 139.7, 139.6, 138.9, 138.2, 133.1, 129.0, 128.0, 127.2, 127.0, 126.9, 114.3, 80.6, 61.3, 57.1, 55.3, 50.4, 44.3, 42.9, 28.3, 24.4, 13.8, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C35H43N3NaO7: 640.2999 found, 640.2984. [α]25 D = −18.00 (c = 0.05 g mL−1, CHCl3).The enantiomeric ratio (er = 98
20) as a colorless solid (25 mg, 81%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 167–169 °C; IR (DCM): 2932, 1655, 813 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.67–7.58 (m, 8H), 7.28–7.00 (m, 6H), 5.41 (s, 1H), 4.82 (t, J = 8.2 Hz, 1H), 4.11–3.96 (m, 4H), 3.88 (br. s, 5H), 2.97–2.91 (m, 1H), 2.21–1.79 (m, 2H), 1.48 (s, 9H), 1.03 (t, J = 7.1 Hz, 3H), 0.85 (t, J = 7.3 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.1, 170.0, 168.2, 159.2, 156.1, 139.7, 139.6, 138.9, 138.2, 133.1, 129.0, 128.0, 127.2, 127.0, 126.9, 114.3, 80.6, 61.3, 57.1, 55.3, 50.4, 44.3, 42.9, 28.3, 24.4, 13.8, 12.1. HRMS (ESI): m/z [M + Na]+ calcd for C35H43N3NaO7: 640.2999 found, 640.2984. [α]25 D = −18.00 (c = 0.05 g mL−1, CHCl3).The enantiomeric ratio (er = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) of the compound 34b-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80
2) of the compound 34b-(D) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 15.50 min, tD = 18.11 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 15.50 min, tD = 18.11 min.
Ethyl (S)-12-((R)-1-(4′′-methoxy-[1,1′:4′,1′′-terphenyl]-4-yl)propyl)-2,2-dimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazatridecan-13-oate (34b-(L))
The compound 34b-(L) was obtained after purification by column chromatography on silica gel (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 80
hexanes = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as a colorless solid (26 mg, 84%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 169–171 °C; IR (DCM): 2932, 1655, 813 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.64–7.57 (m, 8H), 7.25–7.00 (m, 6H), 5.43 (br. s, 1H), 4.82 (t, J = 8.2 Hz, 1H), 4.11–3.96 (m, 4H), 3.88 (br. s, 5H), 2.97–2.91 (m, 1H), 1.95–1.80 (m, 2H), 1.48 (s, 9H), 1.02 (t, J = 7.2 Hz, 3H), 0.84 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.3, 170.3, 168.6, 159.2, 156.2, 139.7, 139.5, 138.9, 138.2, 133.1, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 80.4, 61.2, 57.2, 55.3, 55.3, 50.3, 42.9, 28.3, 24.4, 13.7, 12.0. HRMS (ESI): m/z [M + Na]+ calcd for C35H43N3NaO7: 640.2999 found, 640.2984. [α]25 D = +14.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97
20) as a colorless solid (26 mg, 84%, 0.05 mmol scale); Rf (80% EtOAc/hexane) 0.5; mp: 169–171 °C; IR (DCM): 2932, 1655, 813 cm−1; 1H NMR (400 MHz, CDCl3): δH 7.64–7.57 (m, 8H), 7.25–7.00 (m, 6H), 5.43 (br. s, 1H), 4.82 (t, J = 8.2 Hz, 1H), 4.11–3.96 (m, 4H), 3.88 (br. s, 5H), 2.97–2.91 (m, 1H), 1.95–1.80 (m, 2H), 1.48 (s, 9H), 1.02 (t, J = 7.2 Hz, 3H), 0.84 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (∼101 MHz, CDCl3): δC 171.3, 170.3, 168.6, 159.2, 156.2, 139.7, 139.5, 138.9, 138.2, 133.1, 128.9, 128.0, 127.2, 127.0, 126.8, 114.2, 80.4, 61.2, 57.2, 55.3, 55.3, 50.3, 42.9, 28.3, 24.4, 13.7, 12.0. HRMS (ESI): m/z [M + Na]+ calcd for C35H43N3NaO7: 640.2999 found, 640.2984. [α]25 D = +14.00 (c = 0.05 g mL−1, CHCl3). The enantiomeric ratio (er = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) of the compound 34b-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80
3) of the compound 34b-(L) was determined by HPLC using the Daicel Chiralpak IA column, hexane/i-PrOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 15.04 min, tD = 18.07 min.
20), flow rate 1.0 mL min−1, UV detection at 254 nm, tL = 15.04 min, tD = 18.07 min.
Data availability
The data are available within the article or its ESI.† The crystallographic data for 21a(L) have been deposited at the CCDC under number 2426927 and can be obtained from https://www.ccdc.cam.ac.uk/structures/ (free of charge).Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. A. B. thanks IISER Mohali for funding this research. We thank the central analytical facilities (NMR, HRMS, and X-ray) of IISER Mohali. We thank the central analytical facilities (NMR, HRMS, and X-ray) of IISER Mohali. We also thank the Departmental NMR facility supported by DST-FIST (SR/FST/CS-II/2019/94 (TPN No. 32545)). S. B. thanks IISER Mohali for the PhD fellowship.Notes and references
- Reviews on oligoaryls: (a) N. Sakai, J. Mareda and S. Matile, Acc. Chem. Res., 2005, 38, 79 CrossRef CAS; (b) A. J. Berresheim, M. Müller and K. Müllen, Chem. Rev., 1999, 99, 1747 CrossRef CAS; (c) J. M. Tour, Chem. Rev., 1996, 96, 537 CrossRef CAS; (d) K. Manabe and S. Ishikawa, Chem. Commun., 2008, 3829 RSC; (e) W. Shi, T. Yu and D. Cui, Chin. J. Org. Chem., 2015, 35, 362 CrossRef CAS; (f) S. Kotha, M. Meshram, N. R. Panguluri, V. R. Shah, S. Todeti and M. E. Shirbhate, Chem.–Asian J., 2019, 14, 1356 CrossRef CAS PubMed; (g) C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed; (h) T. Jin and M. Terada, Tetrahedron, 2020, 61, 151514 CrossRef CAS; (i) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS; (j) H. Noguchi, K. Hojo and M. Suginome, J. Am. Chem. Soc., 2007, 129, 758 CrossRef CAS; (k) H. Noguchi, T. Shioda, C.-M. Chou and M. Suginome, J. Am. Chem. Soc., 2008, 10, 377 CAS; (l) A. J. Blake, P. A. Cooke, K. J. Doyle, S. Gair and N. S. Simpkins, Tetrahedron Lett., 1998, 39, 9093 CrossRef CAS; (m) C.-H. Cho, I.-S. Kim and K. Park, Tetrahedron, 2004, 60, 4589 CrossRef CAS; (n) H. Shimizu and K. Manabe, Tetrahedron Lett., 2006, 47, 5927 CrossRef CAS.
- Naturally occurring terphenyls: (a) J.-K. Liu, Chem. Rev., 2006, 106, 2209 CrossRef CAS; (b) W. Yan, Wuringege, S.-J. Li, Z.-K. Guo, W.-J. Zhang, W. Wei, R.-X. Tan and R.-H. Jiao, Bioorg. Med. Chem. Lett., 2017, 27, 51 CrossRef CAS PubMed; (c) X. Wang, A. R. Reynolds, S. I. Elshahawi, K. A. Shaaban, L. O. V. Ponomareva, M. A. Saunders, I. S. Elgumati, Y. Zhang, G. C. Copley, J. C. Hower, M. Sunkara, A. J. Morris, M. K. Kharel, S. G. V. Lanen, M. A. Prendergast and J. S. Thorson, Org. Lett., 2015, 17, 2796 CrossRef CAS PubMed; (d) H. Guo, H. Hu, S. Liu, X. Liu, Y. Zhou and Y. Che, J. Nat. Prod., 2007, 70, 1519 CrossRef CAS PubMed; (e) V. Calí, C. Spatafora and C. Tringali, Eur. J. Org Chem., 2004, 2004, 592 CrossRef; (f) D. N. Quang, T. Hashimoto, M. Nukada, I. Yamamoto, M. Tanaka and Y. Asakawa, Chem. Pharm. Bull., 2003, 51, 1064 CrossRef CAS.
- Bioactive terphenyls: (a) L. Gonzalez-Bulnes, I. Ibanez, L. M. Bedoya, M. Beltran, S. Catalan, J. Alcami, S. Fustero and J. Gallego, Angew. Chem., Int. Ed., 2013, 52, 13405 CrossRef CAS PubMed; (b) C. Medina-Trillo, D. M. Sedgwick, L. Herrera, M. Beltrán, A. Moreno, P. Barrio, L. M. Bedoya, J. Alcami, S. Fustero and J. Gallego, Sci. Rep., 2020, 10, 7190 CrossRef CAS PubMed; (c) M. Roberti, D. Pizzirani, M. Recanatini, D. Simoni, S. Grimaudo, A. D. Cristina, V. Abbadessa, N. Gebbia and M. Tolomeo, J. Med. Chem., 2006, 49, 3012 CrossRef CAS PubMed; (d) J. Ohkanda, J. W. Lockman, M. A. Kothare, Y. Qian, M. A. Blaskovich, S. M. Sebti and A. D. Hamilton, J. Med. Chem., 2002, 45, 177 CrossRef CAS PubMed; (e) M. Tetsuhisa, H. Hirokazu, O. Tadashi, O. Toru, N. Eriko, O. Kouichi, M. Tatanori and H. Masaki, Arzneim. Forsch., 1997, 47, 13 Search PubMed; (f) T. Norikura, K. Futiwara, T. Narita, S. Yamaguchi, Y. Morinaga, K. Iwai and H. Matsue, J. Agric. Food Chem., 2011, 59, 6974 CrossRef CAS PubMed; (g) T. Yamaguchi, Y. Miyaka, A. Miyamura, N. Ishiwata and K. Tatsuta, J. Antibiot., 2006, 59, 729 CrossRef CAS PubMed; (h) L. H. Heitman, R. Narlawar, H. de Vries, M. N. Willemsen, D. Wolfram, J. Brussee and A. P. Ijzerman, J. Med. Chem., 2009, 52, 2036 CrossRef CAS PubMed.
- Bioactive terphenyls: (a) B. E. Haug, W. Stensen and J. S. Svendsen, Bioorg. Med. Chem. Lett., 2007, 17, 2361 CrossRef CAS PubMed; (b) J. Svenson, W. Stensen, B.-O. Brandsdal, B. E. Huag, J. Monrad and J. S. Svendsen, Biochemistry, 2008, 47, 3777 CrossRef CAS PubMed; (c) G. C. Look, C. Vacin, T. M. Dias, S. Ho, T. H. Tran, L. L. Lee, C. Wisner, F. Fang, A. Marra, D. Westmacott, A. E. Hromockyj, M. M. Murphy and J. R. Schullek, Bioorg. Med. Chem. Lett., 2004, 14, 1423 CrossRef CAS PubMed; (d) M. Roberti, D. Pizzirani, M. Recanaatini, D. Simoni, S. Grimaudo, A. D. Crisina, V. Abbadessa, N. Gebbia and M. Tolomeo, J. Med. Chem., 2006, 49, 3012 CrossRef CAS; (e) C. Zhang, J. G. Ondeyka, K. B. Herath, Z. Guan, J. Collado, F. Pelaez, P. S. Leavitt, A. Gurnett, B. Nare, P. Liberator and S. B. Singh, J. Nat. Prod., 2006, 69, 710 CrossRef CAS; (f) Z. Guo, A. Abulaizi, L. Huang, Z. Xiong, S. Zhang, T. Liu and R. Wang, Mar. Drugs, 2022, 20, 679 CrossRef CAS.
- Applications of terphenyls in liquid crystals, polymer/LEDs, MOF, as fluorescence probe, etc.: (a) B. Chen, U. Baumeister, G. Pelzl, M. K. Das, X. Zeng, G. Ungar and C. Tschierske, J. Am. Chem. Soc., 2005, 127, 16578 CrossRef CAS PubMed; (b) M. Pytlarczyk, K. Gaczol, P. Harmata, J. Dziaduszek and J. Herman, J. Mol. Liq., 2021, 335, 116162 CrossRef CAS; (c) A. A. Kiryanov, P. Sampson and A. J. Seed, J. Math. Chem., 2001, 11, 3068 RSC; (d) J. Cai, H. Wang, H. Wang, X. Duan, Z. Wang, Y. Cui, Y. Yang, B. Chen and D. Qian, RSC Adv., 2015, 5, 77417 RSC; (e) S.-Y. Chou and Y. Chen, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 785 CrossRef CAS; (f) A. G. Wright, J. Fan, B. Britton, T. Weissbach, H.-F. Lee, E. A. Kitching, T. J. Peckham and S. Holdcroft, Energy Environ. Sci., 2016, 9, 2130 RSC; (g) L. Li, L. Lv and R.-D. Huang, RSC Adv., 2017, 7, 975 RSC; (h) Y. Liang, B. Han, X. Lu, Y. Zhang and J.-J. Wang, Spectrochim. Acta, Part A, 2025, 326, 125230 CrossRef CAS PubMed; (i) R. Tejpal, M. Kumar and V. Bhalla, Sens. Actuators, B, 2018, 258, 841 CrossRef CAS; (j) V. Bhalla, R. Tejpal and M. Kumar, Dalton Trans., 2012, 41, 403 RSC; (k) A. Krüger, C. Kryschi and D. Schmid, J. Lumin., 1990, 45, 447 CrossRef; (l) Vandana, K. N. Singh, A. Kaur, G. Singh, B. S. Gill, H. Kaur and D. Baliyan, J. Mol. Struct., 2025, 1326, 141113 CrossRef CAS; (m) A. F. Kleman, D. L. Dufek, T. L. Fobe, D. R. McCaslin, B. P. Cary, M. R. Shirts and S. H. Gellman, Org. Lett., 2021, 23, 4855 CrossRef CAS PubMed; (n) H. Sasabe, Y. Seino, M. Kimura and J. Kido, Chem. Mater., 2012, 24, 1404 CrossRef CAS; (o) C.-A. Wu, H.-H. Chou, C.-H. Shih, F.-I. Wu, C.-H. Cheng, H.-L. Huang, T.-C. Chao and M.-R. Tseng, J. Mater. Chem., 2012, 22, 17792 RSC.
- For selected reviews on terphenyl (teraryl) motifs as proteomimetics: (a) C. G. Cummings and A. D. Hamilton, Curr. Opin. Chem. Biol., 2010, 14, 341 CrossRef CAS PubMed; (b) S. Algar, M. Martín-Martínez and R. González-Muñiz, Eur. J. Med. Chem., 2021, 211, 113015 CrossRef CAS PubMed; (c) S. Fletcher and A. D. Hamilton, J. R. Soc. Interface, 2006, 3, 215 CrossRef CAS PubMed.
- Articles on terphenyl (teraryl) motifs as proteomimetics: (a) B. P. Omer, J. T. Ernst and A. D. Hamilton, J. Am. Chem. Soc., 2001, 123, 5382 CrossRef PubMed; (b) O. Kutzki, H. S. Park, J. T. Ernst, B. P. Orner, H. Yin and A. D. Hamilton, J. Am. Chem. Soc., 2002, 124, 11838 CrossRef CAS PubMed; (c) H. Yin, G.-i. Lee, K. A. Sedey, O. Kutzki, H. S. Park, B. P. Orner, J. T. Ernst, H.-G. Wang, S. M. Sebti and A. D. Hamilton, J. Am. Chem. Soc., 2005, 127, 10191 CrossRef CAS PubMed; (d) H. Yin, G. i. Lee, H. S. Park, G. A. Payne, J. M. Rodriguez, S. M. Sebti and A. D. Hamilton, Angew. Chem., Int. Ed., 2005, 44, 2704 CrossRef CAS PubMed; (e) M. Trobe, T. Schreiner, M. Vareka, S. Grimm, B. Wölfl and R. Breinbauer, Eur. J. Org Chem., 2022, 2022, e202101280 CrossRef CAS PubMed; (f) M. Peters, M. Trobe and R. Breinbauer, Chem. –Eur. J., 2013, 19, 2450 CrossRef CAS PubMed; (g) P. Zhao, M. D. Young and C. M. Beaudry, Org. Biomol. Chem., 2015, 13, 6162 RSC; (h) M. Vareka, B. Dahms, M. Lang, M. H. Hoang, M. Trobe, H. Weber, M. M. Hielscher, S. R. Waldvogel and R. Breinbauer, Catalysts, 2020, 10, 340 CrossRef CAS.
- (a) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed; (b) E.-i. Negishi, Angew. Chem., Int. Ed., 2011, 50, 6738 CrossRef CAS PubMed; (c) A. De Meijere, S. Brase and M. Oestreich, Metal-catalyzed Cross-Coupling Reactions and More, Wiley-VCH, Weinheim, 2014 CrossRef; (d) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed.
- (a) L.-C. Campeau and N. Hazari, Organometallics, 2019, 38, 3 CrossRef CAS PubMed; (b) J. Hassan, M. Sevignon, C. Gozzi, E. Schulz and M. Lamaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS PubMed; (c) I. Hussain, J. Capricho and M. A. Yawer, Adv. Synth. Catal., 2016, 358, 3320 CrossRef CAS; (d) A. Biffis, P. Centomo, A. D. Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249 CrossRef CAS PubMed.
- Reviews: Suzuki-Miyaura reaction: (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047 CrossRef CAS.
- Selected papers on the synthesis of teraryls, quateraryls, and oligoaryls via Suzuki-Miyaura reaction: (a) S. A. Kazi, E. M. Campi and M. T. W. Hearn, Tetrahedron, 2018, 74, 1731 CrossRef CAS; (b) C. Minard, C. Palacio, K. Cariou and R. H. Dodd, Eur. J. Org Chem., 2014, 2014, 2942 CrossRef CAS; (c) C.-G. Dong and Q.-S. Hu, J. Am. Chem. Soc., 2005, 127, 10006 CrossRef CAS PubMed; (d) N. Gu, Y. Liu, P. Liu, X. Ma, L. Yan and B. Dai, Chin. J. Chem., 2015, 33, 1189 CrossRef CAS; (e) R. H. Taylor and F.-X. Felpin, Org. Lett., 2007, 9, 2911 CrossRef CAS PubMed; (f) Y. Zhang, J. Han and Z.-J. Liu, J. Org. Chem., 2016, 81, 1317 CrossRef CAS PubMed; (g) K. Manabe, M. Ohba and Y. Matsushima, Org. Lett., 2011, 13, 2436 CrossRef CAS PubMed; (h) K. Manabe and T. Kimura, Org. Lett., 2013, 15, 374 CrossRef CAS PubMed.
- (a) L. Pollegioni and S. Servi, Unnatural Amino Acids: Methods, Protocols, Humana Press, New York, 2012 CrossRef; (b) A. B. Hughes, Amino Acids, Peptides and Proteins in Organic Chemistry: Volume 1 - Origins and Synthesis of Amino Acids, Wiley-VCH, Weinheim, 2009 Search PubMed; (c) A. B. Hughes, Amino Acids, Peptides and Proteins in Organic Chemistry: Volume 3 - Building Blocks, Catalysis and Coupling Chemistry, Wiley-VCH, Weinheim, 2011 Search PubMed; (d) A. B. Hughes, Amino Acids, Peptides and Proteins in Organic Chemistry: Volume 2 - Modified Amino Acids, Organocatalysis and Enzymes, Wiley-VCH, Weinheim, 2009 Search PubMed; (e) M. A. Blaskovich, Handbook on Syntheses of Amino Acids: General Routes for the Syntheses of Amino Acids, Oxford University Press, Oxford, New York, 2010 Search PubMed; (f) M. A. T. Blaskovich, J. Med. Chem., 2016, 59, 10807 CrossRef CAS PubMed; (g) R. Ganguly, B. Sreenivasulu and J. J. Vittal, Coord. Chem. Rev., 2008, 252, 1027 CrossRef CAS; (h) Y. Lu, Curr. Opin. Chem. Biol., 2005, 9, 118 CrossRef CAS PubMed; (i) R. Grandel, U. Kazmaier and F. Rominger, J. Org. Chem., 1998, 63, 4524 CrossRef CAS.
- For selected reviews dealing with the C-H functionalization, see: (a) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (b) C. He, W. G. Whitehurst and M. J. Gaunt, Chem, 2019, 5, 1031 CrossRef CAS; (c) A. Banerjee, S. Sarkar and B. Patel, Org. Biomol. Chem., 2017, 15, 505 RSC; (d) T. Gensch, M. N. Hopkinson, F. Glorius and J. Wencel-Delord, Chem. Soc. Rev., 2016, 45, 2900 RSC; (e) O. Baudoin, Acc. Chem. Res., 2017, 50, 1114 CrossRef CAS PubMed; (f) K. Yang, M. Song, H. Liu and H. Ge, Chem. Sci., 2020, 11, 12616 RSC; (g) F. Kakiuchi and S. Murai, Acc. Chem. Res., 2002, 35, 826 CrossRef CAS PubMed; (h) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (i) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (j) Y. Segawa, T. Maekawa and K. Itami, Angew. Chem., Int. Ed., 2015, 54, 66 CrossRef CAS PubMed; (k) W. Ali, G. A. Oliver, D. B. Werz and D. Maiti, Chem. Soc. Rev., 2024, 53, 9904 RSC; (l) J. He, M. Wasa, K. S. L. Chan, Q. Shao and J.-Y. Yu, Chem. Rev., 2017, 117, 8754 CrossRef CAS PubMed; (m) R. Manoharan and M. Jeganmohan, Asian J. Org. Chem., 2019, 8, 1949 CrossRef CAS; (n) M. M. Mingo, N. Rodriguez, R. G. Arrayas and J. C. Carretero, Chem. Commun., 2022, 58, 2034 RSC; (o) S. A. Babu, R. Padmavathi, S. Suwasia, A. Dalal, D. Bhattacharya, P. Singh and R. Tomar, Stud. Nat. Prod. Chem., 2021, 71, 311 CAS; (p) T. Besset, T. Poisson and X. Pannecoucke, Chem. –Eur. J., 2014, 20, 16830 CrossRef CAS PubMed; (q) A. Mandal, S. Dana, D. Choudhury and M. Baidya, Chem.–Asian J., 2019, 14, 4074 CrossRef CAS PubMed; (r) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef CAS PubMed; (s) M. Miura, T. Satoh and K. Hirano, Bull. Chem. Soc. Jpn., 2014, 87, 751 CrossRef CAS; (t) M.-Z. Lu, J. Goh, M. Maraswami, Z. Jia, J.-S. Tian and T.-P. Loh, Chem. Rev., 2022, 122, 17479 CrossRef CAS PubMed.
- For reviews on bidentate directing group-aided C-H functionalization, see: (a) S. Rej, Y. Ano and N. Chatani, Chem. Rev., 2020, 120, 1788 CrossRef CAS PubMed; (b) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053 CrossRef CAS PubMed; (c) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603 RSC; (d) S. A. Babu, Y. Aggarwal, P. Patel and R. Tomar, Chem. Commun., 2022, 58, 2612 RSC; (e) X. Yang, G. Shan, L. Wang and Y. Rao, Tetrahedron Lett., 2016, 57, 819 CrossRef CAS; (f) S.-F. Ni, G. Huang, Y. Chen, J. S. Wright, M. Li and L. Dang, Coord. Chem. Rev., 2022, 455, 214255 CrossRef CAS; (g) B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle and B.-F. Shi, Chem. Rev., 2021, 121, 14957 CrossRef CAS PubMed; (h) R. K. Rit, M. R. Yadav, K. Ghosh and A. K. Sahoo, Tetrahedron, 2015, 71, 4450 CrossRef CAS.
- For bidentate directing group-aided C-H functionalization, see: (a) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965 CrossRef CAS PubMed; (b) G. Kang, D. A. Strassfeld, T. Sheng, C.-Y. Chen and J.-Q. Yu, Nature, 2023, 618, 519 CrossRef CAS PubMed; (c) A. Seki and Y. Takahashi, Tetrahedron Lett., 2021, 74, 153130 CrossRef CAS; (d) D. Antermite, A. J. P. White, L. Casarrubios and J. A. Bull, ACS Catal., 2017, 82, 9597 Search PubMed; (e) B. Gopalakrishnan, S. A. Babu and R. Padmavathi, Tetrahedron, 2015, 71, 8333 CrossRef CAS; (f) M. B. Calvert and J. Sperry, Org. Biomol. Chem., 2016, 14, 5728 RSC; (g) J. Mio, K. Yang, M. Kurek and H. Ge, Org. Lett., 2015, 17, 3738 CrossRef PubMed; (h) J. Kim, M. Sim, N. Kim and S. Hong, Chem. Sci., 2015, 6, 3611 RSC; (i) B. Gopalakrishnan, S. Mohan, R. Parella and S. A. Babu, J. Org. Chem., 2016, 81, 8988 CrossRef CAS PubMed; (j) R. Parella and S. A. Babu, J. Org. Chem., 2015, 80, 2339 CrossRef CAS PubMed; (k) W. Nack, B. Wang, X. Wu, R. Jio, G. He and G. Chen, Org. Chem. Front., 2016, 3, 561 RSC; (l) S. Biswas, N. R. Bheemireddy, M. Bal, B. F. V. Steijvoort and B. U. W. Maes, J. Org. Chem., 2019, 84, 13112 CrossRef CAS PubMed; (m) W. R. Gutekunst and P. S. Baran, J. Org. Chem., 2014, 79, 2430 CrossRef CAS PubMed; (n) J. Ghouilem, C. Tran, N. Grimblat, P. Retailleau, M. Alami, V. Gandon and S. Messaoudi, ACS Catal., 2021, 11, 1818 CrossRef CAS; (o) Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2014, 136, 898 CrossRef CAS PubMed; (p) P. Hu and T. Bach, Synlett, 2015, 26, 2853 CrossRef CAS; (q) N. Hoshiya, M. Kondo, H. Fukuda, M. Arisawa, J. Uenishi and S. Shuto, J. Org. Chem., 2017, 82, 2535 CrossRef CAS PubMed; (r) C. Reddy, N. Bisht, R. Parella and S. A. Babu, J. Org. Chem., 2016, 81, 12143 CrossRef CAS PubMed; (s) A. Dalal, S. A. Babu and S. Banga, Asian J. Org. Chem., 2024, 13, e202300508 CrossRef CAS.
- For reviews on C-H functionalization of amino acid derivatives, see: (a) T. Brandhofer and O. G. Mancheño, Eur. J. Org Chem., 2018, 2018, 6050 CrossRef CAS; (b) A. Correa, Eur. J. Inorg. Chem., 2021, 2021, 2928 CrossRef CAS; (c) A. F. M. Noisier and M. A. Brimble, Chem. Rev., 2014, 114, 8775 CrossRef CAS PubMed; (d) J. Jeon, C. Lee, I. Park and S. Hong, Chem. Rec., 2021, 21, 3613 CrossRef CAS PubMed; (e) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (f) G. He, B. Wang, W. A. Nack and G. Chen, Acc. Chem. Res., 2016, 49, 635 CrossRef CAS PubMed; (g) S. Shabani, Y. Wu, H. G. Ryan and C. A. Hutton, Chem. Soc. Rev., 2021, 50, 9278 RSC; (h) B.-B. Zhan, M.-X. Jiang and B.-F. Shi, Chem. Commun., 2020, 56, 13950 RSC.
- Papers dealing with C-H functionalization of amino acids, see: (a) M. D. Reddy and E. B. Watkins, J. Org. Chem., 2015, 80, 11447 CrossRef CAS PubMed; (b) B. V. S. Reddy, L. R. Reddy and E. J. Corey, Org. Lett., 2006, 8, 3391 CrossRef CAS PubMed; (c) L. Liu, Y.-H. Liu and B.-F. Shi, Chem. Sci., 2020, 11, 290 RSC; (d) S. Guin, A. Deb, P. Dolui, S. Chakraborty, V. K. Singh and D. Maiti, ACS Catal., 2018, 8, 2664 CrossRef CAS; (e) E. Hernando, J. Villalva, A. M. Martinez, I. Alonso, N. Rodríguez, R. G. Arrayas and J. C. Carretero, ACS Catal., 2016, 6, 6868 CrossRef CAS; (f) M. Fan and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 12152 CrossRef CAS; (g) R. Feng, B. Wang, Y. Liu, Z. Liu and Y. Zhang, Eur. J. Org Chem., 2016, 2016, 139 CrossRef; (h) Y. Weng, X. Ding, J. C. A. Oliveira, X. Xu, N. Kaplaneris, M. Zhu, H. Chen, Z. Chen and L. Ackermann, Chem. Sci., 2020, 11, 9290 RSC; (i) G. He, Y. Zhao, S. Zhang, C. Lu and G. Chen, J. Am. Chem. Soc., 2012, 134, 3 CrossRef CAS PubMed; (j) Q. Bai, Z. Bai and H. Wang, Org. Lett., 2019, 21, 8225 CrossRef CAS PubMed; (k) Y. Jiang, G. Deng, S. Zhang and T.-P. Loh, Org. Lett., 2018, 20, 652 CrossRef CAS PubMed; (l) G. Chen, T. Shigenari, P. Jain, Z. Zhang, Z. Jin, J. He, S. Li, C. Mapelli, M. M. Miller, M. A. Poss, P. M. Scola, K.-S. Yeung and J.-Q. Yu, J. Am. Chem. Soc., 2015, 137, 3338 CrossRef CAS PubMed; (m) L. D. Tran and O. Daugulis, Angew. Chem., Int. Ed., 2012, 51, 5188 CrossRef CAS PubMed.
- Selected papers of our group dealing with the synthesis of unnatural amino acids via C–H arylation of amino acids, see: (a) S. Banga, R. Kaur and S. A. Babu, Eur. J. Org Chem., 2021, 2021, 3641 CrossRef CAS; (b) S. Suwasia and S. A. Babu, Eur. J. Org Chem., 2024, 27, e202400607 CrossRef CAS; (c) P. Patel, S. A. Babu and R. Tomar, Eur. J. Org Chem., 2024, 27, e202400190 CrossRef CAS; (d) S. Banga and S. A. Babu, Eur. J. Org Chem., 2024, 27, e202400272 CrossRef CAS; (e) R. Tomar, D. Bhattacharya and S. A. Babu, Tetrahedron, 2019, 75, 2447 CrossRef CAS; (f) R. Kaur, S. Banga and S. A. Babu, Org. Biomol. Chem., 2022, 20, 4391 RSC; (g) R. Tomar, D. Bhattacharya and S. A. Babu, Asian J. Org. Chem., 2022, 11, e202100736 CrossRef CAS; (h) R. Tomar, S. Suwasia, A. R. Choudhury, S. Venkataramani and S. A. Babu, Chem. Commun., 2022, 58, 12967 RSC; (i) P. Singh and S. A. Babu, Eur. J. Org Chem., 2023, 26, e202300440 CrossRef CAS; (j) A. Dalal, S. Bodak and S. A. Babu, Org. Biomol. Chem., 2021, 22, 1279 RSC.
- Selected reviews dealing with biaryl amino acids, see: (a) L. Feliu and M. Planas, Stud. Nat. Prod. Chem., 2005, 11, 53 CAS; (b) T. Willemse, W. Schepens, H. W. van Vlijmen, B. U. W. Maes and S. Ballet, Catalysts, 2017, 7, 74 CrossRef; (c) P. Lloyd-Williams and E. Giralt, Chem. Soc. Rev., 2001, 30, 145 RSC; (d) O. Skaff, K. A. Jolliffe and C. A. Hutton, J. Org. Chem., 2005, 70, 7353 CrossRef CAS PubMed; (e) Y. Amino and R. M. Williams, Heterocycles, 2019, 99, 83 CrossRef CAS PubMed; (f) S. T. Ahmed, F. Parmeggiani, N. J. Weise, S. L. Flitsch and N. J. Turner, ACS Catal., 2015, 5, 5410 CrossRef CAS; (g) F. M. Gall, D. Hohl, D. Frasson, T. Wermelinger, P. R. E. Mittl, M. Sievers and R. Riedl, Angew. Chem., Int. Ed., 2019, 58, 4051 CrossRef CAS PubMed; (h) E. Moreno, L. A. Nolasco, L. Caggiano and R. F. W. Jackson, Org. Biomol. Chem., 2006, 4, 3639 RSC; (i) W. C. Shieh and J. A. Carlson, J. Org. Chem., 1992, 57, 379 CrossRef CAS; (j) M. J. Burk, J. R. Lee and J. P. Martinez, J. Am. Chem. Soc., 1994, 116, 10847 CrossRef CAS.
- DL-Carboxamides of alanine 3a-(DL), 2-aminobutyric acid 3b-(DL), norvaline 3c-(DL), leucine 3d-(DL), norleucine 3e-(DL), phenylalanine, 3f-(DL), 2-aminooctanoic acid 3g-(DL), linked with 8-aminoquinoline directing group were assembled from their respective racemic amino acids and 8-aminoquinoline. Enantioenriched l-carboxamides of alanine 3a-(L), 2-aminobutyric acid 3b-(L), norvaline 3c-(L), leucine 3d-(L), norleucine 3e-(L), phenylalanine, 3f-(L), linked with 8-aminoquinoline directing group were assembled from their respective enantiopure L-amino acids and 8-aminoquinoline. Enantioenriched D-carboxamides of alanine 3a-(D), norvaline 3c-(D), leucine 3d-(D), norleucine 3e-(D), phenylalanine, 3f-(D), linked with 8-aminoquinoline directing group were assembled from their respective enantiopure D-amino acids and 8-aminoquinoline using standard methods, see ref. 18.
- Single crystal of 21a-(L) was recrystallized from dichloromethane/ diethyl ether. Crystal data. C39H36BN3O5, M = 637.52, Monoclinic, a = 14.619 (3), b = 6.8032 (17), c = 17.129 (3) Å, V = 1689.3 (6) Å3, T = 293 K, space group = P21 (no. 4), Z = 2, 10152 reflections measured, 8670 unique (Rint = 0.066), which were used in all calculations. The final wR(F2) was 0.099 (all data).
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2426927. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra03486h |
| This journal is © The Royal Society of Chemistry 2025 |