Recent progress of MXene as an energy storage material
Yuqiang
Wu
 and
Mengtao
Sun
and
Mengtao
Sun
 *
*
School of Mathematics and Physics, University of Science and Technology Beijing, Beijing, 100086, P. R. China. E-mail: mengtaosun@ustb.edu.cn
First published on 5th January 2024
Abstract
Thanks to its adjustable interlayer distance, large specific surface area, abundant active sites, and diverse surface functional groups, MXene has always been regarded as an excellent candidate for energy storage materials, including supercapacitors and ion batteries. Recent studies have also shown that MXene can serve as an efficient hydrogen storage catalyst. This review aims to summarize the latest research achievements in the field of MXene, especially its performance and application in energy storage. Different synthesis techniques have different effects on the energy storage performance of MXene. In this review, various common synthesis methods and the latest innovations in synthesis methods are discussed. MXene is prone to oxidation, and how to resist oxidation is also an important topic in MXene research. This article introduces the research results on improving the chemical stability of MXene through annealing. In addition, it aims to gain a deeper understanding of the future development and potential of MXene.
1. Introduction
Two-dimensional (2D) materials possess unique physical, chemical, and electronic properties,1 exhibiting excellent conductivity, thermal conductivity, and mechanical strength, among other characteristics. They offer broad prospects for applications in fields such as energy, electronic devices, and materials science.2,3 Notable examples of emerging 2D materials include graphene,4,5 black phosphorus,6,7 borophene8 and transition metal dichalcogenides (TMD).9,10 Graphene has been the most representative and promising material in the field of 2D materials research over the past few years. Its simple structure composed of carbon atoms gives it exceptional properties such as conductivity, thermal conductivity, and mechanical performance.11 However, the limitations of graphene's uniform structure, restricted to a honeycomb hexagonal lattice, make it challenging to modulate its properties within a wide range. This restriction has hindered its development in certain practical applications, particularly in optoelectronic devices. Other common 2D materials also face similar limitations. To overcome the shortcomings of poor tunability in the properties of 2D materials, researchers actively seek highly adjustable materials. Fortunately, the discovery of 2D transition-metal carbides and nitrides (MXene) has filled this gap. MXene consists of a transition metal (M, such as Ti, Zr, V and Mo) and carbon or nitrogen (X), and usually also includes various surface groups (such as –OH, –O, –F and –Cl) forming a diverse composition. The structure of MXene is similar to that of graphene, but in a graphene layer's atomic lattice, metal and carbon (or nitrogen) are mixed, resulting in MXene's unique structure and excellent physical characteristics.12 Since its first discovery in 2011,13 MXene have gained widespread attention and demonstrated extensive potential for applications.14,15The layered structure of MXene is formed by alternating stacks of transition metal carbide or nitride layers, providing a range of unique properties in terms of chemistry, physics, and electronic characteristics. Some MXene are metals, while others are semiconductors. Metal MXene exhibit good electrical conductivity and generally display metallic conductivity characteristics over a wide range of temperature and frequency.16,17 Compared to metals, semiconductors are more commonly used in thermoelectric materials due to their better bandgap engineering capability and typically higher Seebeck coefficients. Semiconductor MXene have extremely high Seebeck coefficients at low temperatures, for example, Sc2C(OH)2 exhibits a Seebeck coefficient of over 2000 μV K−1 at 100 K.18,19
Through various preparation methods and structural manipulation techniques, MXene find applications in multiple fields. In addition to metals and carbon or nitrogen, functional groups are also integral to MXene, referring to different chemical moieties or functional units introduced through chemical modifications on the surface of MXene. These functional groups can modulate the electronic levels, surface activity, chemical stability, and biocompatibility of MXene by altering their surface chemistry and interactions.20 The introduction of functional groups extends the application domains of MXene, such as in catalysis,21–23 sensing,24 energy storage,25 and biomedical fields,26 while offering new possibilities for customized design of MXene in various functional materials and devices. The presence of functional groups is one of the most attractive aspects of MXene, as their tunability enables diversity and customization, allowing MXene to potentially play a role in numerous domains.27,28
In multilayer MXene materials or heterostructures of MXene, the stacking or connections of individual MXene layers are commonly achieved through van der Waals forces, similar to graphite.18,29 van der Waals forces are weak attractive forces that keep the MXene layers closely packed, forming a crystalline structure. This stacking arrangement ensures the overall stability and mechanical strength of multilayer MXene materials.30 However, besides van der Waals connections, other scenarios exist. For instance, under specific conditions, different MXene layers can be connected together by covalent, ionic, or hydrogen bonds. This type of chemical bonding can provide stronger binding forces and may lead to charge transfer or changes in the electronic structure between the layers, thus influencing the physical properties of multilayer MXene.16 Additionally, intercalants can be used to connect multilayer MXene. Intercalants are molecules or ions that can be inserted into the gaps between MXene layers and interact with the MXene layers. This interaction can alter the stacking arrangement of multi-layer MXene and impact its electrical, thermal, or chemical properties.31 The weaker van der Waals forces in MXene may result in the presence of interlayer gaps or pores. These gaps can provide additional spaces for the adsorption of molecules, ions, or other substances, thereby affecting the adsorption capacity or catalytic activity of MXene, making them excellent candidates in fields such as lithium-ion batteries. Furthermore, the relatively weak van der Waals forces make it easier to exfoliate multilayer MXene into monolayer or few-layer structures. This comprehensive review delves into the intriguing world of MXene, exploring its synthesis techniques, remarkable physical properties, and cutting-edge research advancements. With a primary emphasis on its utilization in the energy storage domain, we aim to provide an in-depth analysis of MXene's versatility, promising potential, and significant contributions. By examining the latest research progress, we strive to present a cohesive and enlightening discussion surrounding the synthesis strategies and intricate physical characteristics of MXene, ultimately highlighting its remarkable application in the rapidly evolving realm of energy storage.
2. Synthesis of MXene
The etching method has emerged as a classic approach for MXene synthesis,32 encompassing liquid phase etching, solid phase etching, mixed etching, and external field assisted etching. Over the years, significant strides have been made in furthering the research and development of other synthesis methods, leading to notable advancements in recent times. Through continuous exploration and refinement, a diverse range of alternative synthesis strategies have been investigated, resulting in substantial breakthroughs.2.1 Synthesis of MXene using the classical MAX phase compound etching method
MXene are typically synthesized from MAX phase compounds, where the “M” represents a transition metal element and “A” represents a main group element. By adjusting the combination of raw materials, different types of MXene can be synthesized, greatly expanding the material's diversity and application range. The synthesis process of MXene usually involves chemical etching and exfoliation. Compared to other synthesis methods, the synthesis from MAX phase compounds is relatively simple and easily controllable. In 2012, Naguib et al. reported the exfoliation process of layered transition metal carbide Ti3AlC2,33 as shown in Fig. 1.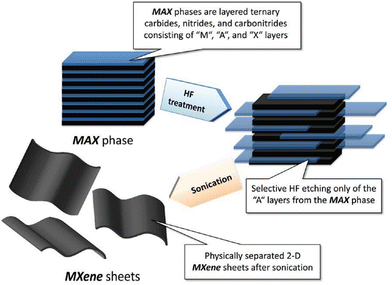 | ||
| Fig. 1 Schematic diagram of HF etching MAX phase compound synthesis of MXene.33 Reproduced from ref. 33 with permission from American Chemical Society, copyright 2023. | ||
Generally, the process of synthesizing MXene from MAX phase compounds includes two steps: etching and exfoliation. During the etching process, the etchant reacts with the main group element layers (A-layer) in the MAX phase compound, dissolving or removing the A-layers, gradually exfoliating the layered structure of the MAX phase compound, and forming MXene structures. The etchant needs to selectively react with the A-layers while not reacting with the layers of MXene. Common etchants include HF, HCl, and others, as shown in Fig. 2, which presents secondary electron SEM micrographs of various MAX phase compounds with or without HF treatment. It is evident that after HF treatment, the MAX phase compounds exhibit significant layering.
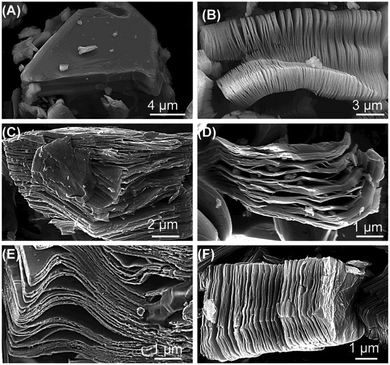 | ||
| Fig. 2 Secondary electron SEM micrographs of various MAX phase compounds with or before HF treatment: (A) Ti3AlC2 before HF treatment, (B) Ti3AlC2 after HF treatment, (C) Ti2AlC after HF treatment, (D) Ta4AlC3 after HF treatment, (E) TiNbAlC after HF treatment, and (F) Ti3AlCN after HF treatment.33 Reproduced from ref. 33 with permission from American Chemical Society, copyright 2023. | ||
The role of exfoliants is to insert into the layered structure of the MAX phase compound and assist in the exfoliation of MXene layers. Exfoliants extend the layered structure of the MAX phase, which is beneficial for separating MXene nanosheets from bulk materials. Exfoliants should be able to insert between the layers of the MAX phase compound and interact with MXene layers without introducing impurities. Common exfoliants include organic solvents and metal chlorides.
Although the synthesis of MXene from MAX phase compounds is commonly carried out, there are still several drawbacks. Firstly, the preparation of MXene from MAX phase compounds typically involves multiple steps of chemical processing. Initially, suitable MAX phase precursor materials need to be selected and pre-treated to obtain single-layer or multi-layer MAX phases. Then, methods such as chemical etching or hydrolysis are employed to exfoliate MXene from the MAX phases. This preparation process is relatively complex, requiring significant time and meticulous operations. Secondly, toxic chemicals such as HF are often used in the synthesis of MXene from MAX phase compounds, posing safety risks to operators and environmental pollution. Additionally, the synthesis of MXene from MAX phase compounds is dependent on the availability and selection of suitable MAX phase precursor materials, and currently, only a fraction of MAX phases can be successfully transformed into MXene. Hence, the choice and availability of materials are somewhat limited. Accurately controlling and quantitatively analyzing surface functional groups on MXene during their synthesis from MAX phase compounds is highly challenging. Moreover, the traditional two-step synthesis method has a low MXene yield (<20%).34 Given the enormous potential applications of MXene in energy, energy storage, electronic devices, etc., it is essential to gradually overcome and improve the preparation methods for MXene.
HF has strong toxicity and high risk. Using HF to etch MAX phase compounds consumes high energy, takes a long time, and generates a large amount of waste that is difficult to recycle. Therefore, it is necessary to find cleaner and safer etchants. In 2014, Ghidiu et al. first used a mixture of HCl and LiF instead of HF as an etchant to achieve safer etching.35 Since then, researchers have begun exploring more efficient and safe etchants. Wu et al. introduced tetrabutylammonium fluoride on the basis of HCl and LiF, reducing the etching time to 2 hours.36,37 The precursor used in the synthesis of MXene also affects the physical properties of the synthesized MXene. In Wu et al.'s work,36 Ti3C2 MXene was etched from the hexagonal Ti3AlC2 precursor. Compared with ordinary Ti3C2 MXene, the interlayer spacing and specific capacitance of the MXene synthesized by Wu et al. increased the interlayer spacing generating more active sites, and the increase in specific capacitance will undoubtedly have a positive effect on the application of supercapacitors.
2.2 FAT method for peeling off multi-layer MXene
In order to address the low yield issue of the traditional two-step synthesis method for MXene, Huang et al. proposed a freeze-and-thaw (FAT) assisted method in 2020, where MXene nanosheets are detached from multilayer MXene using the expansion force generated by freezing water.38 The FAT method is commonly used for the manipulation of chemical substances and the control of chemical reactions. For example, FAT treatment can disrupt the structure of compounds, alter molecular conformations, or promote reactions. The FAT method can be employed for extraction or separation of chemical substances, enhancing reaction rates, or improving reaction selectivity. In the field of 2D material synthesis, FAT is often utilized for the exfoliation of 2D materials. However, although effective, the actual yield of this method has been low. Nevertheless, Huang et al.'s research found that the FAT method exhibits excellent performance in MXene exfoliation. In their experimental study, they found that the yield of folded large-layered MXene (FAT MXene) flakes with distinctive wrinkles can reach 39.0% without ultrasonic treatment. After 1 hour of ultrasound treatment, the yield of MXene can reach 81.4%. Intense ultrasonic treatment can play a role in the exfoliation process of 2D materials. The energy of the sound waves causes deformation, vibration, and cleavage of the material, aiding in the disruption of weak interlayer bonding. Additionally, the vibrational motion generated by intense ultrasonic waves creates a cavitation effect in the liquid, forming regions of negative pressure during each sound wave cycle. When cavitation bubbles rapidly collapse in the liquid, the resulting intense fluid dynamics effects exert impact forces on nearby materials, facilitating the separation and exfoliation of 2D materials.Fig. 3(a) illustrates a schematic diagram of the delaminated-Ti3C2 preparation using the FAT method. Firstly, the etchant selectively reacts with the main group element layers in the MAX phase compound to remove them, leaving a significant gap between the MXene layers. This facilitates the infiltration of a large number of water molecules into the interlayer spaces and their expansion under the FAT process, promoting the delamination of MXene. As water has a maximum density at 4 °C, it is advantageous to maximize the infiltration of water molecules into the interlayer gaps of MXene under 4 °C conditions. The system is then transferred to a −20 °C environment, where the water freezes and expands, leading to an increase in the interlayer spacing of MXene. When the spacing becomes too large, the van der Waals bonds between the layers break, making the MXene easily delaminable. SEM images, HRTEM images, and XRD patterns of different stages are shown in Fig. 3(b)–(i). The layer structure can be clearly observed in Fig. 3(c) and (d), confirming the effect of HF etching. The selected area diffraction (SAED) pattern in Fig. 3(h) displays a distinct hexagonal crystal structure, indicating that the crystal structure of the delaminated MXene remains intact. This forms the basis for the high yield of MXene delamination using the FAT method. The XRD patterns of Ti3AlC2, multilayer-Ti3C2, and delaminated-Ti3C2 are shown in Fig. 3(i). The (104) characteristic diffraction peak of Ti3AlC2 almost disappears, and the (002) diffraction peak shifts from 9.5° to 7.2°, confirming the transition from Ti3AlC2 to multilayer-Ti3C2. The red curve in Fig. 3(i), compared to the blue curve, further shifts the (002) diffraction peak from 7.2° to 5.9°, confirming the successful delamination of MXene.
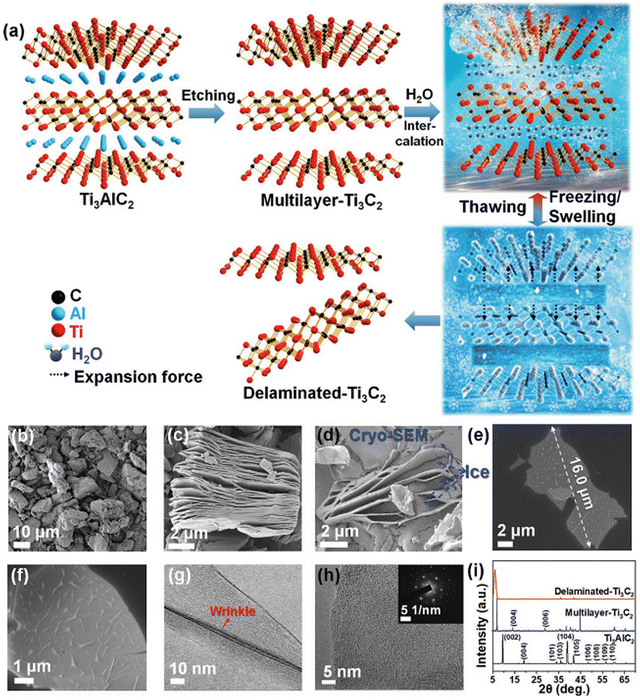 | ||
| Fig. 3 (a) Schematic diagram of the preparation of delayed Ti3C2 using the FAT method, (b) SEM images of Ti3AlC2, (c) SEM images of multilayer Ti3C2, (d) Cryo SEM image of frozen multilayer Ti3C2, (e) and (f) SEM images of delaminated Ti3C2 with objective writing on the surface, (g) and (h) HRTEM images of the Ti3C2Tx 2D sheet and corresponding SAED pattern, and (i) XRD patterns of Ti3AlC2, multilayer-Ti3C2, and delaminated Ti3C2.38 Reproduced from ref. 38 with permission from John Wiley and Sons, copyright 2023. | ||
2.3 Direct synthesis of MXene
In 2023, Wang et al. reported a method for directly synthesizing MXene using reactions between metals and metal halides with graphite, methane, or nitrogen.39 Their synthetic route bypasses the step of the MAX phase compound, offering scalability and excellent atom economy. Excitingly, this direct synthesis route can also produce compounds that have not been synthesized from MAX phases, which is of significant importance for expanding MXene research.
Fig. 4(a) depicts a schematic diagram of Wang et al.'s direct synthesis of Ti2CCl2 using Ti metal, TiCl4, and graphite at 950 °C. Titanium and graphite were ground into fine powders in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 molar ratio and mixed with 1.1 molar equivalents of TiCl4. The mixture was then heated to 950 °C within 20 minutes, and the reaction reached near-maximum yield after two hours. The layer structure of the directly synthesized product can be clearly observed through the scanning electron microscopy (SEM) image of Ti2CCl2 in Fig. 4(b), the energy dispersive X-ray spectroscopy (EDX) elemental mapping of Ti2CCl2 in Fig. 4(c), the high-resolution high-angle annular dark field (HAADF) image of Ti2CCl2 in Fig. 4(d), and the electron energy loss spectroscopy (EELS) elemental maps of Ti2CCl2 in Fig. 4(e).
1.8 molar ratio and mixed with 1.1 molar equivalents of TiCl4. The mixture was then heated to 950 °C within 20 minutes, and the reaction reached near-maximum yield after two hours. The layer structure of the directly synthesized product can be clearly observed through the scanning electron microscopy (SEM) image of Ti2CCl2 in Fig. 4(b), the energy dispersive X-ray spectroscopy (EDX) elemental mapping of Ti2CCl2 in Fig. 4(c), the high-resolution high-angle annular dark field (HAADF) image of Ti2CCl2 in Fig. 4(d), and the electron energy loss spectroscopy (EELS) elemental maps of Ti2CCl2 in Fig. 4(e).
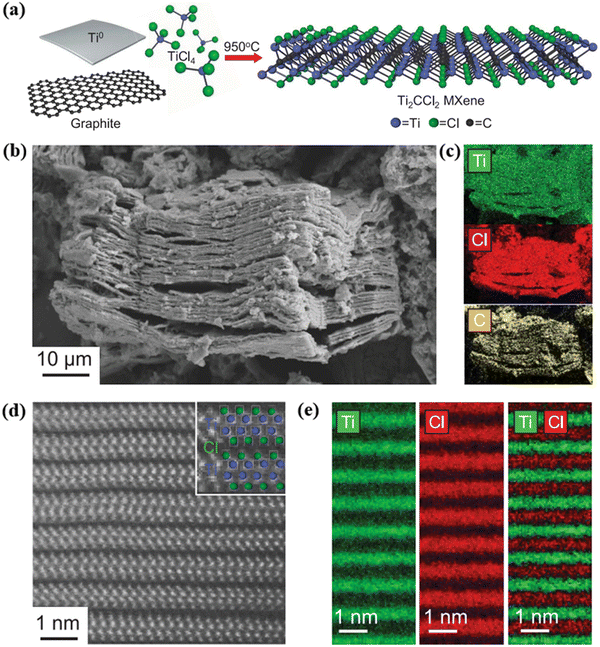 | ||
| Fig. 4 (a) Schematic diagram of the direct synthesis of Ti2CCl2 MXene, (b) SEM image of Ti2CCl2, (c) EDX elemental mapping of Ti2CCl2, (d) HAADF image of Ti2CCl2, and (e) EELS elemental maps of Ti2CCl2.39 Reproduced from ref. 39 with permission from The American Association for the Advancement of Science, copyright 2023. | ||
Chemical vapor deposition (CVD) is a commonly used technique for surface coating and thin film fabrication. This method involves depositing the desired material on a substrate surface by controlling the chemical reactions of gaseous reactants under specific conditions. In CVD, gas or vaporized precursor substances are typically used to generate the desired deposition through processes such as thermal decomposition, dissolution, or chemical reactions on the substrate surface. CVD allows precise control of the deposition process by adjusting various parameters such as temperature, pressure, and reactant gas concentration, making it well-suited for fabricating materials with complex structures or specific performance requirements. CVD can produce uniform and pure thin films or coatings on the substrate surface. Compared to other methods, CVD offers greater flexibility in doping and controlling impurity content in materials. Additionally, CVD is applicable for the fabrication of large-area materials and enables continuous deposition, thus enhancing production efficiency.
As depicted in Fig. 5, Wang et al. successfully synthesized Zr2CCl2 and Zr2CBr2 using the CVD method for the first time, by exposing Zr foil to CH4 and ZrCl4 or ZrBr4 vapors at 975 °C. These compounds could not previously be synthesized using MAX phase compounds. Prior to this, transition metal carbides and nitrides such as Mo2C could be grown using CVD,40 but CVD synthesis of MXene had not been achieved.
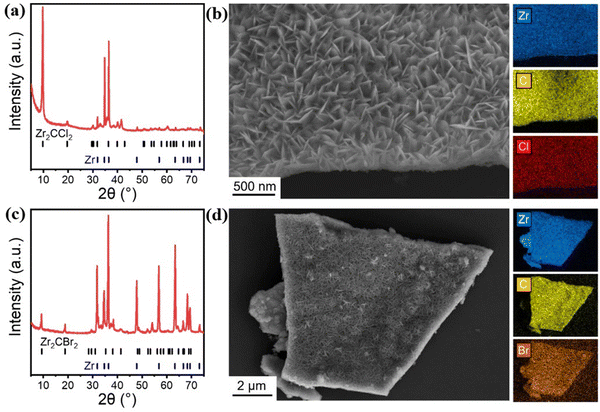 | ||
| Fig. 5 (a) Powder XRD pattern of CVD-Zr2CCl2. (b) SEM-EDX analysis of CVD-Zr2CCl2. (c) Powder XRD pattern of CVD-Zr2CBr2. (d) SEM-EDX analysis of CVD-Zr2CBr2.39 Reproduced from ref. 39 with permission from The American Association for the Advancement of Science, copyright 2023. | ||
There are currently many preparation methods for MXene, and Table 1 shows the advantages and disadvantages of some common preparation methods. The direct synthesis method has the potential to completely replace traditional etching methods. However, there is still a significant gap in the research of direct synthesis methods. Currently, most researchers still use etching methods as the preparation method, and there are still many aspects that need to be explored in the future.
| Preparation method | Advantage | Disadvantage | |
|---|---|---|---|
| Etching | Liquid phase etching | Simple processing, high stripping efficiency | Difficult to handle liquid waste, time consuming, the composition and concentration of the solution need to be strictly controlled |
| Solid phase etching | Suitable for the conversion of soluble precursors, no need to handle liquid waste | Higher temperature required, low yield | |
| Mixed etching | Integrating the advantages of liquid phase etching and solid phase etching, high yield, suitable for large-scale preparation | Still need to handle liquid waste, the process is complex and requires more steps | |
| External field assisted etching | Fast and efficient, can be prepared at lower temperatures | Higher requirements for equipment and materials, the process is complex | |
| Direct synthesis | CVD | High purity, uniformity, and can be prepared on a large area, can precisely control the thickness, composition, and structure of thin films or nanoparticles | Complex equipment, slow speed |
3. Properties of MXene
3.1 Mechanical characteristics of MXene
MXene materials exhibit high hardness, allowing them to resist external pressure and deformation, demonstrating good rigidity. MXene materials usually possess excellent elastic recovery, which means they can return to their original shape after being subjected to external forces. This property makes MXene materials perform well in bending, yielding, and flexing. Under tensile stress, MXene materials can maintain high tensile strength, providing them with excellent endurance in applications that require resistance to tensile stress. MXene materials generally have good toughness, meaning they can maintain a certain level of ductility while under stress, enabling them to withstand significant deformation without fracturing. The specific mechanical strength of MXene materials depends on factors such as their structure, thickness, and fabrication methods. Moreover, in practical applications of MXene, mechanical strength can be further enhanced by altering material composites and adding reinforcing agents. Overall, MXene materials excel in various mechanical performance indicators, providing a solid foundation for their applications in flexible electronics, plastic electronics, strong and tough materials, and other fields.In general, the connection between multilayer MXene is achieved through van der Waals interactions. However, due to the presence of functional groups and the diversity of these groups, interlayer bonding in multilayer MXene can also involve hydrogen bonding, ionic bonding, covalent bonding, and so on. Different types of functional groups introduce different charge characteristics, thereby altering the charge distribution within MXene layers. Functional groups with positive or negative charges can impact the electronic density distribution within MXene layers, thus regulating interlayer charge transfer and distribution. Furthermore, the strength of interlayer interactions in MXene can also be regulated by functional groups. The spatial arrangement and interaction capabilities of functional groups, such as hydrogen bonding and van der Waals forces, affect the stability of interlayer adsorption and stacking. Generally, covalently bonded multilayer MXene exhibit stronger mechanical strength. Therefore, precise control over interlayer interactions in MXene can be achieved by modulating the type, density, and positioning of functional groups, thereby adjusting the structure, properties, and applications of MXene materials. In addition to interlayer interactions, the orientation and compactness of MXene also impact the mechanical strength of MXene films. Generally, larger gaps and defects between single-layer MXene result in a weaker mechanical strength in the assembled MXene films, and in some cases, the mechanical strength of the assembled MXene films can be lower than that of single-layer MXene. Therefore, reducing interlayer gaps and defects during assembly is an important challenge to overcome in assembling MXene films.
In 2021, Wan et al. reported an effective densification strategy using a sequential bridging process involving hydrogen and covalent bonding.31 The most compact hydrogen-bonded MXene (HBM) film was obtained by controlling the carboxymethyl cellulose (CMC) content, with a CMC content of 10 wt%. The boron content was then increased to fabricate sequentially bridged MXene (SBM) films. Covalently bridged MXene (CBM) films were directly prepared from MXene films using the same process as for SBM films. SEM images of the MXene film, HBM film, CBM film, and SBM film are shown in Fig. 6. The porosities of the four films are 15.4 ± 0.6%, 10.5 ± 0.6%, 9.60 ± 0.56%, and 5.35 ± 0.31%, respectively. It is evident from the images that the porosity of the four films gradually decreases, with the MXene film exhibiting larger pores, which clearly hinders the improvement of the film's mechanical strength. On the other hand, the SBM film is the most compact, showing significant improvement compared to the initial MXene film.
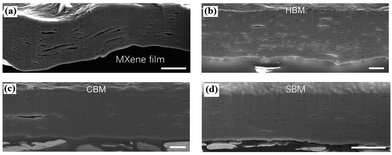 | ||
| Fig. 6 SEM images of (a) MXene film, (b) HBM film, (c) CBM film and (d) SBM film.31 Reproduced from ref. 31 with permission from The American Association for the Advancement of Science, copyright 2023. | ||
After being subjected to tensile stress, existing defects or pores in the film undergo expansion and elongation processes, leading to a decrease in the mechanical strength of the film. When a film is subjected to tensile stress, the material around the defects or pores experiences stress concentration, resulting in internal fracture or plastic deformation of the film. This fracture or plastic deformation further enlarges the size and shape of the defects or pores, causing a reduction in the mechanical strength of the film. Therefore, the presence of defects or pores has a significant impact on the overall mechanical performance of the film, and reducing, repairing, or strengthening these defects can significantly improve the mechanical strength and stability of the film. Fig. 7 shows the mechanical properties of the four films prepared by Wan et al. As shown in Fig. 7(a), the SBM film has the highest tensile strength, while the tensile strength of the MXene film is 87 ± 3 MPa, even lower than that of single-layer MXene. This further proves the negative impact of the presence of pores and defects on the mechanical strength of the film. Fig. 7(b) shows the relationship between the number of failure cycles and the maximum applied stress level for the four films. The MXene film can only withstand 4854 cycles of stretching between 2 and 32 MPa without failure. Compared to the MXene film, the maximum stress level and number of failure cycles gradually increase for the CBM film, HBM film, and SBM film, especially the SBM film, which shows significant improvement in mechanical properties compared to the other three films. The maximum stress level that the SBM film can withstand is six times higher than that of the MXene film, and the number of failure cycles at a stress level that the MXene film cannot reach is a hundred times higher than that at lower stress levels. Fig. 7(c) shows the stress relaxation curve of the four films under a 1.5% strain condition. After relaxation, the MXene film can only maintain 16% of the initial stress, while the SBM film can maintain 67.5% of the initial stress. In the MXene film, the MXene crystallites are prone to sliding during the relaxation process due to weak interlayer connections and the presence of many structural defects. The bridging process strengthens the interactions between MXene layers, effectively reducing the possibility of sliding and improving the mechanical stability and performance of the film. In this way, the SBM film exhibits better performance when dealing with stress relaxation. The high mechanical strength of the SBM film demonstrates the effectiveness of the densification strategy employed by Wan et al., as well as the inherent high mechanical strength of MXene materials.
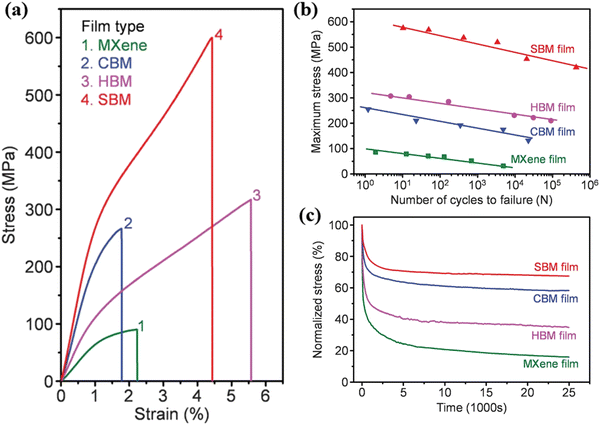 | ||
| Fig. 7 (a) Representative tensile stress–strain curves of the MXene film, CBM film, HBM film, and SBM film. (b) Relationship between failure cycles and maximum applied stress level of MXene film, CBM film, HBM film, and SBM film. (c) Stress relaxation curves of MXene film, CBM film, HBM film, and SBM film under 1.5% strain conditions.31 Reproduced from ref. 31 with permission from The American Association for the Advancement of Science, copyright 2023. | ||
3.2 The chemical stability of MXene
MXene is known for its exceptional mechanical stability, however, its chemical stability remains a major challenge as it is susceptible to oxidation.41,42 This limitation hinders its potential for a wide range of applications, particularly in the field of capacitors and batteries. MXene-based electrochemistry is also difficult to operate in humid environments over extended periods of time. Therefore, it is crucial to investigate the antioxidant properties of MXene to counteract this challenge. In recent years, significant strides have been made in research on the antioxidant performance of MXene.43–45 For instance, the introduction of surface modifications and intercalation techniques has shown promising results in enhancing the chemical stability of MXene. Additionally, the development of MXene-based composites, such as MXene-polymer hybrid materials, promises to further improve the antioxidant properties of MXene while retaining its mechanical strength.Although it is currently possible to slow down the oxidation rate of MXene by controlling its storage conditions,46 these storage conditions are difficult to achieve in practical applications, especially for energy storage devices operating in high-temperature and humid environments, thus finding other ways to resist oxidation is urgently needed.
Ti3C2 has been studied and proven to be used for various purposes, for example, it can work as an electrode for high energy density lithium sulfur batteries47 and as a photothermal material for biomedical treatment.48 However, it is prone to oxidation into TiO2 in humid environments, leading to the destruction of its electrical and photothermal properties. Lee et al. achieved an improvement in the oxidation resistance of MXene through hydrogen annealing.43 They prepared Ti3C2 MXene films with a thickness of 60 nm using LiF/HCl etching of Ti3AlC2 powder. The films were annealed in a vacuum chamber containing hydrogen gas at temperatures ranging from 300 °C to 900 °C. The O 1s regions of the X-ray photoelectron (XPS) spectrum before and after annealing are shown in Fig. 8(a) and (b), which shows the content of various components in the MXene film before and after annealing. Annealing at temperatures above 300 °C can fully remove water molecules from the film,49 which can reduce the interlayer spacing of MXene and thus reduce contact resistance. The water content after annealing is significantly reduced, from 14.0% to 0.9%. The annealed MXene film has good resistance to water penetration, which is also one of the reasons for its resistance to oxidation. Secondly, (–OH) has a high affinity for water, and the destruction of (–OH) by high-temperature annealing can also enhance the antioxidant capacity of MXene films. Fig. 8(a) and (b), also show a significant decrease in (–OH) content, as shown in the following equation43
| Ti3C2(OH)wFx → Ti3C2OyFx + aH2O |
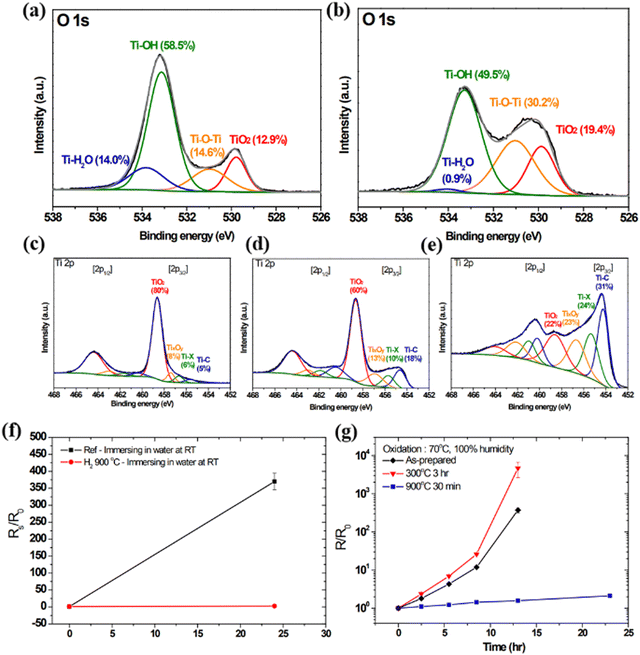 | ||
| Fig. 8 (a) The O 1s regions of the XPS spectra of the (a) as-prepared and (b) hydrogen-annealed samples. (c) The Ti 2p region of the XPS spectra of an as-prepared Ti3C2 MXene film after storage at 70 °C with 100% RH for 1 day. The Ti 2p region of the XPS spectrum of an annealed Ti3C2 MXene film (d) before sputtering and (e) after sputtering, after storage at 70 °C and 100% RH for 1 day. (f) Change in the sheet resistance after immersion of a hydrogen-annealed sample (900 °C) and an as-prepared sample in water at room temperature for 1 day. (g) Time evolution of the sheet resistances for the as-prepared and hydrogen-annealed (300 °C for 3 h and 900 °C for 30 min) MXene films.43 Reproduced from ref. 43 with permission from Royal Society of Chemistry, copyright 2023. | ||
As shown in Fig. 8(c)–(e), the changes in the component content of MXene films before and after annealing at 70 °C with 100% RH for one day are shown. The content of TiO2 is significantly reduced, indicating an improvement in antioxidant capacity. However, the content of TiO2 in annealed MXene shows a significant decrease after sputtering treatment, indicating that annealing treatment is an effective strategy to enhance the antioxidant capacity of MXene. Sheet resistance is an important indicator of the electrochemical properties of MXene films. The oxidation of the film can lead to a significant increase in sheet resistance, as shown in Fig. 8(f) and (g), which shows the relative size of sheet resistance of MXene before and after annealing in a readily oxidizing environment for a period of time. The figure shows that the MXene film annealed at 900 °C for 30 minutes exhibits excellent oxidation resistance compared to the untreated MXene film and the MXene film annealed at 300 °C, and no significant changes in sheet resistance were even observed in water at room temperature for the MXene film annealed at 900 °C, which means that it can work normally at room temperature without worrying about oxidation issues.
The characteristics of surface groups are an important reason for the poor chemical stability of MXene thin films,51 as some surface groups are easily adsorbed or reacted with water or oxygen. Annealing treatment can change the crystal structure and composition of MXene materials, alter the surface groups, and achieve improved chemical stability. At the same time, it can also be used to regulate its electrochemical and mechanical properties. Annealing operations in different gas environments can achieve different results. In 2023, Eom et al. annealed Ti3C2Tx MXene in an ammonia environment to obtain surface nitridation of MXene films. The resulting films maintained high conductivity while achieving higher oxidation stability.50
As shown in Fig. 9(a), Eom et al. annealed the prepared Ti3C2Tx in NH3 and Ar environments to obtain NH3 MXene and Ar MXene, respectively. Raman spectroscopy (Fig. 9(b)) showed the characteristic peaks of each component, with the peak at 200 cm−1 belonging to A1g, 444 cm−1 belonging to TiO2, 540 cm−1 belonging to Ti–N, 1345 cm−1 and 1595 cm−1 belonging to the D and G bands, respectively. Combining Fig. 9(b) and (c), it is shown that TiO2 is produced in Ar MXene, indicating that the MXene film is oxidized during annealing in Ar, while a large number of Ti–N structures are detected in NH3 MXene samples. This indicates that N atoms replace surface groups and combine with Ti atoms to form TiN, which has good conductivity and corrosion resistance, which is beneficial for improving the antioxidant and electrochemical properties of MXene films. Overall, the main components of the as-prepared MXene are Ti, C, O and F. The main components of Ar MXene are Ti, C and O, which are caused by the oxidation process leading to an increase in oxygen element content. The main components of NH3 MXene are Ti, C and N. When annealed in a NH3 environment, the surface of the MXene film undergoes nitridation while retaining the Ti3C2 structure, excluding all O elements, which gives the NH3 MXene film excellent oxidation resistance. Moreover, there are strong D and G bands in the edge region of the Ar MXene sample, indicating that the annealing operation in Ar caused an imbalance in the edge structure, and the oxidation of MXene easily starts from the edge of the MXene film.52 Therefore, protecting the edges of the MXene film is an effective method to reduce the oxidation rate of the MXene film. Furthermore, the edges and inner surface of the MXene annealed in NH3 exhibit uniform chemical states, which also makes it difficult for NH3 MXene to oxidize from the edge of the film.
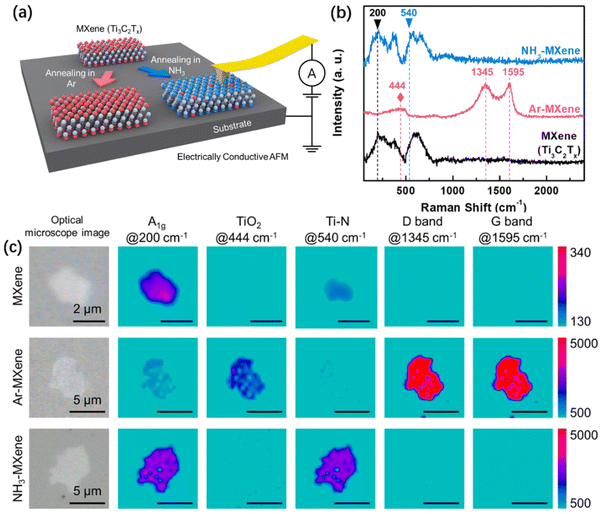 | ||
| Fig. 9 (a) Schematic diagram of MXene annealing. (b) Raman spectra of the as-prepared MXene, Ar MXene, and NH3 MXene. (c) Raman maps of the as-prepared MXene, Ar MXene, and NH3 MXene.50 Reproduced from ref. 50 with permission from Royal Society of Chemistry, copyright 2023. | ||
Fig. 10 shows the excellent oxidation resistance and conductivity of NH3 MXene. The current and current density plots show that Ar MXene has only a slight improvement compared to the as-prepared MXene, while NH3 MXene has a significant improvement, with a conductivity about twice that of Ar MXene films. Fig. 10(j) and (k) show the change in relative resistance over time under strong oxidation conditions, and the change in resistance can represent the change in oxidation degree. From Fig. 10(k), it can be seen that the chemical stability of NH3 MXene is about thirteen times that of Ar MXene, indicating the enormous potential of surface nitridation in improving the chemical stability of MXene thin films.
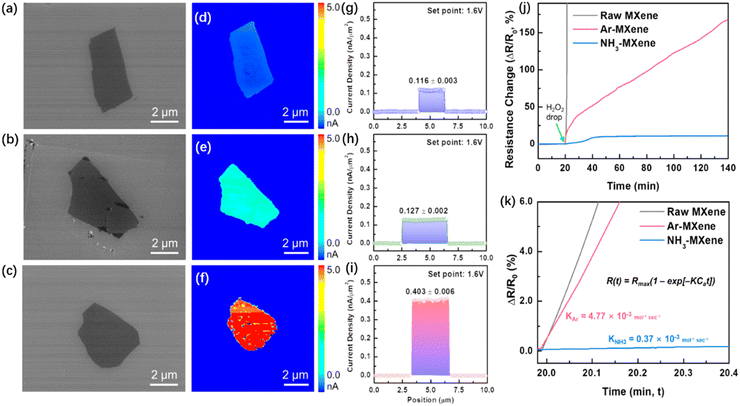 | ||
| Fig. 10 (a)–(c) SEM images of the as-prepared MXene, Ar MXene, and NH3 MXene, (d)–(f) current maps of the as-prepared MXene, Ar MXene, and NH3 MXene obtained using C-AFM under 1.6 V, (g)–(i) current density of MXene single sheets of the as-prepared MXene, Ar MXene, and NH3 MXene, and (j) and (k) resistance changes of MXene samples over time when exposed to a droplet of H2O2 as an oxidant.50 Reproduced from ref. 50 with permission from Royal Society of Chemistry, copyright 2023. | ||
4. Properties and applications of MXene in energy storage
4.1 Electrochemical energy storage performance of MXene
MXene has been proven to be an excellent candidate for high area and volume energy storage due to its good conductivity, abundant active sites, and high intrinsic density.53–55 The large specific surface area and porous structure of MXene materials provide ample storage space for charge, enabling MXene capacitors to achieve a high specific capacitance. The superior conductivity of MXene materials allows for rapid charge transfer within MXene capacitors, resulting in fast charging and discharging rates, facilitating quick energy storage and release. MXene materials exhibit excellent structural and chemical stability, allowing them to withstand numerous charge–discharge cycles without significant performance degradation, thus ensuring the long cycle life and durability of MXene capacitors. They also maintain good electrical performance over a wide temperature range, enabling MXene capacitors to function appropriately in various environments and making them suitable for a wide range of applications. The composition and structure of MXene materials can be tailored by adjusting the preparation conditions, enabling adjustment and optimization of the capacitor's performance. These advantages position MXene materials with great potential in the field of capacitors and have attracted widespread research interest. MXene capacitors can be applied in energy storage systems, mobile devices, electronic devices, and other fields, providing high-performance solutions for energy storage and supply.With its outstanding electrical and mechanical properties, MXene is a highly promising candidate material for capacitor applications. In a study conducted by Huang et al. in 2020, MXene obtained through the FAT method was utilized to fabricate an on-chip all-MXene micro-supercapacitor (MSC),38 as depicted in Fig. 11. The performance of the manufactured MSC device is presented in Fig. 12. Fig. 12(a) and (b) show the electrode thickness-dependent CV and GCD curves, demonstrating that MSC devices with different electrode thicknesses exhibit well-defined rectangular CV curves and triangular GCD profiles. These are indicative of an excellent capacitor performance, offering exceptional operating efficiency. Fig. 12(c) demonstrates that the MXene MSC retains approximately 97.8% of its capacitance after 2000 charge–discharge cycles, underscoring the extensive operational lifespan of this supercapacitor and spotlighting the promising prospects of MXene in supercapacitor devices. Fig. 12(d) and (e) reveal a significant increase in both volumetric and areal capacitance for the MSC with an electrode thickness of 0.4 μm compared to those with electrode thicknesses of 0.2 μm and 0.3 μm. This not only provides insights for the design of MXene MSC devices but also indicates that the capacitance of MXene MSC devices fabricated using the FAT method can be adjusted by modulating the electrode thickness—an operation that is straightforward and underscores the application potential of FAT MXene in the field of supercapacitors.
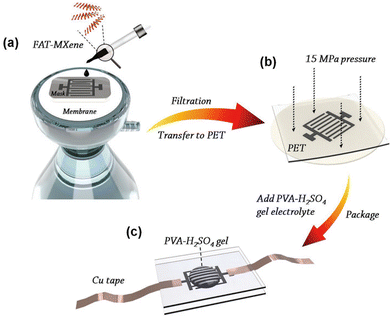 | ||
| Fig. 11 Schematic diagram of the manufacture of MXene MSCs peeled off using the FAT method.38 Reproduced from ref. 38 with permission from John Wiley and Sons, copyright 2023. | ||
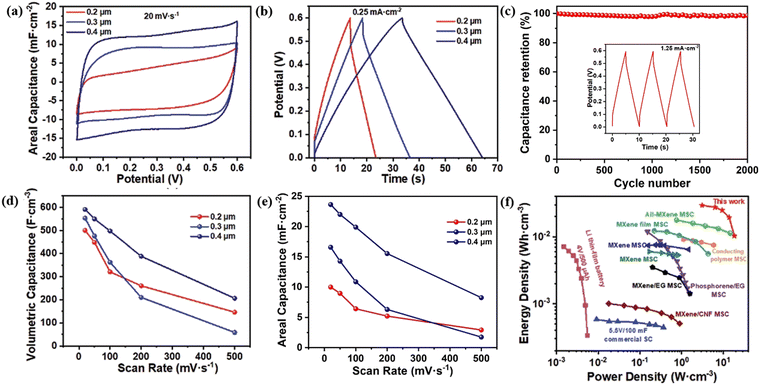 | ||
| Fig. 12 (a) C–V curves of MSC devices with different electrode thicknesses, (b) GCD curves of MSC devices with different electrode thicknesses, (c) capacitance retention rate related to charge discharge cycles, (d) volumetric capacitance of MSCs with different electrode thicknesses related to scanning rate, (e) area capacitance of MSCs with different electrode thicknesses related to scanning rate, and (f) comparison of volumetric capacity and power density of different MSC devices.38 Reproduced from ref. 38 with permission from John Wiley and Sons, copyright 2023. | ||
The structural characteristics of MXene films, including interlayer distance, pore density, and types of functional groups, can have a significant impact on the properties of MXene capacitors. By adjusting the structural characteristics of MXene materials, capacitor performance can be adjusted to achieve a better capacitor performance. By controlling the synthesis conditions, MXene with different structural characteristics can be obtained, which is the most convenient method to regulate its energy storage properties. Recently, Wu et al. introduced HI and synthesized high-density Ti3C2Tx MXene with a dense porous structure, achieving an extremely high surface capacitance density.56 As shown in Fig. 13(f) and (g), the density of MXene synthesized by Wu et al. reached 2.74 g cm−3, and the surface capacitance density reached 18.6 F cm−2, which is much higher than the capacitance density measured in previous research work. This is because the effect of HI makes the MXene layer denser while generating a large number of pores. As shown in Fig. 13(a)–(d), the density of MXene treated with different treatment measures can be observed. The MXene treated with HI exhibits the best density, leading to a higher density. As shown in Fig. 13(e), high-density MXene materials have good development prospects in reducing the volume of capacitors.
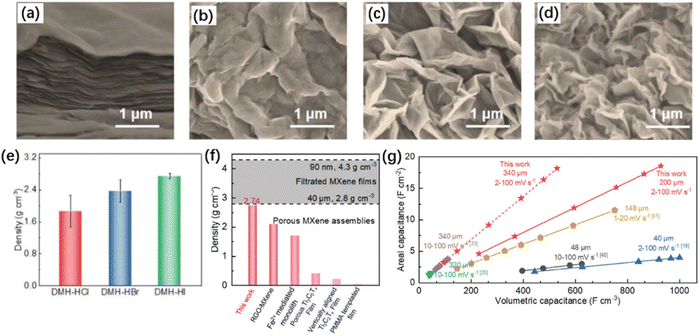 | ||
| Fig. 13 SEM images of (a) MXene film, (b) DMH-HCl, (c) DMH-HBr and (d) DMH-HI. (e) Densities of DMH-HCl, DMH-HBr and DMH-HI. (f) The densities of DMH-4 and previously reported porous MXene assemblies.57–61 (g) The comparison of areal and volumetric capacitance between DMH-4 and other reported MXene-based electrodes with high thickness.56–58,61–63 Reproduced from ref. 56 with permission from John Wiley and Sons, copyright 2023. | ||
Adjusting the structural characteristics of MXene can not only be achieved by intervening in synthesis. Inspired by magic angle graphene, Wu et al. successfully constructed double-layer twisted Ti3C2 (Moiré superlattice MXene),64,65 as shown in Fig. 14. The construction of the Moiré superlattice solved the problem of easy collapse of ordinary Ti3C2 MXene structures, which is an important step in improving the stability of MXene capacitors and opens up new research directions on MXene capacitors. Moreover, Wu et al.'s research results indicate that the Moiré superlattice MXene greatly improves the capacity of Ti3C2 as a supercapacitor electrode under long-term charging and discharging. Overall, interventions can be applied during the synthesis process of MXene to affect its structural characteristics, thereby affecting its energy storage properties.
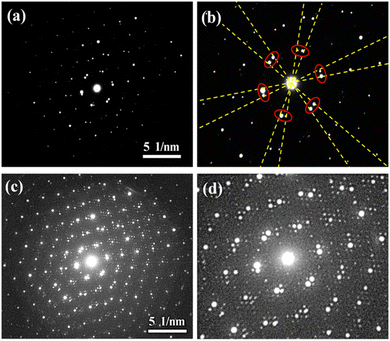 | ||
| Fig. 14 SAED images and local magnification of moiré superlattice Ti3C2 nanosheets.65 Reproduced from ref. 65 with permission from American Chemical Society, copyright 2023. | ||
MXenes have large and controllable interlayer spacing, ultra-high conductivity, diverse synthesis, and abundant surface groups, which make them potential electrode materials. Recently, Li et al. introduced mesoporous carbon onto MXenes and constructed an ordered mesoporous carbon/MXene heterostructure (OMC-g-MXene).66 It serves as a catalyst in Li–S batteries, which can improve the conversion efficiency of sulfur/sulfide and the electrochemical reaction rate.
As shown in Fig. 15(a) and (b), the peak current and area corresponding to the OMC-g-MXene electrode are larger than those corresponding to the other two electrodes, and their precipitation capacity is also higher, indicating that more sulfides are converted in the OMC-g-MXene battery. As shown in Fig. 15(c), the polarization voltage of the OMC-g-MXene battery is the smallest and the peak current is the highest, indicating that it has the highest utilization rate of S. Fig. 15(d) shows that OMC-g-MXene has the highest Li+ diffusion coefficient, while Fig. 15(e) and (f) show that the OMC-g-MXene battery has the fastest ion diffusion performance and electrochemical performance. In summary, the OMC-g-MXene heterostructure can accelerate the diffusion kinetics of Li+, thereby improving the efficiency of Li–S batteries, and the heterostructure constructed by Li et al. plays an excellent role in battery catalysis.
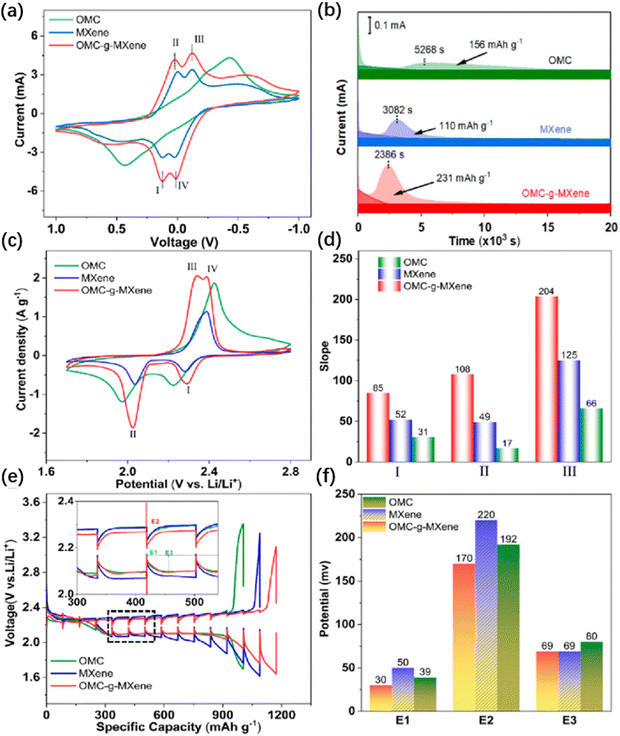 | ||
| Fig. 15 Comparison of the electrochemical performance of different electrode materials in Li–S batteries. (a) CV curves, (b) potential static discharge profiles at 2.05 V, (c) CV profiles of the Li–S cells with catalytic layer, (d) the slope summary of ip vs. the square root of scan rates in different peaks, (e) the GITT of Li–S batteries with the three interlayers at 0.1C, and (f) the corresponding excessive calculated from GITT technology66 Reproduced from ref. 66 with permission from American Chemical Society, copyright 2023. | ||
4.2 MXene enhances hydrogen storage performance
Hydrogen storage involves storing hydrogen gas in specific containers or materials for energy supply and use. Hydrogen storage technologies are crucial for achieving clean energy and sustainable development. MgH2 is an important hydrogen storage material with a high storage capacity. Each gram of MgH2 can release about 7.6 wt% of hydrogen, offering a high theoretical hydrogen storage density. However, MgH2 has slow hydrogen absorption and desorption reactions, requiring high temperatures (typically around 200–300 °C) and long hydrogenation or dehydrogenation times. To improve its hydrogen storage performance, a common approach is to lower the hydrogen absorption and desorption temperatures by adding catalysts or alloying. Additionally, methods such as nanosizing and surface modification have been applied to enhance the hydrogen absorption/desorption rates and cycling stability of MgH2. Despite the challenges in the kinetics and thermodynamics of MgH2 hydrogen storage reactions, it still holds great potential as a hydrogen storage material, especially in the field of hydrogen storage for renewable and sustainable energy systems. Therefore, there is a pressing need to improve the hydrogen storage properties of MgH2. MXene materials possess unique structures and physical properties that make them ideal for hydrogen storage applications. The layered structure of MXene provides a large surface area for hydrogen molecule adsorption and storage, and its high specific surface area increases the density of active adsorption sites, enhancing hydrogen storage capacity. Furthermore, MXene exhibits excellent conductivity, promoting hydrogen molecule adsorption and dissociation, thus accelerating the rate and efficiency of hydrogen storage reactions. The interlayer spacing of MXene materials can be tuned, and controlling the interlayer distance can improve the adsorption and diffusion properties of hydrogen molecules in the MXene layered structure, thereby enhancing the storage capacity and cycling stability of hydrogen storage materials. Additionally, MXene materials possess outstanding chemical stability, maintaining structural integrity in hydrogen storage environments, and avoiding strong reactions with hydrogen and other gases, ensuring long-term reliability and safety during the hydrogen storage process. Moreover, by chemical modification and structural control, the hydrogen storage performance of MXene can be further improved. For example, introducing functional groups or doping other elements can enhance the interaction between MXene and hydrogen molecules, thereby increasing the hydrogen storage capacity and adsorption capability. In conclusion, MXene materials possess unique advantages in hydrogen storage applications.In 2016, Liu et al. reported the excellent catalytic effect of Ti3C2 MXene on the hydrogen storage reaction of MgH2.22 As shown in Fig. 16, with the increase of Ti3C2 content, the onset temperature for rapid dehydrogenation significantly decreases. Pristine MgH2 starts rapid dehydrogenation at a temperature of 278 °C, while the MgH2 + 5 wt% composite material starts at 185 °C, and MgH2 + 7 wt% composite material starts at 180 °C. However, considering the weight of Ti3C2 itself, excessively high Ti3C2 content may reduce the hydrogen storage capacity. After comprehensive evaluation, the MgH2 + 5 wt% composite material demonstrates the best hydrogen storage performance. As shown in Fig. 16(b) and (c), the MgH2 + 5 wt% composite material releases about 5.0 wt% of hydrogen within 10 minutes at a temperature of 250 °C, while pristine MgH2 does not release hydrogen under the same conditions. This signifies that the addition of Ti3C2 optimizes the dehydrogenation capability of MgH2. Not only for dehydrogenation, but also for hydrogen absorption, as shown in Fig. 16(d)–(f), the addition of Ti3C2 significantly improves the hydrogen absorption efficiency of the composite material compared to pristine MgH2. This indicates that Ti3C2 enhances both the hydrogen absorption and dehydrogenation efficiency of MgH2, and the enhancement is reversible. In 2022, Lu et al. conducted similar work.23 They synthesized V2C MXene using the HF etching method, as shown in Fig. 17(a), and added V2C MXene to MgH2 hydrogen storage material. Similar to the results of Liu et al., Lu et al.'s experimental results also demonstrated a significant enhancement in MgH2 hydrogen storage performance with V2C MXene, as shown in Fig. 17(b) and (c).
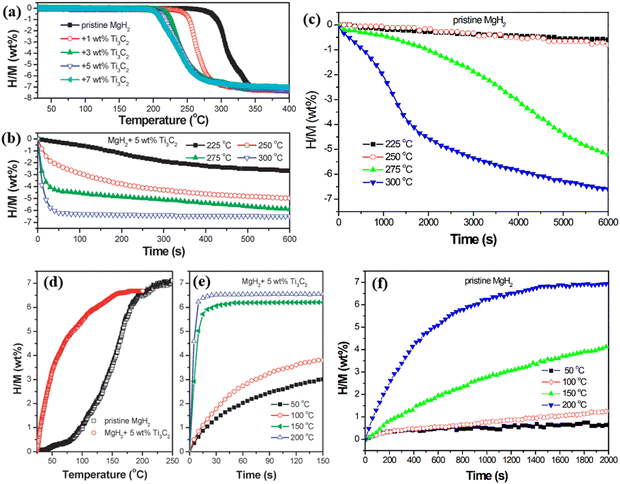 | ||
| Fig. 16 (a) Relationship between dehydrogenation content and temperature of MgH2 with different Ti3C2 doping concentrations. (b) Relationship between dehydrogenation content and time at different temperatures when the Ti3C2 content is 5 wt%. (c) Relationship between pristine MgH2 dehydrogenation content and time at different temperatures. (d) Relationship between hydrogen absorption content and temperature of MgH2 with different Ti3C2 doping concentrations. (e) Relationship between hydrogen absorption content and time at different temperatures when the Ti3C2 content is 5 wt%. (f) The relationship between hydrogen absorption content and time of pristine MgH2 at different temperatures.22 Reproduced from ref. 22 with permission from Royal Society of Chemistry, copyright 2023. | ||
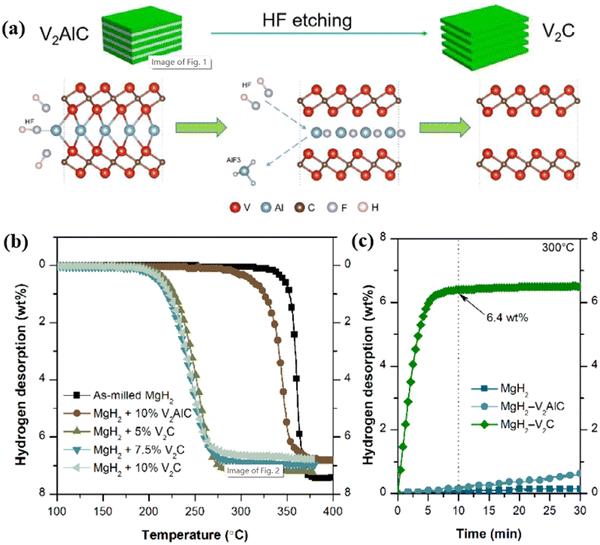 | ||
| Fig. 17 (a) A schematic diagram of HF etching V2AlC to prepare V2C. (b) Changes in the dehydrogenation content of MgH2 with different doping conditions with temperature, and (c) changes in the dehydrogenation content of MgH2, MgH2–V2AlC, and MgH2–V2C with time at 300 °C.23 Reproduced from ref. [23] with permission from Elsevier, copyright 2023. | ||
Building upon the previous two works (Mg–V2C and Mg–Ti3C2), Liu et al. in 2021 prepared V2C and Ti3C2 and introduced them together into MgH2 to adjust its hydrogen desorption/absorption performance.21 The results showed that the synthesized MgH2–V2C–Ti3C2 composite material exhibited better hydrogen storage performance compared to pure MgH2, as shown in Fig. 18(c)–(e). By adding 10 wt% of 2V2C/Ti3C2 into MgH2, the combination of V2C and Ti3C2 facilitated the hydrogen desorption process of MgH2, while Ti3C2 had a more significant impact on the hydrogen absorption process of MgH2 at room temperature compared to V2C. As shown in Fig. 18(a) and (b), Liu et al. proposed a possible mechanism for hydrogen release and absorption in the MgH2–V2C–Ti3C2 system: the combination of V2C and Ti3C2 mainly affected the dehydrogenation process, where hydrogen atoms or molecules may preferentially transfer through the MgH2/V2C/Ti3C2 triple junctions. In the absorption process, hydrogen atoms or molecules may preferentially transfer through the Mg/Ti3C2 interfaces. Microstructural analysis indicated that V2C and Ti3C2 acted as highly efficient catalysts for hydrogen absorption and desorption in MgH2.
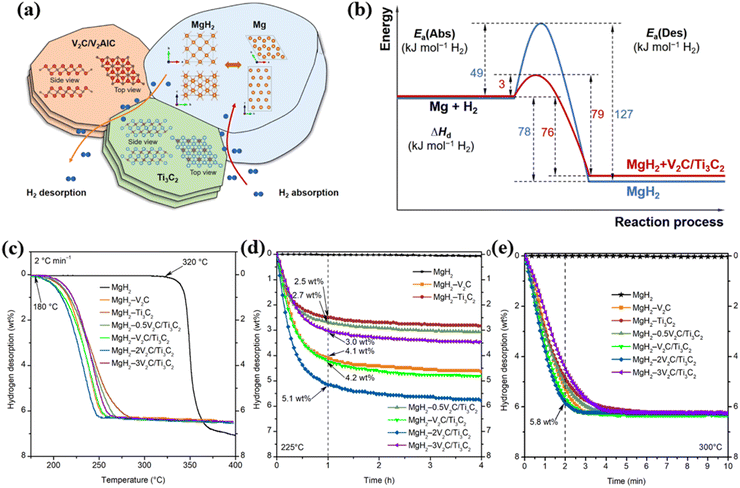 | ||
| Fig. 18 (a) Schematic diagram of the hydrogen absorption and dehydrogenation mechanism of MgH2 V2C/Ti3C2. (b) Energy barrier diagram of MgH2 and MgH2–2V2C/Ti3C2. (c) Temperature dependent curve of dehydrogenation content. (d) Time dependent curve of dehydrogenation content at 225 °C, and (e) time dependent curve of dehydrogenation content at 300 °C.21 Reproduced from ref. 21 with permission from American Chemical Society, copyright 2023. | ||
5. Conclusion and outlook
In summary, in this review, we discussed the energy storage characteristics of MXene, which is widely recognized for use in supercapacitors due to its large specific surface area, rich active sites, and diverse surface groups.MXene has diverse structures, with over a hundred reported species, but only a few widely used for research, with Ti3C2Tx being the most abundant. Therefore, although current experiments have shown that it has excellent physical properties, it is difficult to say that it is the best. MXene still has great potential. This is mainly because the synthesis technology of Ti3C2Tx is the most mature, but if we want to further develop MXene materials, we still need to develop more efficient synthesis technologies in the future to lay the foundation for exploring more MXene materials. Although first-principles calculations can calculate the properties of MXene of various components, current experimental research shows that different experimental methods can lead to different structural characteristics of MXene, and these structural changes are difficult to simulate at the theoretical calculation level. When the synthesis technology is mature enough to easily synthesize various MXenes, more MXenes can be used to study electrochemical and catalytic properties, which is beneficial for researchers to explore better MXenes.
For synthesis technology, the vast majority of current research is based on etching. Although the safety of using HCl and LiF instead of HF is greatly improved, etching methods are still inefficient and uneconomical. However, recently, direct synthesis of MXene has also been explored, and the commonly used CVD method in the synthesis of conventional 2D material is being attempted. The CVD method is economical and efficient in the synthesis of 2D materials, and at present, some other MXene materials have been successfully synthesized using the CVD method. In the future, experimental research on CVD synthesis of various MXene materials is needed to further understand their growth mechanisms and replace traditional etching methods with bottom-up synthesis methods as soon as possible.
An important characteristic of MXene is its easy oxidation, which seriously hinders its practical application. Currently, beneficial explorations have been made on how to slow down the oxidation rate of MXene. Many researchers have used annealing methods to improve the chemical stability of MXene. Annealing operations can change the composition of MXene body and surface groups, thereby achieving inertness to water or oxygen. From this perspective, perhaps DFT calculations can provide some useful attempts.
In short, the research on MXene is still in its early stages, and it is necessary to continue exploring it. The biggest breakthrough is the research on synthesis technology. When an efficient synthesis method is developed, the research on MXene will introduce a significant change in the field of materials science.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 11874407, 91436102 and 1374353), and the Fundamental Research Funds for the Central Universities (06500067).References
- K. Novoselov, A. Geim, S. Morozov, D. Jiang, Y. Zhang, S. Dubonos, I. Grigorieva and A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- X. Zhang and Y. Xie, Chem. Soc. Rev., 2013, 42, 8187–8199 RSC.
- C. Chung, Y. Kim, D. Shin, S. Ryoo, B. Hong and D. Min, Acc. Chem. Res., 2013, 46, 2211–2224 CrossRef CAS PubMed.
- Y. Wang, S. Li, H. Yang and J. Luo, RSC Adv., 2020, 10, 15328–15345 RSC.
- S. Tiwari, S. Sahoo, N. Wang and A. Huczko, J. Sci.: Adv. Mater. Devices, 2020, 5, 10–29 Search PubMed.
- H. Liu, Y. Du, Y. Deng and P. Ye, Chem. Soc. Rev., 2015, 44, 2732–2743 RSC.
- R. Gusmão, Z. Sofer and M. Pumera, Angew. Chem., 2017, 129, 8164 CrossRef.
- Y. Cao, Y. Feng, Y. Cheng, L. Meng and M. Sun, Appl. Phys. Lett., 2023, 122, 231601 CrossRef CAS.
- Y. Feng, F. Shao, L. Meng and M. Sun, ACS Appl. Nano Mater., 2023, 6, 14343–14352 CrossRef CAS.
- S. Manzeli, D. Ovchinnikov, D. Pasquier, O. Yazyev and A. Kis, Nat. Rev. Mater., 2017, 2, 17033 CrossRef CAS.
- J. Zhang, F. Xu, Y. Hong, Q. Xiong and J. Pan, RSC Adv., 2015, 5, 89415–89426 RSC.
- X. Xu, Y. Zhang, H. Sun, J. Zhou, F. Yang, H. Li, H. Chen, Y. Chen, Z. Liu, Z. Qiu, D. Wang, L. Ma, J. Wang, Q. Zeng and Z. Peng, Adv. Electron. Mater., 2021, 7, 2000967 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- R. Pandey, K. Rasool, V. Madhavan, B. Aissa, Y. Gogotsi and K. Mahmoud, J. Mater. Chem. A, 2018, 6, 3522–3533 RSC.
- Y. Ma, Y. Cheng, J. Wang, S. Fu, M. Zhou, Y. Yang, B. Li, X. Zhang and C. Nan, InfoMat, 2022, 4, e12328 CrossRef CAS.
- H. Shin, W. Eom, K. Lee, W. Jeong, D. Kang and T. Han, ACS Nano, 2021, 15, 3320–3329 CrossRef CAS PubMed.
- C. Zhou, X. Zhao, Y. Xiong, Y. Tang, X. Ma, Q. Tao, C. Sun and W. Xu, Eur. Polym. J., 2022, 167, 111063 CrossRef CAS.
- M. Khazaei, M. Arai, T. Sasaki, C.-Y. Chung, N. S. Venkataramanan, M. Estili, Y. Sakka and Y. Kawazoe, Adv. Funct. Mater., 2013, 23, 2185–2192 CrossRef CAS.
- M. Onoue, F. Ishii and T. Oguchi, J. Phys. Soc. Jpn., 2008, 77, 054706 CrossRef.
- K. Gong, K. Zhou, X. Qian, C. Shi and B. Yu, Composites, Part B, 2021, 217, 108867 CrossRef CAS.
- H. Liu, C. Lu, X. Wang, L. Xu, X. Huang, X. Wang, H. Ning, Z. Lan and J. Guo, ACS Appl. Mater. Interfaces, 2021, 13, 13235–13247 CrossRef CAS PubMed.
- Y. Liu, H. Du, X. Zhang, Y. Yang, M. Gao and H. Pan, Chem. Commun., 2016, 52, 705–708 RSC.
- C. Lu, H. Liu, L. Xu, H. Luo, S. He, X. Duan, X. Huang, X. Wang, Z. Lan and J. Guo, J. Magnesium Alloys, 2022, 10, 1051–1065 CrossRef CAS.
- C. Jin and Z. Bai, ACS Sens., 2022, 7, 929–950 CrossRef CAS PubMed.
- M. Zheng and S. Wu, Mater. Lett., 2022, 311, 131570 CrossRef CAS.
- S. Kumar, Y. Lei, N. Alshareef, M. Quevedo-Lopez and K. Salama, Biosens. Bioelectron., 2018, 121, 243–249 CrossRef CAS PubMed.
- Y. Wang, X. Wang, X. Li, Y. Bai, H. Xiao, Y. Liu and G. Yuan, Chem. Eng. J., 2021, 405, 126664 CrossRef CAS.
- B. Ahmed, D. Anjum, Y. Gogotsi and H. Alshareef, Nano Energy, 2017, 34, 249–256 CrossRef CAS.
- W. Song, J. Chen, Z. Li and X. Fang, Adv. Mater., 2021, 33, 2101059 CrossRef CAS PubMed.
- W. Wang, H. Feng, J. Liu, M. Zhang, S. Liu, C. Feng and S. Chen, Chem. Eng. J., 2020, 386, 124116 CrossRef CAS.
- S. Wan, X. Li, Y. Chen, N. Liu, Y. Du, S. Dou, L. Jiang and Q. Cheng, Science, 2021, 374, 96–99 CrossRef CAS PubMed.
- C. Zhou, X. Zhao, Y. Xiong, Y. Tang, X. Ma, Q. Tao, C. Sun and W. Xu, Eur. Polym. J., 2022, 167, 111063 CrossRef CAS.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. Barsoum, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS PubMed.
- J. Xuan, Z. Wang, Y. Chen, D. Liang, L. Cheng, X. Yang, Z. Liu, R. Ma, T. Sasaki and F. Geng, Angew. Chem., Int. Ed., 2016, 55, 14569 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- Q. Wu, Y. Wang, P. Li, S. Chen and F. Wu, Dalton Trans., 2022, 51, 3263 RSC.
- Q. Wu, Y. Xue, P. Li, Y. Wang and F. Wu, Appl. Surf. Sci., 2023, 609, 155329 CrossRef CAS.
- X. Huang and P. Wu, Adv. Funct. Mater., 2020, 30, 1910048 CrossRef CAS.
- D. Wang, C. Zhou, A. Filatov, W. Cho, F. Lagunas, M. Wang, S. Vaikuntanathan, C. Liu, R. Klie and D. Talapin, Science, 2023, 379, 1242–1247 CrossRef CAS PubMed.
- C. Xu, L. Wang, Z. Liu, L. Chen, J. Guo, N. Kang, X. Ma, H. Cheng and W. Ren, Nat. Mater., 2015, 14, 1135–1141 CrossRef CAS PubMed.
- R. Thakur, A. VahidMohammadi, J. Moncada, W. R. Adams, M. Chi, B. Tatarchuk, M. Beidaghi and C. A. Carrero, Nanoscale, 2019, 11, 10716–10726 RSC.
- S. Huang and V. N. Mochalin, Inorg. Chem., 2019, 58, 1958–1966 CrossRef CAS PubMed.
- Y. Lee, S. J. Kim, Y. Kim, Y. Lim, Y. Chae, B. Lee, Y. Kim, H. Han, Y. Gogotsi and C. W. Ahn, J. Mater. Chem. A, 2020, 8, 573 RSC.
- X. Yang, Q. Wang, K. Zhu, K. Ye, C. Wang, D. Cao and J. Yan, Adv. Funct. Mater., 2021, 31, 2101087 CrossRef CAS.
- Q. Wu, P. Li, Y. Wang and F. Wu, Appl. Surf. Sci., 2022, 593, 153380 CrossRef CAS.
- Y. Chae, S. J. Kim, S.-Y. Cho, J. Choi, K. Maleski, B.-J. Lee, H.-T. Jung, Y. Gogotsi, Y. Lee and C. W. Ahn, Nanoscale, 2019, 11, 8387–8393 RSC.
- Y. Dong, S. Zheng, J. Qin, X. Zhao, H. Shi, X. Wang, J. Chen and Z. Wu, ACS Nano, 2018, 12, 2381–2388 CrossRef CAS PubMed.
- G. Liu, J. Zou, Q. Tang, X. Yang, Y. Zhang, Q. Zhang, W. Huang, P. Chen, J. Shao and X. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 40077–40086 CrossRef CAS PubMed.
- A. Feng, Y. Yu, F. Jiang, Y. Wang, L. Mi, Y. Yu and L. Song, Ceram. Int., 2017, 43, 6322–6328 CrossRef CAS.
- W. Eom, H. Shin, W. Jeong, R. B. Ambade, H. Lee and T. H. Han, Mater. Horiz., 2023, 10, 4892 RSC.
- T. Habib, X. Zhao, S. A. Shah, Y. Chen, W. Sun, H. An, J. L. Lutkenhaus, M. Radovic and M. J. Green, npj 2D Mater. Appl., 2019, 3, 8 CrossRef.
- C. J. Zhang, S. Pinilla, N. McEvoy, C. P. Cullen, B. Anasori, E. Long, S.-H. Park, A. Seral-Ascaso, A. Shmeliov, D. Krishnan, C. Morant, X. Liu, G. S. Duesberg, Y. Gogotsi and V. Nicolosi, Chem. Mater., 2017, 29, 4848–4856 CrossRef CAS.
- M. Hu, H. Zhang, T. Hu, B. Fan, X. Wang and Z. Li, Chem. Soc. Rev., 2020, 49, 6666 RSC.
- A. VahidMohammadi, J. Rosen and Y. Gogotsi, Science, 2021, 372, 1165 CrossRef PubMed.
- H. Wang, Y. Wu, X. Yuan, G. Zeng, J. Zhou, X. Wang and J. W. Chew, Adv. Mater., 2018, 30, 1704561 CrossRef PubMed.
- Z. Wu, Y. Deng, J. Yu, J. Han, T. Shang, D. Chen, N. Wang, S. Gu, W. Lv, F. Kang, Y. Tao and Q. Yang, Adv. Mater., 2023, 35, 2300580 CrossRef CAS PubMed.
- M. R. Lukatskaya, S. Kota, Z. Lin, M.-Q. Zhao, N. Shpigel, M. D. Levi, J. Halim, P.-L. Taberna, M. W. Barsoum, P. Simon and Y. Gogotsi, Nat. Energy, 2017, 2, 17105 CrossRef CAS.
- Y. Xia, T. S. Mathis, M. Q. Zhao, B. Anasori, A. Dang, Z. Zhou, H. Cho, Y. Gogotsi and S. Yang, Nature, 2018, 557, 409 CrossRef CAS PubMed.
- T. Shang, Z. Lin, C. Qi, X. Liu, P. Li, Y. Tao, Z. Wu, D. Li, P. Simon and Q. H. Yang, Adv. Funct. Mater., 2019, 29, 1903960 CrossRef.
- Y. Deng, T. Shang, Z. Wu, Y. Tao, C. Luo, J. Liang, D. Han, R. Lyu, C. Qi, W. Lv, F. Kang and Q. H. Yang, Adv. Mater., 2019, 31, 1902432 CrossRef CAS PubMed.
- J. Kong, H. Yang, X. Guo, S. Yang, Z. Huang, X. Lu, Z. Bo, J. Yan, K. Cen and K. K. Ostrikov, ACS Energy Lett., 2020, 5, 2266 CrossRef CAS.
- J. Tang, T. Mathis, X. Zhong, X. Xiao, H. Wang, M. Anayee, F. Pan, B. Xu and Y. Gogotsi, Adv. Energy Mater., 2020, 11, 2003025 CrossRef.
- H. Chen, H. Wang and C. Li, Adv. Mater., 2022, 34, 2205723 CrossRef CAS PubMed.
- Q. Wu, Y. Xue, S. Chao, F. Wu, M. S. Javed, L. Li and W. Zhang, Nano Res., 2023, 16, 5006–5017 CrossRef CAS.
- Q. Wu, Y. Xue, S. Chao, F. Wu, L. Li, M. S. Javed and W. Zhang, ACS Appl. Nano Mater., 2023, 6, 677–684 CrossRef CAS.
- X. Li, Q. Guan, Z. Zhuang, Y. Zhang, Y. Lin, J. Wang, C. Shen, H. Lin, Y. Wang, L. Zhan and L. Ling, ACS Nano, 2023, 17(2), 1653–1662 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
