Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A toolbox for improved recycling of critical metals and materials in low-carbon technologies
Guillaume
Zante
 a,
Christopher E.
Elgar
a,
Christopher E.
Elgar
 a,
Jennifer M.
Hartley
a,
Jennifer M.
Hartley
 ae,
Rudra
Mukherjee
b,
Jeff
Kettle
ae,
Rudra
Mukherjee
b,
Jeff
Kettle
 b,
Louise E.
Horsfall
b,
Louise E.
Horsfall
 c,
Allan
Walton
de,
Gavin D. J.
Harper
c,
Allan
Walton
de,
Gavin D. J.
Harper
 de and
Andrew P.
Abbott
de and
Andrew P.
Abbott
 *ae
*ae
aSchool of Chemistry, University of Leicester, Leicester, LE1 7RH, UK. E-mail: apa1@leicester.ac.uk
bJames Watt School of Engineering, University of Glasgow, Glasgow, G12 8QQ, UK
cInstitute of Quantitative Biology, Biochemistry and Biotechnology, School of Biological Sciences, University of Edinburgh, Edinburgh, EH9 3FF, UK
dSchool of Metallurgy and Materials, University of Birmingham, Birmingham, UK
eThe Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot, UK
First published on 16th January 2024
Abstract
The shift towards renewable energy sources combined with other factors, such as population increase, digitalisation, and a need to decrease carbon footprint, leads to increasing metal consumption. To meet this growing demand and avoid accumulation of waste in landfills, efficient recycling methods are needed. Current pyrometallurgical and hydrometallurgical methods achieve complete digestion of end-of-life materials using high temperatures and high consumption of chemicals, respectively. These methods can be applied to recover critical metals from end-of-life materials but suffer from inherent limitations when it comes to complex end-of-life materials made of interpenetrated layers of metals, inorganics and organics. This critical review describes a set of chemical and physical tools for improved recovery of metals from various waste streams, with a strong focus on the renewable energy sector (wind turbines, solar cells) as well as lithium-ion batteries and catalysts for hydrogen production. These tools target weaknesses at the interfaces between different layers to liberate the valuable metals. Physical methods used for size reduction and separation, ultrasound to process brittle materials, hydrogen decrepitation, selective dissolution and bio-metallurgical methods to process metals are among those reviewed. Management of inorganic and organic fractions is also emphasised, with pyrolysis and solvolysis to process organics and ways to recycle these materials. Limitations and future directions are discussed, providing a comprehensive guide to improve recycling of metals with versatile tools.
Sustainability spotlightClimate action and access to clean energy is heavily reliant on metals. Recycling of metals from renewable energy production and storage devices is highly desirable but is challenging due to the complex architecture of these devices, made of interpenetrated layers of organics, inorganic and dispersed metals. In this critical review, we highlight and discuss different technologies that can potentially reduce the environmental impact of current recycling methods and improve the global recycling rates. This review falls under multiple UN sustainable goals such as goal 12 (responsible consumption and production), goal 7 (affordable and clean energy production) and goal 13 (climate action). |
1 Introduction
Industry 4.0 coupled with an expanding world population is driving an increase in metal consumption and the complexity of material structures. This is exacerbated by the transition from fossil fuels to renewable energy sources1 and the need for devices such as batteries, fuel cells, solar cells, wind turbines, display devices, light emitting diodes (LEDs) lighting as well as the electronics that enable them. There is a need to transition towards Net Zero solutions, and reduce carbon emissions in all areas of our society. This will require widespread technological and societal change and technology transition in many different areas. Future transition pathways will require more sophisticated technologies to enable decarbonisation, with an increasing array of elements employed2 in more sophisticated technological structures than incumbent technologies. The trend toward complicated architectures with thin, multi-layer coatings is causing issues with dilute, complex waste-streams containing dispersed technology critical metals (TCMs). Those elements are increasingly important to the economy due to the aforementioned reasons, and are at risk of supply disruption due to a combination of factors (geopolitical, geological, economic…).Bulk alloys based on iron and aluminium are mainly used for construction, automotive and machinery and are relatively easy to collect and separate by mechanical sorting and pyrometallurgical methods. This makes up the majority of the metal recycling market as these metals are a major component of construction and transportation infrastructures. The main limitation of pyrometallurgical methods is that they are innately energy intensive. Certain other metals such as lead or platinum group metals (PGMs) are used on a lower volume but their environmental toxicity, ease of recycling and high value ensures their recycling efficiency is higher as it is economically profitable to do so and legislation generally ensures they are processed in a circular economy.
Recycling of TCMs as presently conceived, generally includes their liberation from their initial casing using pyrometallurgical or other physical methods (comminution). Further purification and recovery usually requires the use of hydrometallurgical methods. Limitations of these methods are the high consumption of chemicals (particularly water) and the generation of wastes, which emphasises the need for circular processes, where chemicals and water are re-used over several (typically >10) cycles.3
Waste Electronic and Electrical Equipment (WEEE) is one of the fastest growing sources of waste worldwide due to the rapidly increasing disposal of malfunctioning or obsolete electronic devices. WEEE consists of an array of discarded electronic products such as computers, mobile phones, televisions, fridges, medical devices and other electronic appliances that have reached the end of their useful life. An abundant literature is available on metal recycling from WEEE, where authors mainly aim to recover precious metals from printed circuit boards (PCBs). However, WEEE suffers from poor levels of recyclability4,5 which can be linked to high statistical entropy6–8 and low metal content, and ultimately unfavourable economics. The amount of TCMs in WEEE is generally low, and taking into account the low collection rates, absence of detailed material stack information about various state-of-the-art products (adhesives and encapsulants), and difficulties to recycle the products, it unfortunately often leads to almost negligible recovery.9 Recycling these products is however important from an environmental point of view and there is a clear need for an improved regulatory framework as well as technologies to improve collection, sorting and ultimately, recycling rates. To do so, digital products passports10,11 based on blockchain and automatic sorting12 using artificial intelligence are emerging tools that will certainly help improve recycling rates.13
The aforementioned issues are less pronounced for emerging sources of end-of-life materials from clean energy technologies. Solar panels or wind turbines can be found in a single location and do not move throughout their lifetime (their lifespan being predictable), facilitating their collection and producing more constant waste flows. Electric vehicles also provide a source of large concentrations of TCMs and although they are mobile, extended producer responsibility (EPR) is likely to enable their pre-sorting prior to processing when a mature market has established.14 There are calls for EPR to be extended to all aspects of sustainable energy production including wind and solar power, though clearly logistical and legal challenges exist with such long-lifetime products. Most western countries have targets15 for recycling rates, such as those set by the European Union WEEE directive, but in most cases these fall short of defining who is responsible for recycling the products as well as reaching their national targets.
Increased content of critical materials and increased economic importance and value of the metals used (e.g. PGMs for hydrogen production) would certainly lead to economically profitable recycling, lower statistical entropy and higher recyclability of these products. However, valuable metals are also located within complex, multi-layer architectures which are not designed to be recycled. This is why efficient recycling processes with workflows for systemic removal/recycling of protective and encasing layers, and separation from bulk substrates and active materials (like semiconductors) are urgently needed, particularly to reduce demand for carbon-intensive mineral extraction. According to the international energy agency16 and considering a “sustainable development” scenario, the demand for rare earth elements for wind turbines in 2040 would be higher than 10 kt (while it is lower than 5 kt in 2020). Demand for copper and silicon for solar cells would be close to 1000 and 800 kt, respectively (increasing from less than 400 kt for both copper and silicon in 2020). Cumulative demand for battery materials required for electric vehicles (lithium, nickel, cobalt, manganese and graphite) by 2040 could be close to 12, 000 kt while it is lower than 1000 kt in 2020.
Moreover, increased use of these technologies obviously lead to a surge in end-of-life materials generation, with more than 1200 GW h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 of spent batteries available by 2040 and a cumulative amount of up to 78 million tonnes17 of end-of-life solar panels available worldwide by 2050. 421 MW of wind power was decommissioned in Europe in 2018.18 It is estimated that 424 to 3305 GW of wind power will be installed by 2040,19 each of those wind turbines having a lifetime of 20–30 years and containing 25 to 65 kg of NdFeB magnets20 per GW and 10 tonnes of blade materials21 per GW. A significant proportion of PGMs supply comes from recycling,22 with 37 tons of waste PGMs produced from end-of-life vehicles in China alone in 2020.23 The catalysts for proton exchange membrane (PEM) fuel cells need around 300 g of platinum and 700 g for iridium per MW, with an increasing global PEM manufacturing capacity of around 500 MW per year at the moment.16
16 of spent batteries available by 2040 and a cumulative amount of up to 78 million tonnes17 of end-of-life solar panels available worldwide by 2050. 421 MW of wind power was decommissioned in Europe in 2018.18 It is estimated that 424 to 3305 GW of wind power will be installed by 2040,19 each of those wind turbines having a lifetime of 20–30 years and containing 25 to 65 kg of NdFeB magnets20 per GW and 10 tonnes of blade materials21 per GW. A significant proportion of PGMs supply comes from recycling,22 with 37 tons of waste PGMs produced from end-of-life vehicles in China alone in 2020.23 The catalysts for proton exchange membrane (PEM) fuel cells need around 300 g of platinum and 700 g for iridium per MW, with an increasing global PEM manufacturing capacity of around 500 MW per year at the moment.16
With current metallurgical processes, it is challenging to deal with complex materials composed of different layers (metals, inorganics and organics wrapped together). Metal recovery and separation is not straightforward, as metal concentrations and compositions are unknown when compared to traditional ores. Current purification technologies have been developed for separation of critical metals from ores (often separated as by-products of other metals like zinc and copper). However, the composition of end-of-life materials differs greatly from primary minerals. Therefore, a process developed for metal purification within the frame of primary minerals extraction will need to be adjusted (in the best case scenario), in order to take into account differences in the concentration of target metal, nature of competing ions, presence of plastics. In most cases, a new process will be required for each recycled product. Current techniques often oxidise metals, generally after shredding of the end-of-life materials, while the inorganic and organic layers are usually lost. TCMs are often further diluted and lost during the process, which contributes to lower recycling rates.
With both pyrometallurgy and hydrometallurgy, a large amount of energy and/or chemicals is needed to purify the metals, while large investments are needed to treat the hazardous gas or wastewater produced from the process. Both techniques are well-known and have been used for centuries to process minerals, but their application to metal-containing end-of-life materials is challenging, particularly due to the presence of layers of inorganics and organics.
From a sustainability perspective, the main issue with hydrometallurgy is that stoichiometric reagents (usually oxidants) are used. It should be highlighted that aqua-regia or other strong oxidising agents are often used, and this is primarily sulfuric acid which is often from waste sources and hence of lowest cost. A more sustainable and safe approach would be the use of catalytic oxidants, particularly using natural or earth abundant elements or compounds.
Knowing the growing importance of metals, the rise of a circular economy of metals is desirable to reduce the pressure on supply chains, but also to reduce the demand for mining. Although reducing and re-using strategies must be preferred over recycling ones, it is clear that recycling will play a major role in the coming decades, knowing the large number of devices used for clean energy production and storage that will reach their end of life. Reducing the environmental impact of recycling processes is necessary to favour the social acceptance of urban mining and the implantation of new recycling facilities.
This critical review highlights a “toolbox” of processes to deal with the different layers. The tools described herein aim at liberating metals efficiently, avoiding dilution and facilitating purification by targeting the weakness at the interface between different phases. These processes should be designed for circularity (reusing chemicals and/or energy) in order to limit energy and chemical consumption, and to reduce emissions (gases, dusts and volatile chemicals). This review discusses the merits and limitations of each tool and aims to provide guidance amongst the existing and emerging tools to improve recycling of TCMs.
Although most of these techniques could be used for WEEE recycling, the main focus of this paper is on emerging streams of end-of-life materials used for renewable energy production (solar cells, wind turbines), energy storage and electric vehicles (lithium-ion batteries (LiBs)), and PEM fuel cells for hydrogen production as they are more likely to be collected than WEEE. Moreover, a significant proportion of these devices contain organic (polymers) and inorganic (such as fibreglass) materials, which inhibit metal recovery. Their removal is often needed to access the metals, while they often bear little value and possibly have a high impact if dispersed in the environment. This review highlights tools that can be used to treat these non-metallic materials and improve global recycling rates of products.
2 Results and discussion
2.1 Architecture of metal-containing end-of-life materials
Recycling of metals from various end-of-life materials is complicated due to the presence of many diverse and interpenetrated layers of materials. A representative example would be crystalline silicon solar cells made of different layers of inorganics (glass, silicon wafer, silicon nitride), organics (poly-(ethylene-vinyl acetate) (EVA) polymer) and metals (aluminium and silver electrodes, copper wires, and aluminium frames). It is therefore necessary to deal with each of those layers by targeting weaknesses at the interface between different layers, using sustainable processes if possible.The metals are generally reactive, and can be oxidised, although some of them will be more difficult to oxidise than others (high redox potential or formation of passivation layers). Inorganics are generally unreactive, but they are generally of brittle nature and physical methods can be used to remove them. Physical methods are less useful on organics, which are generally not brittle and are also difficult to oxidise. But organics can be removed by pyrolysis, solvent-assisted dissolution or other physical and chemical methods.
It should be mentioned that although metals are the target of the recycling process as they often bear most of the economic value, it is often vital to process the other layers in order to access the metals, as they are wrapped into multiple layers. This is particularly true for LiBs, solar panels, fuel cells, and wind turbine magnets, which share a complex architecture (detailed structure being shown in Fig. 1). This architecture is shared with many different sources of metal-containing end-of-life materials such as PCBs (composite materials and metals), thermoelectrics (ceramics and metals), laminate films (polymers and metals), and others.
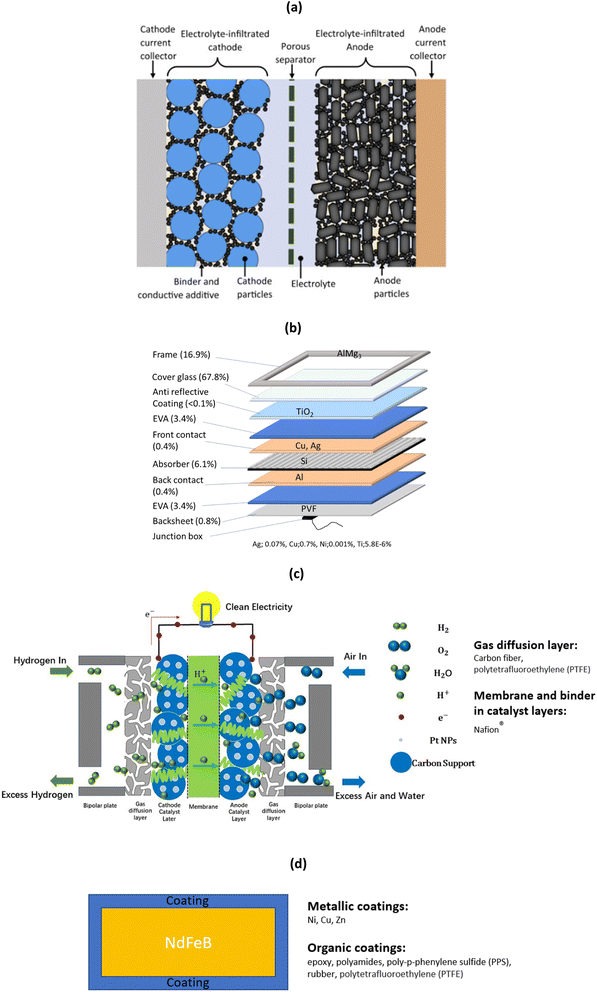 | ||
| Fig. 1 (a) Detailed structure of LiBs (reproduced with permission from ref. 24), (b) solar panels with crystalline solar cells and typical mass percentage of various materials (reproduced with permission from ref. 25). (c) PEM fuel cells for hydrogen production where Pt NPs stands for platinum nanoparticles (reproduced with permission from ref. 26), and (d) coated magnets and commonly used coatings. | ||
There may also be opportunities to up-cycle some of the non-metallic materials into higher value products. It is also necessary to take into account the significant difference in value between different metals within the same waste streams. As most of the process focuses on the expensive precious metals, they often represent a low fraction of the total metallic content and there is a need for selective dissolution methods. An undiscerned dissolution of all metals leads to unnecessary consumption of chemicals and impure product streams. A prudent approach would be to oxidise and dissolve metals selectively, only when needed.
A common first step in most recycling processes would be to characterise the end-of-life material to identify the value and the weakness of the material. The weakness of the material would be a part that can be removed more easily than the others, allowing recovery of the valuable part without having to process the rest of the material.
2.2 State of the art of the recycling techniques
Current metal recycling facilities often combine pyrometallurgy and hydrometallurgy to treat the multi-component metal-containing end-of-life materials and recover TCMs.27,28 Through the action of heat, pyrometallurgy is modifying the three layers and turning them into different products (which is summarised in Fig. 2). End-of-life materials are first treated at high temperatures in a copper smelter, where most of the metals are turned into a copper bullion containing precious metals and copper. The inorganics such as ceramics (as well as some metals) result in a slag which is further processed in a lead blast furnace to produce a lead bullion.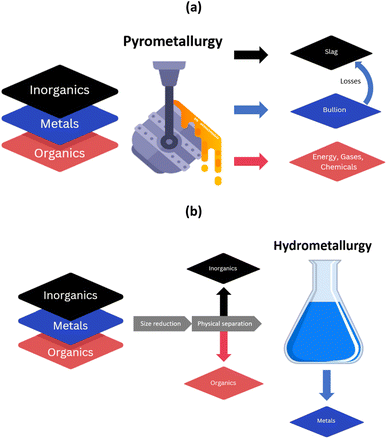 | ||
| Fig. 2 Modification of different layers through the application of (a) pyrometallurgy, and (b) hydrometallurgy. | ||
The organics are used as a source of fuel for the process. If the organic content is too high, removal of organics through physical methods may be required prior to pyrometallurgical treatment. Some of the by-products could be condensed to produce useful chemicals (such as sulphur dioxide to produce sulphuric acid).
Reductants may be present, or they may be added. For some waste streams, e.g. LiBs, the presence of aluminium in the waste stream will act as a reductant, but additional supplementary reductants may be added depending on the metal to be extracted. Hydrometallurgy is then applied for the leaching of the copper bullion, which allows dissolving copper and recovering it by electrowinning. The precious metal residue is then further treated through cupellation (blasting hot air over the molten metal) to recover Au, Ag, Pd and Pt. Similarly, the lead bullion is refined to recover special metals (In, Te, Se) and lead by-products (Sn, Bi, Sb).
Pyrometallurgical processes are energy intensive and they treat without discernment the inorganics, organics and metallic layers. The inorganics often end in the slag and a higher amount of inorganics increases its volume. A larger slag volume leads to higher losses of metals, particularly challenging during smelting of spent LiBs (losses of Li, Mn, Al…). Other elements with a higher volatility (Pb, As, Sn, In, Cd…) can be found in the flue dust, which must be managed.29,30 Degradation of organics at high temperature could lead to the production of valuable chemicals, but also to volatilisation of toxic and hazardous molecules such as brominated flame retardants or polychlorinated biphenyls, often encountered within WEEE plastics, condensers cables, housings, etc. Specific treatment of the gas is necessary to avoid release in the environment of hazardous substances. With this regard, the processing of end-of-life materials containing p-block elements (e.g. Cl, Br, As) is challenging using pyrometallurgy.
Other approaches involve first to shred the end-of-life material to reduce its size, then separate different fractions using physical separation methods prior to application of hydrometallurgical methods for metal purification.31 The complexity of the material may necessitate disassembly of the end-of-life material as a prerequisite. Due to the variability of the feedstock, this step is often done by hand and is labour intensive, although it can be automated to some extent, using desoldering (using mostly high temperatures or oxidising agents), automatic disassembly32 or other thermal, physical or chemical methods.33 This depends on the value and concentration of the TCMs. Studies on LiBs have shown the importance of disassembly rather than shredding on the time and cost/profitability of recycling.34 The complexity of the materials can also make manual disassembly too slow and too expensive depending on the cost of labour which adds a geopolitical aspect to recycling.13,35 Some have posited that in the future “Industrial disassembling” will be a prerequisite to an efficient circular economy of TCMs, in preference to manual disassembly.36
The size of the materials is then reduced, which has multiple benefits. First, it liberates the TCM fraction which is often encapsulated within different layers of inorganics and organics. Secondly, it allows application of physical methods to separate and recover the organics and inorganics, based on the difference in their physical properties. Thirdly, the dissolution of metals from the metallic fraction is more efficient when the size of the particles is smaller. Hence, hydrometallurgy is applied to dissolve the metallic fraction, which is often carried out with harsh stoichiometric chemicals (strong bases, mineral acids, strong oxidisers, etc.). These types of reagents also need neutralising and produce gases which can affect the sustainability of the overall process. The preference for reusable reagents must again be stressed from a sustainability perspective. Some TCMs are found as minor constituents in devices, such as rare-earth elements (REEs) in windscreens, sensors, touchscreens, and headlights in vehicles. These may be too diffuse to warrant dismantling and recovering. This may be an aspect of where responsible innovation13 is required to design out elements which cannot be used sustainably.
Similarly to pyrometallurgy, hydrometallurgical approaches suffer from the indistinct treatment of the different layers. First, the liberation of metals encased in plastics and inorganic layers leads to losses of metals. The size reduction step is energy intensive in common mineral processes, contributing for 36% of the energy consumption of the mining industry in Australia, and more than 1% of Australia's total electricity consumption.37,38 Energy consumption for comminution (crushing and grinding) of gold-containing rocks from underground mines is in the range 15–32 kW h per ton of ore.39 Energy consumption for the crushing of end-of-life Li-ion batteries is in the range 4–12 kW h per ton, depending on the nature of the housing materials, size of the crushed materials and pre-treatment steps applied.40 These numbers seem to indicate that crushing of end-of-life materials is expected to consume less energy than crushing of primary rocks, but size reduction will remain a major contribution to the global energy consumption of the recycling process.
Further losses of metals are possible during the physical separation steps. The dissolution step is often non-selective, which requires further separation of metals with chemical-intensive techniques (ion exchange resins, precipitation, solvent extraction, etc.). Wastewater treatment is then needed after the metals are recovered to deal with dissolved salts, suspended solids, organic solutes and pH issues. For all of these reasons, new processes based on a layer by layer approach are needed, preferably with selective dissolution. Complete digestion of base metals often makes them uneconomic to recover. Sequential delayering processes would be more efficient in order to improve recycling rates and recover not only metals but also inorganic and organic layers.
A typical smelter uses temperatures of 1200 °C,41 while most hydrometallurgical processes are applied at lower temperatures (generally <100 °C). But electricity consumption of hydrometallurgical processes remains an important feature for size reduction and physical separation steps, heating and agitation of leaching solutions, and electrodeposition of metals. A comparative life cycle assessment of pyrometallurgical and hydrometallurgical approaches for the recycling of electronic waste shows that hydrometallurgical processes have a lower carbon footprint42 (18 tons of carbon dioxide per kg of gold, compared to 58 tons of carbon dioxide per kg of gold using pyrometallurgy). Hydrometallurgical processes have a higher impact for most of the environmental factors considered (acidification, eutrophication, ecotoxicity), which is mainly due to the chemicals used such as acids and oxidising agents for the dissolution of metals.
2.3 Physical methods to concentrate metals
It is useful to first identify which size-reduction method is the most appropriate. Hammer mills, shredders and granulators are the most common options. Shear, compression and abrasion forces are used in these processes to break the interfaces and liberate the metals from their organic or inorganic casings. Shredding and grinding of WEEE is a common operation in existing WEEE recycling facilities. Within PCBs, metals like copper are found between layers of resins and their liberation by size reduction is easy. During the shredding of PCBs, aluminium is usually liberated in the coarser fractions. Copper and ferromagnetic metals are liberated completely when the size is further reduced. But interlocking of organics and metals is still an issue with other end-of-life materials like solar cells, where plastic-rich fractions still contain metals after shredding.45 The smaller fractions (<0.25 mm) contain up to 70–80% of silver depending on the nature of the solar cell, which leads to losses in the range 20–30% for silver (the most valuable metal found in crystalline solar cells). The degree of liberation of metals from end-of-life LiBs is high as long as the particle size is reduced down to under 100 μm.46 Reducing the size down to very low small particles may be energy intensive or not possible depending on the nature of the end-of-life material. Moreover, losses of precious metals as high as 40% have been reported during the size reduction steps of WEEE.47
Ball milling could combine size-reduction and oxidation or reduction of metallic species. This mechano-chemical approach allows redox reactions to occur without any solvent. The milling step is done by mixing the end-of-life material with solid chemicals like potassium persulfate, which allows the oxidation of metals from WEEE.48 Other reagents like iron allow the reduction of cobalt from spent LiBs and facilitate metal leaching.49 Other chemical reactions are possible with chemicals such as sodium carbonate to recover gallium from LEDs.50
Fracturing techniques are used in the mining industry to process rocks51 and could be adapted to process end-of-life materials. Microwave heating,52 cryo-milling53 and high-voltage pulses54,55 were reported, mainly to remove inorganics and organics fractions and to liberate the metals. The high voltage pulse crushing technique was reported to recover >90% of Ag and Cu from Si solar panels.56 Other techniques like water jet pulverisation57,58 or laser cutting59 were reported to “cut” selectively the metal-containing parts from the end-of-life material. However, most of these techniques remain expensive and, so far, can hardly compete with the high-capacity industrial machines available for size-reduction.
Although comminution techniques are well established, it is sometimes possible to liberate valuable metals without shredding or grinding, which often leads to losses and dilution of the TCMs. “Soft” delamination techniques could be more sustainable than non-selective shredding to liberate the valuable metals, for example by weakening hydrogen bonding between current collectors and cathode active materials of LiBs,60 or deagglomeration of cathode materials from binders and carbon using the high shear forces in a blender.61 Liberation of the valuable metals from their initial casing is particularly challenging for renewable energy and energy storage materials. For LiBs, short-circuits and management of the potentially hazardous substances (fluorinated lithium salts, organic solvents used as electrolytes) are additional challenges. Preventing fluorinated polymers (like polyvinylidene fluoride (PVDF) or Nafion®) degradation during the recycling process is important for LiBs,62 fuel cells and the backsheet films of photovoltaic modules. Magnets are encased in organic binders63 or coated with other metals64 to prevent corrosion (which is particularly important for offshore wind turbine magnets); removal and separation of this coating is necessary. Solar cells can easily be crushed and sieved, which allows recovery of glass fractions65 efficiently and separating out polymer-rich (>5 mm) and metal-rich fragments (<0.25 mm) according to particle size.45 However, the silicon wafers are inevitably not recovered intact, preventing their direct re-use. “Hot-knife” cutting66 employs elevated temperatures to soften the EVA while a still blade removes the aluminium frame and the junction box from solar cells, allowing copper cable recovery from the junction box. This technology may allow to reduce losses of metals but EVA and backsheet removal is steel needed.
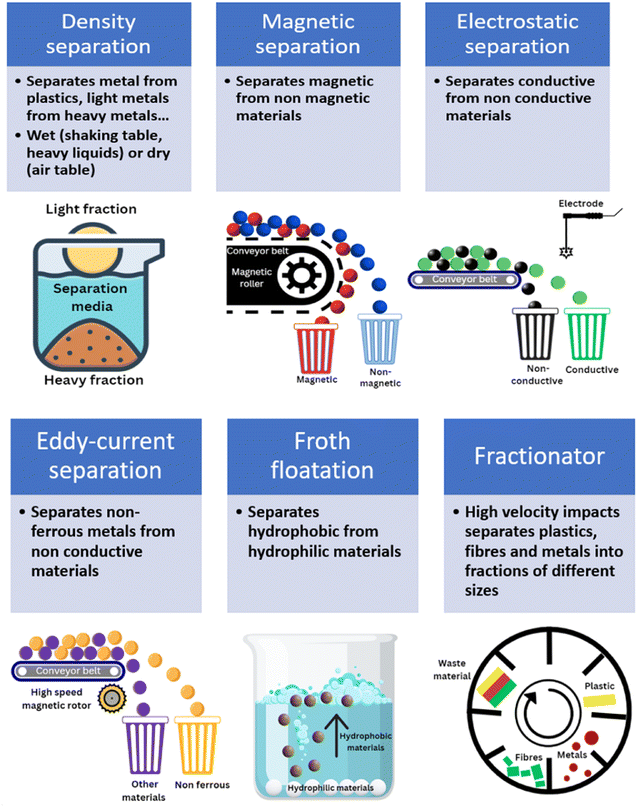
2.3.2.1 Density separation. Physical separation methods are commonly used to separate materials using differences in their physical properties (Fig. 3). Density separation is a simple technique that allows the separation of low-density materials such as plastics from higher-density materials like metals. Perhaps the most efficient and obvious example of this is lead acid battery recycling, where the two solid components, lead and polypropylene (PP), have very different densities. Various techniques are available to sort materials by density. The feasibility of density separation can be estimated by calculating the concentration criterion41 (CC), using eqn (1):
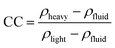 | (1) |
Air tables are the most common within the recycling industry. The materials are fed in the top of the air table, where air is blown vertically from bottom to top. While the high-density fractions will fall, lighter fractions will be blown away by the air stream and separated. Using a shaker table, materials flow through a liquid medium constantly shaken on a table that can be inclined. This wet separation media allows separation of most metals from plastic, while reducing the risks of dust dissemination; it has also shown some merits for concentrating metals from WEEE.67 However, it consumes water and has a relatively low capacity (up to 1 ton per day). It is used more commonly for primary extraction than secondary but there is significant potential to expand the use of this technique.
Heavy liquids typically have a density > 2 g cm−3 and examples include tetrabromoethane and diiodomethane. When separating materials with heavy liquids, materials with a lower density than the liquid will float at the surface, while heavier materials will sink at the bottom of the liquid. Separation of the different phases is easy and the liquid can be reused. However, the toxic nature of some of the heavy liquids used is detrimental to the application of this process. There is room for the application of this process with less hazardous liquids like sodium polytungstate, which is however expensive. Other liquids like sodium silicate68 or some brines69 can reach high densities and can potentially be used as a heavy media for density separation purposes, without the environmental burdens of halogenated solvents. There may be potential to use deep eutectic solvents (DESs) for this application, particularly with metal bromide salts. Both dry70,71 and wet72,73 density separation was reported to remove light components from end-of-life LiBs. Water and brines of different densities allowed glass, metal and plastics separation from crushed solar cells.74 There are significant differences in the densities of materials found in end-of-life materials from clean energy production and storage (displayed in Fig. 1). Those contain high-density metals such as Ag (density75 = 10.5 g cm−3) and lighter polymers (density76 of EVA = 0.95 g cm−3). Separation of intermediate-density materials such as aluminium (density77 = 2.70 g cm−3) is feasible but separating different polymers is more challenging.
2.3.2.2 Magnetic, electrostatic and eddy-current separation. Magnetic separation uses the differences in magnetic susceptibility to separate different metals and materials. The magnetic attraction force41 (FM) depends on the volume of the particles to be separated (V), their magnetic susceptibility (χ), as well as the magnetic field created by the magnetic separator (H) and its gradient (∇H) as described in eqn (2):
| FM = χ × V × ∇H | (2) |
The process is easy to operate and allows separation of ferromagnetic/paramagnetic materials (attracted to magnets) from diamagnetic materials (slightly repelled from magnets). Separation of ferrous materials can be achieved with small magnetic fields (<2 T), while attraction of paramagnetic materials requires stronger magnetic fields41 (10 to 20 T). Magnetic separation provides excellent separation but there is a risk of agglomeration which must be avoided as it leads to loss of metals. Fine particles will increase the risk of agglomeration and reduce the separation efficiency. Dry, low-intensity magnetic separation is generally applied to coarse and highly-magnetic materials (in the range 0.5–6 mm for drum magnetic separators). Wet magnetic separation tends to be used for smaller fractions (<0.5 cm). Size of the feed materials depend on the equipment, with overhead magnets used for ferromagnetic materials as large as 200 mm. Generally, a size reduction step is generally needed regardless of the type of end-of-life materials being considered for recycling.
Application of magnetic separation makes sense within the frame of magnet recycling to separate magnetic materials from other materials. It is also relevant for ferrous materials such as the steel casing78 found in spent LiBs. The presence of cobalt allows magnetic separation of cathode active materials from organic polymers.79 It is also feasible to increase the magnetic susceptibility of cathode active materials to facilitate their separation. By grinding waste lithium–cobalt oxide in the presence of waste polyvinyl chloride (PVC), dechlorination of the polymer is accompanied by the formation of lithium chloride and cobalt chloride,80 according to eqn (3):
| LiCoO2 + 3[–CH2CHCl–]n → LiCl + CoCl2 + CxHyHOz | (3) |
The addition of zero valent iron to the waste PVC and LiCoO2 improves PVC reductive dehalogenation by abstracting chlorine atoms from PVC. Li is preferentially removed from the oxide materials to form lithium chloride (because of its higher charge density than cobalt and its helium-type double shell), leaving iron and cobalt oxide that can be treated thermally to form magnetic oxides (CoFexOy), which can be magnetically separated.81
Electrostatic separators use the differences in conductivity and resistivity of materials to separate them. Shredded materials (with a size generally lower than 5 mm) are usually sent on a conveyor belt and arrive on a rotating drum connected to the ground. In a roll-type corona electrostatic separator, a high-voltage electrostatic field is applied between this drum and one or several electrodes. The materials to be separated are charged by ion bombardment.82 While the conductive materials discharge quickly and are ejected away from the rotating drum, the non-conductive materials remain charged and attracted to the rotating drums which allows their separation. The non-conductive particles are pinned to the electrode by the electric image force (Fi) detach from the rotating electrode if the difference between the centrifugal force (FC) reach a certain value defined by eqn (4):
FC − Fi > Fg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(αd) cos(αd) | (4) |
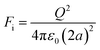 | (5) |
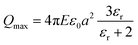 | (6) |
While corona electrostatic separators do not require contact between the materials and the electrodes, triboelectric separators charge the materials by contact, leading to materials losing or gaining electrons depending on their position on the triboelectric series. Positively and negatively charged materials can then be attracted to electrodes. Electrostatic separators generally separate metals from plastics due to their different conductivities. The position of a material in the triboelectric series depends on the amount of negative or positive charge it accumulates, which depends on the nature of the materials and other factors such as size and size roughness of the materials. While polymers like PVC and polytetrafluoroethylene (PTFE) are generally negatively charged, other polymers are usually positively charged, which could allow their separation, although the exact position of PP, polyethylene terephthalate (PET) and polyethylene (PE) in the triboelectric series is still debated.82 Separation of Al and other positively charged polymers from negatively charged PVC and PTFE is possible84,85 and could be interesting within the frame of end-of-life materials from clean energy and energy storage. These materials must first be liberated from their initial casing, which can be achieved with a size reduction step (particle size for triboelectric separation is in the range 1–10 mm).
Eddy-currents allow separation of non-ferrous materials from other materials. By applying a high-frequency magnetic field with a rotating magnet, induced currents create magnetic fields opposite to the first one; non-ferrous metal will be repelled from the magnet. Ferrous metals must be removed prior to eddy-current separation as their temperature tends to increase when they are submitted to eddy-currents. Eddy current separation is applied to particles with size in the range 3–150 mm, and the highest separability is achieved for materials with high conductivity and low density, and was proposed for the separation of current collectors from cathode materials from spent LiBs,86 or Al from solar cells.87
Magnetic separation techniques are often encountered to separate steel casings and remove any magnetic contamination from spent LiBs.70,88 Electrostatic separation has a broader range of application and can be used to separate current collectors or polymers from spent LiBs.89,90 It can also be used to recover 95% of the conductive metals from milled solar cells and separate them from non-conductive fractions (glass and silicon).91 However, glass tends to accumulate with the conductive fraction and polymer separation is challenging, requiring an additional separation step using density or hydrophobicity differences.
2.3.2.3 Froth floatation and fractionation. Within the mining industry, froth floatation is historically used to concentrate metals from low-grade minerals. It allows separation of materials having different hydrophilicity and, as such, has the potential to separate organic layers from end-of-life materials. In froth floatation, hydrophilic particles, wetted by water, stay in the liquid, while more hydrophobic particles will be attached to air bubbles blown in the liquid and can be collected at the surface. Surfactants (called collectors) are used to stabilise air bubbles and enhance the hydrophobicity of target solids through modification of their surface energy. Electrical double layer at the solid/water interface is of primary importance as it controls the absorption of collectors. High surface charge can inhibit the chemisorption of chemically adsorbed collectors, while absorption of physically absorbed collectors depends on the sign and magnitude of the surface charge.92 Froth floatation is a mature technique that can be used for plastics recycling.93 Its use for WEEE recycling concentrates precious metals prior to their purification.94 It has found applications for recovery of cathode materials95,96 and graphite97,98 from spent LiBs, to separate aluminium from silicon99 or recover lead100 from end-of-life solar cells. Polymers like PVDF are used as binders and therefore firmly attached to the cathode active materials. Thermal treatment is therefore needed to remove it. The residue of the thermal treatment can be removed by ultrasonication prior to flotation of hydrophobic materials.101,102 Similarly to all physical separation techniques, complete liberation of the materials is a prerequisite to achieve high separation efficiency. Glass fibres were reported to float during a froth floatation process, possibly due to entrainment related to their morphology or presence of residual hydrophobic resin attached to the fibres.103 Similarly, carbon supports (used as catalysts in fuel cells) will probably float, entraining with them the PGMs attached.
Most physical separation techniques will use one physical property to separate the target fraction from the other components. Fractionator mills will use several of these properties to combine delamination and separation of different fractions. Shredded materials are introduced in a rotating apparatus which submits the materials to high accelerations and decelerations. These high-frequency impacts break the interface of the different layers. While plastics keep their shape, brittle inorganics like fibres are fragmented, and ductile metals aggregate. The output materials have different shapes and sizes, facilitating the subsequent separation of the coarse plastic fraction from the smaller metallic fraction, while precious metals can be found in the finer fractions. Fractionators were proven effective for the processing of conventional WEEE41 (mostly for the separation of plastics, copper and precious metals from PCBs), their use for renewable energy and energy storage end-of-life materials is yet to be demonstrated. Using high accelerations (similar to fractionators), the use of centrifugation was reported to separate lithium-containing materials from carbon-black of spent LiBs.104
Physical techniques are promising for the recycling of emerging end-of-life materials as they are mature and easy to implement on an industrial scale. However, these techniques are used for pre-concentration purposes and the purity of recovered metals is often too low after their application. Moreover, metal losses are inevitable during size reduction and physical separation steps, and emerging waste streams are complex in nature. Breaking metal oxides (the “black mass”) from the cathode of spent LiBs certainly requires hydrometallurgical or pyrometallurgical methods. Physical methods are certainly useful to recover the black mass from the spent vehicle, but other processes are required to recover high-purity lithium carbonate from the black mass.
2.4 Chemical methods to process metals
| 2ZnO + 8Cl− → 2ZnCl42− + O2− + 3e− | (7) |
In the meantime, metals dissolved in the solution are electrochemically reduced at the cathode. Anodic dissolution coupled with cathodic reduction of target metals was applied for precious metals recycling from e-waste107 with DESs as solvents combining complexing ability and electrochemical stability.
Commonly, chemical dissolution is carried out using conventional hydrometallurgical processes, such as either nitric acid or hydrogen peroxide as an oxidising agent in combination with sulfuric acid. Oxidation of the target metal (M) with an oxidising agent such as hydrogen peroxide occurs108 according to eqn (8) and (9), while its oxidation with ferric sulphate can be described with eqn (10):
| M + H2O2 → M+ + HO˙ + OH− | (8) |
| M + HO˙ → M+ + OH− | (9) |
| M + 2Fe3+ → M2+ + 2Fe2+ | (10) |
Oxidation with hydrogen peroxide is attractive since the decomposition products are benign, and the oxidising agent doesn't contaminate the metals. However, ferric ions can be regenerated anodically during an electrodeposition process where the target metal is recovered at the cathode, which leads to lower environmental impact than hydrogen peroxide.42
Metal oxides are also commonly processed using acidic media, with leaching effectiveness based on a combination of proton concentration and the coordinating ligands available. The proton acts on the oxide moiety, and the ligand acts on the metal ion to weaken the metal-oxide bond sufficiently to achieve dissolution. During metal oxide dissolution, with DESs as solvents, an organic acid (HX) is commonly used as a hydrogen bond donor (HBD). The organic acid is first absorbed on the active OH sites of the hydrated metal,109 according to eqn (11):
| M–OH + X → M–OH2+ + Xn− | (11) |
The metal–ligand complex is then formed in solution according to eqn (12), provided that the metal ligand complexes formed are more stable than the metal–OH complex:
| M–OH2+ + Xn− → M–X(n−1) + H2O | (12) |
Using aqueous solutions, large amounts of acids and/or additives are often required to achieve the desired chemistry and reactivity of the target metals, due to competition with the high concentration (55.5 M) of water ligands. These approaches generally lead to non-selective dissolution of metals, consuming a large amount of chemicals but can be applied to a wide range of feedstocks, and is often used when processing low-grade materials. Hydrometallurgical processing is often carried out after pyrometallurgical processing to recover the individual metals from the bullion. Selectivity is usually achieved through the recovery methods, e.g. precipitation, cementation, electrowinning, solvent extraction, or ion exchange. Another option to impart selectivity into a metal dissolution process is to “force” surface passivation onto the less desirable metals. This is a common process that is applied to steels to prevent iron oxide ‘scales’ from forming, but could potentially be expanded to other areas of metal processing. These pickling solutions often involve aqueous solutions made from nitric, sulfuric, or citric acids.110
A greener and more sustainable approach would use a benign and catalytic oxidising agent such as iron chloride or iodine, or to tailor the solvent system to impart selectivity in reactivity, leaching, or solubility. Ionometallurgy uses systems formed of ionic fluids, such as ionic liquids (ILs) or DESs, to process metals. These systems have the benefit of minimal or no water being present, minimising the effect of oxide chemistry.111 They also have inherently high concentrations of ligands, permitting the chemical oxidation of noble metals such as gold,112–114 or palladium.115 Alternatively, the ionic environment can stabilise oxidising species such as iodine116 or bromine117 without the need for additional anions that would be required in aqueous media.
In cases where metal oxides are to be dissolved in DESs, the type of HBD present significantly affects the dissolution process, as acidic HBDs provide both the necessary protons to react with the oxide and ligands to coordinate the liberated metal ions. Depending on the coordination ability of the different HBDs, different metal ion complexes are formed,109 with different stability constants. This can result in either the selective dissolution (or non-dissolution) or selective precipitation of metal salts, as in the case of the processing of LIBs cathode active materials,118,119 or the recovery of metals from flue dust,120,121 lamp phosphors,122 and NdFeB magnets.123 When using ionic fluids to dissolve metal oxides, one of the critical parameters to metal solvation is the anionic component of the system.124,125 However, the downsides to these systems is the high viscosity126–128 and inevitably higher material costs relative to aqueous media. If ILs or DESs are to be used, care must be taken to ensure that the ‘green’ credentials are not overstated, and that the economic benefits are maximised, either by a decrease in the energy needed for the process, reducing the amount of chemical additives required, or through the processing of small, concentrated amounts of high value materials. Systems containing materials where oxide/hydroxide passivation must be minimised will also benefit from the use of ionic fluids, however the high viscosity of ionic fluids will lead to physical losses which must be factored into the overall sustainability of the process.
A middle ground between aqueous solutions and ionic fluids is concentrated brines, which are formed from water and a high concentration of organic or inorganic salt. The benefit of using a brine over an ionic fluid is that the high ligand concentration is maintained, but at viscosity values closer to aqueous solutions. The presence of water does raise the possibility of oxophilic metals passivating, which may be detrimental to complete metal dissolution. However, in some cases, it is interesting to dissolve a base metal layer, which can then liberate less reactive metals from unreactive organic substrates. For example, thermoelectric devices are assembled by sandwiching semiconductor legs between ceramic plates, using copper connectors and tin-based solders. Tailoring the chloride concentration of the brine allows targeted leaching of the solder, liberating the semiconductor legs, as the other elements present would passivate below a certain chloride concentration.129
This selectivity through passivation can also be generated by the addition of water to an ionic fluid containing iodine as the oxidising agent. The metallic portion of PCBs is primarily composed of copper; however multiple layers of different metals are also present in surface coatings and solder. A challenge exists as different generations of PCBs have used different material combinations; however, the most common currently found in WEEE are hot air solder levelling (HASL), which is a tin/lead alloy and electroless nickel immersion gold (ENIG), which contains nickel and a thin layer of gold.130 When no water is present, all PCB metals are easily oxidised together, albeit very slowly due to the poor mass transport caused by the high solvent viscosity. When 40 wt% water is present, the rate of gold oxidation is improved due to the decreased solvent viscosity, but the nickel layer passivates and protects the copper underneath from further oxidation for a period of time.131
Electrochemical dissolution methods take advantage of the differences in reactivity between the target metals. Metals with cathodic redox potentials, such as copper or iron, are much easier to oxidise than those with anodic redox potentials, such as platinum or gold. In aqueous systems, redox potentials are well-characterised,132 however by adding a large number of ligands or through the presence of strongly complexing ligands, the reactivities of the metal complexes formed can be altered. At the extreme end of this ligand concentration scale are the ionic fluids, where the change in chemistry can lead to increased stability of metal ions in solution with respect to the reduced form of the metal. For example, in a system formed from choline chloride and ethylene glycol, Cu+, Ag+, and Au+ form anionic chloride complexes due to the high chloride environment,133 which means that the redox potentials can be up to 1.0 V more cathodic in the ionic fluid compared to in aqueous medium,134 allowing a greater range of metals to be accessible under relatively mild conditions. However, selective electrochemical dissolution is generally not employed in the literature, with the selectivity focus being on the metal recovery step, which is often a chemical method such as precipitation. The bulk of the literature focuses on electropolishing, so this particular “tool” is underutilised and further research must be carried out before it can be applied to complex metal-containing systems.
Importantly, the solvent selected must match the process with regards to material cost of reagents and value of product. The main challenge to selectivity will be to favour oxidation or passivation selectively with cheap, environmentally friendly, and readily available chemicals. Additionally, one must consider the composition of the material when designing the process; if the most valuable metal is embedded in another matrix, then the whole matrix must be dissolved, or some form of comminution must be employed. If the most valuable metal is on the surface of the material, such as with PCBs, then minimising dissolution of the underlayers is important to reduce chemical consumption. Alternatively, the more reactive metals could be oxidised to undercut the valuable and unreactive metals, causing them to slough off and be recovered by simple filtration.
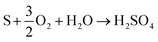 | (13) |
It is also possible to oxidise metallic iron (Fe(0)) and ferrous ion (Fe2+) into ferric ion (Fe3+) using microorganisms, as described in eqn (14).
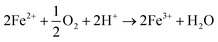 | (14) |
Sulphuric acid is then able to dissolve metals via the acidic mechanisms already described, while ferric ion is able to oxidise metals. Complexolysis is possible with fungi and cyanogenic bacteria. It mainly involves the decarboxylation of glycine to produce cyanide ions, which are then able to form complexes with precious metals like gold. These complexes are then oxidised with oxygen137 according to eqn (15):
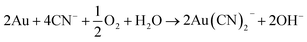 | (15) |
As opposed to conventional cyanide leaching, some bacteria are able to turn toxic cyanides into β-cyanoalanine, which facilitates wastewater treatment. Gold recovery from WEEE was extensively studied using cyanogenic bacteria such as C. violaceum,138,139B. megaterium,140 or P. fluorescens.141
The leaching of metals from end-of-life LiBs is feasible by reduction of Co3+ (within the insoluble Co3+ oxide) into Co2+, which can be achieved using cheap pyrite (FeS2) and sulphur. Both chemicals will be oxidised with oxidising bacteria to produce sulphuric acid and Fe3+ ions. The subsequent reaction of Fe3+ ions with pyrite releases Fe2+ ions which in turn allow formation of soluble sulphate salts of Co and Li.136 Similarly, lanthanide elements (Nd, Pr, Dy) can be leached from end-of-life magnets142 using L. ferrooxidans bacteria, sulphur, and iron. High leaching rates (≥90% for Dy, Nd and Pr) can be obtained in 14 days, ferric ions playing a major role in the leaching efficiency. Multiple studies reported the leaching of PGMs143 from catalysts using bacteria. PGMs having a high redox potential, their leaching is feasible by cyanide leaching,144 similarly to gold leaching depicted in eqn (15). Other strains like Aspergillus niger145 can produce oxalic acid and favour Pt leaching, but the recovery rates remain low (below 40%). Gallium and arsenic leaching from thin film solar cells containing gallium arsenide (GaAs) is feasible using bacteria which can produce organic acids (including amino-acids), which helps solubilise gallium and arsenic in 20–30 days.146
Most of the dissolution reactions involved require nutrients, careful control of the pH and bubbling of compressed air to provide oxygen and CO2 for microbial growth. These experimental conditions will therefore contribute to significant consumption of chemicals for the leaching process. However, comparison of techno economic assessments between biometallurgical and hydrometallurgical processes indicate that the former could be cheaper than the latter for the recovery of metals from end-of-life PCBs147 (0.159 € per kg for biometallurgy, 0.224 € per kg for hydrometallurgy). This process can be made even more economical and sustainable when waste materials are used as nutrients.
Some technological issues need to be addressed for biometallurgical processes, the main ones being potential formation of toxic chemicals like hydrogen cyanide, the presence of organic compounds and heavy metals detrimental to bacterial growth, and precipitation of iron species through iron hydrolysis or jarosite which decreases the leaching efficiency. Biometallurgical leaching suffers from slow kinetics; it typically requires several days to reach significant leaching rates. Different strategies were developed to accelerate the leaching kinetics such as the application of a voltage148 to improve electron transfer reactions, using a weak magnetic field,149 ultrasounds,150 or ultraviolet irradiation151 to induce mutations on the bacteria, this last approach being the only one so far to provide a clear improvement of the leaching kinetics. A more promising approach is the addition of a catalytic agent like activated charcoal,152 or silver.153 In that case too, improvement of kinetics is limited and addition of catalysts could have a negative effect on the growth of microorganisms.
Bacteria can also be used to precipitate or separate metals which have been previously dissolved by bio- or hydrometallurgical means. Multiple bacteria, fungi, plants and extracts are able to precipitate metals through reduction reactions.154 This approach is promising to up-cycle base metals into valuable nanoparticles, or to produce PGM-based catalysts from dissolved PGM solutions,155,156 which can improve the performance of the catalysts as compared to commercial ones.157,158 Bacteria can also be used to produce biomolecules useful for purification of metals. Proteins like lanmodulin have a very high affinity for lanthanides.159 Hence, lanmodulin can be obtained from bacteria, purified, and used for the selective biosorption160 of REEs from WEEE or minerals, eliminating the need for solvents or ion-exchange resins.
Natural molecules like reducing organic acids, sugars or antioxidants can be easily obtained from waste fruit peel,161 waste tea162 or grape seed.163 These molecules were shown to be as efficient as synthetic reducing agents (like hydrogen peroxide) for the dissolution of metals from LiBs cathodes. Hence, microorganisms could be used indirectly through metabolic processes such as fermentation to produce useful chemicals able to solubilise metals. Within this frame, food waste can be considered as a sustainable and cheap source of chemicals for recycling processes.
Overall, biological processes are cheaper and make sense for large volumes of low metal content materials where processing time is less of an issue. Low grade ores such as mine tailings are particularly suited as they represent a high volume and could contain small but significant amounts of critical metals. For the same reasons, low-grade WEEE falls into this category. The biohydrometallurgical purification and recovery of metals from leach liquors is less hampered by slow kinetics and looks promising for reducing chemical consumption during the metal recovery stages. While increasingly used for primary extraction, application to secondary extraction is only in its very early stages of research.
| 2Nd2Fe14B + yH2 → 2Nd2Fe14BHy | (16) |
During hydrogen decrepitation (in the temperature range 25–400 °C), formation of Nd-hydrides and hydrogen diffusion causes expansion of Nd rich regions and cracks within the materials, finally resulting in a demagnetised powder with a size in the range 6–600 μm.166
The hydrogenation–disproportionation–desorption-recombination differs by the higher operation temperature used (in the range 750–950 °C), and the smaller particle size of the powder obtained (down to 0.3 μm (ref. 167 and 168)). In that case, further hydrogenation of Nd2Fe14B leads to its disproportionation into NdH2, Fe2B and Fe, according to eqn (17):
| Nd2Fe14B + 2xH2 → 2NdH2x + 12α-Fe + Fe2B + ΔH | (17) |
Desorption of hydrogen takes place under vacuum at higher temperatures (150 °C for NdFeB phases, up to 700 °C for Nd rich regions). Even higher temperatures also allow recombination of decomposed phases into the original Nd2Fe14B. The obtained product is a powder which is easily separated from the initial casing of the magnet and can then be turned into new magnets by sintering, hot pressing, or pelletising followed by melting and casting.169 Vacuum sintering can take the place of the degassing step. Removal of surface oxides and reduction of oxygen content may be needed since a high oxygen content reduces the sinterability and the magnetic properties of the final material.
The size of the powder obtained depends on the temperature of the hydrogen decrepitation process with room temperatures leading to fine powders (<250 μm) with some larger particles (>500 μm). Hotter hydrogen has a lower solubility in Nd2Fe14B, the lower volume expansion generates larger particles when the process is carried out at 150 °C.164 Milling is sometimes needed in order to reduce particle size and favour densification of the magnet.
When applied to magnets from HDDs169 (coated with Ni or Ni and Cu), hydrogen decrepitation produces a fine, demagnetised powder containing REEs, while metals from the coating can be sieved and separated from the powder. But depending on the nature of the coating and its thickness, pre-processing of the magnets may be needed to allow hydrogen to access these Nd phases and decrepitate them.
As compared to production of magnets from virgin materials, comparative life cycle assessment (LCA) clearly shows a much lower footprint of the hydrogen decrepitation process.170 However, it should be mentioned that applying a nickel coating on the magnet accounts for a significant proportion of the overall environmental footprint, while shredding of the materials leads to massive losses of neodymium (90%). The decrepitation process is so far limited to magnets, but can in theory be applied to other intermetallic compounds for which volume expansion following hydrogen absorption is accompanied by powder formation165 (e.g. LaNi5, SmFe3, TiFe, SmCo5, NdCo5, PrCo5, LaCo5). Other metals show a large volume expansion under hydrogen but either form coarse powders (e.g. Ti, Zr) or keep their integrity (Pd).
2.5 Methods to process inorganic and organic fractions
Ultrasound was first used to improve the leaching rate of lithium into water from spent cathodes following reductive roasting,176 or leaching of the same metals with organic acids177 and hydrogen peroxide. Cavitation was found to improve flotation,178 comminution,179 and leaching of metals from end-of-life PCBs. Leaching of indium and tin from layered materials like liquid crystal displays (LCDs) was improved using either sulfuric180 or hydrochloric181 acid in combination with ultrasound. Application of ultrasound improved precipitation of lithium carbonate by accelerating nucleation and reducing polymerisation of lithium carbonate crystals.182 Leaching rates and/or kinetics of metal dissolution are clearly improved with ultrasound183 in most cases, although it remains unclear if this improvement is of thermal, mechanical, or sonochemical origin. Energy consumption is an obvious limitation during the prolonged use of ultrasonic processes, particularly with high-power, low-frequency ultrasound.
The mechanical effect of ultrasound is particularly useful to break brittle inorganic materials. One could use ultrasound to pulverise the brittle semiconductor legs from thermoelectric materials.129 In that case, water is used as a propagation media for the ultrasound. The process does not produce wastewater, as no metals are dissolved in the propagation media. Contrary to conventional size reduction methods, ultrasound only reduces the size of the most brittle materials (avoiding mixing of highly-brittle semiconductors, brittle ceramics and ductile metals184), which reduces the risk of diluting valuable materials. Hence, the number of steps required for metal purification are lower, while it also reduces the risk of hazardous dust formation.
Ultrasound can also be used as a powerful delamination method. Comparative techno-economic analysis of different processes showed that shredding of end-of-life LiBs was less economically attractive than disassembly followed by delamination of the cathode active materials34 with ultrasound. Delamination is feasible due to the high acoustic pressure induced by cavitation. Using either citric acid or ethylene glycol as wetting agents (which also help the collapse of bubbles), cathode and anode active materials are separated in a few seconds from their current collectors.185 This is a clear advantage, as shredding and leaching of both current collectors and cathodes require additional chemicals and further separation steps to remove aluminium. Removal of lithium–iron phosphate materials from aluminium current collectors was demonstrated using ultrasound,186 which proves that the technique works independently of the nature of the active materials. Interestingly, ultrasound-assisted Fenton oxidation forms hydroxyl radicals, which can possibly degrade the PVDF binder and help recover the cathode materials. Ultrasonic delamination works even better when the binder is partially dissolved, but this objective should not be achieved with toxic or costly solvents like N-methylpyrrolidone187 (NMP). This ultrasonic delamination approach could be helpful for multi-layered end-of-life materials such as fuel cells.188 Application of ultrasonics to solar cells immersed in various solvents like acetic acid, formic acid, butanone and benzyl alcohol,189 allowed removal of polymeric backsheet.
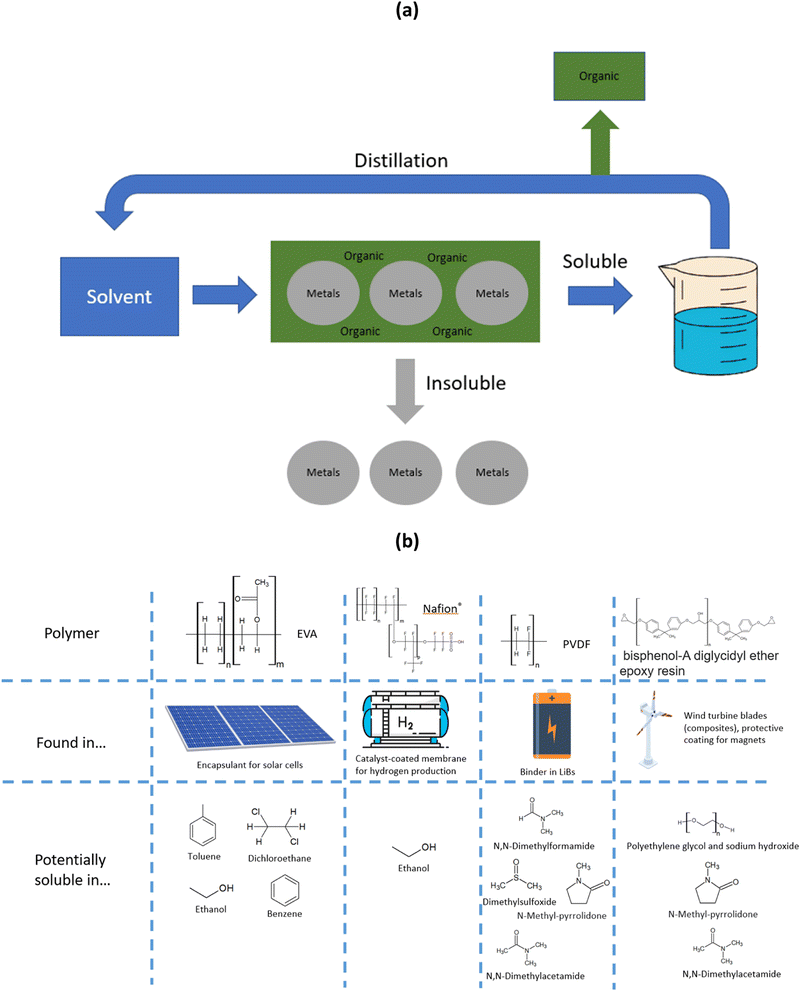 | ||
| Fig. 4 (a) Scheme of organics dissolution process within the frame of metal recycling, and (b) structure and potential solvents for polymers found in end-of-life materials. | ||
Different polymers such as PVC or PET were recycled at the industrial scale by dissolution with organic solvents.190 Care must be taken to avoid hazardous solvents, and select solvents with a low impact on health and the environment.191 Polymer solubility in different solvents can also be predicted up to a certain extent by using the Hansen and Hildebrand solubility parameters.192,193 Evolution of regulation must also be taken into account. The ban on the use of certain plasticizers such as bis(2-ethylhexyl) phthalate made PVC recycling more complicated, as it involves dissolving a banned substance that requires specific authorisation to use.194 In the VinyLoop®process, a butanone–hexane–water mixture was used to dissolve PVC, while the azeotrope formed was regenerated by distillation.190 PET recycling is generally achieved by hydrolysis or glycolysis. PET dissolution from waste textile was achieved at the pilot scale by Worn Again.190 Multiple solvents can be used to dissolve PET (such as aromatic esters or aldehydes), benzyl acetate and ethyl benzoate probably being the best option for health and safety reasons.
Within renewable energy and energy storage end-of-life materials, the most challenging organics are Nafion®, PVDF, EVA and epoxy resins (their structure is shown in Fig. 4(b)).
Nafion® is a sulfonated fluoropolymer (having a PTFE backbone), which is used to conduct cations in PEM fuel cells. This membrane is coated with a catalyst, often made of carbon black particles coated with platinum group metals. Catalyst recovery is possible by dissolution of the Nafion® polymer, which can be achieved with water/alcohol mixtures195 at elevated temperatures (100 °C) but requires working in an autoclave. However, this approach does not take into account the flammability196 of some catalysts (in particular dry and finely divided platinum group metals) which should not be combined with flammable solvents. Moreover, it is possible to dissolve platinum from the catalysts with hot sulfuric acid197 or hydrochloric acid198 and hydrogen peroxide, which may degrade the membrane (particularly the sulfonate groups) but not dissolve it. It is also possible to detach both the hydrophobic catalyst from the membrane and the Pt from the carbon particles by using hydrophobic ILs. Amongst the ILs investigated, the commercial trihexyltetradecyl phosphonium chloride IL allowed high recovery of Pt (>90% after 6 hours at 120 °C) through specific interactions, swelling and wettability of the membrane, the ionomer and the carbon particles composing the catalyst layers of fuel cells.199,200
PVDF is often used as a binder between the current collectors and the cathode active materials of LiBs. Its dissolution has been proven effective to liberate the black mass and separate it from the current collectors. Dissolution can be achieved in 20 minutes at 190 °C using waste cooking oil.201 The fatty acid methyl esters are good solvents for PVDF but they must be modified by transesterification reactions with methanol and sodium hydroxide as a catalyst. However, the process temperature exceeds the melting point of PVDF (around 160 °C). PVDF could also be dissolved in a range of polar solvents202 like N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), NMP, or dimethylacetamide (DMAc). The aforementioned solvents cause problems due to their toxicity, with the exception of DMSO, which is considered safer, but is not innocuous.203 Sustainable alternatives like dihydrolevoglucosenone204 (Cyrene®) have been identified as promising solvents. In that case, large scale availability and cost of the solvent could be an issue. Use of sustainable solvents like gamma-valerolactone for PET recycling by dissolution was demonstrated.205 Both economics and environmental metrics of the process depend on the solvent used but also on the polymer recovery and solvent regeneration method (distillation, anti-solvent precipitation…).
Managing waste fluorinated polymers is complex,206 and their degradation into toxic fluorinated perfluoroalkylated substances is of particular concern. Mineralisation of PVDF (and other fluorinated polymers), where it is decomposed into fluorine ions and carbon dioxide, can be achieved with supercritical water at elevated temperatures (250 °C or more) using either oxygen,207 hydrogen peroxide,208 or potassium permanganate209 as oxidising agents. Degradation of the PVDF starts by hydrogen abstraction from the polymer backbone (eqn (18)), which leads to the formation of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and the release of HF (eqn (19)). The presence of potassium permanganate promotes hydrogen abstraction, while its reaction with water leads to hyperoxide formation ([–CHOOHCF2–]n), which in turns leads to backbone scission (eqn (20)):
C double bond and the release of HF (eqn (19)). The presence of potassium permanganate promotes hydrogen abstraction, while its reaction with water leads to hyperoxide formation ([–CHOOHCF2–]n), which in turns leads to backbone scission (eqn (20)):
| [–CH2CF2–]n → [–CH˙CF2–]n + H˙ | (18) |
[–CH˙CF2–]n + H˙ → [–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF–]n + HF CF–]n + HF | (19) |
| CF2–CH2CF2–CHOOHCF2–CH2CF2 → –CF2–CH2CF2˙ + HCOCF2–CH2CF2 | (20) |
The acid fluoride is easily hydrolysed, subsequent oxidation of end groups and carbon–carbon bond cleavage leads to reduction of PVDF backbone and release of fluoride ions and carbon dioxide.
EVA removal is necessary to liberate the solar cells which contain most of the value (silver electrodes, silicon wafers). Solar cells are completely encapsulated in EVA which protects the wafer from the environment while ensuring mechanical support. EVA dissolution is feasible in a wide range of organic solvents such as acetone, ethanol, toluene, and dichloroethane.210 However, complete dissolution seems too long to be implemented at industrial scale (7 days at 80 °C with most solvents), and initial EVA swelling breaks the wafers, making it impossible to re-use them directly. Process can be made faster (30 minutes at 70 °C) with benzene or toluene (3 mol dm−3) diluted in ethanol as a solvent, and using high intensity ultrasonication211 (900 W). It also allows recovery of the wafer without cracks, as long as the solvent is not degraded. But degradation of the solvent may occur due to the high temperatures reached during ultrasonic irradiation and the formation of radicals during the collapse of bubbles formed under ultrasound. Therefore, clear investigation of the solvent degradation is needed, as well as a clear comparison of electricity consumption, environmental impact and overall cost of the process compared to other EVA removal methods like laser irradiation212 or pyrolysis213 (both techniques allowing recovery of the wafer without breaking it). For all these reasons, delamination of solar cells with gas-expanded liquids214,215 seems more appropriate than dissolution of EVA, although it does not allow intact recovery of wafers. Solar cell delamination is also possible with KOH in ethanol, this solvent allowing a better wettability and ensuring penetration of the hydroxides ions.216 Corrosion of the glass with the same hydroxide ions favours their diffusion between the layers, while EVA is hydrolysed to yield acetic acid and polypropylene, according to eqn (21), separating the glass from the silicon wafer.
[–CH2HC(OC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) OCH3)–]n → nCH3COO− + [–CH2HC(OH)–]n OCH3)–]n → nCH3COO− + [–CH2HC(OH)–]n | (21) |
Composite materials are made from the interpenetration of a matrix material (such as epoxy resins), reinforced with fibreglass or carbon fibres. Their lightweight and mechanical properties made them a material of choice for PCBs or wind turbine blades. Epoxy resins are one of the biggest challenges of wind turbine recycling, with an estimation of around 225![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons a year of fibre-reinforced polymers from wind turbine blades reaching their end-of-life.217 This is also relevant to metal recycling, as magnets are often coated with epoxy resins.218,219 Multiple attempts have been made to break the structure of the epoxy and to recover valuable monomers from the epoxy without decreasing the mechanical performance of the embedded fibres. Epoxy can be decomposed by oxidation with hydrogen peroxide in acetone, which takes 30 minutes at 60 °C and allows to recover clean carbon fibres220 with high mechanical strength. The degradation products formed (which include explosives) are however problematic. Supercritical water and gas-expanded liquids221 can degrade epoxy (probably through hydrolysis), but they could also lead to toxic degradation products like hydrogen sulphide. Epoxy resins can be degraded by a variety of solvents associated with catalysts like polyethylene glycol and sodium hydroxide, which allow ester hydrolysis in less than an hour, at 180 °C and atmospheric pressure.222 Benzyl alcohol and K2CO3 allow depolymerisation of epoxy resin at ambient pressure,223 but at temperatures of 180 °C for ten hours. ZnCl2 in water will preferentially cleave the carbon–nitrogen bonds,224 which would take 6 hours at 250 °C. Concentrated nitric acid (4 M) at 80 °C for 100 hours, allows carbon nitrogen cleavage and nitration of the benzene ring.225 Other solvents like monoethanolamine lead to solid degradation products.226
000 tons a year of fibre-reinforced polymers from wind turbine blades reaching their end-of-life.217 This is also relevant to metal recycling, as magnets are often coated with epoxy resins.218,219 Multiple attempts have been made to break the structure of the epoxy and to recover valuable monomers from the epoxy without decreasing the mechanical performance of the embedded fibres. Epoxy can be decomposed by oxidation with hydrogen peroxide in acetone, which takes 30 minutes at 60 °C and allows to recover clean carbon fibres220 with high mechanical strength. The degradation products formed (which include explosives) are however problematic. Supercritical water and gas-expanded liquids221 can degrade epoxy (probably through hydrolysis), but they could also lead to toxic degradation products like hydrogen sulphide. Epoxy resins can be degraded by a variety of solvents associated with catalysts like polyethylene glycol and sodium hydroxide, which allow ester hydrolysis in less than an hour, at 180 °C and atmospheric pressure.222 Benzyl alcohol and K2CO3 allow depolymerisation of epoxy resin at ambient pressure,223 but at temperatures of 180 °C for ten hours. ZnCl2 in water will preferentially cleave the carbon–nitrogen bonds,224 which would take 6 hours at 250 °C. Concentrated nitric acid (4 M) at 80 °C for 100 hours, allows carbon nitrogen cleavage and nitration of the benzene ring.225 Other solvents like monoethanolamine lead to solid degradation products.226
Brominated epoxy resins from WEEE were dissolved at around 100 °C in NMP227 and DMSO, higher temperatures with DMF,228 or mixtures of propanol and heptane,229 microwaves helping to achieve better extraction rates. Similarly, subcritical acetic acid230 (220 °C) can be used to swell and ultimately decompose brominated epoxy resins. Some ILs such as 1-butyl-3-methyl-imidazolium acetate in combination with ethylene glycol showed promising results for the dissolution of epoxy resins thermosets.231 Other ILs like tributyl ethyl phosphonium diethyl phosphate were unable to dissolve epoxy resins, but were efficient for polymer removal (polyamides, poly-p-phenylene sulfide) from bonded magnets.63
Chemical dissolution of organics could be a powerful separation technique, but only if a non-toxic, recyclable and cheap solvent is available in large quantities to dissolve the polymer under mild conditions, and does not produce harmful by-products. In this regard, dissolution of PVC and some fluorinated polymers seems favourable to avoid formation of gaseous HCl and fluorinated compounds, respectively, when these polymers are submitted to high-temperatures. PVC swelling or dissolution seems particularly indicated to recover copper from PVC-coated copper cables.232 Dissolution of brominated flame retardants for decontamination purposes seems also relevant. For epoxy resins, use of solvents and high temperatures for a prolonged time leads to comparable, if not higher, environmental impact of dissolution as compared to thermal treatment.233
Pyrolysis of fluorinated polymers leads to fluorinated degradation products which are difficult to handle. Acid scrubbing is needed to deal with potential HF formation, while high temperatures are needed to ensure complete destruction of CF4, which takes 1 second at 1440 °C.238 So far, incineration remains the only large-scale solution to ensure complete degradation of fluorinated polymers. Irradiation can also be used to degrade such polymers, which can also be mechanically reprocessed or chemically turned into their monomers.206 Physical processes can be applied to remove fluorinated backsheet from solar cells prior to thermal treatment. This can be achieved using milling machines made for cutting or engraving, which are able to remove the backsheet through superficial abrasion.239
Pyrolysis under nitrogen atmosphere of EVA and PET from solar cells leads to complete conversion of the organics in 30 minutes at 500 °C.240 Liquid decomposition products were mostly long-chain olefins and alkanes (like 1-tridecene, 1-nonadecene, 1-tricosene, and 1-tetradecene), while gaseous products were short-chain olefins and alkanes (like ethane, butane and propane). Pyrolysis possibly allows recuperation of intact silicon wafers,241 which is an additional advantage, but attention must be taken to hazardous and volatile degradation products when dealing with gallium-arsenide solar cells, or the fluorinated polymers from the back sheet of most solar cells.242 Gasification of EVA from solar cells was used to produce cedrene, a medical intermediary used for cancer treatment.243 However, the feedstock used in most studies was shredded solar cells; intact recovery of wafer was demonstrated with pyrolysis, not with gasification.
For epoxy resins, two degradation pathways were identified depending on the temperature of the pyrolysis process. Degradation products will be mostly composed of olefins, alkanes, aromatic compounds, and carboxylic acids.244 The pyrolysis process could be catalytic245 (to break bonds selectively), oxidative,246 or based on superheated steam.247 The degradation temperature and time needed will depend on the atmosphere used, but superheated steam advantageously removes the char produced during pyrolysis, which decontaminates the carbon or glass fibres from composite materials. Another important parameter is the mechanical resistance of the recovered fibres, which is often reduced after thermal treatment. Gasification of epoxies in supercritical water was showed to be promising due to production of OH˙ radicals which improve ring opening and C–O bond rupture.248
Pyrolysis and gasification seems to be more indicated if the degradation products are not harmful, and if there are very large volumes of end-of-life materials to deal with. Both pyrolysis and gasification have been hindered by the use of heterogeneous feedstock. End-of-life solar cells for example could offer a relatively homogeneous feedstock for these processes. So far, high-temperature processes and mechanical shredding are the only relevant large-scale options available for wind turbine blades. Pyrolysis and gasification produces syngas and tars as products which can be used to fuel the process, produce heat, electricity or other chemicals, which is key to reduce their environmental impact. Offshore wind turbines could be pyrolysed near ports and produce valuable chemicals for the chemical industry, often located nearby. Recycling rates, mechanical properties and value of the recovered products must inform techno-economic and life cycle assessments to select which process can be used to deal with the organic fractions. It is however necessary to find relevant options to treat the residue of these processes, like fibreglass.
Waste polymers (plastics) can be mechanically reprocessed,249 burned to produce energy, or up-cycled into value-added products. The last option seems the most promising from an economic point of view. Up-cycling could lead to products such as graphitic or activated carbon,250,251 carbon fibres, foams,252 or silicon carbide,253 for which the raw materials are pyrolysed WEEE. Fig. 5 regroups the value-added and cheaper products that can be obtained from waste graphite, fibreglass, or plastics. Up-cycling of these materials is an interesting option, but the value-added products are often niche applications with a small market. Cheaper products can also be produced using the waste as a raw material, but they offer a much larger market.
Waste plastics from WEEE have been investigated for construction materials produced in bulk quantities like asphalt binder.254 In that case, plastics like acrylonitrile butadiene styrene improved the stiffness and elasticity of the asphalt, possibly due to covalent bonding between plastics and the asphalt promoted by treatment with cumene hydroperoxide. However, high melting temperature plastics like high-impact polystyrene reduced the beneficial interfacial bonding with asphalt. WEEE plastics were also used as coarse aggregate255 for concrete production, but adding more than 10 wt% reduces the mechanical strength of the concrete. Although it reduces the strength of the obtained cement, WEEE residue used as an aggregate could improve the workability and durability256 of the concrete. From a circularity perspective, plastics are immobilised for a long time into construction materials, preventing them from being re-used more than once. The long-term behaviour of such materials is also concerning. It is necessary to anticipate the end of life of concrete containing plastic aggregates, and avoid leakage of microplastics from ageing materials.
Injection moulding can be used to make wood–waste plastic composites257 with improved stiffness. 3D printing filaments258 can be made from WEEE plastics, which gives more flexible filaments and significantly reduces their carbon footprint (around 30% less CO2 when using the waste plastics). WEEE plastics were also investigated as cell culture substrates and showed promising results for cell growth and differentiation due to their surface properties.259
Overall, recycling of waste polymers from WEEE was more studied than polymers from end-of-life materials from renewable energy and energy storage technologies. Recycled EVA was investigated as a raw material to produce bituminous binders.260,261 The incorporation of high contents of EVA (higher than 1 wt%) leads to the formation of polymer rich phases and seems detrimental to the mechanical properties of the bitumen. Incorporation of EVA and its blend with low-density polyethylene (LDPE) and ground tyre rubber could also produce thermoplastic elastomers262 by injection moulding. While LDPE reduces the viscosity of the melt, the EVA copolymer improves the elastomeric properties of the material. Waste EVA could also be turned into vitrimers through a chemical263 or mechanochemical264 approach. Vitrimers are an emerging class of polymers with a dynamic covalent bonding. Exchangeable reactions (like transesterification) occur upon heating the vitrimer at specific temperatures, leading to easily recyclable materials with good mechanical properties.265 Vitrimerisation of epoxy is promising to produce easily recyclable composite materials,266 or recycle epoxy thermosets.267
Fibreglass is another high-volume and low-value material for which direct re-use is sometimes not possible due to reduction of size and mechanical properties during the recycling process. Fibreglass can be treated at high-temperatures (melting with Na2O, then sintering) to produce a glass-ceramic material.268 Fibreglass were also investigated as reinforcement material269 in concrete. Fibres with spherical particles, large size dust or splinters were shown to reduce the mechanical properties of concrete. The presence of oxides like CaO, Al2O3 and SiO2 in glass fibres could improve adhesion and binding of concrete and sometimes improve its mechanical properties.270 However, the incorporation of a high proportion of fibreglass reduces the mechanical properties of mortars.271 3D printing reinforced filaments272 are amongst the products that can be made with waste fibreglass.
Waste graphite was investigated to make conductive electrodes and produce triboelectric generators.273 Graphite can also be up-cycled to higher-value graphene.274 But knowing the large amounts16 of graphite needed for LiBs anodes, the most straightforward market for recycled graphite would be new anodes.13
Amongst the biggest challenges faced for low-value fractions recycling is the lower mechanical properties of recycled products, mismatch between the volume of waste available and the size of the market for recycled products, potential dispersion of hazardous substances, and value of recycled products. While valorisation of WEEE plastics has been studied, management of uncommon polymers such as fluorinated ones or EVA (to a lesser extent) is yet to be addressed and is particularly important knowing the potential environmental damages and the evolution of regulations.
2.6 Limitations and future directions
| Approach | Issue |
|---|---|
| Base metal substrates | Co-deposition of base metals with TCMs could enable selected release through catalytic oxidation i.e. a debondable substrate |
| Debondable adhesives | Permits judicious separation of components without compromising structural integrity |
| Water-miscible adhesives | Enable TCMs to be separated from binders and circumvents high temperature pyrolysis |
| Biocomposites | Enable specific biocatalytic digestion of binders |
| Standardised fixings | Negates the need for unnecessary re-tooling during automated disassembly |
| More physical connections | Allows more rapid disassembly and repair |
| Fewer interfaces | Decreases the number of fixing and amount of adhesive required to construct devices |
| Larger units | Decreases the amount of casing materials and seals |
As such, it is worth addressing why commercial deployment of recycling processes hasn't kept pace with the rapid improvements in research and development over the past 10 years. Although regulation, environmental and health concerns play a role in the development of a circular economy, it is clear that a viable economic model must exist to recover TCMs. Therefore, greater emphasis must be placed on how to incentivise recyclers to recover materials. As materials become more widely used and scarce, it is quite possible that market forces will play a role in encouraging recycling; for example, if the demand for virgin materials escalates, so too will the cost, thus reducing the price differential between virgin and recovered materials. However, relying on market forces can lead to perverse and undesirable consequences and, as such, government intervention may be required to ensure that (i) new products are designed with recycled content, (ii) materials are recycled as a public service, or even (iii) taxes on virgin materials to make recycled materials more economically desirable are introduced.
In addition to the standardisation of recycling methods for existing commercial products, it is important to identify module and device structures that simplify metal recovery from devices without affecting their efficiency and lifetime. For e.g., EVA from silicon solar cells involves peroxide, silane or radiation crosslinking which imparts its high thermal and mechanical durability, but at the same time increases the difficulty in recycling. EVA vitrimers were shown263 to retain 90% of their Young's modulus after 5 iterations of the recycling process as compared to 72% retention of Young's modulus by the thermoplastic EVA. This directly increases the end-of-life value of the panels. At the same time, the modified processes must not affect the key material parameters (in this example, the transmittance of the EVA sheets), so that it is not detrimental to the efficiency of the device (solar cell). If the decrease in the recycling cost fares better than the increase in manufacturing complexity (and hence cost) of the modified process, then it can be a major incentive towards recycling.
For solar panels, most recycling processes aim at recovering glass and the Al frame only. The current mechanical waste sorting practice is to shred the whole module including the frame and junction box or to remove the frame and junction box and shred the solar cell, recovering glass, Al, plastic, some metals and Si. Current plants claim 95% recovery rates, most of this is low grade glass that is used for glass fibre insulation or fillers, the rest being primarily Al from the frame. Future recycling should target recovery of intact silicon wafers and metals, which require a minimum of 30% cost reduction for both mechanical and thermal recycling processes.281 Further, thin-film and flexible solar cells devices might emerge which will require modifications in the recycling process flow. Various solvent based techniques are reported to remove the organic and metal halide layers from perovskite solar cells.282 As the perovskites and other organics are dissolvable in various solvents, mechanical crushing to reduce particle size and subsequent step by step dissolution in various solvents can recover the metal electrodes from these, but the recycling processes must adapt to take into account the environmentally toxic materials (like Pb and Cd) present in these cells.
For wind turbines, decommissioning and transport of such large objects is an economic challenge, particularly the on-site decommissioning of offshore wind turbines. For spent LiBs, the value lies in the recovery of high-purity cobalt, nickel, manganese and lithium salts but their purification is difficult and currently based on complex processes.283 For PEM fuel cells, value lies in the PGM catalysts, the challenge being to liberate them from the anode and cathode catalyst layer, while management of fluorinated polymers is another (potentially costly) issue. In any case, techno-economic assessment is a very important tool to assess which processing route is the most suited for each material and metal content.
In addition to TEA, LCA is an important tool to choose between multiple processes and select the one with the lower environmental impact, which is unfortunately made difficult by the multiple methodologies available and lack of standardisation. Another drawback is the lack of precision when the LCA is performed in the early stages of process development,284 when real-world data from a plant do not exist yet. Furthermore, optimising LCA metrics does not lead to the best performing or lowest cost solutions so methods that combine environmental impact and performance should be considered.285 For chemical processes, it is however possible to get more accurate data from stoichiometric reactions by combining reaction yields with the average values for utility demands.286
As the ease of removal of the polymers directly translates into easier and cheaper access to the metal underneath, the recycling complexity, costs and environmental impact of each alternative/emerging class of encapsulant materials must be compared. In the case of solar cells, non-crosslinking encapsulants like thermoplastic polyolefins and polyvinyl butyral287 (PVB, extensively used in safety glass laminates in automotive industry) or silicone288 based encapsulants are potential alternatives that are possibly easier to dissolve289 or degrade.290
The choice and design of adhesives can reduce the complexity of the recycling process, especially for adhesives that can be removed easily using a trigger mechanism such as ultrasound, magnetic field, electric current, mechanical stress or temperature.291 Adhesives that can be easily dissolved with a chemical are particularly attractive, but would require significant volumes of solvent. At the same time, these alternatives put a limitation on the environments where the devices can be used. For e.g., water-miscible adhesives (particularly as blends) would only require water, but their use would be limited to applications where humidity levels remain low throughout the lifetime of the product. Stress or ultrasound triggered adhesives cannot be used for flexible devices which are expected to withstand high bending stresses in their working environment. Debondable adhesives were shown to improve repairability of small electronics such as smartphones,292 and have a major role to play in other devices such as solar cells293 or LiBs.294
Biocomposites are composites materials made of biodegradable polymer matrices (starch, cellulose, etc.) and/or biofibres of vegetal, animal or mineral origin (hemp, bamboo, etc.). These materials are very attractive since they could make recycling easier.295,296 In addition, there is the emerging area of biodegradable electronics that might also simplify recycling processes,297 enabling recovery of higher value metals and components. In biocomposites, matrix material is selected to avoid epoxies or other petroleum based and non biodegradable polymers, allowing specific biocatalytic digestion of binders, and easy degradation of the polymer matrices. Combustion of these materials does not release additional carbon dioxide, while residual fibreglass after thermal treatment is eliminated. Careful selection of the matrix and reinforcement composite is needed to ensure good mechanical properties to the materials, long durability and low cost.
Finally, the concept of responsible innovation should be highlighted.298 Many items are created using TCMs which are designed for a short linear life; an example of this would be disposable vapes. They are created on a large scale with sufficient lithium to be hazardous but insufficient to make recycling viable. There are other less hazardous batteries that could be used, but irresponsible innovation has led to a large, world-wide, complex waste issue.
3 Conclusion
The transition to a zero-carbon energy system will require the development and deployment of new energy generating and storage technologies. These technologies will use a wider range of critical materials and will be more complex than the technologies used in the era of hydrocarbon-fueled thermal power generation. Increasing dependency on technologies such as wind turbines, solar cells, lithium-ion batteries or catalysts for hydrogen production leads to increased consumption of critical metals. Better recycling processes for these technologies are urgently needed to recover metals more sustainably and with minimal environmental impact.To do so, a set of tools could advantageously complement the conventional hydro- and pyrometallurgical methods by targeting interfacial weaknesses between the different layers. Traditional physical methods of size reduction (comminution), associated with physical separation methods, can be easily implemented to liberate and separate metals from organics and inorganics fractions. High-intensity ultrasonication is useful to delaminate materials and process brittle materials. Organics can be dissolved, pyrolysed or gasified, while metals can be selectively oxidised and dissolved, possibly with biological processes. Hydrogen decrepitation is particularly indicated for magnet recycling. In order to improve recycling rates, low-value fractions like plastics can be turned into construction materials or up-cycled into value-added products. Ultimately, selection of the process applied will depend on techno-economic and life cycle assessments. Given the enormous volumes of technology end-of-life materials that will need to be processed in the future, it could be imagined that future approaches to the treatment of sophisticated technology end-of-life materials, might leverage generic knowledge about the archetypes of mixed material end-of-life materials, combined with automated disassembly and sorting and the application of AI and digital technologies to enable high throughput, high value intelligent recovery from a wide array of materials.
The concept of “Digital Passports”, where physical assets are associated with a digital record that contains details about the material that they contain, are another potential technology that could enable high value sorting of TCMs. Some form of digital asset code, whether it be RFID or QR code, could potentially provide information to future intelligent systems, which could then apply a toolbox approach to select suitable processes for recycling materials streams. Analysis by SystemIQ predicts that such a digital passport could increase profitability for recyclers of lithium ion batteries by 20–30%,299 by optimising pre-processing, material sorting, and aiding in reduced sorting and automated dismantling.
Application of design for recycle principles and responsible innovation are clearly needed to improve recyclability of products before manufacturing. Aspects of design can also substitute components with TCMs for more Earth-abundant alternatives, often with negligible effect on performance.
This paper focuses on a toolbox of technical approaches that can be applied to the challenges of materials criticality and circularity. However, there is also enormous potential to shape the flows of critical materials through a circular economy by shaping the social dimension of the socio-technical systems that utilise them. In some cases, innovation in business models, product service-systems300–302 and new modes of consumption could bolster efforts to commercialise and implement technical improvements to their conservation and recycling. With the application of different approaches being applicable to different materials and production-consumption scenarios, it could be envisaged that a complementary toolbox of business models and product service systems could be synergistic with the push to improve recycling technology, and in many cases, the enabling innovation that facilitates the new approaches outlined in this paper, may be social and not technical in nature.
Abbreviations
| CC | Concentration criterion |
| DESs | Deep eutectic solvents |
| DMAc | Dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| ENIG | Electroless nickel immersion gold |
| EPR | Extended producer responsibility |
| EVA | Poly-(ethylene-vinyl acetate) |
| HASL | Hot air solder levelling |
| HBD | Hydrogen bond donor |
| HDDs | Hard drive disks |
| ILs | Ionic liquids |
| LCA | Life cycle assessment |
| LCDs | Liquid crystals displays |
| LDPE | Low-density polyethylene |
| LEDs | Light emitting diodes |
| LiBs | Lithium-ion batteries |
| NMP | N-methylpyrrolidone |
| PCBs | Printed circuit boards |
| PE | Polyethylene |
| PEM | Proton exchange membrane |
| PET | Polyethylene terephthalate |
| PGMs | Platinum group metals |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethylene |
| PVB | Polyvinyl butyral |
| PVC | Polyvinyl chloride |
| PVDF | Polyvinylidene fluoride |
| REEs | Rare-earth elements |
| TCMs | Technology critical metals |
| TRL | Technology readiness level |
| WEEE | Waste electronic and electric equipment |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the UKRI Interdisciplinary Circular Economy Centre for Technology Metals, Met4Tech project (EP/V011855/1) and the Faraday Institution (Faraday Institution grant code FIRG027, project website https://relib.org.uk), for funding this work. This project has also received funding from the EPSRC SonoCat project (EP/W018632/1). RM and JK acknowledge the support of the EPSRC GEOPIC project (EP/W019248/1).References
- Mining and materials production – Energy Technology Perspectives 2023 – Analysis, https://www.iea.org/reports/energy-technology-perspectives-2023/mining-and-materials-production, accessed August 24, 2023 Search PubMed.
- Securing Technology-Critical Metals for Britain, https://www.birmingham.ac.uk/research/energy/research/centre-strategic-elements-critical-materials/securing-technology-critical-metals-for-britain.aspx, accessed August 24, 2023 Search PubMed.
- K. Binnemans and P. T. Jones, J. Sustain. Metall., 2023, 9, 1–25 CrossRef
.
- X. Zeng and J. Li, J. Cleaner Prod., 2016, 131, 156–162 CrossRef
.
- S. Fang, W. Yan, H. Cao, Q. Song, Y. Zhang and Z. Sun, J. Cleaner Prod., 2018, 182, 624–633 CrossRef
.
- C. Roithner, O. Cencic and H. Rechberger, J. Cleaner Prod., 2022, 331, 129971 CrossRef
.
- X. Tong, H. Yu and T. Liu, J. Cleaner Prod., 2021, 312, 127719 CrossRef
.
- H. Rechberger and T. E. Graedel, Ecol. Econ., 2002, 42, 59–72 CrossRef
.
- B. K. Reck and T. E. Graedel, Science, 2012, 337, 690–695 CrossRef CAS PubMed
.
- R. H. E. M. Koppelaar, S. Pamidi, E. Hajósi, L. Herreras, P. Leroy, H.-Y. Jung, A. Concheso, R. Daniel, F. B. Francisco, C. Parrado, S. Dell'Ambrogio, F. Guggiari, D. Leone and A. Fontana, Sustainability, 2023, 15, 1405 CrossRef
.
- S. F. Jensen, J. H. Kristensen, S. Adamsen, A. Christensen and B. V. Waehrens, Sustain. Prod. Consum., 2023, 37, 242–255 CrossRef
.
- N. M. Charpentier, A. A. Maurice, D. Xia, W.-J. Li, C.-S. Chua, A. Brambilla and J.-C. P. Gabriel, Resour., Conserv. Recycl., 2023, 196, 107033 CrossRef CAS
.
- G. D. J. Harper, E. Kendrick, P. A. Anderson, W. Mrozik, P. Christensen, S. Lambert, D. Greenwood, P. K. Das, M. Ahmeid, Z. Milojevic, W. Du, D. J. L. Brett, P. R. Shearing, A. Rastegarpanah, R. Stolkin, R. Sommerville, A. Zorin, J. L. Durham, A. P. Abbott, D. Thompson, N. D. Browning, B. L. Mehdi, M. Bahri, F. Schanider-Tontini, D. Nicholls, C. Stallmeister, B. Friedrich, M. Sommerfeld, L. L. Driscoll, A. Jarvis, E. C. Giles, P. R. Slater, V. Echavarri-Bravo, G. Maddalena, L. E. Horsfall, L. Gaines, Q. Dai, S. J. Jethwa, A. L. Lipson, G. A. Leeke, T. Cowell, J. G. Farthing, G. Mariani, A. Smith, Z. Iqbal, R. Golmohammadzadeh, L. Sweeney, V. Goodship, Z. Li, J. Edge, L. Lander, V. T. Nguyen, R. J. R. Elliot, O. Heidrich, M. Slattery, D. Reed, J. Ahuja, A. Cavoski, R. Lee, E. Driscoll, J. Baker, P. Littlewood, I. Styles, S. Mahanty and F. Boons, J. Phys.: Energy, 2023, 5, 021501 Search PubMed
.
- L. Dawson, J. Ahuja and R. Lee, Envtl. L. Rev., 2021, 23, 128–143 CrossRef
.
-
Proposal for a Regulation of the European Parliament and of the Council Concerning Batteries and Waste Batteries, Repealing Directive 2006/66/EC and Amending Regulation (EU) No 2019/1020, 2020 Search PubMed
.
- The Role of Critical Minerals in Clean Energy Transitions – Analysis, https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions, accessed August 25, 2023 Search PubMed.
- End-of-life management Solar Photovoltaic Panels, https://www.irena.org/publications/2016/Jun/End-of-life-management-Solar-Photovoltaic-Panels, accessed October 19, 2023 Search PubMed.
- A. Tang, Wind energy in Europe, https://windeurope.org/about-wind/reports/wind-energy-in-europe-outlook-to-2023/, accessed October 19, 2023 Search PubMed.
- S. Hoenderdaal, L. Tercero Espinoza, F. Marscheider-Weidemann and W. Graus, Energy, 2013, 49, 344–355 CrossRef
.
- Y. Yang, A. Walton, R. Sheridan, K. Güth, R. Gauß, O. Gutfleisch, M. Buchert, B.-M. Steenari, T. Van Gerven, P. T. Jones and K. Binnemans, J. Sustain. Metall., 2017, 3, 122–149 CrossRef
.
-
H. Albers, S. Greiner, H. Seifertand and U. Kühne, DEWI Magazin, 2009, 2009, pp. 32–38 Search PubMed
.
- Secondary supplies, https://matthey.com/products-and-markets/pgms-and-circularity/pgm-markets/secondary-supplies, accessed October 19, 2023 Search PubMed.
- H. B. Trinh, J. Lee, Y. Suh and J. Lee, Waste Manage., 2020, 114, 148–165 CrossRef CAS PubMed
.
- J. C. Stallard, L. Wheatcroft, S. G. Booth, R. Boston, S. A. Corr, M. F. L. De Volder, B. J. Inkson and N. A. Fleck, Joule, 2022, 6, 984–1007 CrossRef CAS
.
- T. Maani, I. Celik, M. J. Heben, R. J. Ellingson and D. Apul, Sci. Total Environ., 2020, 735, 138827 CrossRef CAS PubMed
.
- R. Ding, S. Zhang, Y. Chen, Z. Rui, K. Hua, Y. Wu, X. Li, X. Duan, X. Wang, J. Li and J. Liu, Energy and AI, 2022, 9, 100170 CrossRef
.
- A. Tuncuk, V. Stazi, A. Akcil, E. Y. Yazici and H. Deveci, Miner. Eng., 2012, 25, 28–37 CrossRef CAS
.
- C. Hagelüken and J. Kegels, Hydrocarbon engineering, 2004, 9, 31–36 Search PubMed
.
- N. P. Nayak, Mater. Today: Proc., 2022, 50, 2078–2083 CAS
.
- J. Chen, W. Zhang, B. Ma, J. Che, L. Xia, P. Wen and C. Wang, J. Hazard. Mater., 2022, 430, 128497 CrossRef CAS PubMed
.
- M. Chakraborty, J. Kettle and R. Dahiya, IEEE Flex. Electron., 2022, 1, 4–23 Search PubMed
.
- M. Kaya, Waste Manage., 2016, 57, 64–90 CrossRef CAS PubMed
.
- A. A. Maurice, K. N. Dinh, N. M. Charpentier, A. Brambilla and J.-C. P. Gabriel, Sustainability, 2021, 13, 10357 CrossRef CAS
.
- D. Thompson, C. Hyde, J. M. Hartley, A. P. Abbott, P. A. Anderson and G. D. J. Harper, Resour., Conserv. Recycl., 2021, 175, 105741 CrossRef CAS
.
- L. Lander, C. Tagnon, V. Nguyen-Tien, E. Kendrick, R. J. R. Elliott, A. P. Abbott, J. S. Edge and G. J. Offer, Appl. Energy, 2023, 331, 120437 CrossRef
.
- S. Glöser-Chahoud, S. Huster, S. Rosenberg, S. Baazouzi, S. Kiemel, S. Singh, C. Schneider, M. Weeber, R. Miehe and F. Schultmann, Resour., Conserv. Recycl., 2021, 174, 105735 CrossRef
.
- G. R. Ballantyne, M. S. Powell and M. Tiang, 2012.
- A. Somani, T. K. Nandi, S. K. Pal and A. K. Majumder, Int. J. Min. Sci. Technol., 2017, 27, 339–348 CrossRef
.
- Government of Canada, Benchmarking the energy consumption of Canadian underground bulk mines, https://publications.gc.ca/site/eng/287264/publication.html, accessed August 24, 2023.
- L. Wuschke, H.-G. Jäckel, T. Leißner and U. A. Peuker, Waste Manage., 2019, 85, 317–326 CrossRef CAS PubMed
.
-
M. Kaya, in Waste Electrical and Electronic Equipment Recycling, Elsevier, 2018, pp. 33–93 Search PubMed
.
- Z. Li, L. A. Diaz, Z. Yang, H. Jin, T. E. Lister, E. Vahidi and F. Zhao, Resour., Conserv. Recycl., 2019, 149, 20–30 CrossRef
.
- Z. Ruan, M. Li, K. Gao, D. Zhang, L. Huang, W. Xu and X. Liu, ACS Omega, 2019, 4, 9813–9822 CrossRef CAS PubMed
.
- Y. Guo, Y. Li, X. Lou, J. Guan, Y. Li, X. Mai, H. Liu, C. X. Zhao, N. Wang, C. Yan, G. Gao, H. Yuan, J. Dai, R. Su and Z. Guo, J. Mater. Sci., 2018, 53, 13790–13800 CrossRef CAS
.
- Y. Sim, Y. B. Tay, H. K. Pham and N. Mathews, Waste Manage., 2023, 156, 97–106 CrossRef CAS PubMed
.
- A. Vanderbruggen, E. Gugala, R. Blannin, K. Bachmann, R. Serna-Guerrero and M. Rudolph, Miner. Eng., 2021, 169, 106924 CrossRef CAS
.
- P. Jiang, M. Harney, Y. Song, B. Chen, Q. Chen, T. Chen, G. Lazarus, L. H. Dubois and M. B. Korzenski, Procedia Environ. Sci., 2012, 16, 485–490 CrossRef CAS
.
- Z.-Y. Zhang, F.-S. Zhang and T. Yao, Waste Manage., 2017, 68, 490–497 CrossRef CAS PubMed
.
- J. Guan, Y. Li, Y. Guo, R. Su, G. Gao, H. Song, H. Yuan, B. Liang and Z. Guo, ACS Sustainable Chem. Eng., 2017, 5, 1026–1032 CrossRef CAS
.
- B. Swain, C. Mishra, L. Kang, K.-S. Park, C. G. Lee and H. S. Hong, Environ. Res., 2015, 138, 401–408 CrossRef CAS PubMed
.
- S. Kafashi, L. Kuhar, A. Bóna and A. N. Nikoloski, Miner. Process. Extr. Metall. Rev., 2023, 1–16 Search PubMed
.
- R. P. Mahapatra, S. S. Srikant, R. B. Rao and B. Mohanty, Sādhanā, 2019, 44, 209 CrossRef
.
- C. S. Tiwary, S. Kishore, R. Vasireddi, D. R. Mahapatra, P. M. Ajayan and K. Chattopadhyay, Mater. Today, 2017, 20, 67–73 CrossRef CAS
.
- C. L. Duan, Z. J. Diao, Y. M. Zhao and W. Huang, Miner. Eng., 2015, 70, 170–177 CrossRef CAS
.
- Y. Zhao, B. Zhang, C. Duan, X. Chen and S. Sun, Powder Technol., 2015, 269, 219–226 CrossRef CAS
.
- P. Zhao, J. Guo, G. Yan, G. Zhu, X. Zhu, Z. Zhang and B. Zhang, J. Cleaner Prod., 2020, 257, 120442 CrossRef CAS
.
- M. Yang, H. Liu, B. Ye and W. Qian, Process Saf. Environ. Prot., 2021, 148, 805–812 CrossRef CAS
.
- L. Kurz, M. Faryadras, I. Klugius, F. Reichert, A. Scheibe, M. Schmidt and R. Wörner, Batteries, 2021, 7, 29 CrossRef CAS
.
- A. Yoshida, A. Terazono, F. C. Ballesteros, D.-Q. Nguyen, S. Sukandar, M. Kojima and S. Sakata, Resour., Conserv. Recycl., 2016, 106, 48–58 CrossRef
.
- Y. Bai, N. Muralidharan, J. Li, R. Essehli and I. Belharouak, ChemSusChem, 2020, 13, 5664–5670 CrossRef CAS
.
- R. Zhan, T. Payne, T. Leftwich, K. Perrine and L. Pan, Waste Manage., 2020, 105, 39–48 CrossRef CAS PubMed
.
- A. Rensmo, E. K. Savvidou, I. T. Cousins, X. Hu, S. Schellenberger and J. P. Benskin, Environ. Sci.: Processes Impacts, 2023, 25, 1015–1030 RSC
.
- M. A. R. Önal, S. Dewilde, M. Degri, L. Pickering, B. Saje, S. Riaño, A. Walton and K. Binnemans, Green Chem., 2020, 22, 2821–2830 RSC
.
- M. Orefice, A. Eldosouky, I. Škulj and K. Binnemans, RSC Adv., 2019, 9, 14910–14915 RSC
.
- G. Granata, F. Pagnanelli, E. Moscardini, T. Havlik and L. Toro, Sol. Energy Mater. Sol. Cells, 2014, 123, 239–248 CrossRef CAS
.
- NPC incorporated | Global leader of solar module manufacturing equipment, https://www.npcgroup.net/eng/, accessed October 19, 2023 Search PubMed.
- S. Shi, C. Nie, H. Chang, P. Wu, Z. Piao and X. Zhu, J. Cleaner Prod., 2021, 318, 128512 CrossRef CAS
.
- É. F. Rodrigues, A. De Rossi, B. Rovaris, A. Valério, D. de Oliveira and D. Hotza, Waste Biomass Valorization, 2021, 12, 4081–4087 CrossRef
.
- Y. Marcus, ACS Sustainable Chem. Eng., 2017, 5, 11780–11787 CrossRef CAS
.
- C. Hanisch, T. Loellhoeffel, J. Diekmann, K. J. Markley, W. Haselrieder and A. Kwade, J. Cleaner Prod., 2015, 108, 301–311 CrossRef CAS
.
- H. Bi, H. Zhu, L. Zu, S. He, Y. Gao and S. Gao, Waste Manage. Res., 2019, 37, 374–385 CrossRef CAS PubMed
.
- E. Gratz, Q. Sa, D. Apelian and Y. Wang, J. Power Sources, 2014, 262, 255–262 CrossRef CAS
.
- S. P. Barik, G. Prabaharan and B. Kumar, Waste Manage., 2016, 51, 222–226 CrossRef CAS PubMed
.
- M. F. Azeumo, C. Germana, N. M. Ippolito, M. Franco, P. Luigi and S. Settimio, Sol. Energy Mater. Sol. Cells, 2019, 193, 314–319 CrossRef CAS
.
- Silver – Element information, properties and uses | Periodic Table, https://www.rsc.org/periodic-table/element/47/, accessed October 19, 2023 Search PubMed.
- S. Azizi, C. M. Ouellet-Plamondon, P. Nguyen-Tri, M. Fréchette and E. David, Composites, Part B, 2019, 177, 107288 CrossRef CAS
.
- Aluminium – Element information, properties and uses | Periodic Table, https://www.rsc.org/periodic-table/element/13/, accessed October 19, 2023 Search PubMed.
- J. Nan, D. Han and X. Zuo, J. Power Sources, 2005, 152, 278–284 CrossRef CAS
.
- J. Li, G. Wang and Z. Xu, J. Hazard. Mater., 2016, 302, 97–104 CrossRef CAS PubMed
.
- S. Saeki, J. Lee, Q. Zhang and F. Saito, Int. J. Miner. Process., 2004, 74, S373–S378 CrossRef CAS
.
- M.-M. Wang, C.-C. Zhang and F.-S. Zhang, Waste Manage., 2017, 67, 232–239 CrossRef CAS PubMed
.
-
L. Dascalescu, T. Zeghloul and A. Iuga, in WEEE Recycling, Elsevier, 2016, pp. 75–106 Search PubMed
.
- A Table of Electrical Conductivity and Resistivity of Common Materials, https://www.thoughtco.com/table-of-electrical-resistivity-conductivity-608499, accessed October 19, 2023 Search PubMed.
- A. Iuga, L. Calin, V. Neamtu, A. Mihalcioiu and L. Dascalescu, J. Electrost., 2005, 63, 937–942 CrossRef CAS
.
- A. F. Diaz and R. M. Felix-Navarro, J. Electrost., 2004, 62, 277–290 CrossRef CAS
.
- H. Bi, H. Zhu, L. Zu, Y. Gao, S. Gao and Z. Wu, Waste Manage. Res., 2019, 37, 1217–1228 CrossRef CAS PubMed
.
-
Y. R. Smith, J. R. Nagel and R. K. Rajamani, in Energy Technology 2017, ed. L. Zhang, J. W. Drelich, N. R. Neelameggham, D. P. Guillen, N. Haque, J. Zhu, Z. Sun, T. Wang, J. A. Howarter, F. Tesfaye, S. Ikhmayies, E. Olivetti and M. W. Kennedy, Springer International Publishing, Cham, 2017, pp. 379–386 Search PubMed
.
- S. M. Shin, N. H. Kim, J. S. Sohn, D. H. Yang and Y. H. Kim, Hydrometallurgy, 2005, 79, 172–181 CrossRef CAS
.
- A. V. M. Silveira, M. P. Santana, E. H. Tanabe and D. A. Bertuol, Int. J. Miner. Process., 2017, 169, 91–98 CrossRef CAS
.
- T. Zhang, Y. He, F. Wang, L. Ge, X. Zhu and H. Li, Waste Manage., 2014, 34, 1051–1058 CrossRef CAS PubMed
.
- P. Dias, L. Schmidt, L. B. Gomes, A. Bettanin, H. Veit and A. M. Bernardes, J. Sustain. Metall., 2018, 4, 176–186 CrossRef
.
- D. W. Fuerstenau and P. Pradip, Adv. Colloid Interface Sci., 2005, 114–115, 9–26 CrossRef CAS PubMed
.
- O. Kökkılıç, S. Mohammadi-Jam, P. Chu, C. Marion, Y. Yang and K. E. Waters, Adv. Colloid Interface Sci., 2022, 308, 102769 CrossRef PubMed
.
- C. Wang, R. Sun and B. Xing, J. Air Waste Manage. Assoc., 2021, 71, 1483–1491 CrossRef CAS PubMed
.
- T.-O. Folayan, A. L. Lipson, J. L. Durham, H. Pinegar, D. Liu and L. Pan, Energy Technol., 2021, 9, 2100468 CrossRef CAS
.
- R. Zhan, Z. Oldenburg and L. Pan, Sustainable Mater. Technol., 2018, 17, e00062 CrossRef CAS
.
- A. Vanderbruggen, J. Sygusch, M. Rudolph and R. Serna-Guerrero, Colloids Surf., A, 2021, 626, 127111 CrossRef CAS
.
- A. M. Salces, I. Bremerstein, M. Rudolph and A. Vanderbruggen, Miner. Eng., 2022, 184, 107670 CrossRef CAS
.
- S. Harada, Md. A. Uddin, Y. Kato, T. Kawanishi and Y. Hayashi, J. Sustain. Metall., 2019, 5, 551–560 CrossRef
.
- P. Xanthopoulos, S. Bevandić, J. Spooren, K. Binnemans and F. Kukurugya, RSC Adv., 2022, 12, 2351–2360 RSC
.
- G. Zhang, Z. Du, Y. He, H. Wang, W. Xie and T. Zhang, Sustainability, 2019, 11, 2363 CrossRef CAS
.
- G. Zhang, Y. He, Y. Feng, H. Wang and X. Zhu, ACS Sustainable Chem. Eng., 2018, 6, 10896–10904 CrossRef CAS
.
- I. O. Ogunniyi and M. K. G. Vermaak, Miner. Eng., 2009, 22, 378–385 CrossRef CAS
.
- T. Sinn, A. Flegler, A. Wolf, T. Stübinger, W. Witt, H. Nirschl and M. Gleiß, Metals, 2020, 10, 1617 CrossRef CAS
.
- A. P. Abbott, F. Bevan, M. Baeuerle, R. C. Harris and G. R. T. Jenkin, Electrochem. Commun., 2017, 76, 20–23 CrossRef CAS
.
- I. M. Pateli, A. P. Abbott, G. R. T. Jenkin and J. M. Hartley, Green Chem., 2020, 22, 8360–8368 RSC
.
- B. Villemejeanne, S. Legeai, E. Meux, S. Dourdain, H. Mendil-Jakani and E. Billy, J. Environ. Chem. Eng., 2022, 10, 108004 CrossRef CAS
.
- U. Jadhav, C. Su and H. Hocheng, Environ. Sci. Pollut. Res., 2016, 23, 24384–24392 CrossRef CAS PubMed
.
- I. M. Pateli, D. Thompson, S. S. M. Alabdullah, A. P. Abbott, G. R. T. Jenkin and J. M. Hartley, Green Chem., 2020, 22, 5476–5486 RSC
.
- B.-K. Kim, E. J. Lee, Y. Kang and J.-J. Lee, J. Ind. Eng. Chem., 2018, 61, 388–397 CrossRef CAS
.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Green Chem., 2011, 13, 471 RSC
.
- B. May, M. Lexow, N. Taccardi, H.-P. Steinrück and F. Maier, ChemistryOpen, 2019, 8, 15–22 CrossRef CAS PubMed
.
- D. Jones, J. Hartley, G. Frisch, M. Purnell and L. Darras, Palaeont. Electr., 2016, 15, 1–7 Search PubMed
.
- G. R. T. Jenkin, A. Z. M. Al-Bassam, R. C. Harris, A. P. Abbott, D. J. Smith, D. A. Holwell, R. J. Chapman and C. J. Stanley, Miner. Eng., 2016, 87, 18–24 CrossRef CAS
.
- A. Van den Bossche, N. Rodriguez Rodriguez, S. Riaño, W. Dehaen and K. Binnemans, RSC Adv., 2021, 11, 10110–10120 RSC
.
- J. M. Hartley, S. Scott, Z. Dilruba, A. J. Lucio, P. J. Bird, R. C. Harris, G. R. T. Jenkin and A. P. Abbott, Phys. Chem. Chem. Phys., 2022, 24, 24105–24115 RSC
.
- M. Rodríguez, L. Ayala, P. Robles, R. Sepúlveda, D. Torres, F. R. Carrillo-Pedroza, R. I. Jeldres and N. Toro, Metals, 2020, 10, 183 CrossRef
.
- D. L. Thompson, I. M. Pateli, C. Lei, A. Jarvis, A. P. Abbott and J. M. Hartley, Green Chem., 2022, 24, 4877–4886 RSC
.
- S. Tang, J. Feng, R. Su, M. Zhang and M. Guo, ACS Sustainable Chem. Eng., 2022, 10, 8423–8432 CrossRef CAS
.
- P. Zürner and G. Frisch, ACS Sustainable Chem. Eng., 2019, 7, 5300–5308 CrossRef
.
- W. Chen, J. Jiang, X. Lan, X. Zhao, H. Mou and T. Mu, Green Chem., 2019, 21, 4748–4756 RSC
.
- I. M. Pateli, A. P. Abbott, K. Binnemans and N. Rodriguez Rodriguez, RSC Adv., 2020, 10, 28879–28890 RSC
.
- C. Liu, Q. Yan, X. Zhang, L. Lei and C. Xiao, Environ. Sci. Technol., 2020, 54, 10370–10379 CrossRef CAS PubMed
.
- J. Richter and M. Ruck, RSC Adv., 2019, 9, 29699–29710 RSC
.
- E. Daskalopoulou, J. M. Hartley, R. M. Rivera, G. Zante and A. P. Abbott, Phys. Chem. Chem. Phys., 2023, 25, 4854–4861 RSC
.
- G. García, S. Aparicio, R. Ullah and M. Atilhan, Energy Fuels, 2015, 29, 2616–2644 CrossRef
.
- S. Zhang, N. Sun, X. He, X. Lu and X. Zhang, J. Phys. Chem. Ref. Data, 2006, 35, 1475–1517 CrossRef CAS
.
- H. Qin, X. Hu, J. Wang, H. Cheng, L. Chen and Z. Qi, Green Energy Environ., 2020, 5, 8–21 CrossRef
.
- G. Zante, E. Daskalopoulou, C. E. Elgar, R. M. Rivera, J. M. Hartley, K. Simpson, R. Tuley, J. Kettle and A. P. Abbott, RSC Sustainability, 2023, 1, 1025–1034 RSC
.
-
K. Grant, S. Zhang and J. Kettle, in 2023 IEEE Conference on Technologies for Sustainability (SusTech), IEEE, Portland, OR, USA, 2023, pp. 86–90 Search PubMed
.
- J. M. Hartley, S. Scott, R. Marin Rivera, P. Hunt, A. J. Lucio, P. Bird, R. Harris, G. R. T. Jenkin and A. P. Abbott, RSC Sustainability, 2023, 1, 107–116 RSC
.
-
A. J. Bard, R. Parsons and J. Jordan, Standard Potentials in Aqueous Solution, Routledge, 1st edn, 2017 Search PubMed
.
- J. M. Hartley, C.-M. Ip, G. C. H. Forrest, K. Singh, S. J. Gurman, K. S. Ryder, A. P. Abbott and G. Frisch, Inorg. Chem., 2014, 53, 6280–6288 CrossRef CAS PubMed
.
- A. P. Abbott, G. Frisch, S. J. Gurman, A. R. Hillman, J. Hartley, F. Holyoak and K. S. Ryder, Chem. Commun., 2011, 47, 10031 RSC
.
- M. Baniasadi, F. Vakilchap, N. Bahaloo-Horeh, S. M. Mousavi and S. Farnaud, J. Ind. Eng. Chem., 2019, 76, 75–90 CrossRef CAS
.
- B. Xin, D. Zhang, X. Zhang, Y. Xia, F. Wu, S. Chen and L. Li, Bioresour. Technol., 2009, 100, 6163–6169 CrossRef CAS PubMed
.
- S. C. Campbell, G. J. Olson, T. R. Clark and G. McFeters, J. Ind. Microbiol. Biotechnol., 2001, 26, 134–139 CrossRef CAS PubMed
.
- T. D. Chi, J. Lee, B. D. Pandey, K. Yoo and J. Jeong, Miner. Eng., 2011, 24, 1219–1222 CrossRef CAS
.
- G. Natarajan and Y.-P. Ting, Bioresour. Technol., 2014, 152, 80–85 CrossRef CAS PubMed
.
- M. Arshadi and S. M. Mousavi, Bioresour. Technol., 2015, 175, 315–324 CrossRef CAS PubMed
.
- M. A. Faramarzi, M. Stagars, E. Pensini, W. Krebs and H. Brandl, J. Biotechnol., 2004, 113, 321–326 CrossRef CAS PubMed
.
- R. Auerbach, K. Bokelmann, R. Stauber, O. Gutfleisch, S. Schnell and S. Ratering, Miner. Eng., 2019, 134, 104–117 CrossRef CAS
.
- A. Pathak, H. Al-Sheeha, R. Navvamani, R. Kothari, M. Marafi and M. S. Rana, Rev. Environ. Sci. Biotechnol., 2022, 21, 1035–1059 CrossRef CAS
.
- S. Karim and Y.-P. Ting, J. Cleaner Prod., 2020, 255, 120199 CrossRef CAS
.
- H. Malekian, M. Salehi and D. Biria, Waste Manage., 2019, 85, 264–271 CrossRef CAS PubMed
.
- S. Maneesuwannarat, A. S. Vangnai, M. Yamashita and P. Thiravetyan, Process Saf. Environ. Prot., 2016, 99, 80–87 CrossRef CAS
.
-
A. Işildar, Metal Recovery from Electronic Waste: Biological versus Chemical Leaching for Recovery of Copper and Gold, CRC Press, Leiden, the Netherlands, 2018 Search PubMed
.
- V. K. Nguyen, M.-G. Ha, S. Shin, M. Seo, J. Jang, S. Jo, D. Kim, S. Lee, Y. Jung, P. Kang, C. Shin and Y. Ahn, J. Environ. Manage., 2018, 223, 852–859 CrossRef CAS PubMed
.
- M. Qu, J. Chen, Q. Huang, J. Chen, Y. Xu, J. Luo, K. Wang, W. Gao and Y. Zheng, Int. Biodeterior. Biodegrad., 2018, 128, 41–47 CrossRef CAS
.
- S. Vyas and Y.-P. Ting, Environ. Technol. Innovation, 2019, 14, 100310 CrossRef
.
- Y. Dong, H. Lin, H. Wang, X. Mo, K. Fu and H. Wen, Miner. Eng., 2011, 24, 870–875 CrossRef CAS
.
- V. K. Nguyen and J.-U. Lee, Geosci. J., 2014, 18, 355–363 CrossRef CAS
.
- V. K. Nguyen, T. Tran, H.-J. Han, S.-H. Lee and J.-U. Lee, J. Geochem. Explor., 2015, 156, 153–161 CrossRef CAS
.
- N. Pantidos and L. E. Horsfall, J. Nanomed. Nanotechnol., 2014, 5, 1000233 Search PubMed
.
- S. Tarver, D. Gray, K. Loponov, D. B. Das, T. Sun and M. Sotenko, Int. Biodeterior. Biodegrad., 2019, 143, 104724 CrossRef CAS
.
- A. J. Stephen, N. V. Rees, I. Mikheenko and L. E. Macaskie, Front. Energy Res., 2019, 7, 66 CrossRef
.
- Y. Era, J. A. Dennis, S. Wallace and L. E. Horsfall, Green Chem., 2021, 23, 8886–8890 RSC
.
- Y. Era, J. A. Dennis, L. E. Horsfall and S. Wallace, JACS Au, 2022, 2, 2446–2452 CrossRef CAS PubMed
.
- J. A. Mattocks, J. V. Ho and J. A. Cotruvo, J. Am. Chem. Soc., 2019, 141, 2857–2861 CrossRef CAS PubMed
.
- G. J.-P. Deblonde, J. A. Mattocks, D. M. Park, D. W. Reed, J. A. Cotruvo and Y. Jiao, Inorg. Chem., 2020, 59, 11855–11867 CrossRef CAS PubMed
.
- Z. Wu, T. Soh, J. J. Chan, S. Meng, D. Meyer, M. Srinivasan and C. Y. Tay, Environ. Sci. Technol., 2020, 54, 9681–9692 CrossRef CAS PubMed
.
- X. Chen, C. Luo, J. Zhang, J. Kong and T. Zhou, ACS Sustainable Chem. Eng., 2015, 3, 3104–3113 CrossRef CAS
.
- Y. Zhang, Q. Meng, P. Dong, J. Duan and Y. Lin, J. Ind. Eng. Chem., 2018, 66, 86–93 CrossRef CAS
.
- M. Zakotnik, I. R. Harris and A. J. Williams, J. Alloys Compd., 2008, 450, 525–531 CrossRef CAS
.
- T. Takeshita, J. Alloys Compd., 1995, 231, 51–59 CrossRef CAS
.
- X. T. Li, M. Yue, S. X. Zhou, C. J. Kuang, G. Q. Zhang, B. S. Dong and H. Zeng, J. Magn. Magn. Mater., 2019, 473, 144–147 CrossRef CAS
.
-
J. J. Croat, Rapidly Solidified Neodymium-Iron-Boron Permanent Magnets, Woodhead Publishing, 2017 Search PubMed
.
- A. Habibzadeh, M. A. Kucuker and M. Gökelma, ACS Omega, 2023, 8, 17431–17445 CrossRef CAS PubMed
.
- A. Walton, H. Yi, N. A. Rowson, J. D. Speight, V. S. J. Mann, R. S. Sheridan, A. Bradshaw, I. R. Harris and A. J. Williams, J. Cleaner Prod., 2015, 104, 236–241 CrossRef CAS
.
- B. Sprecher, Y. Xiao, A. Walton, J. Speight, R. Harris, R. Kleijn, G. Visser and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 3951–3958 CrossRef CAS PubMed
.
- L. H. Thompson and L. K. Doraiswamy, Ind. Eng. Chem. Res., 1999, 38, 1215–1249 CrossRef CAS
.
-
T. Y. Wu, N. Guo, C. Y. Teh and J. X. W. Hay, in Advances in Ultrasound Technology for Environmental Remediation, Springer Netherlands, Dordrecht, 2013, pp. 5–12 Search PubMed
.
- J. A. Morton, M. Khavari, L. Qin, B. M. Maciejewska, A. V. Tyurnina, N. Grobert, D. G. Eskin, J. Mi, K. Porfyrakis, P. Prentice and I. Tzanakis, Mater. Today, 2021, 49, 10–22 CrossRef CAS
.
- S. V. Sancheti and P. R. Gogate, Ultrason. Sonochem., 2017, 36, 527–543 CrossRef CAS PubMed
.
- R. M. Wagterveld, L. Boels, M. J. Mayer and G. J. Witkamp, Ultrason. Sonochem., 2011, 18, 216–225 CrossRef CAS PubMed
.
- B. Makuza, D. Yu, Z. Huang, Q. Tian and X. Guo, Resour., Conserv. Recycl., 2021, 174, 105784 CrossRef CAS
.
- P. Ning, Q. Meng, P. Dong, J. Duan, M. Xu, Y. Lin and Y. Zhang, Waste Manage., 2020, 103, 52–60 CrossRef CAS PubMed
.
- L. Chen, J. He, L. Zhu, Q. Yao, Y. Sun, C. Guo, H. Chen and B. Yang, Process Saf. Environ. Prot., 2023, 169, 869–878 CrossRef CAS
.
- D. M. dos Santos, D. C. Buzzi, A. B. Botelho Junior and D. C. R. Espinosa, J. Mater. Cycles Waste Manage., 2022, 24, 1991–2001 CrossRef CAS
.
- M. Souada, C. Louage, J.-Y. Doisy, L. Meunier, A. Benderrag, B. Ouddane, S. Bellayer, N. Nuns, M. Traisnel and U. Maschke, Ultrason. Sonochem., 2018, 40, 929–936 CrossRef CAS PubMed
.
- K. Zhang, B. Li, Y. Wu, W. Wang, R. Li, Y.-N. Zhang and T. Zuo, Waste Manage., 2017, 64, 236–243 CrossRef CAS PubMed
.
- C. Zhao, Y. Zhang, H. Cao, X. Zheng, T. Van Gerven, Y. Hu and Z. Sun, Ultrason. Sonochem., 2019, 52, 484–492 CrossRef CAS PubMed
.
- X. Bu, J. K. Danstan, A. Hassanzadeh, A. Behrad Vakylabad and S. C. Chelgani, Miner. Process. Extr. Metall. Rev., 2022, 1–18 CAS
.
- O. Velázquez-Martinez, A. Kontomichalou, A. Santasalo-Aarnio, M. Reuter, A. J. Karttunen, M. Karppinen and R. Serna-Guerrero, Resour., Conserv. Recycl., 2020, 159, 104843 CrossRef
.
- C. Lei, I. Aldous, J. M. Hartley, D. L. Thompson, S. Scott, R. Hanson, P. A. Anderson, E. Kendrick, R. Sommerville, K. S. Ryder and A. P. Abbott, Green Chem., 2021, 23, 4710–4715 RSC
.
- X. Chen, S. Li, Y. Wang, Y. Jiang, X. Tan, W. Han and S. Wang, Waste Manage., 2021, 136, 67–75 CrossRef CAS PubMed
.
- L.-P. He, S.-Y. Sun, X.-F. Song and J.-G. Yu, Waste Manage., 2015, 46, 523–528 CrossRef CAS PubMed
.
- T. Oki, T. Katsumata, K. Hashimoto and M. Kobayashi, Mater. Trans., 2009, 50, 1864–1870 CrossRef CAS
.
- C. Xu, B. Li, X. Yuan, C. Liu, C. Shen and G. Dai, IOP Conf. Ser. Earth Environ. Sci., 2019, 242, 032046 CrossRef
.
- J. Sherwood, Johnson Matthey Technol. Rev., 2020, 64, 4–15 CrossRef CAS
.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef
.
- S. Venkatram, C. Kim, A. Chandrasekaran and R. Ramprasad, J. Chem. Inf. Model., 2019, 59, 4188–4194 CrossRef CAS PubMed
.
- G. Grause, S. Hirahashi, H. Toyoda, T. Kameda and T. Yoshioka, J. Mater. Cycles Waste Manage., 2017, 19, 612–622 CrossRef CAS
.
- Authorisation List – ECHA, https://echa.europa.eu/authorisation-list, accessed August 25, 2023.
- L. Duclos, M. Lupsea, G. Mandil, L. Svecova, P.-X. Thivel and V. Laforest, J. Cleaner Prod., 2017, 142, 2618–2628 CrossRef CAS
.
- T. Chandra and J. P. Zebrowski, J. Chem. Health Saf., 2016, 23, 16–25 CrossRef
.
- F. Xu, S. Mu and M. Pan, Int. J. Hydrogen Energy, 2010, 35, 2976–2979 CrossRef CAS
.
- L. Duclos, L. Svecova, V. Laforest, G. Mandil and P.-X. Thivel, Hydrometallurgy, 2016, 160, 79–89 CrossRef CAS
.
- M. Coudray, E. Billy, H. Mendil-Jakani, P.-H. Haumesser, C. C. Santini and V. Dufaud, Ind. Eng. Chem. Res., 2023, 62, 6355–6366 CrossRef CAS
.
- M. Coudray, E. Billy, S. Pouget, P.-H. Haumesser, C. C. Santini, V. Dufaud and H. Mendil-Jakani, Ind. Eng. Chem. Res., 2023, 62, 6367–6377 CrossRef CAS
.
- M. Wang, Q. Tan, L. Liu and J. Li, ACS Sustainable Chem. Eng., 2020, 8, 7489–7496 CrossRef CAS
.
- M. Li, I. Katsouras, C. Piliego, G. Glasser, I. Lieberwirth, P. W. M. Blom and D. M. de Leeuw, J. Mater. Chem. C, 2013, 1, 7695 RSC
.
- M. Verheijen, M. Lienhard, Y. Schrooders, O. Clayton, R. Nudischer, S. Boerno, B. Timmermann, N. Selevsek, R. Schlapbach, H. Gmuender, S. Gotta, J. Geraedts, R. Herwig, J. Kleinjans and F. Caiment, Sci. Rep., 2019, 9, 4641 CrossRef CAS PubMed
.
- J. E. Marshall, A. Zhenova, S. Roberts, T. Petchey, P. Zhu, C. E. J. Dancer, C. R. McElroy, E. Kendrick and V. Goodship, Polymers, 2021, 13, 1354 CrossRef CAS PubMed
.
- U. S. Chaudhari, D. G. Kulas, A. Peralta, T. Hossain, A. T. Johnson, D. S. Hartley, R. M. Handler, B. K. Reck, V. S. Thompson, D. W. Watkins and D. R. Shonnard, RSC Sustainability, 2023, 1, 1849–1860 RSC
.
- B. Améduri and H. Hori, Chem. Soc. Rev., 2023, 52, 4208–4247 RSC
.
- H. Hori, T. Sakamoto, K. Ohmura, H. Yoshikawa, T. Seita, T. Fujita and Y. Morizawa, Ind. Eng. Chem. Res., 2014, 53, 6934–6940 CrossRef CAS
.
- H. Hori, H. Tanaka, K. Watanabe, T. Tsuge, T. Sakamoto, A. Manseri and B. Ameduri, Ind. Eng. Chem. Res., 2015, 54, 8650–8658 CrossRef CAS
.
- R. Honma, H. Hori, F. R. da Cunha, N. Horiike, L. Steinbach and B. Ameduri, Ind. Eng. Chem. Res., 2019, 58, 13030–13040 CrossRef CAS
.
- T. Doi, I. Tsuda, H. Unagida, A. Murata, K. Sakuta and K. Kurokawa, Sol. Energy Mater. Sol. Cells, 2001, 67, 397–403 CrossRef CAS
.
- Y. Kim and J. Lee, Sol. Energy Mater. Sol. Cells, 2012, 98, 317–322 CrossRef CAS
.
- X. Li, H. Liu, J. You, H. Diao, L. Zhao and W. Wang, Waste Manage., 2022, 137, 312–318 CrossRef CAS PubMed
.
-
P. Dias, P. Dias and H. Veit, in Emerging Photovoltaic Materials, ed. S. K. Kurinec, Wiley, 1st edn, 2018, pp. 61–102 Search PubMed
.
- A. Briand, A. Leybros, O. Doucet, J.-C. Ruiz, P. Fontaine-Giraud, L. Liotaud and A. Grandjean, J. Cleaner Prod., 2023, 410, 137292 CrossRef CAS
.
- É. S. Lovato, L. M. Donato, P. P. Lopes, E. H. Tanabe and D. A. Bertuol, J. CO2
Util., 2021, 46, 101477 CrossRef
.
- Y. Yan, Z. Wang, D. Wang, J. Cao, W. Ma, K. Wei and L. Yun, JOM, 2020, 72, 2624–2632 CrossRef CAS
.
- M.-S. Wu, B. C. Jin, X. Li and S. Nutt, Adv. Manuf.: Polym. Compos. Sci., 2019, 5, 114–127 CAS
.
- P. Muljadi, P. Sardjono and S. Suprapedi, Energy Procedia, 2015, 68, 282–287 CrossRef CAS
.
- O. Gutfleisch, K. Güth, T. G. Woodcock and L. Schultz, Adv. Energy Mater., 2013, 3, 151–155 CrossRef CAS
.
- J. Li, P.-L. Xu, Y.-K. Zhu, J.-P. Ding, L.-X. Xue and Y.-Z. Wang, Green Chem., 2012, 14, 3260 RSC
.
- G. Oliveux, L. O. Dandy and G. A. Leeke, Polym. Degrad. Stab., 2015, 118, 96–103 CrossRef CAS
.
- P. Yang, Q. Zhou, X.-X. Yuan, J. M. N. van Kasteren and Y.-Z. Wang, Polym. Degrad. Stab., 2012, 97, 1101–1106 CrossRef CAS
.
-
K. Shibata and M. Nakagawa, CFRP Recycling Technology Using Depolymerisation under Ordinary Pressure, Hitachi Chemical, 2014 Search PubMed
.
- T. Deng, Y. Liu, X. Cui, Y. Yang, S. Jia, Y. Wang, C. Lu, D. Li, R. Cai and X. Hou, Green Chem., 2015, 17, 2141–2145 RSC
.
- W. Dang, M. Kubouchi, S. Yamamoto, H. Sembokuya and K. Tsuda, Polymer, 2002, 43, 2953–2958 CrossRef CAS
.
- K. El Gersifi, G. Durand and G. Tersac, Polym. Degrad. Stab., 2006, 91, 690–702 CrossRef CAS
.
- S. B. Wath, M. N. Katariya, S. K. Singh, G. S. Kanade and A. N. Vaidya, Chem. Eng. J., 2015, 280, 391–398 CrossRef CAS
.
- H. R. Verma, K. K. Singh and T. R. Mankhand, J. Cleaner Prod., 2016, 139, 586–596 CrossRef CAS
.
- P. Das, Q. Zeng, A. Leybros, J.-C. P. Gabriel, C. Yong Tay and J.-M. Lee, Chem. Eng. J., 2023, 469, 144126 CrossRef CAS
.
- M. Xing, Y. Li, L. Zhao, X. Song, Z. Fu, Y. Du and X. Huang, Waste Manage., 2020, 102, 464–473 CrossRef CAS PubMed
.
- R. L. Pérez, C. E. Ayala, M. M. Opiri, A. Ezzir, G. Li and I. M. Warner, ACS Appl. Polym. Mater., 2021, 3, 5588–5595 CrossRef PubMed
.
- J. Xu, S. Kumagai, T. Kameda, Y. Saito, K. Takahashi, H. Hayashi and T. Yoshioka, Waste Manage., 2019, 89, 27–36 CrossRef CAS PubMed
.
- K. Kawajiri and M. Kobayashi, J. Cleaner Prod., 2022, 378, 134581 CrossRef CAS
.
- D. Chen, L. Yin, H. Wang and P. He, Waste Manage., 2014, 34, 2466–2486 CrossRef CAS PubMed
.
- U. Arena, Waste Manage., 2012, 32, 625–639 CrossRef CAS PubMed
.
- S. M. Alston and J. C. Arnold, Environ. Sci. Technol., 2011, 45, 9386–9392 CrossRef CAS PubMed
.
- J. Dong, Y. Tang, A. Nzihou, Y. Chi, E. Weiss-Hortala and M. Ni, Sci. Total Environ., 2018, 626, 744–753 CrossRef CAS PubMed
.
- W. Tsang, D. R. Burgess and V. Babushok, Combust. Sci. Technol., 1998, 139, 385–402 CrossRef CAS
.
- V. Fiandra, L. Sannino, C. Andreozzi, F. Corcelli and G. Graditi, Waste Manage., 2019, 87, 97–107 CrossRef CAS PubMed
.
- L. Zhang and Z. Xu, Environ. Sci. Technol., 2016, 50, 9242–9250 CrossRef CAS PubMed
.
- R. Wang, E. Song, C. Zhang, X. Zhuang, E. Ma, J. Bai, W. Yuan and J. Wang, RSC Adv., 2019, 9, 18115–18123 RSC
.
- P. Danz, V. Aryan, E. Möhle and N. Nowara, Toxics, 2019, 7, 47 CrossRef CAS PubMed
.
- B. Qin, M. Lin, X. Zhang, Z. Xu and J. Ruan, ACS EST Eng., 2021, 1, 357–362 CrossRef CAS
.
- L. Ge, X. Li, H. Feng, C. Xu, Y. Lu, B. Chen, D. Li and C. Xu, J. Anal. Appl. Pyrolysis, 2023, 170, 105919 CrossRef CAS
.
- T. Wu, W. Zhang, X. Jin, X. Liang, G. Sui and X. Yang, RSC Adv., 2019, 9, 377–388 RSC
.
- C. Ma, D. Sánchez-Rodríguez and T. Kamo, J. Hazard. Mater., 2021, 412, 125329 CrossRef CAS PubMed
.
- K.-W. Kim, J.-S. Jeong, K.-H. An and B.-J. Kim, Ind. Eng. Chem. Res., 2019, 58, 618–624 CrossRef CAS
.
- J. Chen, T. Meng, Q. Wang, Y. Bai, E. Jiaqiang, E. Leng, F. Zhang and G. Liao, Chem. Eng. J., 2022, 433, 133828 CrossRef CAS
.
- V. Sahajwalla and V. Gaikwad, Curr. Opin. Green Sustainable Chem., 2018, 13, 102–107 CrossRef
.
- R. Rajarao, I. Mansuri, R. Dhunna, R. Khanna and V. Sahajwalla, J. Anal. Appl. Pyrolysis, 2014, 105, 14–22 CrossRef CAS
.
- R. R. Rajagopal, L. S. Aravinda, R. Rajarao, B. R. Bhat and V. Sahajwalla, Electrochim. Acta, 2016, 211, 488–498 CrossRef CAS
.
- R. Khanna, M. Ikram-Ul-Haq, R. Cayumil, R. Rajarao and V. Sahajwalla, Fuel Process. Technol., 2015, 134, 473–479 CrossRef CAS
.
- S. Maroufi, M. Mayyas and V. Sahajwalla, ACS Sustainable Chem. Eng., 2017, 5, 4171–4178 CrossRef CAS
.
- M. R. Mohd Hasan, B. Colbert, Z. You, A. Jamshidi, P. A. Heiden and M. O. Hamzah, Constr. Build. Mater., 2016, 110, 79–88 CrossRef CAS
.
- B. T. A. Manjunath, Procedia Environ. Sci., 2016, 35, 731–739 CrossRef CAS
.
- Z. Ullah, M. I. Qureshi, A. Ahmad, S. U. Khan and M. F. Javaid, J. Build. Eng., 2021, 38, 102177 CrossRef
.
- P. F. Sommerhuber, T. Wang and A. Krause, J. Cleaner Prod., 2016, 121, 176–185 CrossRef CAS
.
- V. Gaikwad, A. Ghose, S. Cholake, A. Rawal, M. Iwato and V. Sahajwalla, ACS Sustainable Chem. Eng., 2018, 6, 14432–14440 CrossRef CAS
.
- P. Shi, C. K. Tan, Z. Wu, J.-C. P. Gabriel, M. Srinivasan, J.-M. Lee and C. Y. Tay, Sci. Total Environ., 2022, 807, 151085 CrossRef CAS PubMed
.
- O. González, M. E. Muñoz, A. Santamaría, M. García-Morales, F. J. Navarro and P. Partal, Eur. Polym. J., 2004, 40, 2365–2372 CrossRef
.
- M. García-Morales, P. Partal, F. J. Navarro, F. Martínez-Boza, C. Gallegos, N. González, O. González and M. E. Muñoz, Fuel, 2004, 83, 31–38 CrossRef
.
- L. Mészáros, M. Fejős and T. Bárány, J. Appl. Polym. Sci., 2012, 125, 512–519 CrossRef
.
- H. Guo, L. Yue, G. Rui and I. Manas-Zloczower, Macromolecules, 2020, 53, 458–464 CrossRef CAS
.
- A. Bandegi, T. G. Gray, S. Mitchell, A. Jamei Oskouei, M. K. Sing, J. Kennedy, K. Miller McLoughlin and I. Manas-Zloczower, Mater. Adv., 2023, 4, 2648–2658 RSC
.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed
.
- S. Wang, S. Ma, Q. Li, X. Xu, B. Wang, W. Yuan, S. Zhou, S. You and J. Zhu, Green Chem., 2019, 21, 1484–1497 RSC
.
- L. Yue, M. Amirkhosravi, X. Gong, T. G. Gray and I. Manas-Zloczower, ACS Sustainable Chem. Eng., 2020, 8, 12706–12712 CrossRef CAS
.
- F. A. López, M. I. Martín, F. J. Alguacil, J. Ma. Rincón, T. A. Centeno and M. Romero, J. Anal. Appl. Pyrolysis, 2012, 93, 104–112 CrossRef
.
- D. García, I. Vegas and I. Cacho, Constr. Build. Mater., 2014, 64, 293–300 CrossRef
.
- P. Asokan, M. Osmani and A. D. F. Price, J. Cleaner Prod., 2009, 17, 821–829 CrossRef CAS
.
- C. Piña Ramírez, M. del Río Merino, C. Viñas Arrebola, A. Vidales Barriguete and M. Kosior-Kazberuk, Constr. Build. Mater., 2019, 210, 56–62 CrossRef
.
- A. Rahimizadeh, J. Kalman, K. Fayazbakhsh and L. Lessard, Composites, Part B, 2019, 175, 107101 CrossRef CAS
.
- M. U. Bukhari, A. Khan, K. Q. Maqbool, A. Arshad, K. Riaz and A. Bermak, Energy Reports, 2022, 8, 1687–1695 CrossRef
.
- W. Zhang, Z. Liu, J. Xia, F. Li, W. He, G. Li and J. Huang, Front. Environ. Sci. Eng., 2017, 11, 6 CrossRef
.
- E. Villagrossi and T. Dinon, J. Remanufacturing, 2023, 13, 355–379 CrossRef
.
- I. Kay, S. Farhad, A. Mahajan, R. Esmaeeli and S. R. Hashemi, Energies, 2022, 15, 4856 CrossRef CAS
.
- Y. Lu, W. Pei and K. Peng, Int. J. Adv. Manuf. Technol., 2023, 128, 2825–2843 CrossRef
.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines and P. Anderson, Nature, 2019, 575, 75–86 CrossRef CAS PubMed
.
- A. Fakharuddin, H. Li, F. Di Giacomo, T. Zhang, N. Gasparini, A. Y. Elezzabi, A. Mohanty, A. Ramadoss, J. Ling, A. Soultati, M. Tountas, L. Schmidt-Mende, P. Argitis, R. Jose, M. K. Nazeeruddin, A. R. B. Mohd Yusoff and M. Vasilopoulou, Adv. Energy Mater., 2021, 11, 2101443 CrossRef CAS
.
- Y. Zhou, C. Wang, W. Lu and L. Dai, Adv. Mater., 2020, 32, 1902779 CrossRef CAS PubMed
.
- R. Deng, N. L. Chang, Z. Ouyang and C. M. Chong, Renewable Sustainable Energy Rev., 2019, 109, 532–550 CrossRef CAS
.
- M. S. Chowdhury, K. S. Rahman, V. Selvanathan, A. K. M. Hasan, M. S. Jamal, N. A. Samsudin, Md. Akhtaruzzaman, N. Amin and K. Techato, RSC Adv., 2021, 11, 14534–14541 RSC
.
-
B. Harris, in Nickel-Cobalt-Copper Conference, Alta metallurgical services, Perth, Australia, 2022, pp. 60–79 Search PubMed
.
- F. Piccinno, R. Hischier, S. Seeger and C. Som, J. Cleaner Prod., 2016, 135, 1085–1097 CrossRef CAS
.
- T. W. David and J. Kettle, J. Phys. Chem. C, 2022, 126, 4774–4784 CrossRef CAS
.
- T. Langhorst, B. Winter, D. Roskosch and A. Bardow, ACS Sustainable Chem. Eng., 2023, 11, 6600–6609 CrossRef CAS
.
- A. K. Dhaliwal and J. N. Hay, Thermochim. Acta, 2002, 391, 245–255 CrossRef CAS
.
- M. J. Yun, Y. H. Sim, D. Y. Lee and S. I. Cha, RSC Adv., 2020, 10, 34837–34846 RSC
.
- B. Guner, Y. E. Bulbul and N. Dilsiz, J. Taiwan Inst. Chem. Eng., 2022, 132, 104136 CrossRef CAS
.
- C. Stevens, J. Inorg. Biochem., 1998, 69, 203–207 CrossRef CAS PubMed
.
- K. R. Mulcahy, A. F. R. Kilpatrick, G. D. J. Harper, A. Walton and A. P. Abbott, Green Chem., 2022, 24, 36–61 RSC
.
- A. Parchomenko, S. De Smet, E. Pals, I. Vanderreydt and W. Van Opstal, Sustain. Prod. Consum., 2023, 41, 362–378 CrossRef
.
- T. Radavičius, A. Van Der Heide, W. Palitzsch, T. Rommens, J. Denafas and M. Tvaronavičienė, IRD, 2021, 3, 10–30 CrossRef PubMed
.
- D. L. Thompson, J. M. Hartley, S. M. Lambert, M. Shiref, G. D. J. Harper, E. Kendrick, P. Anderson, K. S. Ryder, L. Gaines and A. P. Abbott, Green Chem., 2020, 22, 7585–7603 RSC
.
- J. Beigbeder, L. Soccalingame, D. Perrin, J.-C. Bénézet and A. Bergeret, Waste Manage., 2019, 83, 184–193 CrossRef CAS PubMed
.
- V. Shanmugam, R. A. Mensah, M. Försth, G. Sas, Á. Restás, C. Addy, Q. Xu, L. Jiang, R. E. Neisiany, S. Singha, G. George, T. Jose E, F. Berto, M. S. Hedenqvist, O. Das and S. Ramakrishna, Compos., Part C: Open Access, 2021, 5, 100138 CAS
.
- G. L. Nogueira, D. Kumar, S. Zhang, N. Alves and J. Kettle, IEEE Trans. Electron Devices, 2023, 70, 1702–1709 CAS
.
- M. B. Rödl, F. Boons and W. Spekkink, Technol. Forecast. Soc. Change, 2022, 174, 121231 CrossRef
.
-
S. Schenck, presented in part at the NAATBATT Lithium Ion Battery Recycling Workshop, The centre, Indianapolis, Indiana, USA, 9/08, 2023 Search PubMed
.
- W. Reim, V. Parida and D. Örtqvist, J. Cleaner Prod., 2015, 97, 61–75 CrossRef
.
- A. Tukker and U. Tischner, J. Cleaner Prod., 2006, 14, 1552–1556 CrossRef
.
- M. Yang and S. Evans, J. Cleaner Prod., 2019, 220, 1156–1166 CrossRef
.
| This journal is © The Royal Society of Chemistry 2024 |