Open Access Article
Open Access ArticleAdvancements in cathode materials for potassium-ion batteries: current landscape, obstacles, and prospects
Titus
Masese
 *ac and
Godwill Mbiti
Kanyolo
*ac and
Godwill Mbiti
Kanyolo
 *ab
*ab
aResearch Institute of Electrochemical Energy, National Institute of Advanced Industrial Science and Technology (AIST), 1-8-31 Midorigaoka, Ikeda, Osaka 563-8577, Japan. E-mail: titus.masese@aist.go.jp; gm.kanyolo@aist.go.jp
bDepartment of Engineering Science, The University of Electro-Communications, 1-5-1 Chofugaoka, Chofu, Tokyo 182-8585, Japan. E-mail: gmkanyolo@mail.uec.jp
cAIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), Sakyo-ku, Kyoto 606-8501, Japan
First published on 4th December 2023
Abstract
The development of advanced energy storage technologies has assumed paramount significance in addressing the escalating demands for sustainable and eco-friendly power sources. Amongst these innovative technologies, potassium-ion batteries (KIBs) have risen to the fore as viable contenders, chiefly owing to their cost-effectiveness and the abundant availability of potassium resources. Nevertheless, the realisation of efficient and high-performance KIBs hinges significantly upon the adept design and appropriate utilisation of cathode materials. Thus, this Perspective provides a comprehensive analysis of the present status, associated challenges, and prospective avenues concerning cathode materials for KIBs. We commence by discussing the significance of KIBs in the context of the global energy landscape and highlight their potential to revolutionise energy storage systems. Subsequently, we delve into cathode materials for KIBs, emphasising their pivotal role in determining the overall performance of these batteries. A systematic survey of the various cathode materials explored to date is presented, primarily encompassing layered oxides, polyanion-based compounds, Prussian analogues and organic moieties. The discussion focuses on the advantages, limitations, and performance metrics of each material class, unveiling the critical insights gained from experimental studies and theoretical investigations. Furthermore, this Perspective sheds light on the prominent challenges and obstacles hindering the widespread adoption of KIBs. These challenges include issues related to limited specific capacities, sluggish kinetics, and performance attenuation during cycling, as well as the scarcity of suitable electrolytes. We offer an authoritative evaluation of the efforts undertaken to address these obstacles, including novel material design strategies, advanced characterisation techniques, and the integration of nanotechnology. Finally, we conclude with a forward-looking perspective, outlining the future directions and potential breakthroughs in electrode materials for KIBs. We emphasise the importance of interdisciplinary collaborations, advancements in computational methods, and fundamental research to foster the development of high-performance and environmentally sustainable KIBs. In conclusion, this Perspective consolidates the current state of electrode materials for KIBs whilst elucidating the challenges that need to be overcome to unlock their full potential. By synthesising the collective knowledge from the latest research endeavours, this Perspective aims to inspire future research and innovation in the pursuit of efficient and scalable KIB technologies.
1 Introduction
Meeting the immense energy demands of our society in a sustainable and environmentally responsible manner has become an urgent global imperative. Over the years, lithium-ion (Li-ion) battery (LIB) technology has reigned as the dominant energy storage system, finding widespread usage in portable electronics and scaling up to large-scale energy storage systems like the electric grid, facilitating the integration of intermittent renewable energy sources such as wind, tidal and solar power.1Fig. 1 shows the anticipated proliferation number of energy devices, encompassing portable electronics to large-scale energy storage systems intended for utilisation in electric vehicles. In this context, the applicability of rechargeable batteries, exemplified by the present-day LIB, is projected to be all-encompassing. However, the projected paucity and uneven global distribution of lithium resources,2 along with the exponential growth of electric vehicles and our growing dependency on renewable energy, have catalysed the pursuit of alternative battery systems to replace or complement commercial Li-ion batteries (LIBs).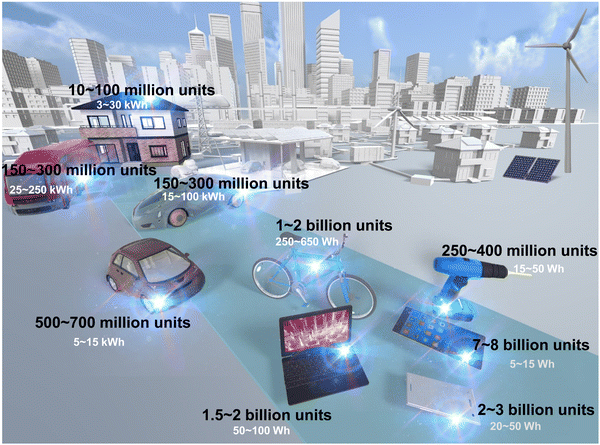 | ||
| Fig. 1 Projected number of energy devices and battery size,3 including those for portable electronics and large-scale energy storage in electric vehicles. Rechargeable batteries, like the current LIB, are expected to be extensively utilised. | ||
1.1 Overall significance of potassium-ion batteries
As a promising contender, potassium-ion (K-ion) batteries (hereafter referred to as KIBs) have emerged as a viable alternative for energy storage,4 offering a range of compelling advantages delved into below:1. The remarkable abundance of potassium resources in the earth's crust (at approximately 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 parts per million (ppm)) compared to lithium (at around 20 ppm) has ignited extensive research focus on potassium-based chemistries as a promising avenue for cost-effective grid-scale energy storage solutions. In reality, the Clarke's number, a significant parameter denoting the relative crustal abundance of elements, exemplifies the remarkable disparity between potassium (2.3 wt%) and lithium (0.006 wt%).6 The prevalence of potassium vastly surpasses that of lithium, emphasising the pronounced variation in their respective occurrences within the Earth's crust (Fig. 2a). Potassium-based salts are thus widely available in substantial quantities worldwide, offering a cost advantage.7 For instance, raw materials such as K2CO3, employed as precursors for the design of electrode materials, are not only abundant but also more economically accessible than Li2CO3. Additionally, potassium resources are more evenly distributed across the globe, eliminating concerns about geopolitical constraints that can impede the supply of lithium. Thus, the sustainable nature of potassium-based alternatives becomes evident, providing a compelling rationale for their exploration in energy storage applications;
000 parts per million (ppm)) compared to lithium (at around 20 ppm) has ignited extensive research focus on potassium-based chemistries as a promising avenue for cost-effective grid-scale energy storage solutions. In reality, the Clarke's number, a significant parameter denoting the relative crustal abundance of elements, exemplifies the remarkable disparity between potassium (2.3 wt%) and lithium (0.006 wt%).6 The prevalence of potassium vastly surpasses that of lithium, emphasising the pronounced variation in their respective occurrences within the Earth's crust (Fig. 2a). Potassium-based salts are thus widely available in substantial quantities worldwide, offering a cost advantage.7 For instance, raw materials such as K2CO3, employed as precursors for the design of electrode materials, are not only abundant but also more economically accessible than Li2CO3. Additionally, potassium resources are more evenly distributed across the globe, eliminating concerns about geopolitical constraints that can impede the supply of lithium. Thus, the sustainable nature of potassium-based alternatives becomes evident, providing a compelling rationale for their exploration in energy storage applications;
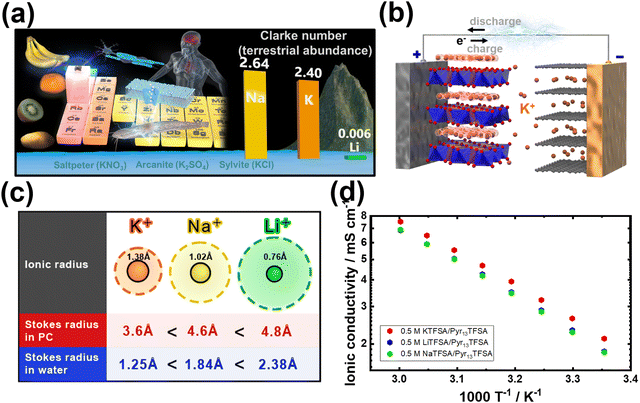 | ||
| Fig. 2 (a) Abundance of potassium resources. (b) Schematic representation of the operation mechanism of potassium-ion battery (KIB), wherein the positive electrode consists of, for instance, layered oxide, and the negative electrode comprises graphite. Analogous to other secondary battery systems (rechargeable batteries), such as those involving lithium ions, the potassium ions efficiently traverse between the electrodes via the electrolyte. (c) Stokes radii of solvated states of alkali metal cations. (d) Arrhenius plots showing the ionic conductivity of pyrrolidinium-based ionic liquid electrolytes based on 1-methyl-1-propylpyrrolidinium bis(trifluoromethanesulphonyl)amide (abbreviated as Pyr13TFSA or Pyr13TFSI) ionic liquids dissolved in KTFSA (KTFSI), NaTFSA and LiTFSA alkali metal salts.5 Figure (d) reproduced from ref. 5 with permission. Copyright 2019 Wiley-VCH. | ||
2. Potassium possesses electrochemical properties that are notably similar to lithium:8
(a) In fact, a potassium-ion battery operates on a mechanism that closely resembles that of a lithium-ion battery (Fig. 2b), wherein potassium ions shuttle between the electrodes through the electrolyte during the charging and discharging processes, in what was initially referred to as a ‘shuttle’ or ‘rocking-chair’ battery technology.
Like other rechargeable batteries, the potassium-ion battery (KIB) comprises an ionically conductive electrolyte containing a potassium-based salt, two current collectors (one negative and one positive), a separator and two electrodes with distinctive chemical potentials. The positive electrode can be constructed using diverse materials, such as layered oxides (e.g., KxCoO2, KCrO2, etc.),9–11 polyanion compounds (e.g., KVPO4F, KFeSO4F),12–19 organic compounds (e.g., poly(anthraquinonyl sulphide (abbreviated as PAQS)),20,21 polyaniline (PANI),22 3,4,9,10-perylene-tetracarboxylic acid-dianhydride (commonly referred to as Pigment Red 224 (C24H8O6) and abbreviated as PTCDA),23etc.), as well as Prussian analogues (e.g., K2MnFe(CN)6, K2FeFe(CN)6, K2NiFe(CN)6, etc.).24–34 Conversely, the negative electrode can be constituted of carbonaceous materials such as graphite, which stands as a prevalent choice due to its proficient facilitation of reversible extraction/insertion of K-ions. As for the electrolyte, customary formulations comprise mixtures of organic carbonate solvents, such as diethyl carbonate or ethyl carbonate, in conjunction with non-coordinating anion salts, prominently exemplified by potassium hexafluorophosphate (KPF6), potassium bis(fluorosulphonyl)imide (KN(SO2F)2 (typically abbreviated as KFSI or KFSA)), potassium trifluoromethanesulphonate (KCF3SO3 (KOTf)), potassium (3-methoxypropyl)((trifluoromethyl)-sulphonyl)imide (abbreviated as KMPSA) and potassium bis(trifluoromethanesulphonyl)imide (KN(CF3SO2)2 (KTFSI or KTFSA)). A slender separator film, designed from insulation material, serves to efficaciously segregate the two electrodes, thereby preventing the occurrence of an internal short circuit.
During the charging phase, a cathodic oxidation half-reaction occurs at the positive electrode (cathode), where a positively charged external power source facilitates the extraction of electrons from the potassium atoms within the cathode material (e.g., KCrO2). Subsequently, these extracted electrons direct potassium ions (K+) through an external circuit to the negative electrode (anode) (e.g., graphite). This process engenders an electrical disequilibrium within the cell, prompting the migration of potassium ions through the electrolyte towards the negatively charged anode. The layered structure of loosely bonded graphene sheets in graphite facilitates a reduction half-reaction, enabling the intercalation of potassium ions into its layers and leading to the formation of potassiated graphite (KC8) in a phenomenon known as potassiation or potassium intercalation/insertion. This potassiation process continues until the graphite achieves full potassiation, resulting in the maximal electrical potential difference between the two electrodes, commonly referred to as the fully charged state. In the discharge process, a converse sequence occurs. Electrons liberated from the graphite anode travel back to the cathodic side, compelling the departure of K+ from the potassiated graphite in a process referred to as depotassiation or potassium de-intercalation/extraction. Concurrently, potassium ions traverse the electrolyte and recombine with the cathode material. This discharge process persists until the graphite is entirely depotassiated, leading to the diminishing of the potential difference between the two electrodes to the minimum voltage level.
(b) Potassium-ion batteries can benefit from the utilisation of electrolyte formulations similar to those used in lithium-ion batteries. For instance, electrolytes such as potassium hexafluorophosphates (KPF6), where potassium replaces lithium in conventional LiPF6-containing electrolytes, can be effectively employed. This compatibility extends to other battery components as well, allowing for the use of graphite and similar materials utilised in the production line of commercial lithium-ion batteries. As a result, the adoption of potassium-ion batteries not only benefits from existing manufacturing infrastructure but also offers potential cost reductions, making potassium-ion technology a promising ‘drop-in’ post-lithium-ion technology.
3. In its solvated state, potassium-ion exhibits a smaller Stokes radius compared to solvated lithium (Fig. 2c). This is primarily due to the larger ionic radius of K+ ions compared to Li+ and Na+ ions.37 As a result, K+ ions experience reduced solvation compared to Na+ and Li+ ions, resulting in a smaller radius for solvated K+ ions. This characteristic leads to weaker Lewis acidity for potassium and facilitates the faster movement of solvated K-ions in electrolytes containing potassium salts (Fig. 2d). The comparatively larger ionic radius of K+ (e.g., 1.38 Å when coordinated octahedrally with ligands) compared to Li+ (0.76 Å) results in a smaller Stokes radius for K+ when solvated in propylene carbonate,38 as illustrated in Fig. 2c. Consequently, K+ exhibits low desolvation energies and enhanced ionic mobility in electrolytes (Fig. 3).39 Indeed, a comparative analysis of the ionic conductivity in K-based electrolytes as opposed to Na- and Li-based counterparts (e.g., APF6 in propylene carbonate, APF6 in ethylene carbonate: dimethyl carbonate, and AFSI in propylene carbonate, where A = K, Na, Li) demonstrates that K-based electrolytes exhibit the highest ionic conductivity, primarily attributed to the weak Lewis acidity of the K+ ions.40–42 Furthermore, the similarities in chemistries between K+ and Li+ ions enable the utilisation of well-established Li-based electrolyte compositions as analogues, which have been instrumental in the exploration of high-performance K-based electrolytes.
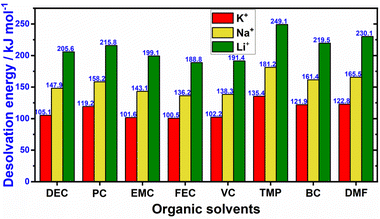 | ||
| Fig. 3 Desolvation energy values of K+, Na+, and Li+ in typical organic solvents. Abbreviations: dimethyl formamide (DMF), butylene carbonate (BC), trimethyl phosphate (TMP), vinylene carbonate (VC), fluoroethylene carbonate (FEC), ethyl methyl carbonate (EMC), propylene carbonate (PC), diethyl carbonate (DEC). The data was derived from ref. 35 and 36. | ||
4. Potassium demonstrates a similar or even lower standard redox potential than lithium in non-aqueous electrolytes commonly used in conventional batteries,44 paving the way for potentially high-voltage battery systems. Notably, nature itself has chosen potassium ions as charge carriers for transmitting synaptic impulses, whilst electric eels employ a combination of potassium and sodium ions in ‘potassium pumps’ to generate remarkably high voltages. From a technical perspective, the electrode potential of K/K+ relative to the standard hydrogen electrode (SHE) (at −2.93 V vs. SHE for K/K+) is comparable to that of Li+/Li (at −3.04 V vs. SHE for Li/Li+) in aqueous solvents. Furthermore, in certain non-aqueous solvents, K+/K exhibits slightly more negative redox potentials than Li+/Li (Fig. 4), resulting in higher voltage outputs and contributing to enhanced energy density in potassium-ion batteries.
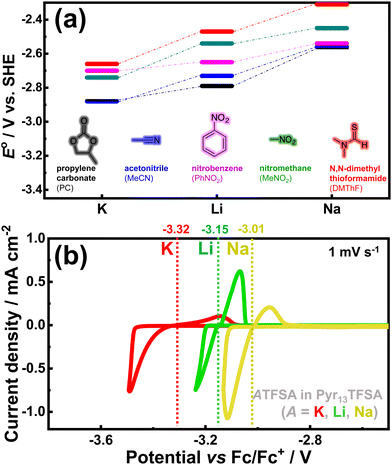 | ||
| Fig. 4 (a) Alkali metal dissolution/deposition potential in organic solvents. Data plotted from ref. 43. (b) Alkali metal dissolution/deposition potential in pyrrolidinium-based ionic liquid electrolytes. Figure (b) reproduced and adapted from ref. 5 with permission. Copyright 2019 Wiley-VCH. | ||
5. Graphite, a commonly used anode material in commercial LIBs, can also serve as a direct anode material in KIBs (Fig. 5a). Remarkably, potassium ions can be reversibly inserted and extracted from graphite due to the inherent stability of the potassium-graphite compound (KC8).44–46 This unique characteristic enables the seamless utilisation of the well-established battery processing systems originally designed for LIBs in the context of KIBs. As a result, the transition and utilisation of graphite-based anodes become significantly easier and more efficient for KIBs compared to other rechargeable battery systems. Graphite anode demonstrates a reversible capacity, amounting to approximately 372 mA h g−1 in a Li cell, attributed to the formation of LiC6 through a series of transitional stages: C ↔ LiC24 ↔ LiC18 ↔ LiC12 ↔ LiC6. In contrast, whilst negligible capacity is observed in a Na cell, K+ can be efficiently and reversibly inserted into graphite in a K cell (as illustrated in Fig. 5a and b). Reversible capacities commensurate with the theoretical capacity of 279 mA h g−1 are commonly achieved in K cells (Fig. 5c), aligning closely with the reversible formation of stable KC8 through a series of transitional stages that are yet polemical:47 C ↔ KC36 ↔ KC24 ↔ KC848 or C ↔ KC24 ↔ KC16 ↔ KC849 or C ↔ KC60 ↔ KC48 ↔ KC36 ↔ KC24/KC16 ↔ KC850 or C ↔ KC48 ↔ KC36 ↔ KC24 ↔ KC851 or C ↔ KC84 ↔ KC72 ↔ KC36 ↔ KC36/KC24 ↔ KC24 ↔ KC8.52 It is indeed pertinent to note that these translational stages occur concurrently with graphite intercalation compounds manifesting electrochromism (Fig. 5d), thereby introducing novel applications of K+ intercalation/de-intercalation electrochemistry within the realm of electrochromic device technologies. Moreover, potassium graphite intercalation compounds, namely KC12n (where n = 2, 3, 4), KC8, etc. can be synthesised through potassium-vapour-based methods, but an alternative approach involves a facile and scalable procedure achieved by combining graphite powder and potassium metal (with sodium as a catalyst) at room temperature (Fig. 5d). Hence, graphite, being an unquestionably inexpensive and extensively utilised anode material in commercial LIBs, can be readily adapted as an anode in KIBs. This noteworthy compatibility implies that the existing infrastructure and supply chain used for manufacturing graphite electrodes in LIBs can be smoothly transitioned to KIBs, thereby facilitating the commercial deployment of KIBs. Graphite can be envisaged as a widely employed anode material in KIBs, owing to its advantageous attributes, including low operation potential, high electronic conductivity, and significant volumetric and gravimetric capacities. The relatively low operation potential of graphite in KIBs (approximately 0.3 V versus (vs.) K/K+, slightly higher than that in Li-cell as shown in Fig. 5a and c) enables the utilisation of the full cell voltage when combined with suitable high-voltage cathode materials. Additionally, this characteristic may mitigate the formation of dendritic potassium metal, a phenomenon typically observed at lower potentials (near 0 V vs. K/K+). Furthermore, K/graphite cells have demonstrated superior rate capabilities compared to Li/graphite cells. Consequently, it becomes feasible to design a KIB system with essentially the same material configuration as current LIBs but with a higher power density.
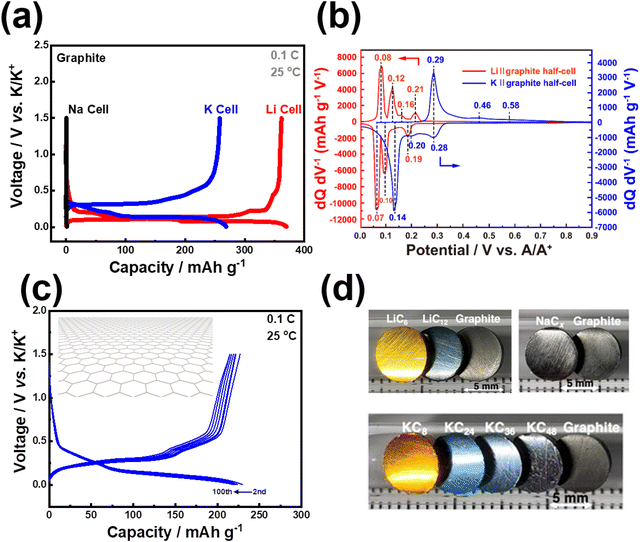 | ||
| Fig. 5 Electrochemical Na+, K+ and Li+ insertion/extraction behaviour in graphite. (a) Voltage–(dis)charge curves of graphite in an electrochemical Li, Na, K half-cell setups. (b) Differential capacity-potential plots of graphite in a Li half-cell and K half-cell showing peaks that correspond to the staging processes. (c) Voltage–(dis)charge profiles of graphite in a K half-cell (1C = 279 mA g−1). (d) Photographs of LiC12, LiC6, KC12n (n = 2, 3, 4), KC8, and graphite pellets. Potassium graphite intercalation compounds display distinct colour with varying potassium content, suggesting that the electrochemical potassiation/depotassiation of graphite could have implications in electrochromic applications. Figure (b) reproduced from ref. 42 with permission. Copyright 2020 American Chemical Society. Figure (d) reproduced and adapted from ref. 53 with permission. Copyright 2023 Wiley-VCH. | ||
6. Aluminium exhibits electrochemical alloying with lithium, forming β-LiAl, when employed as a current collector for the anode in lithium-ion batteries. However, when utilised as a current collector on the anode side of potassium-ion batteries, aluminium does not undergo the same electrochemical alloying reaction.44 As a result, aluminium foil can be utilised as the current collector for the anode in potassium-ion batteries, eliminating the need for expensive copper foils used in the anode side of lithium-ion batteries. This utilisation of low-cost aluminium solely as the current collector in the anode side of potassium-ion batteries not only reduces costs but also decreases the weight of large battery packs. Notably, aluminium boasts a significantly lower density (2.7 g cm−3) in comparison to copper (8.96 g cm−3).
7. In contrast to lithium atoms, potassium atoms exhibit a broader range of coordination geometries with elements like oxygen. This characteristic gives rise to a greater diversity of potassium-based compounds, particularly inorganic compounds, offering a wider array of functionalities compared to lithium-based compounds. Furthermore, potassium-based layered oxides can accommodate potassium-deficient or non-stoichiometric compositions whilst maintaining stability,54–103 which is not commonly observed in lithium-based layered oxides. For instance, the commercially utilised LiCoO2 layered oxide cathode material cannot exist in a stable lithium-deficient form, such as Li0.5CoO2. However, related potassium-deficient layered oxides, such as K0.3CoO2, K0.5CoO2, K0.67CoO2, and so on, can be formed as stable materials. This implies that potassium-based compounds offer a vast landscape of battery materials with diverse electrochemistries.
1.2 Objective and scope of the perspective
Due to the aforementioned advantages, research on potassium-ion batteries (KIBs) has gained significant traction in recent years. Given the larger size of K+ ions in comparison to both Na+ and Li+, considerable research efforts have been dedicated to designing electrode materials that can efficiently accommodate the reversible extraction/insertion of these larger K+ ions. This Perspective presents a comprehensive overview of the progress in non-aqueous KIBs, with a primary focus on high-voltage cathode materials, whilst addressing the challenges that necessitate resolution. Perspectives and directions for the development of high-performance cathode materials in KIBs are also provided, drawing insights from relevant literature and our collective expertise. Additionally, we summarise effective strategies to enhance the electrochemical performance of both electrodes and electrolytes. Ultimately, we deliberate on the prospects for achieving high-performance non-aqueous KIBs. The Perspective aims to offer a holistic view and valuable guidance for researchers engaged in the pursuit of materials for high-voltage (and consequently, high energy density) non-aqueous KIBs. It is worth noting that, as of the present, there exists no universally standardised abbreviation for “potassium-ion battery” in the literature. Various publications have employed either “PIB” or “KIB” as the abbreviated form for “potassium-ion battery”. Given the substantial volume of research papers adopting the abbreviation “KIB” across numerous scientific databases, in this Perspective, we shall abbreviate “potassium-ion battery” as “KIB”.2 High performance cathode materials
Cathode materials for KIBs can be classified broadly into four main groups: (i) Prussian analogues,24–29 (ii) organic compounds, (iii) polyanion-based compounds, and (iv) layered oxides and chalcogenides.104–109 In addition, perovskite compounds (such as K(Mn0.95Ni0.05)F3), conversion compounds (such as CuSO4) and tunnel-structured compounds (such as cryptomelane-type KMn8O16) have emerged,110–113albeit less explored. Fig. 6 illustrates the average voltages attained by various cathode materials, along with their theoretical specific capacities. Hereafter, we will provide a concise summary of the advancements made in the development of high-voltage cathode materials for KIBs, with a particular emphasis on the strengths and weaknesses observed within each category of developed cathode materials.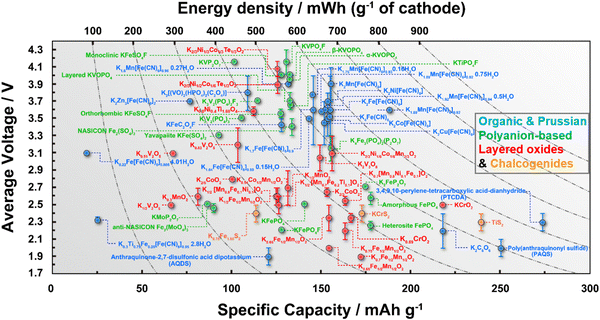 | ||
| Fig. 6 Ragone plots of representative cathode materials for potassium ion batteries. Figure adapted from ref. 114 with permission. | ||
2.1 Prussian analogues
Potassium-based Prussian analogues are hexacyanometallate compounds,24–34 comprising cyanide-bridged coordination polymers formed by reacting transition metal cyanometallates with potassium ions. These compounds possess open three-dimensional (3D) crystalline frameworks and can be represented by the chemical composition KaL[L′(CN)6]1−b·cH2O (0 ≤ a ≤ 2, 0 ≤ b < 1, c ≥ 0), where L and L′ represent various transition metals like Zn, Cu, Ni, Co, Fe, Cr, and Ti, or alkaline-earth metals such as Mg, coordinated to cyano groups. Both L and L′ are typically redox-active, and the fraction of [L′(CN)6] vacancies is denoted as b. By selecting different combinations of L and L′, along with the concentration of interstitial potassium ions, the electrochemistry of Prussian analogues can be tailored for various performance metrics. The structure of a representative hexacyanoferrate, such as K2MnFe(CN)6, is shown in Fig. 7a, demonstrating an open channel structural framework where Fe and Mn are coordinated with C and N, respectively. The open 3D framework structure of Prussian analogues facilitates the easy diffusion of large K-ions, resulting in fast rate capabilities. These compounds have drawn attention owing to their scalable synthesis, high redox potential, and high theoretical capacity. For instance, K2FeFe(CN)6 exhibits a theoretical capacity of 155 mA h g−1, based on the following two-electron reactions:| K2Fe2+[Fe2+(CN)6] ↔ KFe3+[Fe2+(CN)6] + K++ e−, | (1a) |
| KFe3+[Fe2+(CN)6] ↔ Fe3+[Fe3+(CN)6] + K++ e−. | (1b) |
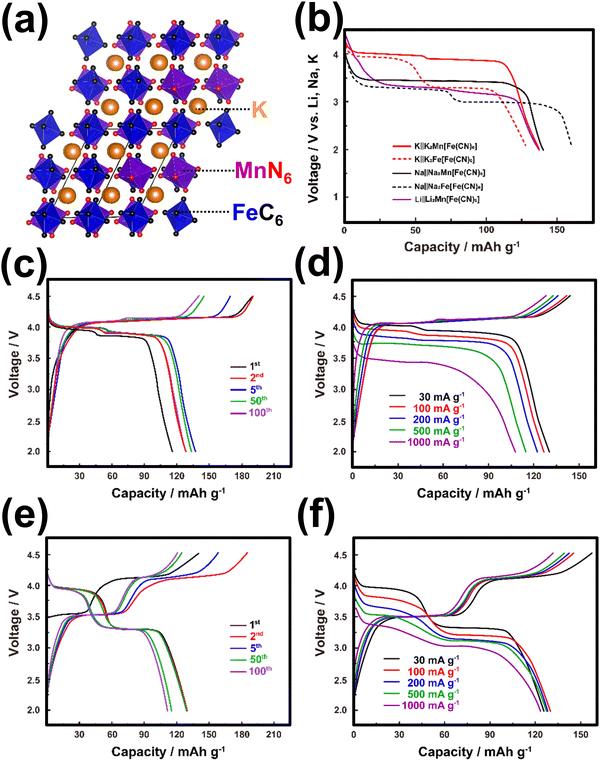 | ||
| Fig. 7 (a) Crystal structural framework of K2MnFe(CN)6. (b) Voltage comparison of Prussian analogues when cycled in K, Na and Li half-cells. (c) Voltage-capacity profiles of K2MnFe(CN)6 and (d) corresponding rate performance. (e) Voltage-capacity profiles of K2FeFe(CN)6 and (f) corresponding rate performance. Figure (b) reproduced with permission from ref. 42. Copyright 2020 American Chemical Society. Figures (c)–(f) reproduced with permission from ref. 115. Copyright 2017 Royal Society of Chemistry. | ||
A significant majority of Prussian analogues, such as K2MnFe(CN)6,115 K2FeFe(CN)6,24,26,115–118 K2CoFe(CN)6,24 K2NiFe(CN)6,24,119 K4Fe(CN)6,120 and others, demonstrate notably high voltages, reaching up to 4 V. These voltages surpass those achieved by their lithium- and sodium-containing counterparts, such as Li2MnFe(CN)6, Na2MnFe(CN)6, Li2FeFe(CN)6 and Na2FeFe(CN)6 (Fig. 7b).121–125 Additionally, Prussian blue analogues exhibit theoretically high gravimetric capacities, as depicted in Fig. 6, which have been experimentally realised. Consequently, Prussian analogues hold immense promise as cathode materials, particularly for high-energy-density KIBs. K2Mn[Fe(CN)6] and K2Fe[Fe(CN)6] demonstrate remarkable electrochemical performance within the Prussian analogues (as shown in Fig. 7c–f). This is attributed to the elevated Mn3+/Mn2+ redox potential, which contributes to the superior characteristics of K2Mn[Fe(CN)6] and its related derivatives. These materials showcase impressive capacities, exceeding 130 mA h g−1, and maintain high average discharge voltages in the range of 3.8–3.9 V versus K/K+. Consequently, they exhibit a relatively high energy density comparable to conventional cathode materials for lithium-ion batteries, such as LiCoO2. However, it should be noted that the presence of high-spin Mn3+ (which is Jahn–Teller active) in K2Mn[Fe(CN)6] introduces structural distortions during successive K+ extraction/insertion cycles. This phenomenon results in K2Mn[Fe(CN)6] experiencing multiple phase transitions, as evidenced by the following transformations: K2Mn2+[Fe2+(CN)6] (monoclinic phase) ↔ KMn2+[Fe3+(CN)6] (cubic phase) ↔ Mn3+[Fe3+(CN)6] (tetragonal phase).42 These structural changes compromise the cyclability of the material. In contrast, K2Fe[Fe(CN)6] and its related derivatives, characterised by high-spin Fe2+ and low-spin Fe3+, exhibit minimal Jahn–Teller distortion. As a consequence, they display robust cycling stability, which is highly desirable for practical applications.
Further advantages of Prussian blue analogues include facile synthesis, non-toxicity, and rapid rate capability.42 Moreover, stable electrochemical performance in non-aqueous electrolytes can be achieved with Prussian analogues, provided that their water content, defects, and particle morphologies are carefully controlled.
However, similar to organic compounds (discussed subsequently), Prussian analogues also exhibit low material densities, typically below 2 g cm−3, which significantly limits their achievable volumetric capacity. Whilst improving their volumetric capacities presents challenges, Prussian analogues, along with organic compounds, may find valuable applications in energy storage where volumetric energy density is of lesser importance. Furthermore, a significant scholarly effort is currently dedicated to addressing a dual set of concerns: water and [Fe(CN)6]4− vacancies. Whilst the intricate role of water in the electrochemical performance of Prussian analogues is well acknowledged, [Fe(CN)6]4− vacancies are believed to exert a deleterious impact on their maximal capacity and structural integrity. This is attributed to their reduction of potassium occlusion capacity and the availability of redox-active Fe2+. To date, the most outstanding performers amongst Prussian blue cathode analogues are those characterised by particles on the nanometric scale (nanoparticles), coupled with minimised [Fe(CN)6]4− vacancies.126
On a different note, the safety considerations, encompassing aspects such as toxicity and thermal stability, remain inadequately understood. It can be contended that significant thermal runaways and the liberation of O2 gas are unlikely to occur in the utilisation of Prussian blue analogues (such as K2MnFe(CN)6) within large-scale battery configurations, given their sole incorporation of cyanide ligands. Nonetheless, the decomposition of Prussian blue analogues does lead to the formation of cyanogen and hydrogen cyanide as byproducts.126 Therefore, a more comprehensive inquiry into their safety and thermal stability becomes imperative.
2.2 Organic compounds
Organic-based cathode materials have attracted substantial interest due to their cost-effectiveness and environmentally friendly nature.42,138–140 These materials consist of Earth-abundant elements, such as hydrogen, carbon, oxygen, and sulphur, which have a minimal environmental impact.139 Moreover, the relatively low atomic weight of the constituent elements bestows organic moieties with theoretically high gravimetric capacities. Additionally, organic compounds possess flexible molecular structures, enabling them to easily accommodate the facile and reversible insertion/extraction of large potassium-ions, thus facilitating fast potassium-ion kinetics.141 Furthermore, the tunability of organic molecules allows for easy functionalisation to adjust their redox electrochemistry, making them versatile for various battery applications. Furthermore, the capability of reversibly inserting/extracting not only cations but also anions in organic molecules extends their potential applications to other high-voltage energy storage systems, such as potassium dual-ion batteries.134A diverse array of organic compounds has been systematically designed and effectively demonstrated to enable the reversible insertion/extraction of K-ions, showcasing considerably higher capacities when compared to cathode materials like layered sulphides, layered oxides, polyanion-based compounds, and Prussian analogues. Organic compounds, including poly(anthraquinonyl sulphide),21 perylene-3,4,9,10-tetracarboxylic dianhydride, poly(pentacenetetrone sulphide),127etc.,141,142 have been extensively investigated as high-capacity cathode materials, showing promise as contenders for flexible KIBs with stable cycle performance. However, most reported organic materials have low average voltages, typically below 3 V, leading to reduced specific energy densities. Imides, anhydrides, quinones, and pyrazinyl compounds are pursued for enabling K+ insertion/extraction through n-type reactions (Fig. 8). These materials accept electrons from electrolytes whilst facilitating K+ cation insertion. However, their n-type redox reactions usually occur at voltages below 3 V, compromising the energy densities of KIBs. To enhance electrochemical performance, molecular fine-tuning is considered to elevate the voltages of organic moieties that undergo n-type redox reactions. It is worth noting that not all organic electrode materials solely undergo K+ insertion/extraction through n-type redox reactions.
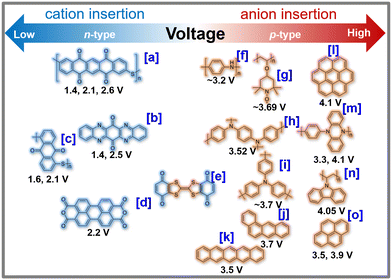 | ||
| Fig. 8 Voltage attained from representative organic cathode materials for potassium-ion batteries. For the sake of clarity, we hereby present the chemical names of each organic moiety as follows. [a] PPTS (poly(pentacenetetrone sulphide)),127 [b] TAPQ (5,7,12,14-tetraaza-6,13-pentacenequinone),128 [c] PAQS (poly(anthraquinonyl sulphide)),21 [d] PTCDA (3,4,9,10-perylene-tetracarboxylic acid dianhydride),23 [e] QTTFQ ((2,3)-(6,7)-bis(1,4-dioxo-1,4-dihydrobenzo)tetrathiafulvalene),129 [f] PANI (polyaniline),130 [g] PTMA (poly(2,2,6,6-tetramethylpiperidinyloxy methacrylate)),131,132 [h] PDPPD (poly(N,N′-diphenyl-p-phenylenediamine)),133 [i] PTPAn (polytriphenylamine),134 [j] p-DPPZ (poly(N-phenyl-5,10-dihydrophenazine)),135 and [k] PVK (poly(N-vinylcarbazole)).136 Figures reproduced with permission from ref. 137. Copyright 2021 Royal Society of Chemistry. | ||
Other organic materials can exhibit high voltages through p-type reactions, losing electrons alongside anion insertion/extraction during battery operations. For example, conductive polymers147,148 like polyaniline and polypyrrole undergo p-type reactions, leading to higher voltages compared to organic materials that undergo n-type redox reactions. Amine-derived polymers (such as poly(N,N′-diphenyl-p-phenylenediamine) (PDPPD), polytriphenylamine (PTPA), poly(N-phenyl-5,10-dihydrophenazine) (p-DPPZ), etc.) exhibit significant potential as viable organic cathode materials, owing to their inherent capacity to potentially yield high specific capacities, coupled with favourable discharge potentials approximating ∼4 V, high (dis)charging rates, and good cyclability. Fig. 9 illustrates the electrochemical performance of polyarylamine poly(N-phenyl-5,10-dihydrophenazine) utilising a meticulously tailored electrolyte composition, resulting in the attainment of exemplary performance parameters.135 Notably, polyarylamine poly(N-phenyl-5,10-dihydrophenazine) exhibits high power and energy density concomitant with good cyclability, thereby establishing crucial foundational criteria warranting contemplation for its prospective practical applications. Polycyclic aromatic hydrocarbons (Fig. 10) have also been extensively investigated for their p-type reactions and show promise as high-voltage organic cathode materials for potassium dual-ion batteries. In fact, organic compounds such as coronene (Fig. 11a), polyaniline, etc., which function via the insertion of anions (e.g., ClO4−, PF6−, FSI−, TFSI−, etc.), demonstrate voltages reaching 4 V, positioning them as high-voltage cathode candidates for potassium dual-ion batteries. Furthermore, the abundance of polycyclic aromatic hydrocarbons, exemplified by compounds like coronene,137 and their utilisation in the scalable synthesis of carbonaceous anode materials, such as graphite, amplify the feasibility of high-voltage dual-ion batteries incorporating polycyclic aromatic hydrocarbons as cathodes coupled with graphite anodes. This innovative approach presents a promising avenue for the development of sustainable energy storage systems exclusively based on hydrocarbons, ushering in a greener and more environmentally conscious energy landscape.
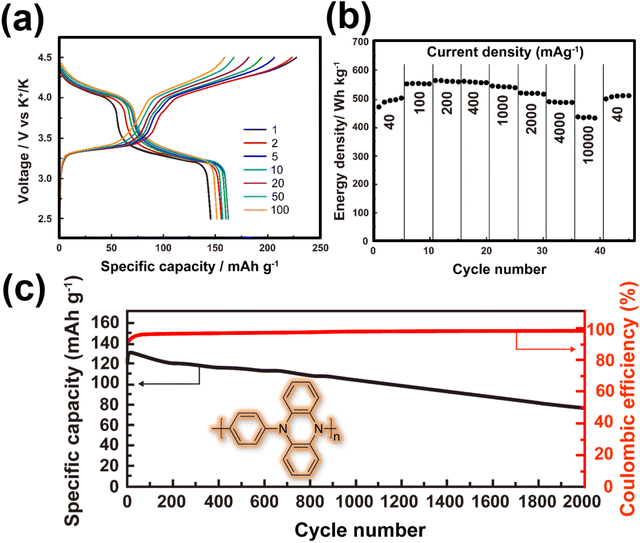 | ||
| Fig. 9 Electrochemical performance of poly(N-phenyl-5,10-dihydrophenazine) as a high-voltage and high-capacity cathode material for KIBs. (a) Voltage-capacity profiles at a current density of 200 mA g−1. The theoretical capacity for poly(N-phenyl-5,10-dihydrophenazine) is approximately 209 mA h g−1. (b) Energy density at various current densities. (c) Cycle performance at a current density of 2 A g−1. A highly concentrated electrolyte entailing 2.2 M KPF6 in diglyme solvent was used in a potassium half-cell setup. Figures reproduced and adapted with permission from ref. 135. Copyright 2019 American Chemical Society. | ||
 | ||
| Fig. 10 Representative polycyclic aromatic hydrocarbons (including supertriphenylene,143 superphenalene143,144 and circumcoronene145,146), which can operate at high voltages via the insertion/extraction of anions such as PF6−, BF4−, FSI−, TFSI−, etc. | ||
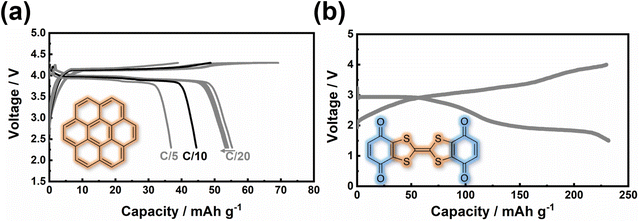 | ||
| Fig. 11 Electrochemical performance of (a) p-type coronene (C24H12) and (b) bipolar (2,3)-(6,7)-bis(1,4-dioxo-1,4-dihydrobenzo)tetrathiafulvalene (QTTFQ) as cathode materials for potassium-ion batteries. The theoretical capacity for C24H12 and QTTFQ is, respectively, 88 mA h g−1 and 441 mA h g−1. A current density of 40 mA g−1 was used in (b). Figures reproduced and adapted from ref. 129 and 137 with permission. Copyright 2019 and 2021 Royal Society of Chemistry. | ||
A significant drawback associated with the utilisation of a majority of organic compounds is their tendency to undergo dissolution into the electrolyte, necessitating the careful selection of compatible electrolytes (such as highly concentrated electrolytes or ionic liquids) and the adoption of special synthetic routes (e.g., oligomerisation or polymerisation).139 Furthermore, a majority of organic compounds tend to exhibit low electronic conductivity. To address this issue, nanosizing of organic compounds and the use of special synthetic routes (e.g., in situ polymerisation) can be envisaged to enhance their electronic conductivity.139 Additionally, hybrid materials based on the fusion of inorganic and organic components, such as K2[(VO)2(HPO4)2(C2O4)]149 and K2[(VOHPO4)2(C2O4)]150 metal–organic phosphates, have demonstrated not only high voltages (reaching up to 4 V) but also fast rate capabilities. Despite the fact that the theoretical capacities of these hybrid materials may be lower than those of pure organic compounds, their exceptional fast rate capabilities and high voltage render them as ideal cathode candidates for the development of high-performance 4 V-class KIBs. However, it is important to note that, similar to Prussian analogues, organic compounds also share the intrinsic drawback of having low tap density (material density), significantly impacting their volumetric energy densities.
Nonetheless, opportunities for improvement persist, particularly in the pursuit of organic molecules with precisely tailored structures that can be engineered into high-capacity cathode materials. An example of such promise (albeit necessitating caution in handling) appears to lie in trinitroaromatic salts, such as C6H2(NO2)3OK which present as nitro-rich organic cathode materials with elevated discharge capacity and voltage due to the strong delocalisation effect between aromatic rings and –NO2.151 Theoretical calculations predict that C6H2(NO2)3OK (potassium picrate) undergoes a 6-electron charge-transfer process, resulting in a high discharge capacity of 606 mA h g−1.151 Octahydroxytetraazopentacene, with a theoretical capacity of close to 520 mA h g−1, has been experimentally reported as a high-capacity organic material.152 Additionally, bipolar organic molecules like tetrathiafulvalene, which enable reversible insertion/extraction of both anions and K+ (e.g., TFSI−, FSI−, PF6−, etc.), can be further explored as high-capacity materials. Fig. 11b shows the electrochemical performance of (2,3)-(6,7)-bis(1,4-dioxo-1,4-dihydrobenzo)tetrathiafulvalene (QTTFQ) which operates via both the p-type and n-type reactions to attain high capacity.129 Moreover, a wide range of organic materials, including N-containing heteroatomic compounds, organosulphur compounds, quinone/phenoxide derivatives, carbonyl compounds, polymeric arylamines, and conductive polymers, hold potential for investigation to design cathode materials with both high capacity and high voltage.139,153–157 Furthermore, the pursuit of organic molecules with high rate capability and electronic conductivity can be anticipated, for instance, through the use of (i) conjugated polymer poly(quinones) with electron-withdrawing groups and (ii) π-conjugated aromatic compounds comprising heteroatoms (such as S, N, and O) with lone electron pairs.
2.3 Polyanion-based compounds
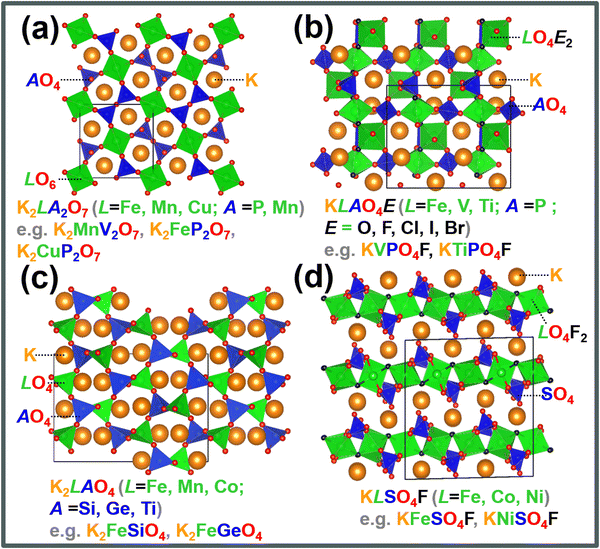
Polyanion-based materials share a similar open three-dimensional (3D) framework with Prussian analogues, characterised by metalloid or non-metal atoms coordinated with oxygen atoms to form polyhedral units.158 The polyhedral units embody a composition of (AO4)n−, with A representing elements such as Si, S, P, Ge, As, B, Mo, W, etc., and n varying from 2 to higher values. These (AO4)n− polyhedra intricately interlink with polyhedral units of transition metal atoms (LOa)n−, where ‘a’ represents an integer such as 4, 5, 6, and so forth, and ‘L’ denotes a transition metal like Ni, Co, Mn, Fe, etc. This arrangement generates voids within the structure, which serve as sites for accommodating or facilitating the movement of potassium ions. The 3D arrangement of K-ions with polyanion units reduces the effective K+–K+ repulsion strength, leading to an elevated working voltage.159 Polyanion-based compounds have undergone extensive investigation in both sodium and lithium-ion batteries, and their material compositions have served as a basis for designing polyanion cathode materials for KIBs. Fig. 12 shows crystal structures of representative polyanion cathode materials for potassium-ion batteries. The inherent open structure of polyanion-based compounds facilitates rapid diffusion of K+ ions and effectively accommodates the significant volume changes that occur during the insertion and extraction of large K-ions. Moreover, polyanion-based materials showcase remarkable thermal stability, making them exceptionally suitable as cathode materials for a wide range of battery applications, spanning from intermediate to high-temperature operations. Notably, they demonstrate theoretically high voltages, with some exceeding 4 V, along with high gravimetric capacities. The high voltage is attributed to a reduction in the covalency of the A–O bonds, a consequence of the pronounced covalent nature of the L–O bonds. This phenomenon (or what is referred to as ‘inductive effect’)160 is contingent upon the electronegativity of the transition metal ‘L’, leading to an augmentation in the redox voltage of the transition metal L ions. Additionally, their material density is relatively higher, generally surpassing 2.5 g cm−3 compared to organic compounds and Prussian analogues.Polyanion-based frameworks, exemplified by KFeSO4F,12–17 KCuSO4F, KVPO4F,18,19 KVOPO4,18 amongst others, boast higher material densities, typically ranging from 2.5 to 3.5 g cm−3 and exhibit high voltages, reaching up to 4 V and even surpassing 4 V in KIBs. Fig. 12 shows crystal structures of representative polyanion cathode materials for KIBs. Their synthesis protocols involve the use of cost-effective salt precursors and appear to be relatively straightforward. For instance, KFeSO4F and related compositions can be synthesised via a solid-state reaction at intermediate temperatures (300–400 °C), yielding various polymorphs: KF + FeSO4F → KFeSO4F.17 KVPO4F can be synthesised at slightly higher temperatures through the following reaction: KF + VPO4 → KVPO4F.159
KVPO4F, characterised by its specific crystal structure with an open framework of KTiOPO4-type (Fig. 12), allowing for rapid diffusion of K+ ions, has emerged as a widely investigated polyanion-based cathode material. It exhibits the ability to reversibly release nearly one K+ per formula unit, equating to approximately 131 mA h g−1, at an average potential of 4.02–4.3 V.18,159 Consequently, KVPO4F offers a material-level specific energy density of 527–563 W h kg−1, comparable to that of LiFePO4, a widely used polyanion-based cathode material for LIBs, which presents an energy density of approximately ∼580 W h kg−1. Related KVPO4F compositions such as (KVPO4O (KVOPO4), KVPO4Cl, etc.) display high redox voltages (Fig. 13). Fig. 14a and b show the voltage-capacity profiles of KVOPO4 and KVPO4F, respectively. The average voltages for KVPO4F and KVOPO4 are reported to be 3.95 V and 4.02 V respectively, ascribed to the V4+/V5+ and V3+/V4+ redox.18 An appreciable irreversible capacity is observed during the initial charging process of KVPO4F and KVOPO4 (Fig. 14a and b), which has been attributed to the substantial decomposition of the electrolyte solution.18
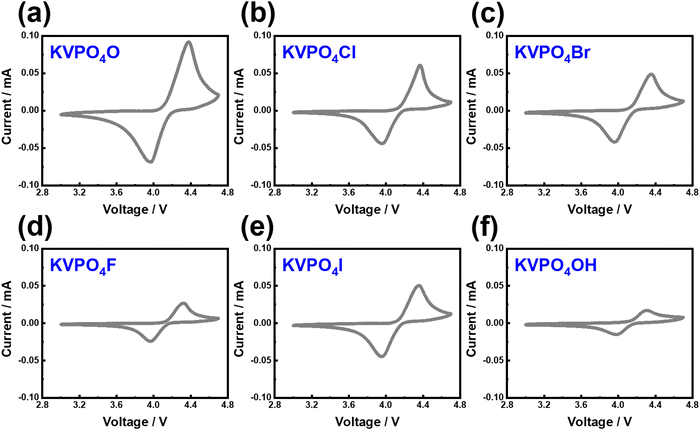 | ||
| Fig. 13 Electrochemical performance of representative potassium-based vanadium phosphates showing high redox voltages. Cyclic voltammograms of (a) KVOPO4, (b) KVPO4Cl, (c) KVPO4Br, (d) KVPO4F, (e) KVPO4I and (f) KVPO4OH. The measurements were taken at a scanning speed of 0.1 mV s−1 at a cut-off voltage of 3.0–4.6 V. Potassium half-cells were used with 0.5 M KTFSI in Pyr13TFSI ionic liquid as the electrolyte. Figures reproduced and adapted from ref. 114 with permission. | ||
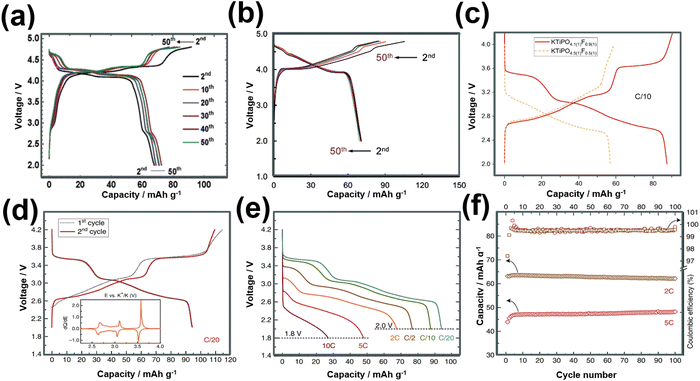 | ||
| Fig. 14 Voltage-capacity profiles of (a) KVOPO4 (α-polymorph) and (b) KVPO4F. The theoretical capacity of KVOPO4 and KVPO4F is 133.3 mA h g−1 and 131.4 mA h g−1, respectively. (c) Voltage profile comparisons of KTiPO4F and KTiPO4.5F0.5. (d) Voltage profiles of KTiPO4F at C/20 rate, (e) corresponding rate performance and (f) cycle performance at various current densities. The theoretical capacity of KTiPO4F and KTiPO4.5F0.5 is 133.4 mA h g−1 and 134.4 mA h g−1, respectively. Figures (a) and (b) reproduced from ref. 18 with permission. Copyright 2017 Royal Society of Chemistry. Figures (c), (d)–(f) reproduced with permission from ref. 180. Copyright 2020 Springer Nature. | ||
Researchers have been striving to narrow the energy density gap between KVPO4F and LiFePO4 by exploring compounds such as K3V2(PO4)2F3,161 K3V2(PO4)3,162K3V2O2(PO4)2F,163etc.,164–166 which exhibit more than one K+ per formula unit as high-capacity polyanion-based materials. Notably, the feasibility of extracting more than two K+ per formula unit in K3V2(PO4)2F3 has been demonstrated by harnessing the electrochemical activity of the third potassium in K3V2(PO4)2F3, leading to the formation of KxV2(PO4)2F3.161 Vanadium-based polyanion compounds are poised to attract significant interest in the forthcoming years due to vanadium's capability to adopt various oxidation states, with oxidation states of 3+, 4+, and 5+ (V3+, V4+ and V5+) being particularly common in battery materials. This unique property enables vanadium to engage in multi-redox reactions, facilitating the reversible extraction/insertion of more than one K+ ion, as demonstrated in compositions like K6V2(PO4)4.167 The Ragone plot in Fig. 6 showcases the average voltages achieved by various polyanion-based cathode compounds reported thus far for KIBs. Vanadium-based orthophosphates (e.g., K3V3(PO4)4,168 K3V3(PO4)4H2O,169 K3V2(PO4)3162,170), fluorophosphates (KV0.5Ti0.5PO4F,171 KVPO4F,18,159 K1.5VOPO4F0.5,172 K3V2O2(PO4)2F,163 KVPO4F0.5O0.5173), K3(VO)(HV2O3)(PO4)2(HPO4),174 oxyphosphates (KVOPO4),18,175 and pyrophosphates (KVP2O7)176,177 exhibit high voltages, reaching 4 V and even beyond, whilst maintaining fast rate capabilities and good cyclability. Mixed polyanion compounds, such as K6(VO)2(V2O3)2(PO4)4(P2O7),178,179 also exhibit high voltages surpassing 4 V.
Titanium-based fluorophosphates like KTiPO4F exhibit voltages of 3.6–3.7 V (Fig. 14c), which is remarkably high for titanium redox transitions (Ti3+/Ti4+), attributed to the KTiOPO4-type framework it adopts.180 It should be noted here that sustaining the Ti3+ oxidation state necessitates the maintenance of inert or reducing conditions at every stage encompassing synthesis and electrode material preparation of KTiPO4F. Nonetheless, KTiPO4F demonstrates intricate voltage-capacity profiles, characterised by three distinct reversible processes at 3.6, 3.1, and 2.7 V (Fig. 14d), with all three processes exhibiting flat voltage profiles that indicate a biphasic transition. The utilisation of carbon-coated KTiPO4F has been experimentally validated to deliver a noteworthy capacity of 94 mA h g−1 (equivalent to the extraction of 0.8 K+) whilst maintaining high cycle stability at a rate of C/20 along with good rate performance (Fig. 14e and f). This exceptional performance is attributed to the remarkably elevated potential for the Ti4+/Ti3+ redox activity, which has been hypothesised to originate from a synergistic interplay between the material's structural attributes (e.g., electrostatic interactions, vacancy/charge ordering) and chemical properties (e.g., inductive effect) specific to the KTiOPO4-type KTiPO4F.181,182 Such distinctive features position KTiPO4F as a promising high-energy-density cathode material candidate. Whilst the related Mn- and Fe-based compositions of the phosphates may show inferior voltages, with some (e.g., K2FeP2O7, K2MnP2O7, etc.)165 being deemed electrochemically inactive, their sulphate counterparts, such as KFeSO4F, demonstrate high voltages (>3.5 V) with both good rate capabilities and cycle stability (Fig. 15), positioning them not only as cost-effective but also as high-voltage cathode candidates for sustainable and high-energy-density KIBs.
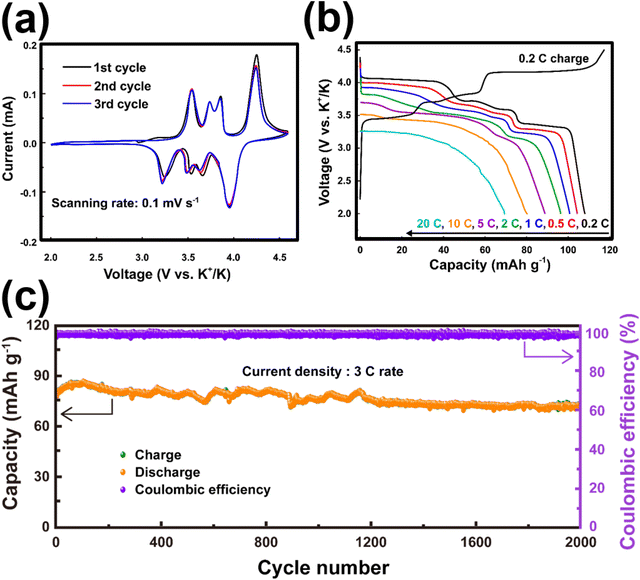 | ||
| Fig. 15 Electrochemical performance of KFeSO4F. (a) Cyclic voltammogram of KFeSO4F taken at a scanning rate of 0.1 mV s−1. (b) Rate performance of KFeSO4F and (c) cycle performance at a current density commensurate to 3C. The theoretical capacity for KFeSO4F is 127.6 mA h g−1 (1C = 127.6 mA g−1). Figures reproduced from ref. 14 with permission. Copyright 2022 Elsevier. | ||
A significant challenge faced with polyanion-based compounds lies in their intrinsically low electronic conductivities. Addressing this limitation requires the incorporation of significant quantities of conductive materials, such as carbon, to fully harness and optimise their electrochemical performance. However, this practice can lead to reduced volumetric energy densities. Many polyanion-based cathode materials investigated by us and other research groups, such as langbeinite-type K2M2(SO4)3 (M = Cu, Co, Ni, Mn, Fe), KM(PO3)3, K2MV2O7 (M = Mn, Co), K2MP2O7,165 KMP2O7 (M = Fe, Mn, Ti, Cr, V), K2VOP2O7,183 KMPO4, KMnVO4, fedotovite-type K2Cu3O(SO4)3,184 KFePO4F, K3Fe2(PO4)3, buetschliite-type K2Mn(SeO3)2, yavapaiite-type KFe(SO4)2, KFeGeO4, KFeSiO4, amongst others, require both nanosizing and carbon nanocoating to fully exploit their electrochemical behaviour; otherwise, many of them may appear essentially ‘electrochemically inactive’ or behave as insulators. Nanosizing, carbon-coating, and doping have also been utilised to enhance the electronic conductivity of polyanion-based compounds such as KFePO4,185 K3V(PO4)2,186 K3V2(PO4)3,162,187 KFe0.95Mg0.05SO4F,16 K3−xRbxV2(PO4)3 (x = 0.03, 0.05 and 0.07),188etc. As illustrated in Fig. 6, polyanionic compounds typically exhibit lower theoretical gravimetric capacities than Prussian analogues, which may compromise their energy density. However, the high voltages demonstrated by polyanion-based compounds can potentially counterbalance their intrinsic low electronic conductivities. As a result, a careful material screening process becomes essential, with a focus on identifying polyanion-based compounds that exhibit not only high voltages (>4.5 V) but also high gravimetric capacities (>130 mA h g−1). Fig. 16 showcases the projected voltages in polyanion cathode materials, underpinned by computational screening, alongside their corresponding theoretical capacities. The realm of theoretical computations unveils a plethora of polyanion compounds that manifest both high voltages and high theoretical capacities.167,176,189–192 Additionally, electrochemical ion-exchange utilising sodium- or rubidium-based polyanion compounds can serve as another strategy for obtaining new potassium-based polyanion compounds with favourable electrochemical properties, as demonstrated in the design of K3V2(PO4)2F3.161
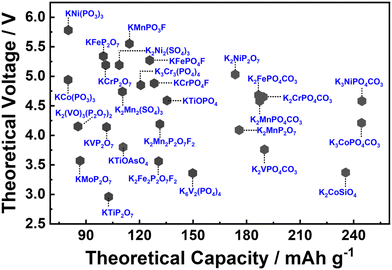 | ||
| Fig. 16 Theoretical voltages calculated for polyanion compounds for potassium-ion batteries. Graph plotted based on the data derived from ref. 167, 176 and 189–192. | ||
Moreover, most polyanionic compounds tend to exhibit lower theoretical capacities compared to both Prussian blue analogues and organic compounds, primarily due to their heavy polyanion formula weights. Nonetheless, ample opportunities remain to explore high-capacity polyanion-based materials, such as pyrophosphates (e.g., K2MnP2O7,165 K2FeP2O7,165 K2NiP2O7, K2CoP2O7, K2MnP2O7F2,167etc.), orthophosphates (e.g., K6V2(PO4)4167), silicates (including various emerging slow-release fertilisers,193,194e.g., K2FeSiO4 (Fig. 17a), K2MnSiO4, etc.), vanadates (e.g., K2MnV2O7,195 K2CoV2O7196), borates (e.g., K2Fe2B2O7 (isostructural with K2Al2B2O7),197,198 KFeBO3, KMnBO3, etc.), and sulphates (e.g., K2Fe(SO4)2), most of which involve two K+ ions per formula unit, resulting in a K-to-transition metal content ratio of ≥2.
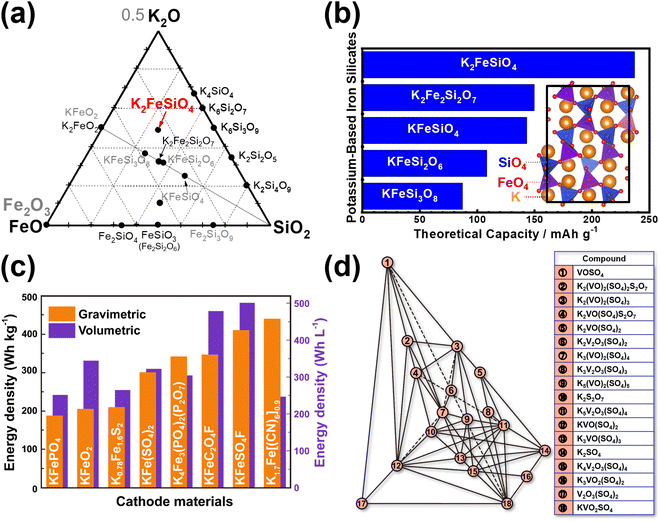 | ||
| Fig. 17 Exploration of new polyanion-based cathode compound for potassium-ion batteries. (a) Ternary phase diagram of K2O–FeO(Fe2O3)–SiO2 showing new silicate-based polyanion compounds (such as K2FeSiO4). (b) Theoretical specific capacities of new iron-based silicates that can be explored as high-capacity cathode materials. Inset shows the crystal structure of cristobalite-type K2FeSiO4. (c) Plot showing gravimetric and volumetric energy densities attainable with iron-based cathode materials reported in literature. (d) K2O–V2O5–V2O4–SO3 ternary phase diagram unveiling new vanadium-based sulphates that can be assessed as high-voltage cathode materials. (c) Reproduced and adapted from ref. 14 with permission. Copyright 2022 Elsevier. (d) Adapted from ref. 199 and 200 with permission. Copyright 2007 Springer Nature. | ||
Iron-based polyanion compounds, exemplified by K2FeSiO4, exhibit significant theoretical capacities (Fig. 17b) and thus hold promise for integration into the composition of iron-based materials tailored for utilisation as high-energy-density cathode material systems (Fig. 17c). Whilst we acknowledge the apparent impracticality of vanadium-based polyanionic compounds due to vanadium's toxicity, it is worth noting that delving into unconventional vanadium-based materials can furnish valuable insights into maximising the potential of cathode materials for KIBs. Our preliminary exploration of the K2O–V2O5–V2O4–SO3 phase system (depicted in Fig. 17d)199,200 has brought to light a suite of novel vanadium-based polyanion cathode materials, exemplified by compounds like K2VO(SO4)2,201 KV(SO4)2202 and V2O3(SO4)2.203 These materials exhibit noteworthy attributes, including high voltages (up to 4 V) and high theoretical capacities, opening vistas for further investigation. The lability of the polyanion moiety also contributes to the polymorphism exhibited by polyanionic compounds such as KFeSO4F17 and K2CuP2O7,204,205 leading to a rich variety of crystal structures with distinct electrochemical performance. Notably, polyanionic compounds offer a diverse array of crystal structures compared to Prussian analogues, thereby providing tunable electrochemistry that can be exploited to optimise both their volumetric energy densities and rate capabilities. Furthermore, the electrochemistry of polyanion compounds, such as pyrophosphates,176 has revealed captivating optical characteristics. For instance, the extraction of potassium from compounds like KMoP2O7, KVP2O7, and KTiP2O7 induces noticeable colour shifts,176 suggesting potential applications in electrochromic devices.
The broad class of fluorosulphates and fluorophosphates holds significant potential for further exploration. Preliminary investigations have already demonstrated the feasibility of designing new fluorosulphates through solid-state reactions involving inexpensive potassium salts, such as KCl, KBr, KI, K2SO4, KOH, and others. For instance, derivative compositions of new fluorosulphates and fluorophosphates can be designed via the following reactions at intermediate temperatures: KA + FeSO4 → KFeSO4A (A = Cl, Br, I, OH); KA + NiSO4 → KNiSO4A (A = Cl, Br, I, OH); KA + CoSO4 → KCoSO4A (A = Cl, Br, I, OH); KA + NiSO4 → KNiSO4A (A = Cl, Br, I, OH); KA + FePO4 → KFePO4A (A = Cl, Br, I, OH); KA + VPO4 → KVPO4A (A = Cl, Br, I, OH); KA + VOSO4 → KVOSO4A (A = F, Cl, Br, I, OH); K2SO4 + VOSO4 → K2VO(SO4)2; KF + VOSO4 → KVO(SO4)F. Additionally, oxyphosphates, such as KVOPO4, can be synthesised through carbothermal reduction processes at intermediate temperatures: K2CO3 + V5+OPO4 + C → KV4+OPO4.
Furthermore, the exploration of mixed polyanion compounds, replicating chemical compositions like KFePO4NO3,206 K4Fe3(PO4)2(P2O7),207 K4[Mn2Fe](PO4)2(P2O7),208 K2M3(SO4)3(OH)2(H2O)2 (M = Co, Fe), KFe3(SO4)2(OH)6,209 (K(Mo2PO6))(P2O7)2,210 K3(VO)(V2O3)(PO4)2(HPO4),211 K3(VO)(HV2O3)(PO4)2(HPO4),211 K2Mn3(VO4)2(CO3),212etc., presents an opportunity to further enhance the electrochemical performance of polyanion-based materials. The broad class of mixed polyanion compounds encompasses polyoxyanion groups such as PO4–CO3, C2O4–SO4, PO4–NO3, SO4–OH, PO4–SO4, SO4–S2O3, BO3–B2O5, PO4–P2O7, and others,213 offering ample room for exploration and optimisation. In addition, the exploration of high- and medium-entropy polyanion compounds (e.g., K3Mn2/3V2/3Ti2/3(PO4)3) can provide a pathway for designing new polyanion frameworks with enhanced rate capabilities, as demonstrated for their Na-based analogues.214
2.4 Layered oxides and chalcogenides
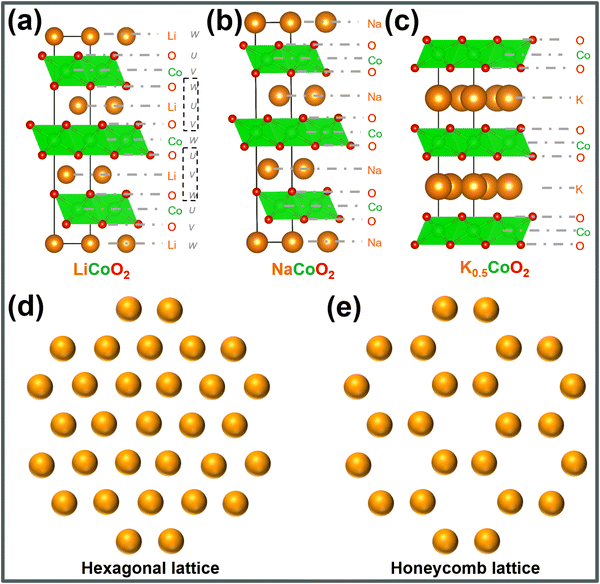
Layered oxides, characterised by the composition AxLO2 (where A represents an alkali metal, L denotes primarily 3d transition metals, and 0 < x < 4/3), have been subject to extensive investigation as potential cathode materials for both lithium-ion and sodium-ion batteries. This interest stems from the initial demonstration of electrochemical Li and Na extraction/insertion in the case of layered LiCoO2 and NaCoO2 back in 1980.215,216 Notably, potassium-based layered oxides, such as KxMO2, were already a subject of exploration during the 1960s and 1970s.217,218 Despite the discovery of these potassium-based layered oxides, it was not until 2016 that the electrochemical K-ion extraction/insertion in non-aqueous K cells was officially reported.219Potassium-based layered oxide cathodes, emulating compositions found in sodium- and lithium-ion batteries, have been actively pursued as potential cathode materials for KIBs.62,220–230 Typically, these layered oxides, adopting the general composition of AxLO2, consist of alkali metal atoms (A) arranged between transition metal oxide slab layers (LO6 octahedra). As a result, various stacking sequences are observed (simply classifiable using the Hagenmuller–Delmas notation', or more elaborately using the face-centred cubic (FCC) notation), primarily influenced by (i) the type of alkali metal atom or transition metal present within the slab and (ii) the specific synthesis conditions. Due to the larger ionic radius of K+ compared to the transition-metal ions in the slab, defects involving the mixing of transition-metal ions and K+ are averted. This stands in stark contrast to Li-based layered oxides, where defects arise due to the similar ionic radius of Li+ and transition metal ions, leading to their mixing. Moreover, the larger ionic radius of K+ fosters weaker interlayer bonds, thereby facilitating facile 2D cationic diffusion during battery operation. This characteristic enables efficient movement of potassium ions within the layered framework.
Fig. 18a–c depict the crystal structures of archetypal layered frameworks (namely, LiCoO2, NaCoO2 and K0.5CoO2) which can be categorised within the Delmas notation as O3-, P3-, and P2-type, respectively. The numeric labels (1, 2, 3,…) correspond to the recurrent transition metal slabs that are oriented perpendicular to the layers of alkali metal within the unit cell. In contrast, the designations “P” or “O” are indicative of the alkali metal atoms' coordination with oxygen ligands, manifesting either as prismatic or octahedral arrangements. Table 1 shows representative layered cathode oxides classified in Delmas notation, for brevity. Within the framework of the FCC notation, the three-dimensional (3D) lattice configuration, exemplified for instance by LiCoO2, manifests as an ordered UOVCoWOULiVOWCoUOVLiWOUCoVOWLiUO sequence. This pattern iterates consistently to give rise to the layered structure of the material. The intrinsic value of adopting the FCC notation resides in its capacity to inherently encode the coordination phenomena between oxygen species and cationic entities. Concurrently, this notation provides an implicit exposition of information pertaining to the spatial arrangement of ions in the two-dimensional (2D) lattice layers.235 Furthermore, the predominant occupation sites for alkali cations encompass either hexagonal lattices or honeycomb lattices, as schematically shown in Fig. 18d and e. The latter configuration is prominently observed within alkali-deficient or alkali-poor layered frameworks.
| Delmas' notation | Layered compounds |
|---|---|
| O3-type | KCrS2, KCrO2 |
| P2-type | K2Ni2TeO6 (K2/3Ni2/3Te1/3O2), K0.6CoO2, K0.5Mn0.7Fe0.2Ti0.1O2, K0.6Mn0.8Ni0.1Ti0.1O2, |
| K2/3Ni1/3Co1/3Te1/3O2 (K2NiCoTeO6), K0.75[Ni1/3Mn2/3]O2, K0.75[Mn0.8Ni0.1Fe0.1]O2, | |
| K0.83[Ni0.05Mn0.95]O2, K0.21MnO2, K0.7[Cr0.85Sb0.15]O2, K0.76Fe0.2Mg0.1Mn0.7O2, | |
| K5/9Mn7/9Ti2/9O2, K0.44Ni0.22Mn0.78O2, K0.75[Ni1/3Mn2/3]O2, K0.83[Ni0.05Mn0.95]O2, | |
| K0.6Co0.67Mn0.33O2, K0.6Co0.66Mn0.17Ni0.17O2, K0.41CoO2, K0.65Fe0.5Mn0.5O2, | |
| K0.56Ni0.52Sb0.48O2, K0.86Co0.62Sb0.38O2 | |
| P3-type | K2Ni2TeO6, K0.5Mn0.8Co0.1Ni0.1O2, K0.5Mn0.7Ni0.3O2, K0.6Cr0.6Ti0.4O2, K0.45Mn0.5Co0.5O2 |
| K0.70[Cr0.86Sb0.14]O2, K0.5MnO2, K0.69CrO2, K0.54[Co0.5Mn0.5]O2, K0.5[Mn0.8Fe0.1Ni0.1]O2, | |
| K1/2Mn5/6Mg1/12Ni1/12O2, K0.45Mn1−xFexO2 (x = 0, 0.1, 0.2, 0.3, 0.4 and 0.5), K0.8CrO2, | |
| K0.5[Ni0.1Mn0.9]O2, K0.5[Mn0.85Ni0.1Co0.05]O2, K0.4Fe0.1Mn0.8Ti0.1O2, K0.45Co1/12Mg1/12Mn5/6O2, | |
| K0.45Mn0.9Mg0.1O2, K0.39CrO2, K0.23MnO2, K0.54Mn0.78Mg0.22O2, K0.45Ni0.1Fe0.1Mn0.8O2, | |
| KxMn0.7Ni0.3O2 (x = 0.4–0.7), K0.4Fe0.1Mn0.8Ti0.1O2, K0.5Mn0.7Co0.2Fe0.1O2, | |
| K0.5Mn0.72Ni0.15Co0.13O2, K0.45Mn0.9Al0.1O2, K0.67Mn0.83Ni0.17O2, K0.5Mg0.15[Mn0.8Mg0.05]O2, | |
| K0.45Ni0.1Co0.1Mg0.05Mn0.75O2, K0.45Ni0.1Co0.1Al0.05Mn0.75O2, K0.48Mn0.4Co0.6O2 | |
| K0.45Rb0.05Mn0.85Mg0.15O2, K0.35Mn0.8Fe0.1Cu0.1O2, K0.5Mn0.8Fe0.2O2, K0.3Mn0.95Co0.05O2 | |
| K0.5Ni0.5Sb0.5O2, K0.5Co0.5Sb0.5O2, K0.5Mn0.67Fe0.33O1.95N0.05 | |
Layered transition metal oxides, such as KxCrO2 (x = 0.69, 0.8, and 1),67,78,232 KxMnO2 (x = 0.3, 0.5 and 0.7),85,236 KxCoO2 (x = 0.44 and 0.6),65,66 K0.48Mn0.4Co0.6O2,237 KxFe0.5Mn0.5O2 (x = 0.45, 0.65 and 0.7),68,238 K0.45Mn0.5Co0.5O2,60 K0.5Mn0.8Co0.1Ni0.1O2,55 K0.67Ni0.17Co0.17Mn0.66O2,239 K1.39Mn3O6,240 KxV2O5,68,241–246 K2V3O8,247 K0.72Li0.27Ni0.6Co0.2Mn0.2O2,248 K0.7Fe0.05Co0.1Mn0.75Ni0.05V0.05O2,225etc., as well as layered chalcogenides (e.g., KCrS2,231 Cu0.11TiSe2 superconductor249 and K0.78Fe1.60S2250), are considered promising cathode candidates owing to their theoretically high energy density and two-dimensional K+ diffusion pathways. However, the large size of K+ ions leads to increased K+–K+ electrostatic repulsion within the layered structure, resulting in multiple phase transitions and low operating voltages.64Fig. 19a shows the voltage profiles of LiCoO2, Na2/3CoO2 and K0.41CoO2.66 Layered oxides exhibit voltage profiles with a staircase-like pattern, the amplitude of which is contingent upon the separation distance between the layers. The interlayer spacing is subject to modulation by the size of the alkali atom intercalated within the slab. As illustrated in Fig. 19a, the inclusion of potassium atoms with substantial atomic radii within KxCoO2 results in increased interlayer distances, consequently impacting the ionicities of the transition metal constituents, thereby exerting a discernible influence upon the redox potentials. Moreover, vacancies are created or annihilated in the course of alkali ion intercalation or de-intercalation processes, thereby instigating a reconfiguration of alkali atom coordination within the layered framework. Illustrated in Fig. 19b are the distinct phases of transitions (in Delmas' notation) in representative layered oxides that entail alterations in the manner by which alkali metal atoms are coordinated with the oxygen atoms belonging to the adjacent transition metal slabs. These multiple transitions transpire as a consequence of fluctuations in the alkali atom content. Fig. 19c delineates the voltage profiles exhibited by K0.5MnO2, illuminating the nature of phase transitions inherent in the course of K+ intercalation and de-intercalation processes. The sequence of phase transformations during the K+ de-intercalation or extraction process of K0.5MnO2 encompasses an initial P3 to O3 biphasic transition, subsequently progressing to another biphasic transition from O3 to a heretofore uncharacterised phase (designated as “X”). Computational results further predict the unidentified phase to assume an O1 configuration. X-ray diffraction investigations, as depicted in Fig. 19d, unveil the reversibility of the phase evolution during K+ intercalation or insertion processes, signifying an absence of supplementary phase transitions. This observation implies a reversible intercalation and deintercalation process of potassium in K0.5MnO2.
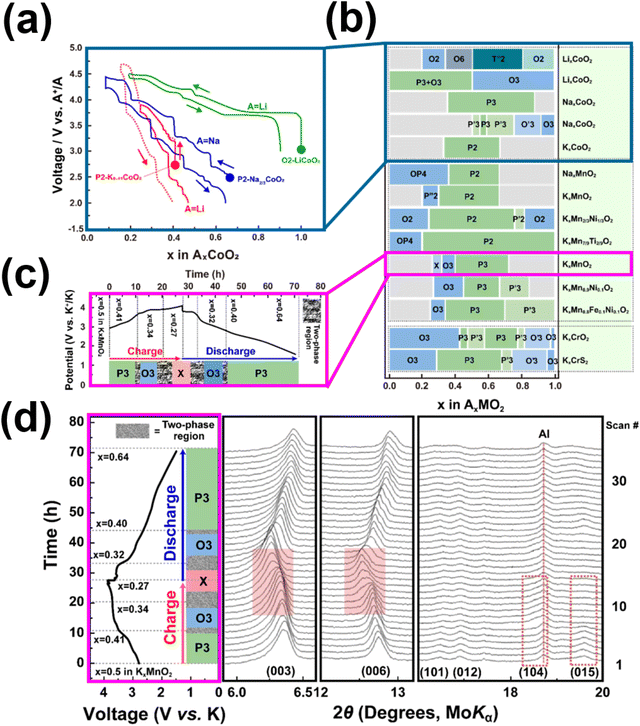 | ||
| Fig. 19 (a) Voltage profiles comparison of LiCoO2, Na2/3CoO2 and K0.41CoO2. (b) Phase transition in the stacking order of the slabs noted in layered oxides and sulphides during alkali-ion extraction. (c) Structural changes during K+ extraction/insertion process of K0.5MnO2. (d) In situ X-ray diffraction data of K0.5MnO2 during initial charging and discharging processes. Figure (a) reproduced with permission from ref. 66. Copyright 2017 Royal Society of Chemistry. Figure (b) reproduced and adapted from ref. 229 with permission. Copyright 2021 Elsevier. Figures (c) and (d) reproduced from ref. 64 with permission. Copyright 2017 Wiley-VCH. | ||
Fig. 20a shows the voltage-capacity profiles of nanostructured K0.5MnO2 during successive cycles, along with electrochemical performance when subjected to various current densities (Fig. 20b).75 The enhancement of electrochemical performance in layered oxides can be achieved through meticulous optimisation of particle morphology. Fig. 20c further showcases the voltage-capacity profiles of K0.6CoO2 microcubes, revealing their augmented electrochemical performance relative to both microspheres and microparticles (depicted in Fig. 20d).56 The K0.6CoO2 microcubes demonstrate a substantial reversible capacity coupled with superior capabilities in terms of rate performance and long-term cycle stability (Fig. 20e). These improvements are attributed to the distinctive morphology inherent to the K0.6CoO2 microcubes.56
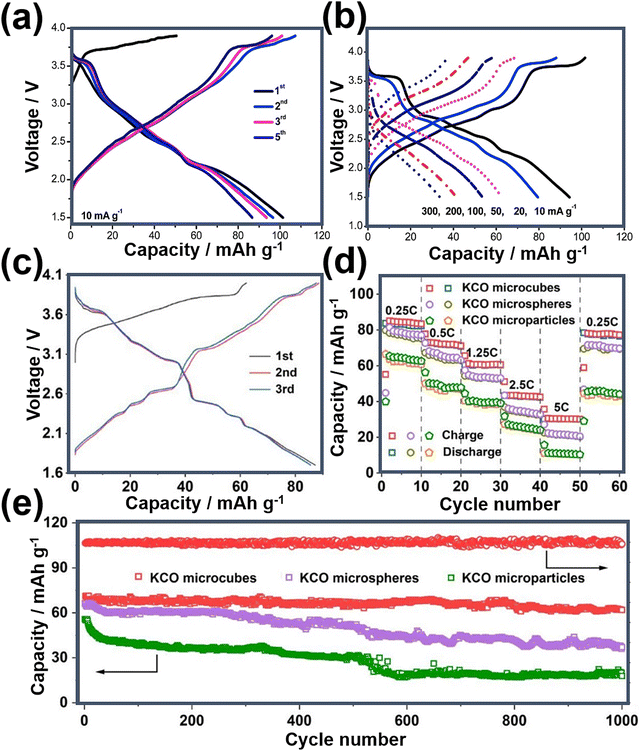 | ||
| Fig. 20 (a) Voltage-capacity profiles of K0.5MnO2 and (b) corresponding rate performance. (c) Voltage-capacity profiles of K0.6CoO2 at a current density equivalent to C/4 (1C = 80 mA g−1). (d) Rate performance of nanoarchitectured K0.6CoO2 (abbreviated as KCO) and (e) cycle performance at a current density commensurate to C/2. Figures (a) and (b) reproduced with permission from ref. 75. Copyright 2019 Elsevier. Figures (c), (d) and (e) reproduced with permission from ref. 56. Copyright 2023 American Chemical Society. | ||
Whilst a vast majority of documented potassium-based layered oxides predominantly adopt the P2- and P3-type frameworks, which proffer notable advantages over O-type frameworks in relation to rate capabilities, they do, however, manifest diminished operational voltages (typically falling below 3 V). As previously alluded, the conspicuous Devil's staircase-like voltage profiles (akin to the Cantor–Vitali mathematical function251–254) are a consequence of the substantial size of the K+ ion, which increases the electrostatic repulsion interactions between adjacent K+ ions.64 A strategic avenue for the amelioration of operational voltages within the domain of potassium-based layered oxides is to address this K+–K+ electrostatic repulsion phenomenon directly, accomplished through judicious structural design of the layered oxide framework. An illustrative exemplar involves the manipulation of K+ ion concentrations, yielding a P3-type K0.7Mn0.7Ni0.3O2 variant characterised by a disordered vacancy/K+ architecture, thereby evincing an elevated voltage plateau vis-à-vis its ordered K0.4Mn0.7Ni0.3O2 counterpart.93 An alternative strategy encompasses the conception of a corrugated layered structure, whereby potassium atoms are sufficiently segregated so as to mitigate the repulsive K+–K+ interactions throughout successive K+ intercalation and de-intercalation processes.
A series of corrugated layered titanates,255 including K0.8M0.8Ti1.2O4 (M = V, Cr, Mn, Fe and Co), K0.6Cr0.6Ti0.4O2, etc.,256 with longer K+–K+ distances (of ∼3.9 Å) compared to conventional KxMO2 layered oxides' (typically ∼3.0 Å),232 have been found to effectively mitigate the K+–K+ electrostatic repulsion, resulting in high voltages with minimal phase transitions. For example, a representative layered titanate such as K0.8Ni0.4Ti1.6O4 (Fig. 21a) exhibits a high working voltage of 3.6 V.256Fig. 21b and c show the voltage-capacity profiles of K0.8Ni0.4Ti1.6O4 and K0.8Co0.8Ti1.2O4.256 Although further material optimisation are required to enhance the capacity, these layered oxides display high voltages. Furthermore, our preliminary results indicate that layered stannates, like KxNi0.5Sn0.5O2 (x < 1), demonstrate high operating voltages of approximately 3.9 V, positioning related layered oxide compositions as promising high-voltage cathode materials that warrant further exploration.
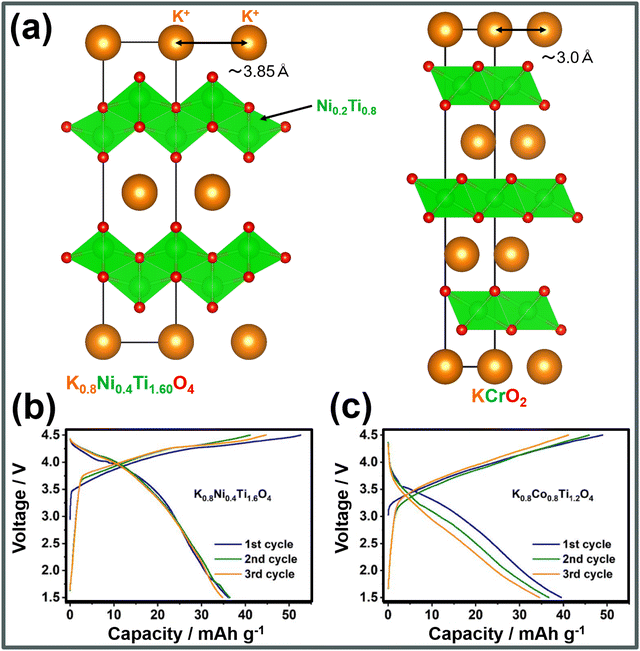 | ||
| Fig. 21 Electrochemical performance of representative corrugated layered titanate cathode materials. (a) Comparison of the structural framework of corrugated layered titanates with that of conventional layered oxides, in terms of the K+–K+ distances. (b) Voltage-capacity profiles of K0.8Ni0.4Ti1.6O4 and (c) K0.8Co0.8Ti1.2O4. Figures (b) and (c) reproduced from ref. 256 with permission. Copyright 2022 American Chemical society. | ||
Honeycomb layered oxides,257 characterised by a honeycomb arrangement of mainly transition metal atoms in the slab, represent an underrated class of cathode materials with exceptional properties. These materials not only exhibit high material densities, high voltages, and fast ion kinetics but also demonstrate excellent thermal stability, a feature distinct from conventional layered oxides.257 Notable potassium-based honeycomb layered oxides include K2Ni2TeO6 (or equivalently as K2/3Ni2/3Te1/3O2),72,258 K2Ni1.5Co0.5TeO6 (K2/3Ni1/2Co1/6Te1/3O2),5,72 K4NiTeO6 (K4/3Ni1/3Te1/3O2), K4Ni0.5Co0.5TeO6 (K4/3Ni1/6Co1/6Te1/3O2), amongst others. Fig. 22 displays high-resolution transmission electron microscopy (TEM) images of honeycomb layered K2NiCoTeO6, acquired through distinct zone axes. Clearly discernible within these TEM micrographs is the honeycomb arrangement of Ni/Co around Te atoms within the transition metal slab, as evidenced by the recurring –Te–Ni/Co–Ni/Co–Te– sequences. This spatial configuration becomes particularly pronounced when examining the TEM images taken along the100 zone axis (as illustrated in Fig. 22a and b). Notably, the K atoms incorporated within K2NiCoTeO6 exhibit a prismatic coordination with oxygen atoms, thereby unveiling a P2-type layered framework. This mode of coordination is unequivocally substantiated by the TEM depictions captured along the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction (as presented in Fig. 22c and d). Furthermore, the TEM images divulge that potassium atoms also adopt a honeycomb lattice, as depicted in Fig. 18e. Honeycomb layered tellurates exhibit K–K distances approximating 3.0 Å. Consequently, the expectation of elevated voltages similar to those observed in corrugated layered titanates such as K0.8Ni0.4Ti1.6O4 and K0.8Co0.8Ti1.2O4,256 which possess significantly longer K+–K+ distances, should not be readily assumed. Cyclic voltammograms (Fig. 23a) of honeycomb layered tellurates with the composition of K2Ni2−xCoxTeO6 (x = 0, 0.25, 0.50 and 0.75) display redox voltages reaching 4 V, bringing to the fore honeycomb layered oxides as promising high-voltage cathode materials. Fig. 23b and c further show the voltage profiles of honeycomb layered K2NiCoTeO6 (K2/3Ni1/3Co1/3Te1/3O2) and K2Ni1.5Co0.5TeO6 (K2/3Ni1/2Co1/6Te1/3O2), demonstrating the honeycomb layered tellurates to exhibit high voltages reaching 4 V. The high voltage attained can be ascribed to the ‘inductive effect’160 imposed by the more electronegative [TeO6]6− on the Ni/Co redox couple.
0] direction (as presented in Fig. 22c and d). Furthermore, the TEM images divulge that potassium atoms also adopt a honeycomb lattice, as depicted in Fig. 18e. Honeycomb layered tellurates exhibit K–K distances approximating 3.0 Å. Consequently, the expectation of elevated voltages similar to those observed in corrugated layered titanates such as K0.8Ni0.4Ti1.6O4 and K0.8Co0.8Ti1.2O4,256 which possess significantly longer K+–K+ distances, should not be readily assumed. Cyclic voltammograms (Fig. 23a) of honeycomb layered tellurates with the composition of K2Ni2−xCoxTeO6 (x = 0, 0.25, 0.50 and 0.75) display redox voltages reaching 4 V, bringing to the fore honeycomb layered oxides as promising high-voltage cathode materials. Fig. 23b and c further show the voltage profiles of honeycomb layered K2NiCoTeO6 (K2/3Ni1/3Co1/3Te1/3O2) and K2Ni1.5Co0.5TeO6 (K2/3Ni1/2Co1/6Te1/3O2), demonstrating the honeycomb layered tellurates to exhibit high voltages reaching 4 V. The high voltage attained can be ascribed to the ‘inductive effect’160 imposed by the more electronegative [TeO6]6− on the Ni/Co redox couple.
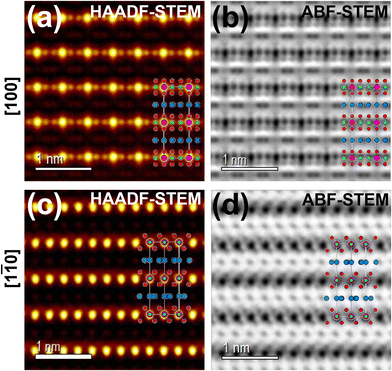 | ||
Fig. 22 High-resolution transmission electron microscopy images of honeycomb layered Rb(5%)-doped K2NiCoTeO6 (K2/3Ni1/3Co1/3Te1/3O2) taken at various zone axes. (a) High-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM) image taken along100 zone axis and (b) corresponding annular bright-field (ABF)–STEM image. (c) HAADF–STEM image taken along [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] zone axis and (d) corresponding ABF–STEM image. K atoms are shown in blue, Ni/Co and Te atoms are shown in green and pink, respectively. Oxygen atoms are shown in red. 0] zone axis and (d) corresponding ABF–STEM image. K atoms are shown in blue, Ni/Co and Te atoms are shown in green and pink, respectively. Oxygen atoms are shown in red. | ||
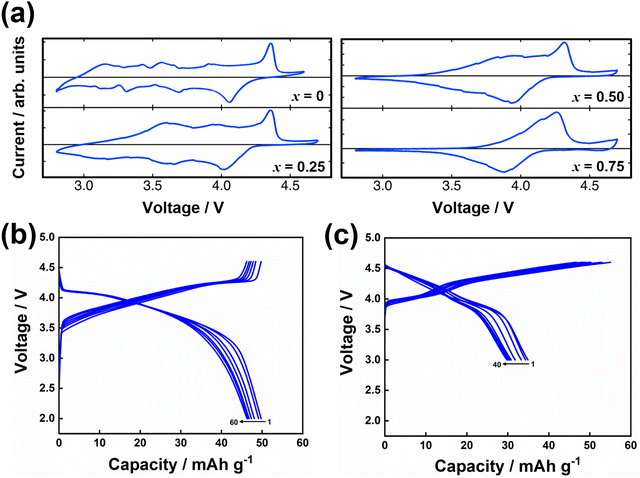 | ||
| Fig. 23 (a) Cyclic voltammograms of K2Ni2−xCoxTeO6 (K2/3Ni(2−x)/3Cox/3Te1/3O2) taken at a scanning speed of 0.1 mV s−1 and at a cut-off voltage of 2.8–4.7 V. 0.5 M KTFSI in Pyr13TFSI ionic liquid was used as the electrolyte in potassium half-cells. (b) Voltage-capacity profiles of honeycomb layered K2/3Ni1/2Co1/6Te1/3O2 using 0.5 M KTFSI in Pyr13TFSI ionic liquid as the electrolyte in K half-cells. A current density commensurate to C/10 was applied (1C = 127.8 mA g−1). (c) Voltage-capacity profiles of honeycomb layered K2/3Ni1/3Co1/3Te1/3O2 (5% Rb-doped) using 1 M KFSI in Pyr13FSI ionic liquid as the electrolyte in K half-cells. Tungsten foils were used as the current collector to avert corrosion from FSI−. A current density commensurate to C/10 was applied (1C = 127.8 mA g−1). Figure (a) reproduced and adapted with permission from ref. 5. Copyright 2019 Wiley-VCH. | ||
In addition to the achieved high voltages, honeycomb layered tellurates also manifest considerable material density (tap density). This notable characteristic bodes favourably for their prospective application as materials with high energy density, pending the complete optimisation of their capacity. However, the presence of chalcogens like tellurium in these honeycomb layered oxide compositions may hinder their practical realisation due to cost and scarcity concerns. Future research endeavours could be directed towards exploring tellurium-free honeycomb layered oxide compositions, such as the bismuthates, antimonates, tungstenates, molybdenates, ruthenates, etc., which offer the potential for both high voltages and high theoretical capacities.257 Moreover, from a pedagogical perspective, the distinctive crystalline structure and inherent structural symmetries of these materials facilitate the prevalence of 2D atomistic interactions within their honeycomb layered heterostructures. This characteristic opens up opportunities for exploring unconventional magnetic phenomena, such as Heisenberg-Kitaev interactions, and investigating novel emergent properties, including quantum geometries and topologies.257–266 The unique properties of these materials provide valuable insights into fundamental physics principles and can serve as excellent educational tools to foster a deeper understanding of advanced materials and their intriguing behaviours.
A significant challenge associated with many layered oxide materials is their delicate handling requirements, necessitating a controlled atmosphere, such as storage in argon-purged glove boxes or dry-rooms, due to their high sensitivity to air and moisture (hygroscopic nature). Nevertheless, their inherent instability can be effectively mitigated and controlled through meticulous tuning of their chemical composition. Partial substitution of constituent transition metal atoms has emerged as a promising and viable approach, as evident in Na-analogues like NaNi2/3Sb1/3O2 (Na3Ni2SbO6). In this case, even a minor amount of substitution with elements such as Mn, Mg, or Ru has been found to significantly improve the structural stability of the material.267–269 A similar strategy can be adopted to improve the stability of hygroscopic potassium-based layered oxides. Additionally, the concept of utilising multiple transition metals in equivalent proportions, commonly referred to as ‘high-entropy oxides’,270,271 has recently emerged as a novel approach for designing stable layered oxides with distinctive physicochemical properties. Whilst this concept presents exciting new possibilities for designing and implementing potassium-based layered oxides, it is still in its nascent stages with substantial room for further exploration and development. Moreover, the application of plasma-enhanced sintering methodologies has demonstrated significant efficacy in augmenting the structural stability of layered oxides, exemplified by K0.6Ni0.2Co0.3Mn0.5O2,272 particularly when exposed to moisture.
A vast majority of potassium-based layered oxides and chalcogenides suffer from potassium deficiency, which limits their capacity to bring a sufficient amount of potassium into a potassium-ion battery (KIB). To overcome this limitation, one promising approach is the investigation of stoichiometric layered oxides (e.g., KCrO2232 and KCrS2231) or potassium-rich layered oxides, as found in, for instance, the aforementioned honeycomb layered oxide compositions or potassium hypomanganates such as K3MnO4.273 Another strategy involves exploring materials that operate through the combined participation of both transition metal cation and oxygen/sulphide anion redox chemistry, enabling the full utilisation of the capacity in both potassium-rich and potassium-deficient layered oxide cathode materials. It is noteworthy that reversible anion-redox chemistry has been observed in certain layered oxide cathode materials, such as K0.4Fe0.5Mn0.5O2238 and K0.78Fe1.60S2.250
These approaches, including high-throughput materials screening,274–276 hold promise in addressing the potassium deficiency challenge and unlocking the full potential of layered oxide cathode materials for high-performance KIBs. Further research in this direction is crucial for advancing the field of potassium-ion battery technology and enhancing its practical applications in energy storage.
2.5 Approaches to enhance material performance.
Fig. 24 below presents a summary of the distinct classes of cathode materials employed in rechargeable KIBs, along with the corresponding challenges to be addressed, and their respective solutions.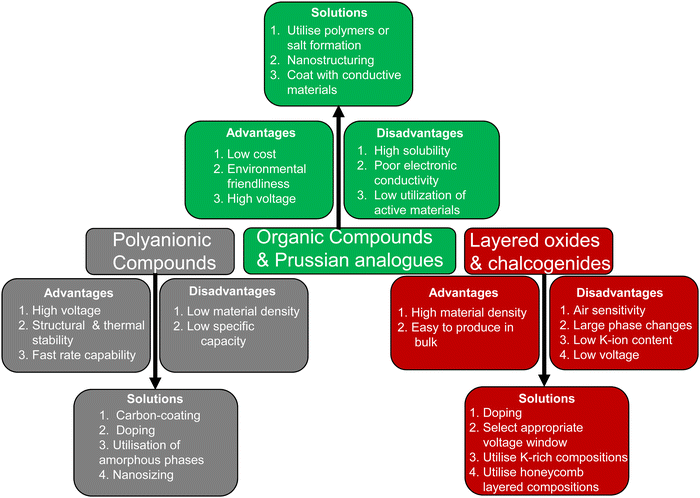 | ||
| Fig. 24 Pros/cons of various positive electrode (cathode) materials for KIBs, along with their solutions. Figures adapted from ref. 4 with permission. Copyright 2020 Wiley-VCH. | ||
3 High-energy-density potassium-ion battery full cell design
As the quest for alternative energy storage systems gains momentum, the exploration of potassium-ion batteries (KIBs) as potential replacements for lithium-ion batteries has witnessed a notable surge. This comprehensive Perspective serves to expound on the current state of research endeavours in the domain of KIBs, focusing specifically on the advancements and challenges pertaining to cathode materials. In tandem with the intensive recent research, KIBs have emerged as highly promising contenders for next-generation battery technology, boasting attributes such as high voltage, high power capabilities, and cost-effectiveness. Nevertheless, to enable their practical implementation, further enhancements in energy density, cyclability, high-power operation, and safety must be achieved.Within the confines of this Perspective, we hereafter also envisage innovative design strategies for both anode materials and electrolyte formulations, which play a pivotal role in realising a feasible potassium-ion battery. Additionally, we impart valuable perspectives and directions for designing materials tailored to suit KIBs, derived from our own experience and insights garnered from pertinent literature. It is our aspiration that these perspectives will not only underscore the potential of KIBs as a competitive energy-storage technology but also illuminate the pathways leading to its actualisation. We endeavour to elucidate the accessibility of the knowledge and strategies indispensable for the development of this technology. Nevertheless, it is imperative to clarify that the perspectives delineated herein represent the viewpoints of the authors and should not be misconstrued as an exhaustive compendium of strategies pursuable in the pursuit of high-performance KIBs.
3.1 Suitable anode materials for high-voltage cathode materials
The exploration of various anode materials that can be readily coupled with high-voltage/high energy density cathode materials for KIBs has yielded significant advancements.51,306–334Fig. 25a shows exemplars of anode materials reported for KIBs. Ragone plots showing the average voltages attained by selected anode materials along with theoretical capacities are shown in Fig. 25b. Fig. 25c shows the theoretical gravimetric and volumetric capacities attainable with alloy-based anode materials compared to carbonaceous materials such as carbon. Carbonaceous materials, including graphite and its derivatives such as hard carbon (non-graphitisable carbon (Fig. 26a)) and soft carbon (graphitisable carbon), emerge as promising candidates for anode materials.335–359 This is attributed to their relatively low cost, high surface area, and ease of surface modification. Notably, graphite stands out as a viable option for achieving high-voltage KIBs, as its potassiation/depotassiation voltage plateau lies above 0.1 V versus K/K+ (Fig. 5). Additionally, the design of hierarchical porous 2D and 3D nanostructures from graphene/graphite is a straightforward process, and further appropriate structural modifications hold the potential to enhance its energy density. Nonetheless, practical application of graphite anodes demands further improvement in terms of reaction kinetics and managing volume expansion during potassiation. Addressing these challenges would be crucial to unlocking the full potential of graphite as an anode material in high-performance potassium-ion batteries.360 As research continues, advancements in anode materials are essential for achieving significant progress in KIB technology and meeting the demands of next-generation energy storage applications.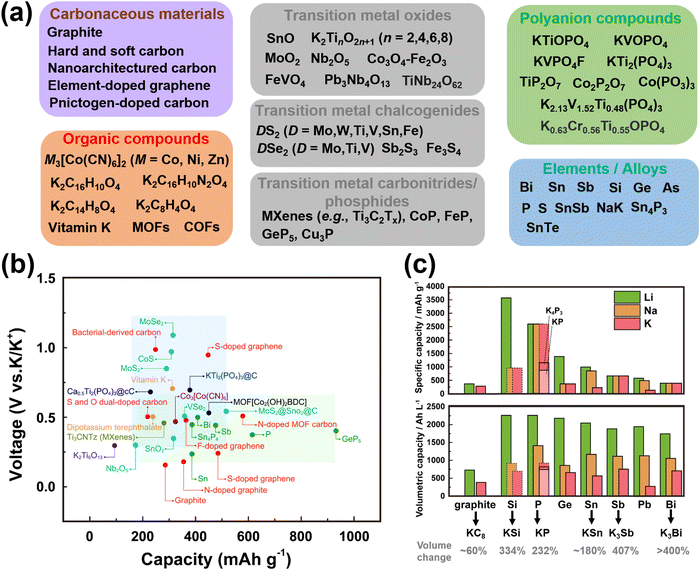 | ||
| Fig. 25 (a) Exemplars of anode materials for potassium-ion batteries.297–305 (b) Ragone plot showing the average voltages attained by selected anode materials along with their theoretical capacities. (c) Theoretical capacities of alloy-based anode materials compared to graphite in potassium-, sodium- and lithium-ion battery configurations, and (d) corresponding volumetric capacities. Figures adapted with permission.42 Copyright 2020 American Chemical Society. | ||
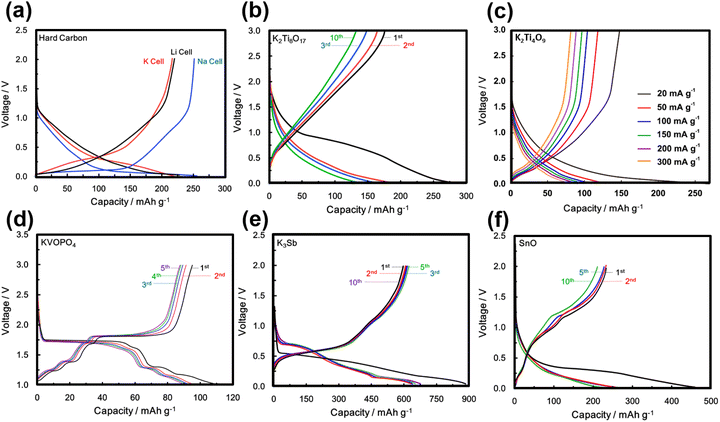 | ||
| Fig. 26 Voltage-capacity profiles of (a) hard carbon (in potassium-ion, sodium-ion, and lithium-ion battery configurations),42 (b) K2Ti8O17,361 (c) K2Ti4O9 under various current densities,362 (d) KVOPO4,42 (e) Sb363 and (f) SnO.364 Figures (a) and (d) reproduced from ref. 42 with permission. Copyright 2020 American Chemical Society. Figure (b) reproduced from ref. 361 with permission. Copyright 2016 Royal Society of Chemistry. Figure (c) reproduced from ref. 362 with permission. Copyright 2017 American Chemical Society. Figure (e) reproduced from ref. 363 with permission. Copyright 2015 American Chemical Society. Figure (f) reproduced from ref. 364 with permission. Copyright 2018 American Chemical Society. | ||
Layered titanates, including K2Ti2O5, K2Ti3O7, K2Ti4O9, K2Ti6O13, K2Ti8O17, etc., operating through topotactic K+ insertion/extraction mechanisms similar to graphite, hold significant promise as anode materials.361,365–368 These materials offer high capacity attainable through Ti4+/Ti3+ redox reactions, with low volume change and relatively high operation potentials at around 0.5 V. Fig. 26b and c show the voltage-capacity plots for K2Ti8O17 and K2Ti4O9. One notable advantage of layered titanates over graphite and other carbonaceous materials is their higher material densities (tap densities), which contributes to their potential for enhanced performance in terms of volumetric energy density. To fully exploit the electrochemical capabilities of layered titanates, thoughtful electrode design strategies, such as nanostructuring, are imperative. These strategies can optimise the electrochemical performance of the materials, enabling them to achieve their full potential as anode materials for potassium-ion batteries. Additionally, titanium-based polyanion compounds like KTiOPO4369 and KTi2(PO4)3,370,371 whilst exhibiting theoretically low capacities, can play a pivotal role in ensuring the thermal stability of layered titanates during high-temperature operation. Moreover, the incorporation of polyanion-based compounds can further elevate the material densities and, consequently, improve the volumetric energy densities of layered titanates. For the successful development of polyanion-based anodes, it is essential to design materials with a reasonable operating potential and satisfactory capacity, striking a balance between these properties to ensure their effective utilisation in KIBs. Fig. 26d displays the voltage-capacity profiles of a representative polyanionic compound (i.e., KVOPO4) evaluated also as an anode material for KIBs. Through systematic research and innovative design, layered titanates and polyanion-based compounds offer exciting prospects for advancing the performance of anode materials in KIBs.
Anodes operating via alloying reactions, such as those based on materials like Bi and Sn, show great promise as candidates for KIBs due to their high capacity.373–375Fig. 26e shows the voltage-capacity plots of Sb as an anode material for KIBs, operating via alloying reaction to form K3Sb. However, certain challenges associated with these alloy anodes need to be addressed. These challenges include the low initial Coulombic efficiency, high operating potential, and significant volume expansion (see Fig. 25c) observed during the potassiation/depotassiation processes. Resolving these issues is crucial to fully harness the potential of alloy anodes and to enhance their performance in KIBs. Apart from alloying reactions, anodes operating via conversion reactions (such as SnO (shown in Fig. 26f)) offer alternative pathways for high-capacity anode materials in KIBs. Additionally, anode materials that undergo cumulative alloying and conversion reaction (such as Sb2Se3)376 or cumulative (de)intercalation and conversion processes can be utilised to increase their energy density.7
Similarly, organic anodes (Fig. 27), including conjugate carboxylates and π-conjugated 2D and 3D covalent organic frameworks (COFs),142,377–380 hold great potential for high-capacity anode applications. The challenges that require addressing include the dissolution of active materials into the electrolyte and the inadequate electronic conductivity found in a majority of organic anodes. Organic anodes can be particularly well-suited for flexible organic KIBs, enabling innovative and versatile designs for next-generation energy storage devices. As research progresses, addressing the challenges faced by alloying anodes and exploring the potential of conversion-based and organic anodes will undoubtedly contribute to the development of high-performance KIBs and advance the field of energy storage technology.
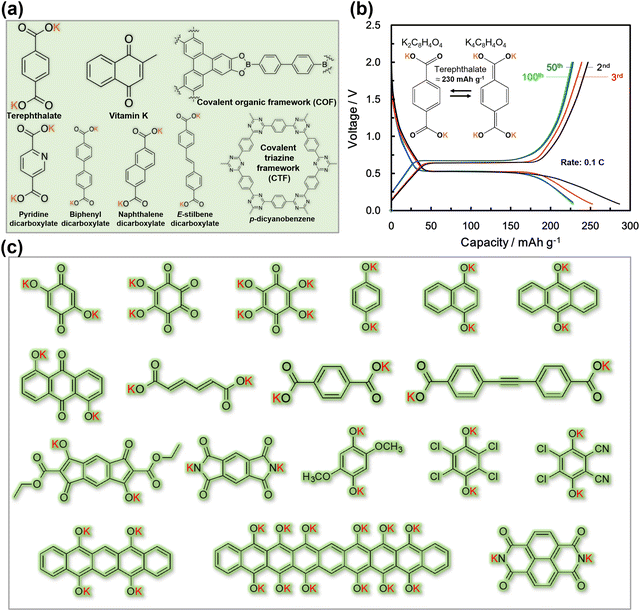 | ||
| Fig. 27 (a) Representative organic moieties that have been explored as anode materials for potassium-ion batteries. (b) Voltage-capacity profiles of potassium terephthalate (K2C8H4O4) demonstrating good cyclability and rate performance. (c) Exemplars of new carboxylate organic compounds that can be explored as anode materials for potassium-ion batteries. Figure (b) reproduced from ref. 372 with permission. Copyright 2017 Royal Society of Chemistry. | ||
In light of its scalability and suitability for commercialisable potassium-ion battery (KIB) anodes, graphite emerges as a promising candidate. The existing infrastructure established for manufacturing graphite electrodes in lithium-ion batteries (LIBs) can be leveraged and transitioned to accommodate KIBs, streamlining the commercialisation process for this novel battery system. Nevertheless, the key determinant lies in understanding and optimising the solid–electrolyte interphase layer and comprehending the K+ extraction/insertion mechanism. Significant research efforts should be directed towards unraveling the intricacies of the solid–electrolyte interphase (SEI),381–384 its interplay with the additives, electrolytes, and binders employed, as well as the mechanism of its growth and formation. The controlled, reliable, and safe growth of SEI on the graphite surface is pivotal to facilitating the adoption of graphite as an anode material for KIBs. Moreover, several theoretical models, such as the Daumas–Hérold model and Rüdorff–Hofmann model,385–387 have been proposed to describe the intercalation process of alkali ions like K+ into graphite (also referred to as the ‘staging’ process). However, these models still lack comprehensive understanding and need further exploration. Exploring the potential formation of ripplocations388–392 during the process of potassium-ion intercalation and de-intercalation in graphite, through techniques such as transmission electron microscopy, can provide captivating insights. A holistic understanding of the mechanism governing electrochemical K+ extraction/insertion mechanism of graphite is crucial,393 including insights into the influence of current rate on the staging processes and the nature of phases present. Furthermore, addressing concerns related to high current-rate performance and safety of graphite in KIBs is of paramount importance. This is a major area of concern that persists in LIBs and requires careful examination and mitigation to ensure the successful and safe deployment of graphite in KIBs. In conclusion, whilst graphite shows promise as a viable anode material for KIBs, extensive research is imperative to elucidate and optimise the SEI formation,394 K-ion insertion/extraction mechanisms, and ensure high current-rate performance and safety, thereby facilitating the successful integration of graphite in practical potassium-ion battery systems.393
3.2 Safe and compatible electrolytes for high-voltage cathode materials
Aside from electrodes, another pivotal aspect of battery technologies lies in the electrolyte, which profoundly governs their performance and lifespan. Ever since the discovery of reversible electrochemical K+ insertion/extraction in graphite, a plethora of electrolytes have been subjected to thorough investigation to unveil their physicochemical properties.36,54,383,395–399 To date, an array of electrolyte systems has been extensively studied, encompassing organic-solvent-based electrolytes, ionic liquid electrolytes, aqueous electrolytes, and solid-state electrolytes. Notably, research endeavours have predominantly concentrated on organic liquid electrolytes.Amongst the potassium salts employed in organic-solvent-based electrolytes, noteworthy candidates include potassium trifluoromethanesulphonate (KCF3SO3), potassium perchlorate (KClO4), potassium tetrafluoroborate (KBF4), potassium bis(trifluoromethylsulphonyl)imide (typically abbreviated as KTFSI, KTf2N or KTFSA), potassium bis(fluorosulphonyl)imide (KFSI, Kf2N or KFSA), potassium hexafluorophosphate (KPF6), potassium (3-methoxypropyl)((trifluoromethyl)-sulphonyl)imide (abbreviated as KMPSA), potassium bis(2-(2-(2-methoxyethoxy)ethoxy)ethyl) phosphate (KTEEP), potassium (bis(2-(2-ethoxy)ethoxy)ethyl) phosphate (KDEEP), and more recently potassium hexafluoropropane-1,3-disulfonimide (KHFDF).400 Notably, the molar solubility of KPF6, KTFSI, and KFSI salts in organic solvents (Fig. 29a), suchlike propylene carbonate (PC), far exceeds that of KClO4 and KBF4, both of which at room temperature exhibit almost insolubility in PC solvent. Moreover, the pronounced interactions between BF4− and K+ detrimentally impact the conductivity of KBF4-based electrolytes, rendering them less suitable. Similarly, KClO4 is sparingly used due to safety concerns pertaining to the reduction of ClO4− ions. Thus, it can be inferred that KBF4 and KClO4 are not ideal potassium salts for practical KIBs. It is pertinent to note that commonly utilised potassium salts typically entail fluorinated anions, as the electron-withdrawing attributes of fluorine atoms and the delocalised charge of the anions engender high solubility of these salts in organic non-aqueous (aprotic) solvents. Table 2 shows the physicochemical properties of representative potassium salts utilised in KIB electrolytes.54,382
| Potassium salt | Decomposition temperature (°C) | Conductivity (mS cm−1) (room temperature) | Solubility | Cost | Toxicity |
|---|---|---|---|---|---|
| KClO4 | 610 | 1.1 | Hardly dissolves in propylene carbonate | Low | High |
| KBF4 | 530 | 0.2 | Hardly dissolves in propylene carbonate | Low | High |
| KPF6 | 575 | 5.75 | 0.9 mol kg−1 in propylene carbonate | Low | Low |
| KCF3SO3 | 238.5 | — | 22 mol L−1 | High | Slight |
| KN(SO2F)2 (KFSI) | 102 | 7.2 | 7.5 mol kg−1 in dimethoxyethane | High | Slight |
| KN(CF3SO2)2 (KTFSI) | 198–203 | 6.1 | 6 mol kg−1 in dimethoxyethane | High | Slight |
Imides, such as KTFSI and KFSI, have garnered considerable traction owing to their exceptional solubility in both ether and ester solvents. Electrolytes based on KFSI or KTFSI have exhibited superior ionic conductivity and enhanced electrochemical stability compared to KPF6-based electrolytes, thus positioning them as prospective candidates amongst mainstream electrolytes for KIBs. However, it should be noted that typical KTFSI-based and KFSI-based electrolytes exhibit severe corrosion on the aluminium current collector at high voltages, particularly exceeding 4.0 V. Additionally, the use of ether solvents renders the electrolytes more susceptible to decomposition at relatively low voltages owing to the elevated highest occupied molecular orbital energy levels of ether molecules, impeding their application in high-voltage full-cell designs. Fortunately, both challenges can be circumvented through the adoption of highly concentrated electrolytes280 or the implementation of current collectors based on tungsten or titanium nitride, albeit acknowledging the potential cost implications.
Despite their relatively high ionic conductivity, KPF6 salts suffer from certain drawbacks, including susceptibility to hydrolysis and inadequate thermal stability. Thus, achieving a delicate balance amongst these properties becomes imperative. Electrolytes comprising binary salts, such as KFSI–KPF6 in organic solvents like ethylene carbonate (EC) and diethylene carbonate (DEC), have demonstrated the ability to amalgamate the benefits of both KPF6 and KFSI salts,401 resulting in enhanced stability at high potentials and reduced viscosity. Furthermore, the solid electrolyte interphase (SEI) formed in KPF6/KFSI electrolyte exhibits reduced thickness compared to that formed in KPF6 single-salt electrolytes, thus ensuring favourable cyclability when employing binary-salt electrolytes.396 Notably, research on binary-salt electrolytes for KIBs remains an unexplored territory, offering ample opportunities for further investigation and advancements.
Electrolyte optimisation strategies, such as the incorporation of additives like fluoroethylene carbonate (FEC), trimethylene sulphate,402 ethylene sulphate, potassium difluorophosphate, vinylene carbonate, sulphate esters of 1,3,2-dioxathiolane 2,2-dioxide,402,403 dimethylsulfamoyl fluoride,404 1,3-propane sultone,403 butylene sulphite403 amongst others, hold immense potential to enhance the electrochemical performance of potassium-ion battery (KIB) electrolytes. Despite their higher cost, FEC stands out as a dominant additive for high-voltage battery electrolytes, attributed to its robust oxidation resistance owing to the fluorine-containing structure. To fully exploit the potential benefits of various additives, particularly FEC, in the formation of SEI and the manipulation of K+ solvation structures, further in-depth exploration is warranted. Numerous challenges persist in the quest to develop an ideal organic liquid electrolyte with exemplary reliability. Future research efforts can be directed towards controlling and mitigating side reactions between the electrolyte and potassium metal.405 Additionally, the mechanism behind SEI layer formation and charge transfer kinetics in KIBs remains enigmatic, necessitating extensive investigations in the forthcoming studies. Given the high reactivity of potassium, the integration of advanced characterisation techniques, such as in situ transmission electron microscopy (TEM), cryo-electron microscopy, and in situ magnetometry,406 in conjunction with computation, can offer valuable insights into the charge transfer kinetics, microstructures, and reaction mechanism of SEI.407 Moreover, the advent of cutting-edge technologies like machine interaction, artificial intelligence, and big data analysis holds the potential to drive further advancements in electrolyte chemistry and the elucidation of SEI phenomena, as well as the development of compatible electrode materials for KIBs. By harnessing these multidisciplinary approaches, the path towards achieving highly efficient and reliable potassium-ion batteries can be substantially expedited.
A significant challenge faced by KIBs revolves around safety concerns, particularly in light of the negative publicity surrounding battery safety issues. However, the development of a reliable and safe KIB, even at a cost-to-performance ratio lower than LIBs, holds the potential to establish dominance in certain applications. One approach to ensure the safety and thermal stability of liquid aprotic electrolytes for KIBs involves the incorporation of functional additives such as flame retardants ((Fig. 29b)),408–413 for example, triethylphosphate ((C2H5)3PO4), trimethylphosphate ((CH3)3PO4), tributylphosphate ((C4H9)3PO4) and tris(2,2,2-trifluoroethyl) phosphate ((C6H6)F9PO4), into the organic solvents. This strategy helps to guarantee improved safety without compromising performance. Alternatively, ionic liquids present promising attributes in terms of better thermodynamic stability and wider electrochemical stability windows,414 in comparison to both organic liquid and aqueous electrolytes. Furthermore, ionic liquids demonstrate exceptionally high solubilities with a diverse range of organic, inorganic, and polymer materials. This unique property enables the amalgamation of various cations and anions, thus bestowing unparalleled flexibility in the design of KIBs for a myriad of applications. However, the widespread adoption of ionic liquid-based electrolytes for KIBs has been hindered by their high cost. Despite this challenge, there have been some reports on the utilisation of KFSI- and KTFSI-based ionic liquid electrolytes, such as 1 M KFSI in Pyr13FSI, 0.5 M KTFSI in Pyr13TFSI, and 0.3 M KTFSI in Pyr14TFSI, amongst others.5,72,415–423 For brevity, 1-butyl-3-methylpyrrolidinium and 1-propyl-3-methylpyrrolidinium have been abbreviated as Pyr14 and Pyr13, respectively. Table 3 shows the physicochemical properties of representative ionic liquid electrolytes for KIBs.5,424 0.5 M KTFSI in Pyr13TFSI ionic liquid demonstrates large electrochemical window, as shown in the cyclic and linear sweep voltammograms (Fig. 28a). Moreover, 0.5 M KTFSI in Pyr13TFSI ionic liquid shows stable deposition and dissolution of potassium with relatively low overvoltage (Fig. 28b).
| Concentration (M) | Potassium salt | Ionic liquid | Conductivity (room temperature) (mS cm−1) | Decomposition temperature (°C) | Electrochemical stability window (V) |
|---|---|---|---|---|---|
| 0.5 | KTFSI | PyrH4TFSI | 1.49 | 300 | 4 |
| 1 | KFSI | Pyr13FSI | 4.8 | 307 | 5.72 |
| 0.5 | KTFSI | Pyr14TFSI | 7.96 | 350 | 6 |
| 0.5 | KTFSI | Pyr13TFSI | 2.1 | 417 | 6.01 |
 | ||
| Fig. 28 Electrochemical stability of 0.5 M KTFSI in Pyr13TFSI ionic liquid at 298 K. (a) Cyclic and linear sweep voltammograms conducted at a scan rate of 1 mV s−1 to assess the cathodic (in red) and anodic (in grey) limit. (b) Voltage profiles during galvanostatic deposition and dissolution of potassium in potassium symmetric cells performed at a current density of 6.4 μA cm−2. Figures adapted from ref. 5 with permission. Copyright 2019 Wiley-VCH. | ||
Fig. 29c illustrates the configuration of typical ionic liquids, solvents, and salts integrated into the design of liquid-based electrolytes.396,425 Ionic liquids investigated for use in KIBs predominantly employ pyrrolidinium cations, coupled with imide anions such as FSI− and TFSI−. The lowered melting point of these ionic liquids can be attributed to the asymmetry of their ions, which hinders the compact arrangement of the ions, thus imparting them with remarkable thermal stability. However, it is noteworthy that pyrrolidinium-based ionic liquids often exhibit high viscosity. In contrast, quaternary ammonium-based ionic liquids have displayed enhanced electrochemical stability when utilised in LIBs, yet their potential as electrolytes for KIBs remains largely unexplored.426
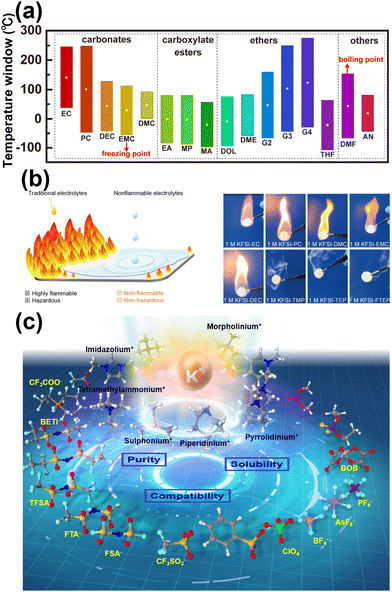 | ||
| Fig. 29 (a) Representative organic solvents utilised in the formulation of liquid electrolytes for KIBs. (b) A visual representation depicting the contrasting hazardous attributes between non-flammable electrolytes based on phosphate esters and conventional/traditional electrolytes composed of ethers and carbonates. Experimental examination involving flame tests conducted on diverse electrolytes is also shown. (c) Selection of organic and inorganic salts for the purpose of formulating ionic liquids for KIBs. Ionic liquids, comprising of organic cations like piperidinium, imidazolium, pyrrolidinium, amongst others, are conjoined with either organic or inorganic anions such as ClO4−, PF6−, and BF4−. Notably, organic or inorganic anions are represented in yellow, whilst organic cations are denoted in black. During the formulation of appropriate ionic liquids for high-voltage operation, considerations encompassing factors like salt purity, solubility, and compatibility with the electrode materials are imperative. Abbreviations: ethylene carbonate (EC), propylene carbonate (PC), diethyl carbonate (DEC), ethyl methyl carbonate (EMC), dimethyl carbonate (DMC), ethyl acetate (EA), methyl propionate (MP), methyl acetate (MA), 1,3-dioxolane (DOL), 1,2-dimethoxyethane (DME), diglyme (G2), triglyme (G3), tetraglyme (G4), tetrahydrofuran (THF), N,N-dimethylformamide (DMF), acetonitrile (AN), trimethyl phosphate (TMP), triethyl phosphate (TEP), tris(2,2,2-trifluoroethyl) phosphate (FTEP). Figures reproduced with permission. Figure (b) reproduced from ref. 411 with permission. Copyright 2023 Royal Society of Chemistry. Figure (c) reproduced and adapted from ref. 257 Copyright 2021 Royal Society of Chemistry. | ||
The drawbacks associated with ionic liquids can potentially be offset by the additional functional capabilities they offer when utilised in KIBs. These benefits include a wide applicable temperature range, high safety, and exceptional durability. One area of focus for future research is to address the cost issues related to ionic liquid electrolytes. An effective approach for cost reduction involves exploring more economical reaction pathways, exemplified by the implementation of ion exchange resins as an alternative to conventional batch metathesis reactions.427 This methodological shift has demonstrated its efficacy in enhancing the cost-efficiency of deploying ionic liquids, particularly within sectors such as timber processing. A similar strategy can also be applied to the scalable design of ionic liquids for batteries. Another strategy to curtail the cost involves blending ionic liquids with organic solvents like propylene carbonate, which may meet the cost requirements whilst preserving the desirable properties of the ionic liquid electrolytes. Additionally, exploring the use of potassium single cation molten salts, which exclusively contain K+ as the cationic species, presents another avenue to lower the cost of employing pyrrolidinium-based ionic liquids. However, it is essential to note that potassium single cation molten salts generally operate at elevated temperatures, necessitating further developments in room-temperature single cation molten salts. Recent advancements have shown that single cation molten salts composed of an almost equimolar mixture of potassium (fluorosulphonyl)(trifluoromethylsulphonyl)amide (KFTA) and potassium bis(fluorosulphonyl)amide (KFSA) exhibit low melting points of 67 °C.428 This demonstrates the feasibility of preparing single cation molten salt electrolytes that can function at room temperature with potential adjustments to the potassium salt compositions (Fig. 30a). In fact, it has been demonstrated that a combination of potassium bis(fluorosulphonyl)amide (KFSA) and potassium (3-methoxypropyl)((trifluoromethyl)sulphonyl)amide (KMPSA) can form a liquid state at remarkably low temperatures, as low as −13 °C.429 This discovery represents the emergence of a room-temperature ionic liquid exclusively composed solely of K+ cations, marking a significant milestone in the field. Anticipated future efforts are expected to be dedicated to mitigating the limitations associated with ionic liquids, including their elevated viscosities and relatively higher costs in comparison to conventional organic solvents. Additionally, it is worth noting that elevated temperatures can potentially unlock the latent performance of certain ionic liquids that exhibit suboptimal ionic conductivities at room temperature.
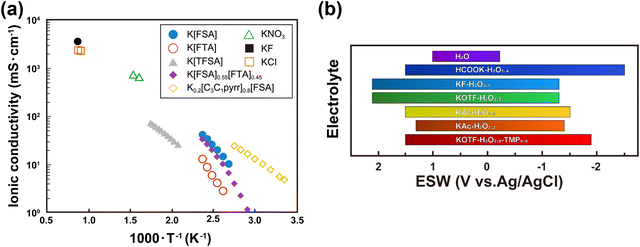 | ||
| Fig. 30 (a) Arrhenius plot showcasing the ionic conductivity for chosen potassium single cation molten salts and room-temperature ionic liquids. Abbreviation: N-methyl-N-propylpyrrolidinium (C3C1pyrr). (b) Electrochemical stability window (ESW) of representative electrolytes for aqueous KIBs. Abbreviation: potassium acetate (KAc), potassium trifluoromethanesulphonate (KOTF). Figure (a) reproduced from ref. 428 with permission. Copyright 2020 American Chemical Society. Figure (b) reproduced from ref. 430 with permission. Copyright 2023 Wiley-VCH. | ||
Aqueous electrolytes (Fig. 30b), utilising water as the solvent along with cost-effective salts like nitrates and sulphates, offer significant cost reduction and inherent safety advantages compared to non-aqueous electrolytes.431–433 However, their applicability in organic electrolytes is limited due to their extreme insolubility in organic solvents. One of the key advantages of aqueous electrolytes over non-aqueous counterparts is their high ionic conductivity, contributing to excellent power density. Leveraging these advantages, aqueous KIBs have the potential to serve as high-power energy storage systems for grid-scale applications. Nonetheless, a notable disparity exists between aqueous KIBs and organic liquid electrolyte-based KIBs concerning energy density. The relatively lower energy density of aqueous KIBs primarily stems from the narrow electrochemical stability windows of aqueous electrolytes, resulting in limited working voltage. Conventionally, the electrochemical stability windows of typical aqueous electrolytes, thermodynamically restricted to 1.23 V, are narrower compared to those of organic electrolytes. Beyond this electrochemical stability window, water electrolysis gives rise to the evolution of oxygen or hydrogen, or both, adversely impacting the performance of aqueous KIBs. Moreover, the phase transition of water due to variations in temperature imposes limitations on the application of aqueous KIBs under elevated or sub-zero temperatures, consequently restricting the operational temperature windows of aqueous electrolytes. To overcome these challenges, it becomes imperative to explore novel water-in-salt-electrolytes (abbreviated as WiSE or WiS) that exhibit high electrode compatibility and ensure a wide electrochemical stability range.434–438 By delving into WiSE technology, promising solutions can be envisioned to enhance the energy density and expand the temperature operability of aqueous KIBs, thereby propelling their practical adoption for diverse energy storage applications.
The advancement of all solid-state KIBs represents a burgeoning field,440–442 inspired by the progress seen in LIB research, offering inherent safety benefits. A recent breakthrough involves an all-solid-state battery architecture comprising Prussian blue KxFe[Fe(CN)6] as the cathode, K–C composite as the anode, and β/β′′-Al2O3 solid electrolyte.439 This design has demonstrated remarkable attributes, showcasing high energy density, excellent capacity retention, and satisfactory electrochemical performance across a wide temperature range of −20 °C to 120 °C (Fig. 31).439 Despite the existence of polymer electrolytes exhibiting high K+ conductivity, the literature on solid-state potassium-ion conductors remains relatively scarce.443–476 As a result, the current focus of research revolves around enhancing the ionic conductivity of solid-state potassium-ion conductors. However, it is essential to note that other pivotal electrochemical properties often remain insufficiently characterised, including electrochemical stability, ion selectivity, mechanical properties, and assembly techniques. As the field of all solid-state KIBs continues to progress, further investigations into these unexplored areas hold promise for bolstering the performance and reliability of these advanced energy storage systems. A comprehensive understanding of these key properties will pave the way for the development of safer, more efficient, and practical all-solid-state KIB technologies.
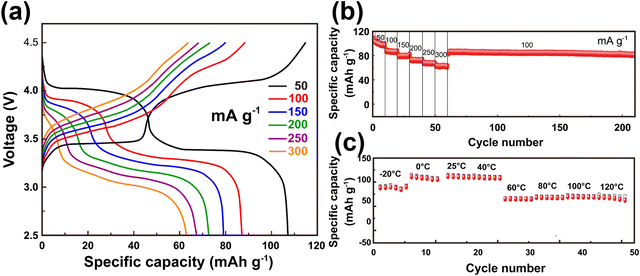 | ||
| Fig. 31 Electrochemical performance of an all-solid-state potassium battery setup comprising Prussian blue KxFe[Fe(CN)6] as cathode, K–C composite as the anode and β/β′′-Al2O3 as solid electrolyte. (a) Voltage-capacity profiles at various current densities. (b) Cycle performance at various current densities. (c) Cycle performance at various temperature ranges. Figures reproduced with permission.439 Copyright 2023 Wiley-VCH. | ||
Indeed, the development of solid-state electrolytes has been a pivotal direction in the advancement of LIBs, and it is anticipated that a similar trend will unfold in the domain of KIBs.444 (Fig. 32a–c) show the ionic conductivity data of various solid-state K+ conductors, as documented within the existing literature. Notably, some of these materials (such as KSi2P3, β/β′′-Al2O3, K3−2xBaxSbSe4 (x = 0, 0.2, 0.3 and 0.4), K2+xZr1−xYxCl6 (x = 0–1), etc.) exhibit appreciable ionic conductivity,452,456–458,460–472,477–480 even under ambient room temperature conditions. Given the relatively large ionic radius of K+, facilitating K+ mobility within solid materials presents a significant challenge. To expedite research progress in this area, a synergistic approach that combines theoretical computations for materials screening with experimental studies is envisaged.191 Such an integrated strategy can help identify suitable solid-state electrolytes with diverse dimensional frameworks (1D, 2D, or 3D) that exhibit high K+ ionic conductivity.481–487 However, similar to the challenges faced in LIBs and sodium-ion batteries, most solid electrolytes for KIBs may suffer from low ionic conductivity and substantial interphasial resistance with the electrodes. To overcome these hurdles, ionogel-electrolyte membranes, which encompass solid matrices combined with ionic liquids, offer a promising avenue for exploration. These ionogel-electrolyte membranes possess desirable characteristics, including non-flammability, non-volatility, and superior electrochemical and physicochemical properties.488 By leveraging such innovative materials, advancements in the development of solid-state electrolytes for KIBs are poised to accelerate, leading to enhanced performance and safety in potassium-ion battery technologies. Nevertheless, potassium metal's extreme reactivity (1s22s22p63s23p64s1) can raise safety issues when utilised as anode material. Designing all-solid-state KIBs using anode materials such as Sn composites, graphite or pre-potassiated graphite53 has the potential to showcase their promise, mirroring the achievements seen in all-solid-state LIBs.
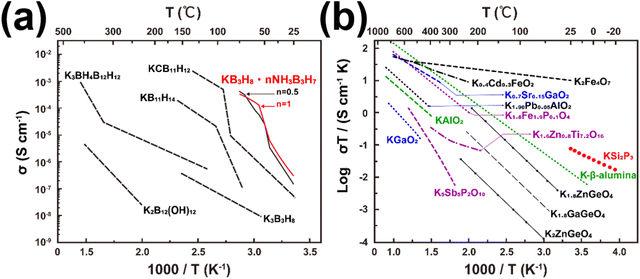 | ||
| Fig. 32 Exemplars of inorganic compounds that can be utilised as solid-state potassium-ion conductors. (a) Borohydrides.452 (b) Oxides (including cristoballite-type polyanion frameworks), antiperovskite phosphides and sulphides. Graph plotted from data derived from ref. 456–458, 460–472, 477 and 478. Figure (a) reproduced from ref. 425 with permission. Copyright 2022 American Chemical Society. | ||
3.3 Full cell design
Various cathode materials have been employed in the design of high-voltage KIBs. Several high-performance KIB full cells have been developed, primarily falling within the following main categories: (i) non-aqueous batteries, exemplified by configurations such as K2Mn[Fe(CN)6] cathode//1 M KFSA in Pyr13FSA ionic liquid electrolyte//graphite anode;281,489 (ii) dual-ion batteries, featuring arrangements like graphite cathode//5 M KFSA in ethylene carbonate/dimethylene carbonate electrolyte//graphite anode;490 (iii) aqueous batteries, comprising K2FeFe(CN)6 cathode//21 M KCF3SO3 water-in-salt electrolyte//KTi2(PO4)3 anode;491,492 (iv) all-organic flexible batteries, consisting of, for instance, potassium terephthalate (K4C8H4O4) as anode//1 M KPF6 in dimethoxyethanne as electrolyte//[N,N′-bis(2-anthraquinone)]-perylene-3,4,9,10-tetracarboxydiimide as an insoluble organic cathode;493 and (v) all-solid-state batteries, which encompass combinations like K2FeFe(CN)6 cathode//K2Fe4O7 solid–electrolyte//K metal anode.454 Notably, non-aqueous KIB battery prototypes have exhibited energy densities comparable to those of LIBs, as illustrated in Fig. 33a. In particular, Fig. 33b and c demonstrate the electrochemical performance of a high-energy-density KIB full cell, employing graphite as the anode and Prussian blue (K2MnFe(CN)6) as the cathode, revealing minimal capacity decay and remarkable rate capability.126Fig. 34 further demonstrates the electrochemical performance of a KIB full cell, employing graphite as the anode and KFeSO4F polyanion-based compound as the cathode, revealing good rate capabilities along with noteworthy capacity retention following prolonged (dis)charge cycles.14 These electrochemical performances substantiate the potential of KIBs as promising, high-performance, and cost-effective alternatives to traditional LIBs.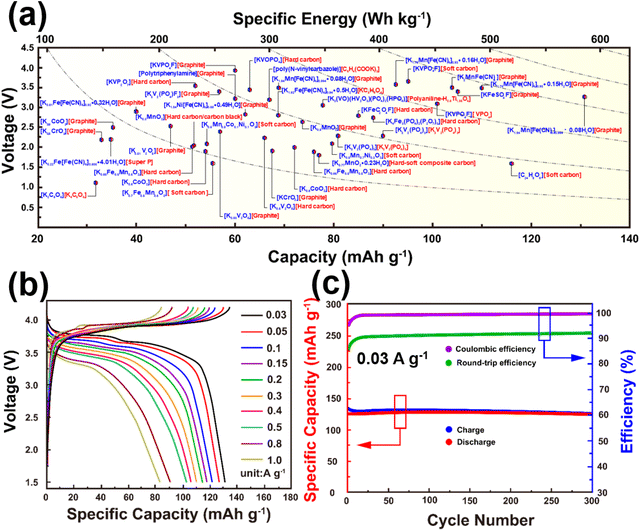 | ||
| Fig. 33 (a) Ragone plot showing the energy densities, at a material level, attained by representative KIB full cells reported in existing literature. Cathode and anode materials are shown in blue and red, respectively. (b) Rate performance of a KIB full cell comprising K2MnFe(CN)6 as cathode and graphite as anode. (c) Corresponding cycle performance. Figures (b) and (c) reproduced and adapted from ref. 126 with permission. Copyright 2021 Springer Nature. | ||
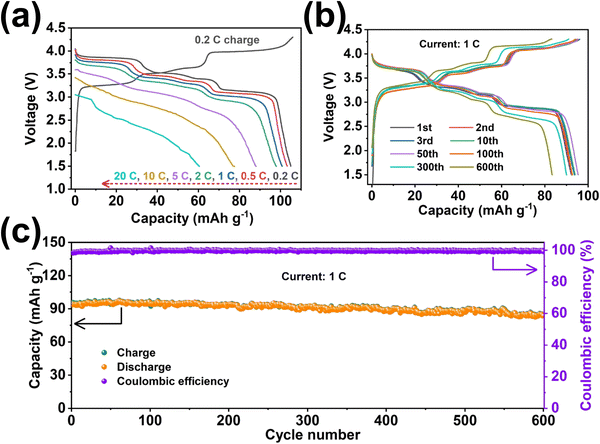 | ||
| Fig. 34 Electrochemical performance of a KIB full cell, comprising KFeSO4F as cathode and graphite as anode material. (a) Voltage-capacity plots showing the rate performance. (b) Rate performance at a current density commensurate to 1C (1C = 127.6 mA g−1). (c) Cycle performance. Figures reproduced from ref. 14 with permission. Copyright 2022 Elsevier. | ||
The operation of a potassium dual-ion battery involves the simultaneous insertion of K+ ions into the anode and anions (such as ClO4−, PF6−, FSI−, TFSI−, FTFSI−, CF3SO3−, BETI−, BF4−, etc.) into the cathode during the charging process. Subsequently, during the discharging process, both the anions and K+ cations are extracted from the cathode and anode, returning to the electrolyte to combine once again. Remarkably, the high working voltage (>4.5 V) of potassium dual-ion batteries can reach as high as 5.0 V, depending on the specific anion employed.422,490,494–499 Beyond their attainable high-voltage characteristics, potassium dual-ion batteries possess the advantages of being low-cost and delivering high capacities with excellent cyclability, rendering them sustainable high-energy-density battery systems. The most extensively researched dual-ion battery configurations utilise carbon materials (such as graphite) as both the cathode and anode. Fig. 35 illustrates the electrochemical performance of a potassium dual-ion battery featuring K metal as the anode, ionic liquid (1 M KFSI in Pyr13FSI) as the electrolyte, and graphite as the cathode. To mitigate severe corrosion, typically observed in KFSI-based electrolytes at high voltages (>4.0 V), on the aluminium current collector, tungsten foil was employed as the current collector in this particular system.
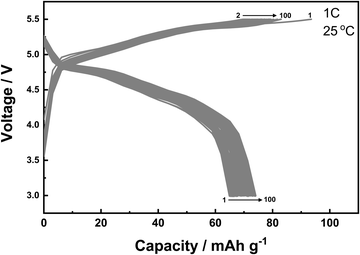 | ||
| Fig. 35 Voltage-capacity profiles of a dual-ion battery setup, comprising graphite as the cathode and potassium metal as anode. A 1 M KFSI in Pyr13FSI ionic liquid was used as the electrolyte. Tungsten foils were used as the current collectors to avert corrosion typically observed in KFSI-based electrolytes. FSI− can intercalate into graphite to form C6[FSI]0.5;490 thus attaining a maximum intercalation capacity of 186 mA h g−1. (1C = 186 mA g−1). | ||
Demonstrations of KIBs prototypes with high voltage, resulting in elevated energy density and high power output, show promising results. KIBs offer a notable advantage over LIBs and sodium-ion batteries, owing to the wealth of commercial knowledge and extensive research derived from the widespread commercialization and implementation of LIBs, along with the ongoing investigation into sodium-ion batteries. Particularly, the expertise in high-voltage cathode materials discovery and characterisation from previous research will significantly expedite the material development and prototyping processes for high-performance KIBs. Learning from past mistakes, both commercial and academic, presents an opportunity to advance KIB technology further. The pursuit of practical high-performance KIB materials is currently employing a cornucopia of ideas, philosophies, and strategies, ranging from drawing parallels with LIB and sodium-ion battery materials to computation-guided screening and direct syntheses of novel functional materials. The vast compositional space of electrode and electrolyte materials applicable to realising KIB full cells underscores the importance of leveraging knowledge derived from LIBs and sodium-ion batteries to make informed decisions on material selection and chemical systems to be pursued by researchers. An under-explored area in KIB research remains the cost analyses500 and environmental impact assessment. Although limited in scope for nascent KIBs, life-cycle assessment (LCA) proves to be a valuable tool for exploring potential combinations of high-energy-density electrodes, compatible electrolyte materials, and production processes with minimal environmental impact, ultimately leading to lower costs. Fig. 36 provides a succinct overview of the methodologies employable in research concerning potassium-ion batteries.
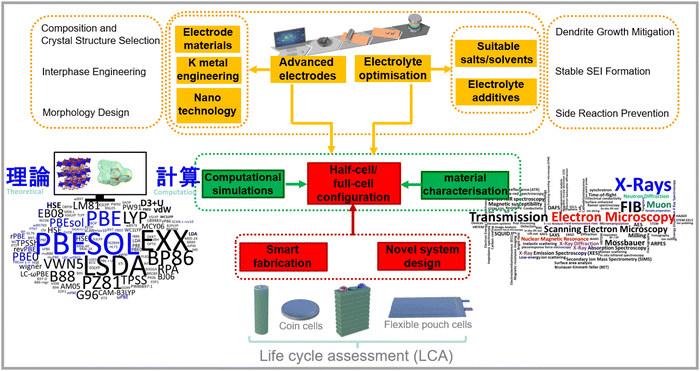 | ||
| Fig. 36 Overview of the methodologies employed in research concerning potassium-ion batteries. Figure adapted from ref. 6 with permission. Copyright 2019 American Association for the Advancement of Science. | ||
Furthermore, it is imperative to address practical considerations in the evaluation of KIB prototypes.501 Currently, relying solely on material-level and half-cell tests for performance assessment proves to be inconsistent and inadequate. Assembling full-battery devices introduces greater complexity, making it essential to recognise that evaluating electrodes at the half-battery level is tantamount to overestimating their practical application. Crucial details relating to battery assembly are often overlooked during experiments, such as the mass ratio of inactive components (current collectors, binders), electrolyte loading, electrode thickness, and active material loading. Consequently, a shift in focus towards performance evaluation based on KIB full battery measurements through configuration optimisation becomes necessary. This approach would provide a more comprehensive understanding of their real-world capabilities and limitations. Notably, early demonstrations of high-energy density KIB full cells and other potassium battery variants (Fig. 37) demonstrate promising potential for this sustainable battery class, which may serve as a captivating addition to the future energy storage landscape. In summary, the exciting prospects of KIBs and related potassium-based batteries lie in their potential contributions to a sustainable and diversified energy storage mix in the future. However, ensuring accurate performance evaluation at the full-battery level is crucial to realising their true capabilities and optimising their practical application.
 | ||
| Fig. 37 Diversity of energy storage systems based on potassium-ion technology. Such diversification has the potential to facilitate the expansion into a variety of distinct target markets. | ||
Anode and cathode material combinations, as detailed in laboratory-scale full-cell potassium-ion battery (KIB) configurations (several of which are illustrated in Fig. 33a), have undergone comprehensive assessment through a pragmatic lens, encompassing pouch cell design.501 Notably, non-aqueous KIB configurations leveraging graphite as the anode material have been shown to exhibit a propensity for yielding high energy densities. The peak of energy density achievement particularly in non-aqueous KIBs at the pouch cell level has been reported to culminate at approximately 150.0 W h kg−1 (attained by employing KVPO4F as the cathode material and graphite as the anode material).501 This significant metric is on par with prevailing lithium-ion battery (LIB) cells, where lithium iron phosphate (LiFePO4) serves as the cathode material and graphite as the anode material. The projected energy density enhances the potential for potassium-ion batteries to assert dominance within the mid- to low-tier market sectors, driven by their inherent cost-effectiveness. However, it should be acknowledged that the energy densities elucidated within the scope of laboratory-based cell configurations (such as those in Fig. 33a) lack a comprehensive consideration of the cumulative mass contributions of individual constituents comprising a KIB full-cell architecture—namely electrolytes, anodes (negative electrodes), cathodes (positive electrodes), separators, current collectors, and packaging casings. Therefore, the disclosed energy density values (Fig. 33a), whilst illustrative of maximal potential at the material level, are likely to exhibit much lower energy density values upon real-world full-cell evaluation, reflecting the multifaceted weight considerations intrinsic to practical implementations.
Fig. 38 displays the energy density of aqueous and non-aqueous potassium-ion batteries (KIBs) at the pouch cell level, calculated using various electrode combinations, as reported in ref. 501. Specifically, the figure illustrates that aqueous KIBs exhibit significantly lower energy density (<50 W h kg−1) compared to non-aqueous KIBs due to their lower operating voltages. Achieving an energy density of >150 W h kg−1 is a realistic prospect for non-aqueous KIB systems. Whilst this projected energy density still falls short of that achieved by current lithium-ion batteries (LIBs), further enhancements in energy density are expected through battery assembly engineering and optimisation of electrode materials. Notably, KIBs that use graphite as an anode and polyanion-based cathode materials, such as KVPO4F, demonstrate the highest energy density in terms of both high voltage and high capacity. Other cathode candidates include Prussian analogues, organic moieties, and manganese-based layered oxides, which show comparable energy density. Prussian analogues exhibit higher operating voltages but with lower specific capacities. Whilst research and development in practical KIBs are still in their early stages, there is ample room for exploration within the extensive electrode materials database to design high-energy-density KIBs surpassing >150 W h kg−1.
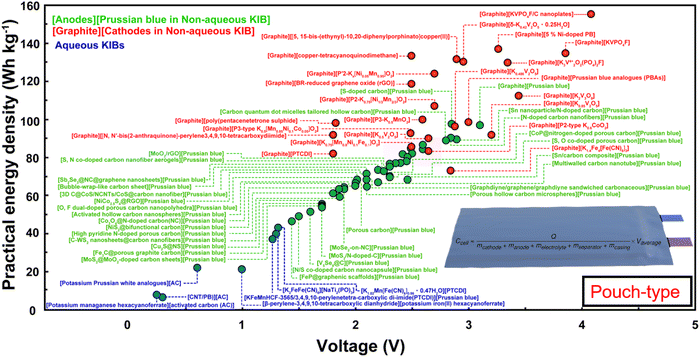 | ||
| Fig. 38 Practical energy density projected based on reported KIB full cell material combinations. Graph plotted from data derived from ref. 501. In some instances, Prussian blue has been abbreviated as ‘PB’ for brevity's sake. Figure adapted with permission.501 Copyright 2022 Springer Nature. | ||
4 Conclusion
This Perspective highlights significant advancements in high-performance cathode materials for potassium-ion batteries (KIBs), enabling readers to recognise the potential benefits of KIB technology when complementing lithium-ion battery (LIB) technology in high-voltage and high-power applications (Fig. 39a). Owing to the larger size of K+ compared to Li+, KIBs can be envisaged to effectively complement LIBs (Fig. 39b) in large-scale energy storage applications, addressing the limited paucity of terrestrial lithium reserves. One strategy to enhance KIB energy density involves designing suitable cathode materials and stable electrolytes for enduring long-term high-voltage operation. The practical evaluation of potassium-ion battery (KIB) full-cell designs envisions the possibility of achieving high voltage (in the 4 V range) and elevated energy density (>150 W h kg−1),501 positioning KIBs as competitive high-performance energy storage systems. Whilst KIB prototypes (designed at the laboratory scale) show promising electrochemical performance, proactive efforts from researchers are necessary to ensure the development of a commercially viable KIB and elevate the technology readiness level. This approach is crucial to meet the diverse energy demands of our society. | ||
| Fig. 39 (a) Applications envisioned for potassium-ion battery technology. Figure adapted from ref. 7 with permission. (b) Volumetric and gravimetric energy densities of rechargeable batteries. Figure adapted from ref. 124 with permission. | ||
Furthermore, there is untapped potential in exploring the fundamental scientific principles and technological applications of multifunctional potassium-based cathode materials, particularly their honeycomb layered counterparts.257,262 The field of KIBs holds uncharted territory, driving avant-garde innovation in K-ion technology. This journey encompasses a wide spectrum of discoveries, from novel phenomena in fundamental physics and chemistry, such as geometric and topological effects, to unprecedented degenerate cationic states triggering phase transitions through geometric distortions.235 Additionally, groundbreaking technologies like Kitaev materials502,503 in quantum computing504 and feasible applications in the realms of semiconductors and superconductors are emerging.505 The saying ‘strike while the iron is hot’, which resonated even in the Iron Age, finds its parallel in the realm of KIBs, urging researchers in this field to seize the opportunities that the era of potassium holds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support of AIST Edge Runners Funding, Japan Society for the Promotion of Science (JSPS KAKENHI Grant Number 23K04922), TEPCO Memorial Foundation and Japan Prize Foundation.References
- Z. Yang, J. Zhang, M. C. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS
.
-
S. Ash, Mineral commodity summaries 2019, US Geological Survey Reston, VA, 2019 Search PubMed
.
- C. Vaalma, D. Buchholz, M. Weil and S. Passerini, Nat. Rev. Mater., 2018, 3, 1–11 CrossRef
.
- R. Rajagopalan, Y. Tang, X. Ji, C. Jia and H. Wang, Adv. Funct. Mater., 2020, 30, 1909486 CrossRef CAS
.
- K. Yoshii, T. Masese, M. Kato, K. Kubota, H. Senoh and M. Shikano, ChemElectroChem, 2019, 6, 3901–3910 CrossRef CAS
.
- W. Zhang, Y. Liu and Z. Guo, Sci. Adv., 2019, 5, eaav7412 CrossRef CAS PubMed
.
- X. Min, J. Xiao, M. Fang, W. A. Wang, Y. Zhao, Y. Liu, A. M. Abdelkader, K. Xi, R. V. Kumar and Z. Huang, Energy Environ. Sci., 2021, 14, 2186–2243 RSC
.
- A. Eftekhari, Z. Jian and X. Ji, ACS Appl. Mater. Interfaces, 2017, 9, 4404–4419 CrossRef CAS PubMed
.
- X. Zhang, Z. Wei, K. N. Dinh, N. Chen, G. Chen, F. Du and Q. Yan, Small, 2020, 16, 2002700 CrossRef CAS
.
- Z.-X. Huang, Z.-Y. Gu, Y.-L. Heng, E. H. Ang, H.-B. Geng and X.-L. Wu, Chem. Eng. J., 2023, 452, 139438 CrossRef CAS
.
- M. G. T. Nathan, H. Yu, G.-T. Kim, J.-H. Kim, J. S. Cho, J. Kim and J.-K. Kim, Adv. Sci., 2022, 9, 2105882 CrossRef CAS
.
- L. Lander, G. Rousse, A. M. Abakumov, M. Sougrati, G. Van Tendeloo and J.-M. Tarascon, J. Mater. Chem. A, 2015, 3, 19754–19764 RSC
.
- C. Ling and F. Mizuno, J. Mater. Chem. A, 2013, 1, 8000–8006 RSC
.
- J. Liao, Q. Hu, Y. Du, J. Li, L. Duan, J. Bao and X. Zhou, Sci. Bull., 2022, 67, 2208–2215 CrossRef CAS PubMed
.
- J. Dong, J. Liao, X. He, Q. Hu, Y. Yu and C. Chen, Chem. Commun., 2020, 56, 10050–10053 RSC
.
- P. R. Kumar, T. Hosaka, T. Shimamura, D. Igarashi and S. Komaba, ACS Appl. Energy Mater., 2022, 5, 13470–13479 CrossRef CAS
.
- N. Recham, G. Rousse, M. T. Sougrati, J.-N. Chotard, C. Frayret, S. Mariyappan, B. C. Melot, J.-C. Jumas and J.-M. Tarascon, Chem. Mater., 2012, 24, 4363–4370 CrossRef CAS
.
- K. Chihara, A. Katogi, K. Kubota and S. Komaba, Chem. Commun., 2017, 53, 5208–5211 RSC
.
- S. S. Fedotov, N. R. Khasanova, A. S. Samarin, O. A. Drozhzhin, D. Batuk, O. M. Karakulina, J. Hadermann, A. M. Abakumov and E. V. Antipov, Chem. Mater., 2016, 28, 411–415 CrossRef CAS
.
- I. Gomez, O. Leonet, J. Alberto Blazquez, H.-J. Grande and D. Mecerreyes, ACS Macro Lett., 2018, 7, 419–424 CrossRef CAS PubMed
.
- Z. Jian, Y. Liang, I. A. Rodrguez-Pérez, Y. Yao and X. Ji, Electrochem. Commun., 2016, 71, 5–8 CrossRef CAS
.
- H. Wang, J. Lin and Z. X. Shen, J. Sci.: Adv. Mater. Devices, 2016, 1, 225–255 Search PubMed
.
- Y. Chen, W. Luo, M. Carter, L. Zhou, J. Dai, K. Fu, S. Lacey, T. Li, J. Wan and X. Han,
et al.
, Nano Energy, 2015, 18, 205–211 CrossRef CAS
.
- X. Wu, Z. Jian, Z. Li and X. Ji, Electrochem. Commun., 2017, 77, 54–57 CrossRef CAS
.
- A. Eftekhari, Z. Jian and X. Ji, ACS Appl. Mater. Interfaces, 2017, 9, 4404–4419 CrossRef CAS
.
- A. Eftekhari, J. Power Sources, 2004, 126, 221–228 CrossRef CAS
.
- Q. Song, Z. Li, S. Gan, W.-S. Dong, W. Wang, J. Zhang and Q. Yu, J. Mater. Chem. A, 2023, 11, 1532–1550 RSC
.
- S. Zhao, Z. Guo, K. Yan, X. Guo, S. Wan, F. He, B. Sun and G. Wang, Small Struct., 2021, 2, 2000054 CrossRef CAS
.
- P. Padigi, J. Thiebes, M. Swan, G. Goncher, D. Evans and R. Solanki, Electrochim. Acta, 2015, 166, 32–39 CrossRef CAS
.
- J. Cattermull, K. Sada, K. Hurlbutt, S. J. Cassidy, M. Pasta and A. L. Goodwin, Chem. Mater., 2022, 34, 5000–5008 CrossRef CAS PubMed
.
- R. Ma, Z. Wang, Q. Fu, W. Zhou, Y. Mo, J. Tu, Z. Wang, P. Gao, C. Fan and J. Liu, J. Energy Chem., 2023, 83, 16–23 CrossRef CAS
.
- Z. Li, B. Ozdogru, B. Bal, M. Bowden, A. Choi, Y. Zhang, H. Wang, V. Murugesan, V. G. Pol and Ö. Ö. Çapraz, Adv. Energy Mater., 2023, 13, 2301329 CrossRef CAS
.
- Y. Bai, K. Yuchi, X. Liu, S. Tian, S. Yang, X. Qian, B. Ma, M. Fang, Y. Liu and Z. Huang,
et al.
, Eur. J. Inorg. Chem., 2023, e202300246 CrossRef CAS
.
- L. Xue, Y. Li, H. Gao, W. Zhou, X. Lu, W. Kaveevivitchai, A. Manthiram and J. B. Goodenough, J. Am. Chem. Soc., 2017, 139, 2164–2167 CrossRef CAS PubMed
.
- M. Okoshi, Y. Yamada, S. Komaba, A. Yamada and H. Nakai, J. Electrochem. Soc., 2016, 164, A54 CrossRef
.
- L. Ni, G. Xu, C. Li and G. Cui, Exploration, 2022, 20210239 CrossRef
.
- E. Nightingale Jr, J. Phys. Chem., 1959, 63, 1381–1387 CrossRef
.
- Y. Matsuda, H. Nakashima, M. Morita and Y. Takasu, J. Electrochem. Soc., 1981, 128, 2552 CrossRef CAS
.
- M. Okoshi, Y. Yamada, S. Komaba, A. Yamada and H. Nakai, J. Electrochem. Soc., 2016, 164, A54 CrossRef
.
- S. Amara, J. Toulc'Hoat, L. Timperman, A. Biller, H. Galiano, C. Marcel, M. Ledigabel and M. Anouti, ChemPhysChem, 2019, 20, 581–594 CrossRef CAS PubMed
.
- Y. R. Dougassa, J. Jacquemin, L. El Ouatani, C. Tessier and M. Anouti, J. Phys. Chem. B, 2014, 118, 3973–3980 CrossRef CAS PubMed
.
- T. Hosaka, K. Kubota, A. S. Hameed and S. Komaba, Chem. Rev., 2020, 120, 6358–6466 CrossRef CAS PubMed
.
- Y. Marcus, Pure Appl. Chem., 1985, 57, 1129–1132 CrossRef CAS
.
- S. Komaba, T. Hasegawa, M. Dahbi and K. Kubota, Electrochem. Commun., 2015, 60, 172–175 CrossRef CAS
.
- Z. Jian, W. Luo and X. Ji, J. Am. Chem. Soc., 2015, 137, 11566–11569 CrossRef CAS PubMed
.
- W. Luo, J. Wan, B. Ozdemir, W. Bao, Y. Chen, J. Dai, H. Lin, Y. Xu, F. Gu and V. Barone,
et al.
, Nano Lett., 2015, 15, 7671–7677 CrossRef CAS PubMed
.
- X. Wang and H. Wang, Adv. Powder Mater., 2022, 1, 100057 CrossRef
.
- Z. Jian, W. Luo and X. Ji, J. Am. Chem. Soc., 2015, 137, 11566–11569 CrossRef CAS PubMed
.
- W. Luo, J. Wan, B. Ozdemir, W. Bao, Y. Chen, J. Dai, H. Lin, Y. Xu, F. Gu and V. Barone,
et al.
, Nano Lett., 2015, 15, 7671–7677 CrossRef CAS PubMed
.
- J. Liu, T. Yin, B. Tian, B. Zhang, C. Qian, Z. Wang, L. Zhang, P. Liang, Z. Chen and J. Yan,
et al.
, Adv. Energy Mater., 2019, 9, 1900579 CrossRef
.
- L. Fan, R. Ma, Q. Zhang, X. Jia and B. Lu, Angew. Chem., 2019, 131, 10610–10615 CrossRef
.
- H.-J. Liang, B.-H. Hou, W.-H. Li, Q.-L. Ning, X. Yang, Z.-Y. Gu, X.-J. Nie, G. Wang and X.-L. Wu, Energy Environ. Sci., 2019, 12, 3575–3584 RSC
.
-
A. Iyo, H. Ogino, S. Ishida and H. Esaki, Dramatically Accelerated Formation of Graphite Intercalation Compounds Catalyzed by Sodium, 2023 Search PubMed
.
- Y. Xu, T. Ding, D. Sun, X. Ji and X. Zhou, Adv. Funct. Mater., 2023, 33, 2211290 CrossRef CAS
.
- L. Duan, Y. Xu, Z. Zhang, J. Xu, J. Liao, J. Xu, Y. Sun, Y. He and X. Zhou, J. Mater. Chem. A, 2021, 9, 22820–22826 RSC
.
- Z. Zhang, Q. Hu, J. Liao, Y. Xu, L. Duan, R. Tian, Y. Du, J. Shen and X. Zhou, Nano Lett., 2023, 23, 694–700 CrossRef CAS
.
- L. Duan, J. Xu, Y. Xu, R. Tian, Y. Sun, C. Zhu, X. Mo and X. Zhou, J. Energy Chem., 2023, 76, 332–338 CrossRef CAS
.
- Y.-S. Xu, J.-C. Gao, X.-S. Tao, Y.-G. Sun, Y. Liu, A.-M. Cao and L.-J. Wan, ACS Appl. Mater. Interfaces, 2020, 12, 15313–15319 CrossRef CAS PubMed
.
- A. K. Pandey, B. D. Campéon, I. Konuma and N. Yabuuchi, Energy Adv., 2023, 2, 98–102 RSC
.
- Z. Zhang, J. Sun, L. Duan, Y. Du, J. Li, J. Shen and X. Zhou, J. Mater. Chem. A, 2022, 10, 554–560 RSC
.
- W. Ko, J. Kim, J. Kang, H. Park, Y. Lee, J. Ahn, B. Ku, M. Choi, H. Ahn and G. Oh,
et al.
, Mater. Today Energy, 2023, 101356 CrossRef CAS
.
- Y.-S. Xu, Y.-N. Zhou, Q.-H. Zhang, M.-Y. Qi, S.-J. Guo, J.-M. Luo, Y.-G. Sun, L. Gu, A.-M. Cao and L.-J. Wan, Chem. Eng. J., 2021, 412, 128735 CrossRef CAS
.
- F. Li, X. Gu, S. Wu, S. Dong, J. Wang, P. Dai, L. Li, D. Liu and M. Wu, Electrochim. Acta, 2023, 439, 141571 CrossRef CAS
.
- H. Kim, D.-H. Seo, J. C. Kim, S.-H. Bo, L. Liu, T. Shi and G. Ceder, Adv. Mater., 2017, 29, 1702480 CrossRef PubMed
.
- H. Kim, J. C. Kim, S.-H. Bo, T. Shi, D.-H. Kwon and G. Ceder, Adv. Energy Mater., 2017, 7, 1700098 CrossRef
.
- Y. Hironaka, K. Kubota and S. Komaba, Chem. Commun., 2017, 53, 3693–3696 RSC
.
- J.-Y. Hwang, J. Kim, T.-Y. Yu, S.-T. Myung and Y.-K. Sun, Energy Environ. Sci., 2018, 11, 2821–2827 RSC
.
- T. Deng, X. Fan, J. Chen, L. Chen, C. Luo, X. Zhou, J. Yang, S. Zheng and C. Wang, Adv. Funct. Mater., 2018, 28, 1800219 CrossRef
.
- J. U. Choi, J. Kim, J.-Y. Hwang, J. H. Jo, Y.-K. Sun and S.-T. Myung, Nano Energy, 2019, 61, 284–294 CrossRef CAS
.
- J. U. Choi, J. Kim, J. H. Jo, H. J. Kim, Y. H. Jung, D.-C. Ahn, Y.-K. Sun and S.-T. Myung, Energy Storage Mater., 2020, 25, 714–723 CrossRef
.
- T. Masese, K. Yoshii, M. Kato, K. Kubota, Z. Huang, H. Senoh and M. A. Shikano, Chem. Commun., 2019, 55, 985–988 RSC
.
- T. Masese, K. Yoshii, Y. Yamaguchi, T. Okumura, Z. Huang, M. Kato, K. Kubota, J. Furutani, Y. Orikasa, H. Senoh and O. Rechargeable, Nat. Commun., 2018, 9, 1–12 CrossRef CAS
.
- J. H. Jo, J. U. Choi, Y. J. Park, Y. H. Jung, D. Ahn, T.-Y. Jeon, H. Kim, J. Kim and S.-T. Myung, Adv. Energy Mater., 2020, 10, 1903605 CrossRef CAS
.
- J.-Y. Hwang, J. Kim, T.-Y. Yu, H.-G. Jung, J. Kim, K.-H. Kim and Y.-K. Sun, J. Mater. Chem. A, 2019, 7, 21362–21370 RSC
.
- B. Peng, Y. Li, J. Gao, F. Zhang, J. Li and G. Zhang, J. Power Sources, 2019, 437, 226913 CrossRef CAS
.
- L. Liu, J. Liang, W. Wang, C. Han, Q. Xia, X. Ke, J. Liu, Q. Gu, Z. Shi and S. Chou,
et al.
, ACS Appl. Mater. Interfaces, 2021, 13, 28369–28377 CrossRef CAS
.
- C.-L. Liu, S.-H. Luo, H.-B. Huang, X. Liu, Y.-C. Zhai and Z.-W. Wang, Chem. Eng. J., 2019, 378, 122167 CrossRef CAS
.
- N. Naveen, S. C. Han, S. P. Singh, D. Ahn, K.-S. Sohn and M. Pyo, J. Power Sources, 2019, 430, 137–144 CrossRef CAS
.
- J. U. Choi, Y. J. Park, J. H. Jo, Y. H. Jung, D.-C. Ahn, T.-Y. Jeon, K.-S. Lee, H. Kim, S. Lee and J. Kim,
et al.
, Energy Storage Mater., 2020, 27, 342–351 CrossRef
.
- M. K. Cho, J. H. Jo, J. U. Choi and S.-T. Myung, ACS Appl. Mater. Interfaces, 2019, 11, 27770–27779 CrossRef CAS PubMed
.
- J. Hao, K. Xiong, J. Zhou, A. M. Rao, X. Wang, H. Liu and B. Lu, Energy Environ. Mater., 2022, 5, 261–269 CrossRef CAS
.
- X. Zhang, D. Yu, Z. Wei, N. Chen, G. Chen, Z. X. Shen and F. Du, ACS Appl. Mater. Interfaces, 2021, 13, 18897–18904 CrossRef CAS
.
- J. Liang, C. Lin, X. Meng, M. Liang, J. Lai, X. Zheng, Q. Huang, L. Liu and Z. Shi, J. Mater. Chem. A, 2021, 9, 17261–17269 RSC
.
- C.-L. Liu, S.-H. Luo, H.-B. Huang, Y.-C. Zhai and Z.-W. Wang, ChemElectroChem, 2019, 6, 2308–2315 CrossRef CAS
.
- T. Liu, S. Hou, Y. Li, S. Xue, J. Hu, H. Fu, C. Yang and L. Zhao, J. Energy Chem., 2022, 64, 335–343 CrossRef CAS
.
- M. G. T. Nathan, N. Naveen, W. B. Park, K.-S. Sohn and M. Pyo, J. Power Sources, 2019, 438, 226992 CrossRef CAS
.
- C.-L. Liu, S.-H. Luo, H.-B. Huang, Y.-C. Zhai and Z.-W. Wang, Electrochim. Acta, 2018, 286, 114–122 CrossRef CAS
.
- R. Huang, Q. Xue, J. Lin, X. Zhang, J. Zhou, F. Wu, L. Li and R. Chen, Nano Res., 2022, 1–7 CAS
.
- M. G. T. Nathan, W. B. Park, N. Naveen, S. Park, K.-S. Sohn and M. Pyo, J. Electrochem. Soc., 2020, 167, 100507 CrossRef CAS
.
- R. Dang, Q.-B. Yan, E. Zhao, N. Li, K. Wu, Z. Chen, Z. Wu, X. Liu, Z. Hu and X. Xiao, Sci. China: Mater., 2022, 65, 1741–1750 CrossRef CAS
.
- J. Feng, S.-H. Luo, L. Yang, K. Cai, Y. Dou, Q. Wang, Y. Zhang and X. Liu, Energy Storage, 2022, 4, e277 CrossRef CAS
.
- Y.-S. Xu, Q.-H. Zhang, D. Wang, J.-C. Gao, X.-S. Tao, Y. Liu, Y.-G. Sun, L. Gu, B.-B. Chang and C.-T. Liu,
et al.
, Energy Storage Mater., 2020, 31, 20–26 CrossRef
.
- Z. Xiao, J. Meng, F. Xia, J. Wu, F. Liu, X. Zhang, L. Xu, X. Lin and L. Mai, Energy Environ. Sci., 2020, 13, 3129–3137 RSC
.
- X. Zhang, Y. Yang, X. Qu, Z. Wei, G. Sun, K. Zheng, H. Yu and F. Du, Adv. Funct. Mater., 2019, 29, 1905679 CrossRef CAS
.
- W. Zhong, X. Liu, Q. Cheng, T. Tan, Q. Huang, Q. Deng, J. Hu and C. Yang, Appl. Phys. Rev., 2021, 8, 031412 CAS
.
- Q. Deng, F. Zheng, W. Zhong, Q. Pan, Y. Liu, Y. Li, G. Chen, Y. Li, C. Yang and M. Liu, Chem. Eng. J., 2020, 392, 123735 CrossRef CAS
.
- Y. Huang, X. Zhang, H. Lin, Z. Wei, Y. Zeng, X. Ge, W. Zhang, X. Wang, X. Jin and Z. X. Shen,
et al.
, Chem. Eng. J., 2023, 453, 139571 CrossRef CAS
.
- P. Bai, K. Jiang, X. Zhang, J. Xu, S. Guo and H. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 10490–10495 CrossRef CAS PubMed
.
- R.-J. Luo, X.-L. Li, J.-Y. Ding, J. Bao, C. Ma, C.-Y. Du, X.-Y. Cai, X.-J. Wu and Y.-N. Zhou, Energy Storage Mater., 2022, 47, 408–414 CrossRef
.
- R. Dang, N. Li, Y. Yang, K. Wu, Q. Li, Y. L. Lee, X. Liu, Z. Hu and X. Xiao, J. Power Sources, 2020, 464, 228190 CrossRef CAS
.
- Z. Caixiang, J. Hao, J. Zhou, X. Yu and B. Lu, Adv. Energy Mater., 2023, 13, 2203126 CrossRef CAS
.
- J. Lv, B. Wang, J. Hao, H. Ding, L. Fan, R. Tao, H. Yang, J. Zhou and B. Lu, eScience, 2023, 3, 100081 CrossRef
.
- Q. Zhang, C. Didier, W. K. Pang, Y. Liu, Z. Wang, S. Li, V. K. Peterson, J. Mao and Z. Guo, Adv. Energy Mater., 2019, 9, 1900568 CrossRef
.
- J. Liao, Y. Han, Z. Zhang, J. Xu, J. Li and X. Zhou, Energy Environ. Mater., 2021, 4, 178–200 CrossRef CAS
.
- W. Li, Z. Bi, W. Zhang, J. Wang, R. Rajagopalan, Q. Wang, D. Zhang, Z. Li, H. Wang and B. Wang, J. Mater. Chem. A, 2021, 9, 8221–8247 RSC
.
- L. Wu, M. Gu, Y. Feng, S. Chen, L. Fan, X. Yu, K. Guo, J. Zhou and B. Lu, Adv. Funct. Mater., 2022, 32, 2109893 CrossRef CAS
.
- H. Kim, H. Ji, J. Wang and G. Ceder, Trends Chem., 2019, 1, 682–692 CrossRef CAS
.
- X. Zhang, D. Yang, X. Rui, Y. Yu and S. Huang, Curr. Opin. Electrochem., 2019, 18, 24–30 CrossRef CAS
.
- S. Wang, F. Chen, L.-M. Zhang, Y.-X. Li, N.-Q. Ren, K. Cao, J.-C. Xiao and C.-H. Chen, Nanoscale, 2022, 14, 5347–5355 RSC
.
- S. Wang, B. Cui, Q. Zhuang, Y. Shi and H. Zheng, Nanoscale Res. Lett., 2019, 14, 1–11 CrossRef PubMed
.
- Y. Lee, J.-K. Yoo, H. Park, W. Ko, J. Kang, J. H. Jo, G. Yoon, H.-G. Im, H. Yashiro and S.-T. Myung,
et al.
, J. Mater. Chem. A, 2021, 9, 5475–5484 RSC
.
- Z. Tai, M. Shi, S. Chong, Y. Chen, Q. Tan, C. Shu and Y. Liu, Sustainable Energy Fuels, 2019, 3, 736–743 RSC
.
- S. Wang, Y. Chen, B. Cui, B. Li, S. Wang, Y. Cui, Z. Ju and Q. Zhuang, Appl. Surf. Sci., 2020, 514, 145954 CrossRef CAS
.
-
Y. E. A. Sakurai, Post lithium ion secondary battery development, NTS Inc., Tokyo, 2023, pp. 12–28 Search PubMed
.
- X. Bie, K. Kubota, T. Hosaka, K. Chihara and S. Komaba, J. Mater. Chem. A, 2017, 5, 4325–4330 RSC
.
- S. Chong, Y. Chen, Y. Zheng, Q. Tan, C. Shu, Y. Liu and Z. Guo, J. Mater. Chem. A, 2017, 5, 22465–22471 RSC
.
- J. Liao, Q. Hu, Y. Yu, H. Wang, Z. Tang, Z. Wen and C. Chen, J. Mater. Chem. A, 2017, 5, 19017–19024 RSC
.
- X. Ma, Y. Guo, C. Yu, X. Chen, L. Gui, N. Cheng, J. Sun, P. Chen, J. Chen and Z. Zi,
et al.
, J. Alloys Compd., 2022, 904, 164049 CrossRef CAS
.
- S. Chong, Y. Wu, S. Guo, Y. Liu and G. Cao, Energy Storage Mater., 2019, 22, 120–127 CrossRef
.
- Y. Pei, C. Mu, H. Li, F. Li and J. Chen, ChemSusChem, 2018, 11, 1285–1289 CrossRef CAS PubMed
.
- Y. Lu, L. Wang, J. Cheng and J. B. Goodenough, Chem. Commun., 2012, 48, 6544–6546 RSC
.
- X. Bie, K. Kubota, T. Hosaka, K. Chihara and S. Komaba, J. Power Sources, 2018, 378, 322–330 CrossRef CAS
.
- L. Wang, J. Song, R. Qiao, L. A. Wray, M. A. Hossain, Y.-D. Chuang, W. Yang, Y. Lu, D. Evans and J.-J. Lee,
et al.
, J. Am. Chem. Soc., 2015, 137, 2548–2554 CrossRef CAS
.
- K. Kubota, M. Dahbi, T. Hosaka, S. Kumakura and S. Komaba, Chem. Rec., 2018, 18, 459–479 CrossRef CAS
.
- X. Wu, S. Qiu, Y. Liu, Y. Xu, Z. Jian, J. Yang, X. Ji and J. Liu, Adv. Mater., 2022, 34, 2106876 CrossRef CAS
.
- L. Deng, J. Qu, X. Niu, J. Liu, J. Zhang, Y. Hong, M. Feng, J. Wang, M. Hu and L. Zeng,
et al.
, Nat. Commun., 2021, 12, 2167 CrossRef CAS
.
- M. Tang, Y. Wu, Y. Chen, C. Jiang, S. Zhu, S. Zhuo and C. Wang, J. Mater. Chem. A, 2019, 7, 486–492 RSC
.
- S.-Y. Yang, Y.-J. Chen, G. Zhou and Z.-W. Fu, J. Electrochem. Soc., 2018, 165, A1422 CrossRef CAS
.
- M. Kato, T. Masese, M. Yao, N. Takeichi and T. Kiyobayashi, New J. Chem., 2019, 43, 1626–1631 RSC
.
- H. Gao, L. Xue, S. Xin and J. B. Goodenough, Angew. Chem., 2018, 130, 5547–5551 CrossRef
.
- W. Guo, Y.-X. Yin, S. Xin, Y.-G. Guo and L.-J. Wan, Energy Environ. Sci., 2012, 5, 5221–5225 RSC
.
- T. Zhou, W. Jin, W. Xue, B. Dai, C. Feng, X. Huang, P. Théato and Y. Li, J. Power Sources, 2021, 483, 229136 CrossRef CAS
.
- F. A. Obrezkov, A. F. Shestakov, V. F. Traven, K. J. Stevenson and P. A. Troshin, J. Mater. Chem. A, 2019, 7, 11430–11437 RSC
.
- L. Fan, Q. Liu, Z. Xu and B. Lu, ACS Energy Lett., 2017, 2, 1614–1620 CrossRef CAS
.
- F. A. Obrezkov, V. Ramezankhani, I. Zhidkov, V. F. Traven, E. Z. Kurmaev, K. J. Stevenson and P. A. Troshin, J. Phys. Chem. Lett., 2019, 10, 5440–5445 CrossRef CAS PubMed
.
- C. Li, J. Xue, A. Huang, J. Ma, F. Qing, A. Zhou, Z. Wang, Y. Wang and J. Li, Electrochim. Acta, 2019, 297, 850–855 CrossRef CAS
.
- K. Minami, T. Masese and K. Yoshii, New J. Chem., 2021, 45, 4921–4924 RSC
.
- Y. Chen, W. Luo, M. Carter, L. Zhou, J. Dai, K. Fu, S. Lacey, T. Li, J. Wan and X. Han,
et al.
, Nano Energy, 2015, 18, 205–211 CrossRef CAS
.
- R. R. Kapaev and P. A. Troshin, J. Mater. Chem. A, 2020, 8, 17296–17325 RSC
.
- J. J. Shea and C. Luo, ACS Appl. Mater. Interfaces, 2020, 12, 5361–5380 CrossRef CAS
.
- S. Xu, Y. Chen and C. Wang, J. Mater. Chem. A, 2020, 8, 15547–15574 RSC
.
- W. Zhang, W. Huang and Q. Zhang, Chem. – Eur. J., 2021, 27, 6131–6144 CrossRef PubMed
.
- M. Radhakrishnan, S. Prabhu, M. Arockiaraj and M. Arulperumjothi, Int. J. Quantum Chem., 2022, 122, e26818 CrossRef CAS
.
- Ž. Tomovi, M. D. Watson and K. Müllen, Angew. Chem., Int. Ed., 2004, 43, 755–758 CrossRef PubMed
.
- A. Soncini, E. Steiner, P. W. Fowler, R. W. Havenith and L. W. Jenneskens, Chem. – Eur. J., 2003, 9, 2974–2981 CrossRef CAS
.
- I. A. Popov and A. I. Boldyrev, Eur. J. Org. Chem., 2012, 3485–3491 CrossRef CAS
.
- K. Namsheer and C. S. Rout, RSC Adv., 2021, 11, 5659–5697 RSC
.
- A. Duan, Z. Wang, X. Huang and Y. Li, Angew. Chem., Int. Ed., 2023, e202302754 CAS
.
- J. Liao, Q. Hu, J. Mu, X. He, S. Wang and C. Chen, Chem. Commun., 2019, 55, 659–662 RSC
.
- A. S. Hameed, A. Katogi, K. Kubota and S. Komaba, Adv. Energy Mater., 2019, 9, 1902528 CrossRef CAS
.
- Y. Wang, X. Zhao, Y. Wang, W. Qiu, E. Song, S. Wang and J. Liu, ACS Appl. Mater. Interfaces, 2022, 15, 1129–1137 CrossRef PubMed
.
- A. Slesarenko, I. K. Yakuschenko, V. Ramezankhani, V. Sivasankaran, O. Romanyuk, A. V. Mumyatov, I. Zhidkov, S. Tsarev, E. Z. Kurmaev and A. F. Shestakov,
et al.
, J. Power Sources, 2019, 435, 226724 CrossRef CAS
.
- R. Shi, S. Jiao, Q. Yue, G. Gu, K. Zhang and Y. Zhao, Exploration, 2022, 20220066 CrossRef
.
- J. Hu, Y. Hong, M. Guo, Y. Hu, W. Tang, S. Xu, S. Jia, B. Wei, S. Liu and C. Fan,
et al.
, Energy Storage Mater., 2023, 56, 267–299 CrossRef
.
- X. Zhu, R. N. Ali, M. Song, Y. Tang and Z. Fan, Polymers, 2022, 14, 5538 CrossRef CAS PubMed
.
- V. Ramezankhani, I. K. Yakuschenko, S. Vasilyev, T. A. Savinykh, A. V. Mumyatov, I. S. Zhidkov, E. V. Shchurik, E. Z. Kurmaev, A. F. Shestakov and P. A. Troshin, J. Mater. Chem. A, 2022, 10, 3044–3050 RSC
.
- B. Tian, J. Zheng, C. Zhao, C. Liu, C. Su, W. Tang, X. Li and G.-H. Ning, J. Mater. Chem. A, 2019, 7, 9997–10003 RSC
.
- C. Masquelier and L. Croguennec, Chem. Rev., 2013, 113, 6552–6591 CrossRef CAS PubMed
.
- H. Kim, D.-H. Seo, M. Bianchini, R. J. Clément, H. Kim, J. C. Kim, Y. Tian, T. Shi, W.-S. Yoon and G. Ceder, Adv. Energy Mater., 2018, 8, 1801591 CrossRef
.
- A. Manthiram and J. Goodenough, J. Power Sources, 1989, 26, 403–408 CrossRef CAS
.
- X. Lin, J. Huang, H. Tan, J. Huang and B. Zhang, Energy Storage Mater., 2019, 16, 97–101 CrossRef
.
- J. Han, G.-N. Li, F. Liu, M. Wang, Y. Zhang, L. Hu, C. Dai and M. Xu, Chem. Commun., 2017, 53, 1805–1808 RSC
.
- Z. Zhang, R. Wang, Z. Chen, X. Liu, Z. Liu, J. Zeng, X. Zhao, K. Peng, Q. Yao and X. Zhang,
et al.
, Chem. Eng. J., 2022, 436, 135235 CrossRef CAS
.
- K.-Y. Zhang, Z.-Y. Gu, E. H. Ang, J.-Z. Guo, X.-T. Wang, Y. Wang and X.-L. Wu, Mater. Today, 2022, 54, 189–201 CrossRef CAS
.
- T. Hosaka, T. Shimamura, K. Kubota and S. Komaba, Chem. Rec., 2019, 19, 735–745 CrossRef CAS PubMed
.
- H. He, K. Cao, S. Guo, W. Yao, F. Chen and C.-H. Chen,
et al.
, J. Power Sources, 2023, 564, 232862 CrossRef CAS
.
- J. Wang, B. Ouyang, H. Kim, Y. Tian, G. Ceder and H. Kim, J. Mater. Chem. A, 2021, 9, 18564–18575 RSC
.
- S. Li, M. Li, R. Shen, Z. Liu, X. Lv, J. Su, H. Kuai and Y. Wen, J. Alloys Compd., 2023, 172446 CrossRef CAS
.
- T. Jenkins, J. A. Alarco and I. D. Mackinnon, ACS Omega, 2021, 6, 1917–1929 CrossRef CAS PubMed
.
- H.-X. Kuai, J.-F. Lu, X.-Y. Lv, Y.-F. Long and Y.-X. Wen, Ionics, 2022, 28, 3817–3831 CrossRef CAS
.
- X. Yang, D. Yan, T. Chou and J. C. Kim, J. Mater. Chem. A, 2023, 11, 14304–14310 RSC
.
- H. Park, W. Ko, Y. Lee, J. Kang, J. Ahn, J.-K. Yoo and J. Kim, J. Mater. Chem. A, 2021, 9, 11802–11811 RSC
.
- L. Larbi, R. Wernert, P. Fioux, L. Croguennec, L. Monconduit and C. Matei Ghimbeu, ACS Appl. Mater. Interfaces, 2023, 15, 18992–19001 CrossRef CAS PubMed
.
- J. Liao, C. Chen, Q. Hu, Y. Du, Y. He, Y. Xu, Z. Zhang and X. Zhou, Angew. Chem., 2021, 133, 25779–25786 CrossRef
.
- J. Li, Y. Zheng, K. San Hui, K. Wang, C. Zha, D. A. Dinh, J. Tu, Z. Shao and K. N. Hui, Energy Storage Mater., 2023, 102852 CrossRef
.
- W. B. Park, S. C. Han, C. Park, S. U. Hong, U. Han, S. P. Singh, Y. H. Jung, D. Ahn, K.-S. Sohn and M. Pyo, Adv. Energy Mater., 2018, 8, 1703099 CrossRef
.
- X.-D. He, J.-Y. Liao, T. Chen, L.-M. Zhang, X. Ding, J.-R. Wang, S. Wang, Z.-Y. Wen and C.-H. Chen, J. Alloys Compd., 2021, 884, 161126 CrossRef CAS
.
- I. V. Tereshchenko, D. A. Aksyonov, A. Zhugayevych, E. V. Antipov and A. M. Abakumov, Solid State Ionics, 2020, 357, 115468 CrossRef CAS
.
- M. Ohara, A. S. Hameed, K. Kubota, A. Katogi, K. Chihara, T. Hosaka and S. Komaba, Chem. Sci., 2021, 12, 12383–12390 RSC
.
- S. S. Fedotov, N. D. Luchinin, D. A. Aksyonov, A. V. Morozov, S. V. Ryazantsev, M. Gaboardi, J. R. Plaisier, K. J. Stevenson, A. M. Abakumov and E. V. Antipov, Nat. Commun., 2020, 11, 1484 CrossRef CAS PubMed
.
- O. Yakubovich, N. Khasanova and E. Antipov, Minerals, 2020, 10, 524 CrossRef CAS
.
- A. M. Abakumov, S. S. Fedotov, E. V. Antipov and J.-M. Tarascon, Nat. Commun., 2020, 11, 4976 CrossRef CAS PubMed
.
- H. He, K. Cao, S. Zeng, J. Si, Y. Zhu and C.-H. Chen, J. Power Sources, 2023, 587, 233715 CrossRef CAS
.
- L. Lander, G. Rousse, D. Batuk, C. V. Colin, D. A. Dalla Corte and J.-M. Tarascon, Inorg. Chem., 2017, 56, 2013–2021 CrossRef CAS PubMed
.
- I. Sultana, M. M. Rahman, S. Mateti, N. Sharma, S. Huang and Y. Chen, Batteries Supercaps, 2020, 3, 450–455 CrossRef CAS
.
- J. Bodart, N. Eshraghi, T. Carabin, B. Vertruyen, R. Cloots, F. Boschini and A. Mahmoud, J. Power Sources, 2020, 480, 229057 CrossRef CAS
.
- H.-X. Kuai, J.-F. Lu, X.-Y. Lv, Y.-F. Long and Y.-X. Wen, Ionics, 2022, 28, 3817–3831 CrossRef CAS
.
- S. Zheng, S. Cheng, S. Xiao, L. Hu, Z. Chen, B. Huang, Q. Liu, J. Yang and Q. Chen, J. Alloys Compd., 2020, 815, 152379 CrossRef CAS
.
- J. Huang, X. Cai, H. Yin, Y. Li, W. Lin, S. Huang and Y. Zhang, J. Phys. Chem. Lett., 2021, 12, 2721–2726 CrossRef CAS PubMed
.
- A. Bocchini, U. Gerstmann, T. Bartley, H.-G. Steinrück, G. Henkel and W. Schmidt, Phys. Rev. Mater., 2022, 6, 105401 CrossRef CAS
.
- R. Eremin, N. Kabanova, Y. A. Morkhova, A. Golov and V. Blatov, Solid State Ionics, 2018, 326, 188–199 CrossRef CAS
.
- K. Li, X. Fan, D. J. Singh and W. Zheng, J. Energy Chem., 2021, 54, 377–385 CrossRef CAS
.
- L. Wu and M. Liu, Ind. Eng. Chem. Res., 2007, 46, 6494–6500 CrossRef CAS
.
- W. Yuan, Q. Zhang, J. Kano and F. Saito,
et al.
, Powder Technol., 2014, 260, 22–26 CrossRef CAS
.
- H. B. Yahia, E. Gaudin and J. Darriet, Z. Naturforsch. B, 2007, 62, 873–880 CrossRef
.
- H. B. Yahia, R. Essehli, I. Belharouak and E. Gaudin, Mater. Res. Bull., 2015, 71, 7–10 CrossRef
.
- Y. Wang and R. Li, J. Solid State Chem., 2010, 183, 1221–1225 CrossRef CAS
.
- Z.-G. Hu, T. Higashiyama, M. Yoshimura, Y. K. Yap, Y. Mori and T. Sasaki, Jpn. J. Appl. Phys., 1998, 37, L1093 CrossRef CAS
.
- V. Krasilnikov and V. Bamburov, Dokl. Chem., 2007, 247–250 CrossRef CAS
.
- V. Krasilnikov, Russ. J. Inorg. Chem., 2007, 52, 258–262 CrossRef
.
- X. Ge, Y. Chen, S. Liu, X. Yang and K. Feng, J. Solid State Electrochem., 2022, 26, 1627–1636 CrossRef CAS
.
- R. Fehrmann, B. Krebs, G. Papatheodorou, R. W. Berg and N. J. Bjerrum, Inorg. Chem., 1986, 25, 1571–1577 CrossRef CAS
.
- S. F. Linnell, J. L. Payne, D. M. Pickup, A. V. Chadwick, A. R. Armstong and J. T. Irvine, J. Mater. Chem. A, 2020, 8, 19502–19512 RSC
.
- A. ElMaadi, A. Boukhari and E. Holt, J. Alloys Compd., 1995, 223, 13–17 CrossRef CAS
.
- R. Nath, D. Kasinathan, H. Rosner, M. Baenitz and C. Geibel, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 134451 CrossRef
.
- H. Yaghoobnejad Asl and A. Choudhury, Chem. Mater., 2016, 28, 5029–5036 CrossRef CAS
.
- H. Park, H. Kim, W. Ko, J. H. Jo, Y. Lee, J. Kang, I. Park, S.-T. Myung and J. Kim, Energy Storage Mater., 2020, 28, 47–54 CrossRef
.
- J. Kang, H. Park, W. Ko, Y. Lee, J. Ahn, J.-K. Yoo, S. H. Song, H. Kim and J. Kim, J. Mater. Chem. A, 2021, 9, 9898–9908 RSC
.
- F. Wang, S. Liu, Q. Jiang, K. Feng, X. Yang, X. Li, H. Zhang, M. Xia and H. Zhang, J. Power Sources, 2020, 452, 227835 CrossRef CAS
.
- S. Dai, T. Feng, C. Chen, X. Li and M. Wu, J. Electrochem. Soc., 2020, 167, 110517 CrossRef CAS
.
- J. Vaughey, W. T. Harrison and A. J. Jacobson, J. Solid State Chem., 1994, 110, 305–313 CrossRef CAS
.
- O. V. Yakubovich, E. V. Yakovleva, A. N. Golovanov, A. S. Volkov, O. S. Volkova, E. A. Zvereva, O. V. Dimitrova and A. N. Vasiliev, Inorg. Chem., 2013, 52, 1538–1543 CrossRef CAS PubMed
.
- A. Zhao, Y. Fang, X. Ai, H. Yang and Y. Cao, J. Energy Chem., 2021, 60, 635–648 CrossRef CAS
.
- L. Zhu, M. Wang, S. Xiang, D. Sun, Y. Tang and H. Wang, Adv. Energy Mater., 2023, 2302046 CrossRef CAS
.
- C. Delmas, C. Fouassier and P. Hagenmuller, Phys. B + C, 1980, 99, 81–85 CrossRef CAS
.
- J.-J. Braconnier, C. Delmas, C. Fouassier and P. Hagenmuller, Mater. Res. Bull., 1980, 15, 1797–1804 CrossRef CAS
.
- R. Hoppe and H. Sabrowsky, Z. Anorg. Allg. Chem., 1965, 339, 144–154 CrossRef
.
- C. Fouassier, C. Delmas and P. Hagenmuller, Mater. Res. Bull., 1975, 10, 443–449 CrossRef CAS
.
- C. Vaalma, G. A. Giffin, D. Buchholz and S. Passerini, J. Electrochem. Soc., 2016, 163, A1295 CrossRef CAS
.
- J. C. Pramudita, D. Sehrawat, D. Goonetilleke and N. Sharma, Adv. Energy Mater., 2017, 7, 1602911 CrossRef
.
- Y.-S. Xu, S.-J. Guo, X.-S. Tao, Y.-G. Sun, J. Ma, C. Liu and A.-M. Cao, Adv. Mater., 2021, 33, 2100409 CrossRef CAS PubMed
.
- Z. Xiao, F. Xia, L. Xu, X. Wang, J. Meng, H. Wang, X. Zhang, L. Geng, J. Wu and L. Mai, Adv. Funct. Mater., 2022, 32, 2108244 CrossRef CAS
.
- W. Li, Z. Bi, W. Zhang, J. Wang, R. Rajagopalan, Q. Wang, D. Zhang, Z. Li, H. Wang and B. Wang, J. Mater. Chem. A, 2021, 9, 8221–8247 RSC
.
- H. Park, Y. Lee, W. Ko, M. Choi, B. Ku, H. Ahn, J. Kim, J. Kang, J.-K. Yoo and J. Kim, Batteries Supercaps, 2023, 6, e202200486 CrossRef CAS
.
- X. Ding, Y. Wang, X. Wang, L. Geng, C. Guo, W. Liu, H. Wang, C. Sun and C. Han, Chem. Eng. J., 2023, 466, 143331 CrossRef CAS
.
- M. G. T. Nathan, H. Yu, G.-T. Kim, J.-H. Kim, J. S. Cho, J. Kim and J.-K. Kim, Adv. Sci., 2022, 9, 2105882 CrossRef CAS PubMed
.
- J. Liao, Y. Han, Z. Zhang, J. Xu, J. Li and X. Zhou, Energy Environ. Mater., 2021, 4, 178–200 CrossRef CAS
.
- Z. Liu, H. Su, Y. Yang, T. Wu, S. Sun and H. Yu, Energy Storage Mater., 2021, 34, 211–228 CrossRef
.
- Z. Wu, J. Zou, S. Chen, X. Niu, J. Liu and L. Wang, J. Power Sources, 2021, 484, 229307 CrossRef CAS
.
- B. Pandit, E. S. Goda, M. Ubaidullah, S. F. Shaikh, U. T. Nakate, A. P. Khedulkar, D. Kumar and R.-A. Doong,
et al.
, Ceram. Int., 2022, 48, 28856–28863 CrossRef CAS
.
- N. Naveen, W. B. Park, S. P. Singh, S. C. Han, D. Ahn, K.-S. Sohn and M. Pyo, Small, 2018, 14, 1803495 CrossRef PubMed
.
- H. Kim, D.-H. Seo, A. Urban, J. Lee, D.-H. Kwon, S.-H. Bo, T. Shi, J. K. Papp, B. D. McCloskey and G. Ceder, Chem. Mater., 2018, 30, 6532–6539 CrossRef CAS
.
- O. Smirnova, V. Nalbandyan, M. Avdeev, L. Medvedeva, B. Medvedev, V. Kharton and F. Marques, J. Solid State Chem., 2005, 178, 172–179 CrossRef CAS
.
- L. Duan, H. Tang, X. Xu, J. Liao, X. Li, G. Zhou and X. Zhou, Energy Storage Mater., 2023, 62, 102950 CrossRef
.
-
G. M. Kanyolo and T. Masese, Advances in Honeycomb Layered Oxides: Part II-Theoretical advances in the characterisation of honeycomb layered oxides with optimised lattices of cations, 2022 Search PubMed
.
- C.-L. Liu, S.-H. Luo, H.-B. Huang, Y.-C. Zhai and Z.-W. Wang, Chem. Eng. J., 2019, 356, 53–59 CrossRef CAS
.
- K. Sada and P. Barpanda, Chem. Commun., 2020, 56, 2272–2275 RSC
.
- T. Masese, K. Yoshii, K. Tada, M. Kato, S. Uchida, K. Kubota, T. Ina, T. Okumura, Z.-D. Huang and J. Furutani,
et al.
, Energy Technol., 2020, 8, 2000039 CrossRef CAS
.
- C. Liu, S. Luo, H. Huang, Z. Wang, A. Hao, Y. Zhai and Z. Wang, Electrochem. Commun., 2017, 82, 150–154 CrossRef CAS
.
- S. Zhao, K. Yan, P. Munroe, B. Sun and G. Wang, Adv. Energy Mater., 2019, 9, 1803757 CrossRef
.
- M. Clites, J. L. Hart, M. L. Taheri and E. Pomerantseva, ACS Energy Lett., 2018, 3, 562–567 CrossRef CAS
.
- B. Tian, W. Tang, C. Su and Y. Li, ACS Appl. Mater. Interfaces, 2018, 10, 642–650 CrossRef CAS PubMed
.
- K. Yuan, R. Ning, M. Bai, N. Hu, K. Zhang, J. Gu, Q. Li, Y. Huang, C. Shen and K. Xie, Energy Technol., 2020, 8, 1900796 CrossRef CAS
.
- A. Bhatia, J.-P. Pereira-Ramos, N. Emery and R. Baddour-Hadjean, Chem. Mater., 2021, 33, 5276–5289 CrossRef CAS
.
- X. Niu, J. Qu, Y. Hong, L. Deng, R. Wang, M. Feng, J. Wang, L. Zeng, Q. Zhang and L. Guo,
et al.
, J. Mater. Chem. A, 2021, 9, 13125–13134 RSC
.
- Y. Wu, G. Chen, X. Wu, L. Li, J. Yue, Y. Guan, J. Hou, F. Shi and J. Liang, J. Semiconductors, 2023, 44, 041701 CrossRef
.
- J. H. Jo, J.-Y. Hwang, J. U. Choi, H. J. Kim, Y.-K. Sun and S.-T. Myung, J. Power Sources, 2019, 432, 24–29 CrossRef CAS
.
- W. Sohn, J. S. Chae, G. H. Lim and K. C. Roh, J. Alloys Compd., 2022, 898, 162904 CrossRef CAS
.
- L. Wu, M. Gu, Y. Feng, S. Chen, L. Fan, X. Yu, K. Guo, J. Zhou and B. Lu, Adv. Funct. Mater., 2022, 32, 2109893 CrossRef CAS
.
- S. C. Han, W. B. Park, K.-S. Sohn and M. Pyo, Inorg. Chem. Front., 2020, 7, 2023–2030 RSC
.
- D. Sumner, Canadian Mathematical Bull., 1964, 7, 65–75 CrossRef
.
-
S. Troscheit, Mathematical Proceedings of the Cambridge Philosophical Society, 2014, pp. 295–311 Search PubMed
.
-
K. J. Falconer, Mathematical Proceedings of the Cambridge Philosophical Society, 2004, pp. 167–174 Search PubMed
.
- R. Darst, Proc. Am. Math. Soc., 1993, 119, 105–108 Search PubMed
.
- D. Groult, C. Mercey and B. Raveau, J. Solid State Chem. France, 1980, 32, 289–296 CrossRef CAS
.
- J. Liao, Q. Hu, X. Sheng, Z. Zhang, Y. Xu, X. Mo and X. Zhou, ACS Mater. Lett., 2022, 4, 1653–1659 CrossRef CAS
.
- G. M. Kanyolo, T. Masese, N. Matsubara, C.-Y. Chen, J. Rizell, Z.-D. Huang, Y. Sassa, M. Månsson, H. Senoh and H. Matsumoto, Chem. Soc. Rev., 2021, 50, 3990–4030 RSC
.
- T. Masese, Y. Miyazaki, G. Mbiti Kanyolo, T. Takahashi, M. Ito, H. Senoh and T. Saito, ACS Appl. Nano Mater., 2021, 4, 279–287 CrossRef CAS
.
- G. M. Kanyolo and T. Masese, J. Phys. Commun., 2022, 6, 041001 CrossRef
.
-
G. M. Kanyolo and T. Masese, Reproducing the asymptotic behaviour of galaxy rotation curves by a novel constraint in general relativity, 2021 Search PubMed
.
- T. Masese, G. M. Kanyolo, Y. Miyazaki, M. Ito, N. Taguchi, J. Rizell, S. Tachibana, K. Tada, Z.-D. Huang and A. Alshehabi,
et al.
, Adv. Sci., 2022, 2204672 Search PubMed
.
-
G. M. Kanyolo, T. Masese, A. Alshehabi and Z.-D. Huang, Advances in honeycomb layered oxides: Part I-Syntheses and Characterisations of Pnictogen-and Chalcogen-Based Honeycomb Layered Oxides, 2022 Search PubMed
.
- G. Kanyolo and T. Masese, Sci. Rep., 2020, 10, 1–13 CrossRef PubMed
.
- G. Kanyolo and T. Masese, Sci. Rep., 2022, 12, 1–14 CrossRef PubMed
.
- T. Masese, Y. Miyazaki, J. Rizell, G. Kanyolo, C. Chen, H. Ubukata, K. Kubota, K. Sau, T. Ikeshoji, Z. Huang and O. Mixed, Nat. Commun., 2021, 12, 1–16 CrossRef PubMed
.
- G. Kanyolo and T. Masese, Partition function for quantum gravity in 4 dimensions as a 1/N expansion, preprint: hal-03335930 technical report, 2021.
- Y. Kee, N. Dimov, A. Staykov and S. Okada, Mater. Lett., 2016, 183, 187–190 CrossRef CAS
.
- Y. Kee, B. Put, N. Dimov, A. Staykov and S. Okada, Batteries Supercaps, 2020, 3, 390–391 CrossRef CAS
.
- L. Xiao, Z. Din, Q. Huang, C. Chen, Y. Feng, C. Liang, P. Gao and W. Wei, Acta Mater., 2020, 199, 34–41 CrossRef CAS
.
- D. Bérardan, S. Franger, A. Meena and N. Dragoe, J. Mater. Chem. A, 2016, 4, 9536–9541 RSC
.
- C. M. Rost, E. Sachet, T. Borman, A. Moballegh, E. C. Dickey, D. Hou, J. L. Jones, S. Curtarolo and J.-P. Maria, Nat. Commun., 2015, 6, 1–8 Search PubMed
.
- Y. Cai, X. Zeng, D. Pang, Y. Gu, J. Qi, T. Masese, F. Chang, Y. Li, S. Wang and Z. Li,
et al.
, Materialia, 2023, 27, 101674 CrossRef CAS
.
- A. Sagot, L. Stievano and V. Pralong, ACS Appl. Energy Mater., 2023, 6, 7785–7789 CrossRef CAS
.
- S. Park, S. Park, Y. Park, M. H. Alfaruqi, J.-Y. Hwang and J. Kim, Energy Environ. Sci., 2021, 14, 5864–5874 RSC
.
- R. Maleki, M. Asadnia and A. Razmjou, Adv. Intell. Syst., 2022, 4, 2200073 CrossRef
.
- M.-F. Ng, Y. Sun and Z. W. Seh, Energy Adv., 2023, 2, 449–464 RSC
.
- Y. Chen and C. Wang, Acc. Chem. Res., 2020, 53, 2636–2647 CrossRef CAS PubMed
.
- A. Zhou, W. Cheng, W. Wang, Q. Zhao, J. Xie, W. Zhang, H. Gao, L. Xue and J. Li, Adv. Energy Mater., 2021, 11, 2000943 CrossRef CAS
.
- W. Shu, C. Han and X. Wang, Adv. Funct. Mater., 2023, 2309636 Search PubMed
.
- T. Hosaka, K. Kubota, H. Kojima and S. Komaba, Chem. Commun., 2018, 54, 8387–8390 RSC
.
- H. Onuma, K. Kubota, S. Muratsubaki, T. Hosaka, R. Tatara, T. Yamamoto, K. Matsumoto, T. Nohira, R. Hagiwara and H. Oji,
et al.
, ACS Energy Lett., 2020, 5, 2849–2857 CrossRef CAS
.
- Y. Liu, Z.-Y. Gu, Y.-L. Heng, J.-Z. Guo, M. Du, H.-J. Liang, J.-L. Yang, K.-Y. Zhang, K. Li and X.-L. Wu, Green Energy Environ., 2023 DOI:10.1016/j.gee.2023.10.004
.
- L. Wu, J. Sun, Y. Zhang, S. Jin, Y. Kong and J. Xu, Inorg. Chem., 2010, 49, 2715–2720 CrossRef CAS PubMed
.
- L. Wu, B. Wang, Y. Zhang, L. Li, H. Wang, H. Yi, Y. Kong and J. Xu, Dalton Trans., 2014, 43, 13845–13851 RSC
.
- Y. Yao, K. Satoh, T. Takahashi and T. Yoneyama, Soil Sci. Plant Nutrition, 2006, 52, 133 Search PubMed
.
- Y. Luo, Z. Xia, H. Liu and Y. He, Opt. Mater., 2014, 36, 723–726 CrossRef CAS
.
- E. Arroyabe and V. Kahlenberg, Eur. J. Mineral., 2011, 23, 101–110 CrossRef CAS
.
- Z. Leng, D. Zhang, H. Bai, P. She, J. Zhao and Z. Tang, Opt. Mater., 2021, 118, 111293 CrossRef CAS
.
- Y. Cai, W. Liu, F. Chang, S. Jin, X. Yang, C. Zhang, L. Bai, T. Masese, Z. Li and Z.-D. Huang, ACS Appl. Mater. Interfaces, 2023, 15, 48277–48286 CrossRef CAS PubMed
.
- V. S. K. Sungjemmenla, C. B. Soni, V. Kumar and Z. W. Seh, Energy Technol., 2022, 10, 2200421 CrossRef
.
- E. M. Erickson, W. Li, A. Dolocan and A. Manthiram, ACS Appl. Mater. Interfaces, 2020, 12, 16451–16461 CrossRef CAS PubMed
.
- K. Edstroem, T. Gustafsson and J. O. Thomas, Electrochim. Acta, 2004, 50, 397–403 CrossRef CAS
.
- H. Maleki Kheimeh Sari and X. Li, Adv. Energy Mater., 2019, 9, 1901597 CrossRef CAS
.
- S. Malmgren, K. Ciosek, M. Hahlin, T. Gustafsson, M. Gorgoi, H. Rensmo and K. Edström, Electrochim. Acta, 2013, 97, 23–32 CrossRef CAS
.
- K. Nie, Y. Hong, J. Qiu, Q. Li, X. Yu, H. Li and L. Chen, Front. Chem., 2018, 6, 616 CrossRef CAS
.
- M. Gauthier, T. J. Carney, A. Grimaud, L. Giordano, N. Pour, H.-H. Chang, D. P. Fenning, S. F. Lux, O. Paschos and C. Bauer,
et al.
, J. Phys. Chem. Lett., 2015, 6, 4653–4672 CrossRef CAS PubMed
.
- H. Liu, Y. He, K. Cao, S. Wang, Y. Jiang, X. Liu, K.-J. Huang, Q.-S. Jing and L. Jiao, Small, 2021, 17, 2008133 CrossRef CAS PubMed
.
- K. Cao, S. Wang, Y. Jia, D. Xu, H. Liu, K.-J. Huang, Q.-S. Jing and L. Jiao, Chem. Eng. J., 2021, 406, 126902 CrossRef CAS
.
- Y. Chen, H. Qin, J. Zhou, T. Yang, B. Sun, Y. Ni, H. Wang, S. A. Redfern, M. Miao and H.-Q. Lin,
et al.
, J. Phys. Chem. Lett., 2022, 13, 7439–7447 CrossRef CAS PubMed
.
- X. L. Huang, F. Zhao, Y. Qi, Y.-A. Qiu, J. S. Chen, H. K. Liu, S. X. Dou and Z. M. Wang, Energy Storage Mater., 2021, 42, 193–208 CrossRef
.
- Q. Yang, Z. Tai, Q. Xia, W. Lai, W. Wang, B. Zhang, Z. Yan, J. Peng, H. Yang and H. Liu,
et al.
, J. Mater. Chem. A, 2021, 9, 8378–8385 RSC
.
- E. J. Canto-Aguilar, M. A. Oliver-Tolentino, G. Ramos-Sánchez and I. González, Electrochim. Acta, 2021, 371, 137828 CrossRef CAS
.
- A. P. Vijaya Kumar Saroja, Z. Wang, H. R. Tinker, F. R. Wang, P. R. Shearing and Y. Xu, SusMat, 2023, 3, 222–234 CrossRef CAS
.
- C. He, J. Zou, C.-F. Sun and Q. Liu, Mater. Lett., 2023, 134627 CrossRef CAS
.
- X. Niu, Y. Zhang, L. Tan, Z. Yang, J. Yang, T. Liu, L. Zeng, Y. Zhu and L. Guo, Energy Storage Mater., 2019, 22, 160–167 CrossRef
.
- Y. Wu, H.-B. Huang, Y. Feng, Z.-S. Wu and Y. Yu, Adv. Mater., 2019, 31, 1901414 CrossRef CAS
.
- Y. Liu, Y.-X. Lu, Y.-S. Xu, Q.-S. Meng, J.-C. Gao, Y.-G. Sun, Y.-S. Hu, B.-B. Chang, C.-T. Liu and A.-M. Cao, Adv. Mater., 2020, 32, 2000505 CrossRef CAS
.
- I. Sultana, M. M. Rahman, Y. Chen and A. M. Glushenkov, Adv. Funct. Mater., 2018, 28, 1703857 CrossRef
.
- X. Yuan, B. Zhu, J. Feng, C. Wang, X. Cai and R. Qin, Chem. Eng. J., 2021, 405, 126897 CrossRef CAS
.
- K.-X. Lei, J. Wang, C. Chen, S.-Y. Li, S.-W. Wang, S.-J. Zheng and F.-J. Li, Rare Met., 2020, 39, 989–1004 CrossRef CAS
.
- S. Imtiaz, I. S. Amiinu, Y. Xu, T. Kennedy, C. Blackman and K. M. Ryan, Mater. Today, 2021, 48, 241–269 CrossRef CAS
.
- H. Gao, X. Guo, S. Wang, F. Zhang, H. Liu and G. Wang, EcoMat, 2020, 2, e12027 CrossRef CAS
.
- W. Li, Z. Li, C. Zhang, W. Liu, C. Han, B. Yan, S. An and X. Qiu, Solid State Ionics, 2020, 351, 115319 CrossRef CAS
.
- J. Wang, H. Wang, X. Zang, D. Zhai and F. Kang, Batteries Supercaps, 2021, 4, 554–570 CrossRef CAS
.
- G. Luo, X. Feng, M. Qian, W. Zhang, W. Qin, C. Wu and L. Pan, Mater. Chem. Front., 2023, 7, 3011–3036 RSC
.
- K.-H. Nam, V. Ganesan, K. H. Chae and C.-M. Park, Carbon, 2021, 181, 290–299 CrossRef CAS
.
- Z. Li, J. Wen, Y. Cai, F. Lv, X. Zeng, Q. Liu, T. Masese, C. Zhang, X. Yang and Y. Ma,
et al.
, Adv. Funct. Mater., 2023, 2300582 CrossRef CAS
.
- Y. Xu, J. Zhang and D. Li, Chem. – Asian J., 2020, 15, 1648–1659 CrossRef CAS PubMed
.
- S. Liu, L. Kang, J. Henzie, J. Zhang, J. Ha, M. A. Amin, M. S. A. Hossain, S. C. Jun and Y. Yamauchi, ACS Nano, 2021, 15, 18931–18973 CrossRef CAS PubMed
.
- J. Zheng, Y. Wu, Y. Sun, J. Rong, H. Li and L. Niu, Nano-Micro Lett., 2021, 13, 1–37 CrossRef CAS
.
- Z. Hong, H. Maleki, T. Ludwig, Y. Zhen, M. Wilhelm, D. Lee, K.-H. Kim and S. Mathur, J. Energy Chem., 2021, 62, 660–691 CrossRef CAS
.
- Y. Huang, R. Haider, S. Xu, K. Liu, Z.-F. Ma and X. Yuan, Energy Storage Mater., 2022, 51, 327–360 CrossRef
.
- N. Khan, G. Han and S. A. Mazari, J. Electroanal. Chem., 2022, 907, 116051 CrossRef CAS
.
- Q. Xue, D. Li, Y. Huang, X. Zhang, Y. Ye, E. Fan, L. Li, F. Wu and R. Chen, J. Mater. Chem. A, 2018, 6, 12559–12564 RSC
.
- J. Rehman, X. Fan, A. Laref, V. A. Dinh and W. Zheng, J. Alloys Compd., 2021, 865, 158782 CrossRef CAS
.
- G. Suo, Y. Cheng, J. Zhang and S. M. Ahmed, ChemNanoMat, 2021, 7, 1291–1308 CrossRef CAS
.
- S. Yu, S.-O. Kim, H.-S. Kim and W. Choi, Int. J. Energy Res., 2019, 43, 7646–7654 CrossRef CAS
.
- L. Zhang, W. Wang, S. Lu and Y. Xiang, Adv. Energy Mater., 2021, 11, 2003640 CrossRef CAS
.
- J. Wang, H. Wang, X. Zang, D. Zhai and F. Kang, Batteries Supercaps, 2021, 4, 554–570 CrossRef CAS
.
- T. Yamamoto, A. Yadav and T. Nohira, J. Electrochem. Soc., 2022, 169, 050507 CrossRef CAS
.
- J. Wang, Z. Liu, J. Zhou, K. Han and B. Lu, ACS Mater. Lett., 2021, 3, 1572–1598 CrossRef CAS
.
- L. Xue, H. Gao, W. Zhou, S. Xin, K. Park, Y. Li and J. B. Goodenough, Adv. Mater., 2016, 28, 9608–9612 CrossRef CAS PubMed
.
- S. Dong, Y. Song, Y. Fang, K. Zhu, K. Ye, Y. Gao, J. Yan, G. Wang and D. Cao, Carbon, 2021, 178, 1–9 CrossRef CAS
.
- Y.-Z. Fang, R. Hu, K. Zhu, K. Ye, J. Yan, G. Wang and D. Cao, Adv. Funct. Mater., 2020, 30, 2005663 CrossRef CAS
.
- X. Li, J. Li, L. Ma, C. Yu, Z. Ji, L. Pan and W. Mai, Energy Environ. Mater., 2022, 5, 458–469 CrossRef CAS
.
- H. Lei, J. Li, X. Zhang, L. Ma, Z. Ji, Z. Wang, L. Pan, S. Tan and W. Mai, InfoMat, 2022, 4, e12272 CrossRef CAS
.
- Y.-Y. Zhu, Y.-H. Wang, Y.-T. Wang, T.-J. Xu and P. Chang, Carbon Energy, 2022, 4, 1182–1213 CrossRef CAS
.
- M. Shen, H. Ding, L. Fan, A. M. Rao, J. Zhou and B. Lu, Adv. Funct. Mater., 2023, 2213362 CrossRef CAS
.
- F. Yuan, Y. Li, D. Zhang, Z. Li, H. Wang, B. Wang, Y. Wu and Y. A. Wu, Inorg. Chem. Front., 2023, 10, 2547–2573 RSC
.
- A. K. Thakur, M. S. Ahmed, J. Park, R. Prabakaran, S. Sidney, R. Sathyamurthy, S. C. Kim, S. Periasamy, J. Kim and J.-Y. Hwang, Int. J. Energy Res., 2022, 46, 4033–4070 CrossRef CAS
.
- Y.-h Wu, X.-n Wu, Y.-y Guan, Y. Xu, F.-n Shi and J.-y Liang, New Carbon Mater., 2022, 37, 852–874 CrossRef CAS
.
- T. Zhu, B. Mai, P. Hu, C. Cai, B. Xing, Z. Wei, C. Chen, H. Fan, M. Li and X. Wang, ACS Appl. Energy Mater., 2023, 6, 2370–2377 CrossRef CAS
.
- W.-J. Deng, X.-D. He, L.-M. Zhang, J.-R. Wang and C.-H. Chen, Energy Technol., 2021, 9, 2100644 CrossRef CAS
.
- J. Zou, C. He, J. Bao, C.-F. Sun and Y. Li, Ionics, 2023, 1–7 Search PubMed
.
- Y. Liu, Y.-X. Lu, Y.-S. Xu, Q.-S. Meng, J.-C. Gao, Y.-G. Sun, Y.-S. Hu, B.-B. Chang, C.-T. Liu and A.-M. Cao, Adv. Mater., 2020, 32, 2000505 CrossRef CAS PubMed
.
- X. Wu, Y. Chen, Z. Xing, C. W. K. Lam, S.-S. Pang, W. Zhang and Z. Ju, Adv. Energy Mater., 2019, 9, 1900343 CrossRef
.
- Z. Jian, S. Hwang, Z. Li, A. S. Hernandez, X. Wang, Z. Xing, D. Su and X. Ji, Adv. Funct. Mater., 2017, 27, 1700324 CrossRef
.
- Z. Tai, Q. Zhang, Y. Liu, H. Liu and S. Dou, Carbon, 2017, 123, 54–61 CrossRef CAS
.
- L. Wang, J. Yang, J. Li, T. Chen, S. Chen, Z. Wu, J. Qiu, B. Wang, P. Gao and X. Niu,
et al.
, J. Power Sources, 2019, 409, 24–30 CrossRef CAS
.
- Z. Xing, Y. Qi, Z. Jian and X. Ji, ACS Appl. Mater. Interfaces, 2017, 9, 4343–4351 CrossRef CAS PubMed
.
- X. Yuan, B. Zhu, J. Feng, C. Wang, X. Cai and R. Qin, Chem. Eng. J., 2021, 405, 126897 CrossRef CAS
.
- J. Zhou and S. Guo, SmartMat, 2021, 2, 176–201 CrossRef CAS
.
- X. Wang, K. Han, D. Qin, Q. Li, C. Wang, C. Niu and L. Mai, Nanoscale, 2017, 9, 18216–18222 RSC
.
- X. Li, J. Li, L. Ma, C. Yu, Z. Ji, L. Pan and W. Mai, Energy Environ. Mater., 2022, 5, 458–469 CrossRef CAS
.
- M. Sha, L. Liu, H. Zhao and Y. Lei, Carbon Energy, 2020, 2, 350–369 CrossRef CAS
.
- X. He, J. Liao, Z. Tang, L. Xiao, X. Ding, Q. Hu, Z. Wen and C. Chen, J. Power Sources, 2018, 396, 533–541 CrossRef CAS
.
- Q. Wang, C. Gao, W. Zhang, S. Luo, M. Zhou, Y. Liu, R. Liu, Y. Zhang, Z. Wang and A. Hao, Electrochim. Acta, 2019, 324, 134902 CrossRef CAS
.
- Y. Feng, S. Chen, D. Shen, J. Zhou and B. Lu, Energy Environ. Mater., 2021, 4, 451–457 CrossRef CAS
.
- Z. Wu, J. Zou, S. Shabanian, K. Golovin and J. Liu, Chem. Eng. J., 2022, 427, 130972 CrossRef CAS
.
- F. Yuan, J. Hu, Y. Lei, R. Zhao, C. Gao, H. Wang, B. Li, F. Kang and D. Zhai, ACS Nano, 2022, 16, 12511–12519 CrossRef CAS
.
- J. Han, M. Xu, Y. Niu, G.-N. Li, M. Wang, Y. Zhang, M. Jia and C. ming Li, Chem. Commun., 2016, 52, 11274–11276 RSC
.
- Y. Dong, Z.-S. Wu, S. Zheng, X. Wang, J. Qin, S. Wang, X. Shi and X. Bao, ACS Nano, 2017, 11, 4792–4800 CrossRef CAS PubMed
.
- W. D. McCulloch, X. Ren, M. Yu, Z. Huang and Y. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 26158–26166 CrossRef CAS PubMed
.
- M. Shimizu, R. Yatsuzuka, T. Koya, T. Yamakami and S. Arai, ACS Appl. Energy Mater., 2018, 1, 6865–6870 CrossRef CAS
.
- K. Cao, H. Liu, W. Li, C. Xu, Q. Han, Z. Zhang and L. Jiao, J. Electroanal. Chem., 2019, 841, 51–55 CrossRef CAS
.
- C. Liu, H. Wang, S. Zhang, M. Han, Y. Cao, S. Liu, Z. Yang, A. Chen and J. Sun, Nanoscale, 2020, 12, 11427–11434 RSC
.
- B. Kishore, G. Venkatesh and N. Munichandraiah, J. Electrochem. Soc., 2016, 163, A2551 CrossRef CAS
.
- S. Zhao, L. Dong, B. Sun, K. Yan, J. Zhang, S. Wan, F. He, P. Munroe, P. H. Notten and G. Wang, Small, 2020, 16, 1906131 CrossRef CAS PubMed
.
- P. R. Kumar, K. Kubota, D. Igarashi and S. Komaba, J. Phys. Chem., 2021, 125, 24823–24830 CAS
.
- N. Voronina, J. H. Jo, A. Konarov, J. Kim and S.-T. Myung, Small, 2020, 16, 2001090 CrossRef CAS PubMed
.
- J. Han, Y. Niu, S.-J. Bao, Y.-N. Yu, S.-Y. Lu and M. Xu, Chem. Commun., 2016, 52, 11661–11664 RSC
.
- K. Lei, F. Li, C. Mu, J. Wang, Q. Zhao, C. Chen and J. Chen, Energy Environ. Sci., 2017, 10, 552–557 RSC
.
- I. Sultana, M. M. Rahman, Y. Chen and A. M. Glushenkov, Adv. Funct. Mater., 2018, 28, 1703857 CrossRef
.
- J. Huang, X. Lin, H. Tan and B. Zhang, Adv. Energy Mater., 2018, 8, 1703496 CrossRef
.
- I. Sultana, T. Ramireddy, M. M. Rahman, Y. Chen and A. M. Glushenkov, Chem. Commun., 2016, 52, 9279–9282 RSC
.
- S. Chong, M. Ma, L. Yuan, S. Qiao, S. Dong, H. Liu and S. Dou, Energy Environ. Mater., 2023, e12458 CrossRef CAS
.
- T. Sun, J. Xie, W. Guo, D.-S. Li and Q. Zhang, Adv. Energy Mater., 2020, 10, 1904199 CrossRef CAS
.
- L. Zhu, G. Ding, L. Xie, X. Cao, J. Liu, X. Lei and J. Ma, Chem. Mater., 2019, 31, 8582–8612 CrossRef CAS
.
- E. R. Wolfson, L. Schkeryantz, E. M. Moscarello, J. P. Fernandez, J. Paszek, Y. Wu, C. M. Hadad and P. L. McGrier, ACS Appl. Mater. Interfaces, 2021, 13, 41628–41636 CrossRef CAS PubMed
.
- J. Sun, Y. Xu, A. Li, R. Tian, Y. Fei, B. Chen and X. Zhou, ACS Appl. Nano Mater., 2022, 5, 15592–15599 CrossRef CAS
.
- H. Wang, D. Zhai and F. Kang, Energy Environ. Sci., 2020, 13, 4583–4608 RSC
.
- F. Yuan, Z. Li, D. Zhang, Q. Wang, H. Wang, H. Sun, Q. Yu, W. Wang and B. Wang, Adv. Sci., 2022, 9, 2200683 CrossRef PubMed
.
- X. Zhang, J. Meng, X. Wang, Z. Xiao, P. Wu and L. Mai, Energy Storage Mater., 2021, 38, 30–49 CrossRef
.
- H. Wang, J. Hu, J. Dong, K. C. Lau, L. Qin, Y. Lei, B. Li, D. Zhai, Y. Wu and F. Kang, Adv. Energy Mater., 2019, 9, 1902697 CrossRef CAS
.
- N. Daumas and A. Hérold, Sci. Paris C, 1969, 268, 373 CAS
.
- A. Lerf, Dalton Trans., 2014, 43, 10276–10291 RSC
.
- W. Rüdorff and U. Hofmann, Z. Anorg. Allg. Chem., 1938, 238, 1–50 CrossRef
.
- A. Kushima, X. Qian, P. Zhao, S. Zhang and J. Li, Nano Lett., 2015, 15, 1302–1308 CrossRef CAS PubMed
.
- M. Barsoum and G. Tucker, Scr. Mater., 2017, 139, 166–172 CrossRef CAS
.
- J. G. McHugh, P. Mouratidis and K. Jolley, Phys. Rev. B, 2021, 103, 195436 CrossRef CAS
.
- H. O. Badr and M. W. Barsoum, Carbon, 2023, 201, 599–615 CrossRef CAS
.
- M. W. Barsoum, Front. Mater., 2020, 7, 146 CrossRef
.
- Y. Lei, D. Han, J. Dong, L. Qin, X. Li, D. Zhai, B. Li, Y. Wu and F. Kang, Energy Storage Mater., 2020, 24, 319–328 CrossRef
.
- J. Park, Y. Jeong, M. H. Alfaruqi, Y. Liu, X. Xu, S. Xiong, M.-G. Jung, H.-G. Jung, J. Kim and J.-Y. Hwang,
et al.
, ACS Energy Lett., 2021, 7, 401–409 CrossRef
.
- M. Zhou, P. Bai, X. Ji, J. Yang, C. Wang and Y. Xu, Adv. Mater., 2021, 33, 2003741 CrossRef CAS PubMed
.
- Y. Liu, C. Gao, L. Dai, Q. Deng, L. Wang, J. Luo, S. Liu and N. Hu, Small, 2020, 16, 2004096 CrossRef CAS PubMed
.
- Y. Lei, L. Qin, R. Liu, K. C. Lau, Y. Wu, D. Zhai, B. Li and F. Kang, ACS Appl. Energy Mater., 2018, 1, 1828–1833 CrossRef CAS
.
- J. Li, Y. Hu, H. Xie, J. Peng, L. Fan, J. Zhou and B. Lu, Angew. Chem., 2022, 134, e202208291 CrossRef
.
- L. Fan, Y. Hu, A. M. Rao, J. Zhou, Z. Hou, C. Wang and B. Lu, Small Methods, 2021, 5, 2101131 CrossRef CAS PubMed
.
- Y. Hu, L. Fan, A. M. Rao, W. Yu, C. Zhuoma, Y. Feng, Z. Qin, J. Zhou and B. Lu, Natl. Sci. Rev., 2022, 9, nwac134 CrossRef CAS PubMed
.
- T. Hosaka, T. Matsuyama, K. Kubota, S. Yasuno and S. Komaba, ACS Appl. Mater. Interfaces, 2020, 12, 34873–34881 CrossRef CAS
.
- T. Hosaka, T. Fukabori, T. Matsuyama, R. Tatara, K. Kubota and S. Komaba, ACS Energy Lett., 2021, 6, 3643–3649 CrossRef CAS
.
- M. Zhou, Y. Fan, Y. Gao, Z. Ma, Z. Liu, W. Wang, H. A. Younus, Z. Chen, X. Wang and S. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 44429–44438 CrossRef CAS PubMed
.
- Z. T. Gossage, T. Hosaka, T. Matsuyama, R. Tatara and S. Komaba, J. Mater. Chem. A, 2023, 11, 914–925 RSC
.
- J. Popovic, J. Electrochem. Soc., 2022, 169, 030510 CrossRef CAS
.
- Z. Zhao, H. Zhang, F. Li, L. Zhao, Q. Li and H. Li, Nano Lett., 2022, 22, 10102–10110 CrossRef CAS PubMed
.
- H. Wang, D. Zhai and F. Kang, Energy Environ. Sci., 2020, 13, 4583–4608 RSC
.
- G. Liu, Z. Cao, L. Zhou, J. Zhang, Q. Sun, J.-Y. Hwang, L. Cavallo, L. Wang, Y.-K. Sun and J. Ming, Adv. Funct. Mater., 2020, 30, 2001934 CrossRef CAS
.
- G. Zeng, S. Xiong, Y. Qian, L. Ci and J. Feng, J. Electrochem. Soc., 2019, 166, A1217–A1222 CrossRef CAS
.
- S. Ji, J. Li, J. Li, C. Song, S. Wang, K. Wang, K. S. Hui, C. Zha, Y. Zheng and D. A. Dinh,
et al.
, Adv. Funct. Mater., 2022, 32, 2200771 CrossRef CAS
.
- L. Fan, H. Xie, Y. Hu, Z. Caixiang, A. M. Rao, J. Zhou and B. Lu, Energy Environ. Sci., 2023, 16, 305–315 RSC
.
- X. Feng, Z. Zhang, R. Li, W. Xiong, B. Yu, M. Wang, J. Chen, Z. Ma, B. Guo and Y. Huang,
et al.
, Sustainable Energy Fuels, 2022, 6, 2198–2206 RSC
.
- Y. Gu, S. Fang, L. Yang and S.-I. Hirano, ACS Appl. Energy Mater., 2021, 4, 4919–4927 CrossRef CAS
.
- Y. Zheng, D. Wang, S. Kaushik, S. Zhang, T. Wada, J. Hwang, K. Matsumoto and R. Hagiwara, EnergyChem, 2022, 4, 100075 CrossRef CAS
.
- T. Yamamoto, R. Matsubara and T. Nohira, J. Chem. Eng. Data, 2021, 66, 1081–1088 CrossRef CAS
.
- T. Yamamoto, A. Yadav and T. Nohira, J. Electrochem. Soc., 2022, 169, 050507 CrossRef CAS
.
- T. Yamamoto and T. Nohira, Chem. Rec., 2023, e202300169 CrossRef CAS PubMed
.
- T. Yamamoto, Electrochemistry, 2022, 90, 101005 CrossRef CAS
.
- W. Zhang, H. Tian, J. Wang, H. Sun, J. Wang and W. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 38887–38894 CrossRef CAS PubMed
.
- H. Sun, P. Liang, G. Zhu, W. H. Hung, Y.-Y. Li, H.-C. Tai, C.-L. Huang, J. Li, Y. Meng and M. Angell,
et al.
, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 27847–27853 CrossRef CAS PubMed
.
- M. Fiore, S. Wheeler, K. Hurlbutt, I. Capone, J. Fawdon, R. Ruffo and M. Pasta, Chem. Mater., 2020, 32, 7653–7661 CrossRef CAS
.
- K. Beltrop, S. Beuker, A. Heckmann, M. Winter and T. Placke, Energy Environ. Sci., 2017, 10, 2090–2094 RSC
.
- T. Yamamoto, K. Matsumoto, R. Hagiwara and T. Nohira, J. Phys. Chem. C, 2017, 121, 18450–18458 CrossRef CAS
.
- M. Arnaiz, A. Bothe, S. Dsoke, A. Balducci and J. Ajuria, J. Electrochem. Soc., 2019, 166, A3504 CrossRef CAS
.
- W. Zhou, M. Zhang, X. Kong, W. Huang and Q. Zhang, Adv. Sci., 2021, 8, 2004490 CrossRef CAS PubMed
.
- Y. Chen, S. Liu, Z. Bi, Z. Li, F. Zhou, R. Shi and T. Mu, Green Energy Environ., 2023 DOI:10.1016/j.gee.2023.05.002
.
- I. A. Shkrob, L. A. Robertson, Z. Yu, R. S. Assary, L. Cheng, L. Zhang, E. Sarnello, X. Liu, T. Li and A. P. Kaur,
et al.
, J. Mol. Liq., 2021, 334, 116533 CrossRef CAS
.
- H. Yamamoto, C.-Y. Chen, K. Kubota, K. Matsumoto and R. Hagiwara, J. Phys. Chem. B, 2020, 124, 6341–6347 CrossRef CAS PubMed
.
- L. Schkeryantz, P. Nguyen, W. D. McCulloch, C. E. Moore, K. C. Lau and Y. Wu, J. Phys. Chem. C, 2022, 126, 11407–11413 CrossRef CAS
.
- J. Chen, S. Lei, S. Zhang, C. Zhu, Q. Liu, C. Wang, Z. Zhang, S. Wang, Y. Shi and L. Yin,
et al.
, Adv. Funct. Mater., 2023, 2215027 CrossRef CAS
.
- X. Zhang, T. Xiong, B. He, S. Feng, X. Wang, L. Wei and L. Mai, Energy Environ. Sci., 2022, 15, 3750–3774 RSC
.
- L. Jiang, Y. Lu, C. Zhao, L. Liu, J. Zhang, Q. Zhang, X. Shen, J. Zhao, X. Yu and H. Li,
et al.
, Nat. Energy, 2019, 4, 495–503 CrossRef CAS
.
- J. Han, A. Mariani, S. Passerini and A. Varzi, Energy Environ. Sci., 2023, 16, 1480–1501 RSC
.
- P. Kulkarni, D. Ghosh and R. G. Balakrishna, Sustainable Energy Fuels, 2021, 5, 1619–1654 RSC
.
- D. P. Leonard, Z. Wei, G. Chen, F. Du and X. Ji, ACS Energy Lett., 2018, 3, 373–374 CrossRef CAS
.
- H. Chen, Z. Zhang, Z. Wei, G. Chen, X. Yang, C. Wang and F. Du, Sustainable Energy Fuels, 2020, 4, 128–131 RSC
.
- L. Droguet, A. Grimaud, O. Fontaine and J.-M. Tarascon, Adv. Energy Mater., 2020, 10, 2002440 CrossRef CAS
.
- D. Xiao, L. Zhang, Z. Li, H. Dou and X. Zhang, Energy Storage Mater., 2022, 44, 10–28 CrossRef
.
- J.-F. Wu, W. Zhou, Z. Wang, W.-W. Wang, X. Lan, H. Yan, T. Shi, R. Hu, X. Cui and C. Xu,
et al.
, Adv. Mater., 2023, 2209833 CrossRef CAS PubMed
.
- H. Fei, Y. Liu, Y. An, X. Xu, J. Zhang, B. Xi, S. Xiong and J. Feng, J. Power Sources, 2019, 433, 226697 CrossRef CAS
.
- G. Du, M. Tao, D. Liu, M. K. Aslam, Y. Qi, J. Jiang, Y. Li, S.-J. Bao and M. Xu, J. Colloid Interface Sci., 2021, 582, 932–939 CrossRef CAS PubMed
.
-
T. Masese and G. M. Kanyolo, Storing Energy, Elsevier, 2022, pp. 265–307 Search PubMed
.
- J. Zheng, H. Fang, L. Fan, Y. Ren, P. Jena and Y. Wu, J. Phys. Chem. Lett., 2021, 12, 7120–7126 CrossRef CAS PubMed
.
- H. Aziam, B. Larhrib, C. Hakim, N. Sabi, H. B. Youcef and I. Saadoune, Renewable Sustainable Energy Rev., 2022, 167, 112694 CrossRef CAS
.
- H. Fei, Y. Liu, Y. An, X. Xu, G. Zeng, Y. Tian, L. Ci, B. Xi, S. Xiong and J. Feng, J. Power Sources, 2018, 399, 294–298 CrossRef CAS
.
- E. Manarin, F. Corsini, S. Trano, L. Fagiolari, J. Amici, C. Francia, S. Bodoardo, S. Turri, F. Bella and G. Griffini, ACS Appl. Polym. Mater., 2022, 4, 3855–3865 CrossRef CAS PubMed
.
- M. Elmanzalawy, E. Sanchez-Ahijón, O. Kisacik, J. Carretero-González and E. Castillo-Martnez, ACS Appl. Energy Mater., 2022, 5, 9009–9019 CrossRef CAS
.
- A. D. Khudyshkina, P. A. Morozova, A. J. Butzelaar, M. Hoffmann, M. Wilhelm, P. Theato, S. S. Fedotov and F. Jeschull, ACS Appl. Polym. Mater., 2022, 4, 2734–2746 CrossRef CAS
.
- A. D. Khudyshkina, A. J. Butzelaar, Y. Guo, M. Hoffmann, T. Bergfeldt, M. Schaller, S. Indris, M. Wilhelm, P. Théato and F. Jeschull, Electrochim. Acta, 2023, 454, 142421 CrossRef CAS
.
- Y. Zhang, A. Bahi, F. Ko and J. Liu, Small, 2022, 18, 2107186 CrossRef CAS PubMed
.
- J. Zheng, B. Perry and Y. Wu, ACS Mater. Au, 2021, 1, 92–106 CrossRef CAS PubMed
.
- Y. Zhang, P. Qiu, J. Zheng, X. Chen, X.-M. Chen, S. Li, C. Ji, Y. Wu and X. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 17378–17387 CrossRef CAS PubMed
.
- J. Shao, J. Zheng, L. Qin, S. Zhang, Y. Ren and Y. Wu, Angew. Chem., 2022, 134, e202200606 CrossRef
.
- H. Yuan, H. Li, T. Zhang, G. Li, T. He, F. Du and S. Feng, J. Mater. Chem. A, 2018, 6, 8413–8418 RSC
.
- Y.-J. Gu, Y.-A. Lo, A.-C. Li, Y.-C. Chen, J.-H. Li, Y.-S. Wang, H.-K. Tian, W. Kaveevivitchai and C.-W. Kung, ACS Appl. Energy Mater., 2022, 5, 8573–8580 CrossRef CAS
.
- E. Burmakin, B. Antonov and G. S. Shekhtman, Inorg. Mater., 2010, 46, 540–544 CrossRef CAS
.
- G. Nechaev and E. Burmakin, Russ. J. Electrochem., 2011, 47, 1411–1414 CrossRef CAS
.
- E. Burmakin and G. Nechaev, Russ. J. Electrochem., 2013, 49, 1001–1003 CrossRef CAS
.
- V. R. Jeedi, K. K. Ganta, Y. Mallaiah, R. Swarnalatha, S. N. Reddy and A. S. Chary, J. Polym. Res., 2022, 29, 64 CrossRef CAS
.
- G. Nechaev and E. Burmakin, Russ. J. Electrochem., 2011, 47, 457–460 CrossRef CAS
.
- E. Burmakin, G. Nechaev and G. S. Shekhtman, Russ. J. Electrochem., 2008, 44, 1386–1392 CrossRef CAS
.
- E. Burmakin, G. Nechaev, G. S. Shekhtman and S. Plaksin, Russ. J. Electrochem., 2009, 45, 934–937 CrossRef CAS
.
- E. Burmakin and G. S. Shekhtman, Inorg. Mater., 2008, 44, 882–885 CrossRef CAS
.
- N. V. Proskurnina, V. I. Voronin, G. S. Shekhtman, L. N. Maskaeva, N. A. Kabanova, A. A. Kabanov and V. A. Blatov, J. Phys. Chem. C, 2017, 121, 21128–21135 CrossRef CAS
.
- T. Takahashi and K. Kuwabara, Electrochim. Acta, 1978, 23, 375–379 CrossRef CAS
.
- S. Yamamoto, S. Tamura and N. Imanaka, J. Alloys Compd., 2006, 418, 226–229 CrossRef CAS
.
- Y. W. Kim, A. Oda and N. Imanaka, Electrochem. Commun., 2003, 5, 94–97 CrossRef CAS
.
- J.-M. Doux, N. Stephant, A. L. G. La Salle, O. Joubert, D. Guyomard and E. Quarez, CrystEngComm, 2019, 21, 594–601 RSC
.
- J.-M. Doux, L. Leguay, A. L. G. La Salle, O. Joubert and E. Quarez, Solid State Ionics, 2018, 324, 260–266 CrossRef CAS
.
- E. Wang and M. Greenblatt, Chem. Mater., 1991, 3, 542–546 CrossRef CAS
.
- C. Delmas, A. Maazaz and P. Hagenmuller, Solid State Ionics, 1983, 9, 83–88 CrossRef
.
- A. Maazaz and C. Delmas, Solid State Ionics, 1982, 6, 261–266 CrossRef CAS
.
- J. Zheng, L. Schkeryantz, G. Gourdin, L. Qin and Y. Wu, ACS Appl. Energy Mater., 2021, 4, 4156–4164 CrossRef CAS
.
- B. Babu, C. Neumann, S. Muench, M. Enke, L. Medenbach, C. Leibing, A. Lex-Balducci, A. Turchanin, U. S. Schubert and A. Balducci, Energy Storage Mater., 2023, 56, 342–350 CrossRef
.
- V. Madhani, M. S. Rathore and D. Kumar, High Performance Polymers, 2023, 35, 28–35 CrossRef CAS
.
- A. Kotronia, K. Edström, D. Brandell and H. D. Asfaw, Adv. Energy Sustainability Res., 2022, 3, 2100122 CrossRef CAS
.
- J. Grins and M. Nygren, Solid State Ionics, 1983, 9, 869–876 CrossRef
.
- A. Haffner, A.-K. Hatz, O. E. Zeman, C. Hoch, B. V. Lotsch and D. Johrendt, Angew. Chem., Int. Ed., 2021, 60, 13641–13646 CrossRef CAS PubMed
.
- J. Shao, H. Ao, L. Qin, J. Elgin, C. E. Moore, Y. Khalifa, S. Zhang and Y. Wu, Adv. Mater., 2023, 2306809 CrossRef CAS PubMed
.
- Y. Okada, A. Nasu, T. Kimura, H. Tsukasaki, S. Mori, H. Ben Yahia, K. Motohashi, A. Sakuda and A. Hayashi, Chem. Mater., 2023, 35, 7422–7429 CrossRef CAS
.
- B. He, A. Ye, S. Chi, P. Mi, Y. Ran, L. Zhang, X. Zou, B. Pu, Q. Zhao and Z. Zou,
et al.
, Sci. Data, 2020, 7, 153 CrossRef CAS PubMed
.
- V. Gulino, A. Wolczyk, A. A. Golov, R. A. Eremin, M. Palumbo, C. Nervi, V. A. Blatov, D. M. Proserpio and M. Baricco, Inorg. Chem. Front., 2020, 7, 3115–3125 RSC
.
- R. A. Eremin, P. N. Zolotarev, A. A. Golov, N. A. Nekrasova and T. Leisegang, J. Phys. Chem. C, 2019, 123, 29533–29542 CrossRef CAS
.
- L. Fiedler, K. Shah, M. Bussmann and A. Cangi, Phys. Rev. Mater., 2022, 6, 040301 CrossRef CAS
.
- A. P. Shevchenko, R. A. Eremin and V. A. Blatov, CrystEngComm, 2020, 22, 7298–7307 RSC
.
- R. Xiao, H. Li and L. Chen, J. Mater. Chem. A, 2020, 8, 5157–5162 RSC
.
- Y. Wang, S. Wu, W. Shao, X. Sun, Q. Wang, R. Xiao and H. Li, J. Materiomics, 2022, 8, 1038–1047 CrossRef
.
- S. Wang, Y. Jiang and X. Hu, Adv. Mater., 2022, 34, 2200945 CrossRef CAS PubMed
.
- M. Fiore, S. Wheeler, K. Hurlbutt, I. Capone, J. Fawdon, R. Ruffo and M. Pasta, Chem. Mater., 2020, 32, 7653–7661 CrossRef CAS
.
- K. V. Kravchyk, P. Bhauriyal, L. Piveteau, C. P. Guntlin, B. Pathak and M. V. Kovalenko, Nat. Commun., 2018, 9, 1–9 CrossRef CAS PubMed
.
- Y. Li, W. Deng, Z. Zhou, C. Li, M. Zhang, X. Yuan, J. Hu, H. Chen and R. Li, J. Mater. Chem. A, 2021, 9, 2822–2829 RSC
.
- J. Ge, X. Yi, L. Fan and B. Lu, J. Energy Chem., 2021, 57, 28–33 CrossRef CAS
.
- Y. Hu, W. Tang, Q. Yu, X. Wang, W. Liu, J. Hu and C. Fan, Adv. Funct. Mater., 2020, 30, 2000675 CrossRef CAS
.
- B. Ji, F. Zhang, X. Song and Y. Tang, Adv. Mater., 2017, 29, 1700519 CrossRef PubMed
.
- J. Zhu, Y. Li, B. Yang, L. Liu, J. Li, X. Yan and D. He, Small, 2018, 14, 1801836 CrossRef PubMed
.
- L. Fan, Q. Liu, S. Chen, K. Lin, Z. Xu and B. Lu, Small, 2017, 13, 1701011 CrossRef PubMed
.
- A. Yu, Q. Pan, M. Zhang, D. Xie and Y. Tang, Adv. Funct. Mater., 2020, 30, 2001440 CrossRef CAS
.
- B. Ji, F. Zhang, N. Wu and Y. Tang, Adv. Energy Mater., 2017, 7, 1700920 CrossRef
.
- M. Zhang, J. Zhong, W. Kong, L. Wang, T. Wang, H. Fei, H. Luo, J. Zhu, J. Hu and B. Lu, Energy Environ. Mater., 2021, 4, 413–420 CrossRef CAS
.
- Z. Yan and M. Obrovac, J. Power Sources, 2020, 464, 228228 CrossRef CAS
.
- L. Sun, G. Li, S. Zhang, S. Liu, J. Yuwono, J. Mao and Z. Guo, Sci. China: Chem., 2022, 1–9 Search PubMed
.
- S. Trebst and C. Hickey, Phys. Rep., 2022, 950, 1–37 CrossRef CAS
.
- S. M. Winter, A. A. Tsirlin, M. Daghofer, J. van den Brink, Y. Singh, P. Gegenwart and R. Valent, J. Phys.: Condens. Matter, 2017, 29, 493002 CrossRef PubMed
.
- A. Kitaev, Ann. Phys., 2006, 321, 2–111 CAS
.
- Y.-Z. You, I. Kimchi and A. Vishwanath, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 085145 CrossRef
.
| This journal is © The Royal Society of Chemistry 2024 |